Abstract
Introduction
Levels of lipoprotein (LP) (a) are useful marker for risk stratification of cardiovascular disease. This genetic biomarker is suggestive of patient predisposition to acute coronary event. The present study was to study correlation of LP(a) levels and plaque morphology in very young patients (<35 years) with acute coronary syndrome (ACS).
Methods
A prospective, single-center, observational study consisting of very young patients with ACS and fit for optical coherence tomography (OCT) guided invasive coronary angiography was conducted at tertiary-care centre. LP(a) levels were compared between healthy controls and very young ACS patients. Correlation of LP(a) levels and plaque characteristics in very young ACS patients was done using OCT imaging.
Results
Out of enrolled 80 subjects, 40 were very young ACS and 40 were matched healthy controls. In very young patients, plaque rupture and erosion were mechanism of ACS in 67.5% and 32.5% patients, respectively. Mean levels of LP(a) were 28.10 ± 13.96 nmol/l in healthy controls and 47.19 ± 29.85 nmol/l in very young patients with ACS (p = 0.022). Among very young ACS patients, patients with LP(a) levels<75 nmol/l and ≥75 nmol/l had mean thin cap fibroatheroma thickness of 117.08 ± 52.542 μm and 95.00 ± 36.286 μm, respectively (p = 0.2355).
Conclusion
Higher levels of LP(a) were seen in younger patients with ACS compared with matched healthy individuals. Plaque rupture was the commonest mechanism of ACS in very young ACS patients. Patients with high LP(a) levels had lesser thickness of fibrous cap in OCT imaging compared with low levels of LP(a).
Keywords: Acute coronary syndrome, Coronary artery disease, Lipoprotein (a), Optical coherence tomography, Plaque
1. Introduction
Acute coronary syndrome (ACS) continues to be a significant cause of cardiac mortality and morbidity around the world. This clinical condition may be triggered by rupture or erosions of atherosclerotic plaques or calcified nodules. Plaque rupture, the most common cause of acute luminal coronary thrombosis, is characterized by highly thrombogenic, red cell–rich necrotic core material with an overlying thin disrupted fibrous cap.1 Thin-cap fibroatheroma (TCFA), defined as fibroatheroma less than 65 μm, is a precursor lesion of plaque rupture.2 Although TCFA is synonymous with the concept of a “vulnerable plaque” in angiographic and autopsy studies, the plaque progression mechanism is complex, and may involve both local and systemic factors. Vulnerable plaques are those with a large, thin inflamed fibrous cap encasing a very large lipid core and densely infiltrated by macrophages and lymphocytes. However, only a small percentage of these vulnerable plaques progress to rupture. The pathophysiology and morphological characteristics of TCFA that progress to rupture are not clearly understood.3
Optical coherence tomography (OCT) is an intravascular imaging modality that uses near-infrared light to construct high resolution intracoronary images. High resolution (10–20 μm) of this imaging modality enables detailed assessment of the histologic hallmarks of culprit atherosclerotic plaque morphology as well as total cholesterol burden in the setting of ACS patients. OCT findings for target lesion and TCFA show a poor correlation with clinical presentation of coronary syndromes, specifically so in patients with acute myocardial events when compared to those with stable angina.4,5 Lesion plaque burden has been well correlated with acute events. In this study, we intend to correlate, plaque morphology rather than plaque burden for acute events. Thus, greater understanding of plaque morphology can guide an appropriate patient-specific preventive and therapeutic approach in ACS.
Cardiovascular risk factors are equally important for the development of atherosclerotic lesions both the young and elderly populations. Varying prevalence of these risk factors among young patients has drawn attention to other more frequently prevalent risk factors such as cholesterol ester transfer protein (CETP) gene, hepatic lipase gene, lipoprotein lipase gene, apo A1 gene, apo E gene and apo B gene.6 There is evidence in literature for increased serum LP(a) levels in young patients with coronary artery disease.7, 8, 9 LP(a) is reported to be an independent risk factor for ACS in young individuals in whom the higher levels of LP(a) show a three-fold increase the risk of ACS.10 In long term follow up of INTERHEART study, it was found that the higher Lp(a) concentrations were associated with an increased risk of MI and carried an especially high population burden in South Asians and Latin Americans.11 India has the highest burden of ACS in the world11 and an earlier onset of ACS is reported in Asian Indians.6 Hence, against this background this study assessed the correlation of LP(a)levels and plaque morphology in very young(<35 years) Indian patients with ACS.12 LP(a)high levels have been correlated to increased thrombogenicity. Thus, it looks wise to look for any evidence of changes in coronary atheroma due to elevated levels of LP(a), which might lead to more thrombogenicity or vulnerability for plaque rupture.
2. Materials and methods
2.1. Study design
This prospective, observational, single-center study was conducted in a tertiary care centre. The study was conducted according to the Declaration of Helsinki and the study protocol was approved by the independent ethics committee prior to study commencement (study registration number: ECR/262/Inst/UP/2013/RR-16). All patients provided written informed consent prior to enrolment in the study.
2.2. Patient population
In our study, we included two groups: i) very young (<35 years) ACS patients and (ii) age-matched healthy controls. The baseline demographic and clinical characteristics are shown in Table 1. The diagnosis of ACS was established if the patients met one or more of the following criteria: (i) acute cardiac chest pain or angina equivalent consistent with moderate to high-risk unstable angina or myocardial infarction, lasting more than 10 min duration 72 h before invasive examination and (ii) evidence of ACS requiring catheterization documented by elevated enzymes (CK-MB or increase or decrease in high-sensitivity Troponin I/T > 99th percentile) and/or (iii) ECG with ST-depression >1 mm in ≥2 contiguous leads after the J-point and/or transient ST-elevation >1 mm in ≥2 contiguous leads lasting <30 min or ST-elevation acute coronary syndrome with onset <24 h previously and chest pain >30 min, ST-elevation >1 mm in ≥2 contiguous leads or new left bundle block. Only those patients fit for invasive coronary angiography as per pre-catheterization protocol of the institute were included in the study.
Table 1.
The baseline characteristics of study ACS group with ≤35 years.
| Clinical characteristics | N% | |
|---|---|---|
| Diabetes mellitus | 8 (18.6) | |
| Hypertension | 2 (4.7) | |
| Previous history of CAD | 1 (2.3) | |
| SM | 21 (48.8) | |
| TC | 21 (48.8) | |
| Family history |
6 (14.0) |
|
| Lipid profile | Mean ± SD | |
| LDL-C | 81.63 ± 38.86 | |
| HDL-C | 37.80 ± 8.31 | |
| TG | 150.73 ± 52.27 | |
| Lp(a) | 61.20 ± 55.88 | |
| LDL-C: low density lipoprotein; HDL-C: high density lipoprotein; Lp(a): lipoprotein | ||
| Number of Vessels Involved | N% | |
| Single vessel disease | 33 (82.5) | |
| Triple vessel disease | 4 (10) | |
| Double vessel disease | 3 (7.5) | |
Patients with familial dyslipidaemia; congenital heart disease, valvular heart disease; cardiomyopathies; clinically significant liver or renal dysfunction or other significant systemic disease; human immunodeficiency virus infection (HIV); hepatitis B surface antigen (HBS); hepatitis C virus (HCV); tuberculosis; malignancy; refractory ventricular arrhythmia requiring pharmacologic or defibrillator therapy; active infection; acute psychotic disease; or cardiogenic shock or heart failure requiring intubation, inotropes, diuretics or mechanical circulation support were excluded from the study.
2.3. Study assessments
LP(a) levels were measured, and OCT was performed for the study duration. LP(a) levels were compared between healthy controls and very young ACS patients. LP(a)was measured once after admission through National Accreditation Board for Hospitals & Healthcare Providers accredited lab. It was measured by ELISA technique using monoclonal antibodies based on immunoturbidimetric principle against apo-a moiety of Lp(a) in fasting state because of its falsely elevated level after meals.13
OCT imaging: OCT images were obtained with a frequency-domain OCT system and the Dragon Fly catheter (Ilumien Optis, St. Jude Medical, St. Paul, Minnesota, USA). While clearing the blood from the culprit artery by contrast injection, the automated pullback was performed at a speed of 20 mm/s. Plaque rupture was diagnosed as presence of rupture or discontinuity in the fibrous cap. Plaque erosion was diagnosed as presence of an intact fibrous cap with attached thrombus, irregularity of the lumen of the culprit lesion in absence of thrombus, or lesions with underlying plaque attenuated by thrombus. Lesions such as bridging in the left anterior descending coronary artery or intimal dissection which did not meet any of the above criteria were categorized as others. Fibrous plaque was identified as a homogeneous plaque with high backscatter. Fibroatheromaous plaque was identified as low backscatter plaque with diffuse border and attenuation. TCFA was defined as plaque with lipid content with the thinnest part of the fibrous cap measuring <65 μm.
2.4. Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables as counts and percentages. Categorical variables were compared with the chi-square square test or Fisher's exact test. A p-value <0.05 was considered statistically significant. Data was analysed with SPSS software version 19.0 (SPSS. Inc., Chicago, IL, USA.).
3. Results
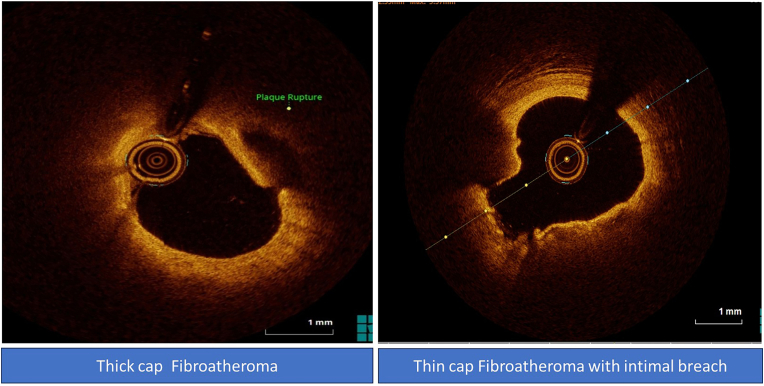
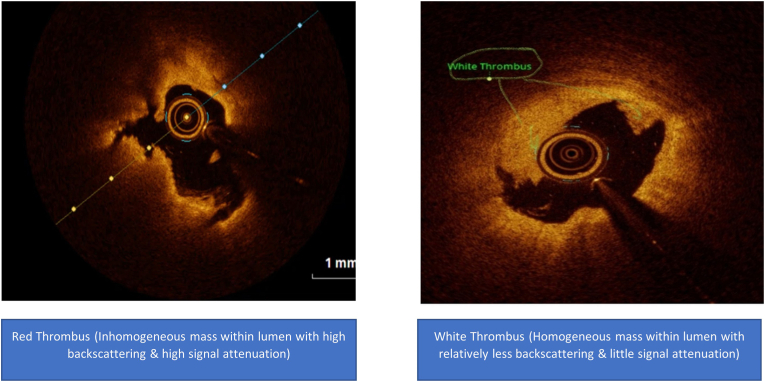
A total of 80 subjects were enrolled, 40 patients were very young ACS and 40 were age matched healthy subjects. In very young ACS patients, the mechanism of ACS was plaque rupture in 27 (67.5%) patients and plaque erosion in 13 (32.5%) patients. Nine (29.0%) patients had TCFA whilst 22 (71.0%) patients had thick cap fibroatheroma (Fig. 1). Red, white, and red and white thrombus was observed in 14 (45.2%), 7 (22.6%), and 10 (32.3%) patients respectively(Fig. 2) (Table 2).
Fig. 1.
OCT images of thin and thick fibroatheroma.
Fig. 2.
OCT images of red and white thrombus.
Table 2.
OCT characteristics.
| N | |
|---|---|
| Plaque rupture | 25 |
| Plaque erosion | 12 |
| Myocardial Bridging | 0 |
| Intimal Dissection |
0 |
| N % | |
| Fibroatheromaous | 34 (85) |
| Fibrous | 4 (10) |
| Intimal Flap with Intra Mural Hematoma | 1 (2.50) |
| Bridging |
1 (2.50) |
| N % | |
| Thin cap fibro atheroma | 9 (26.47) |
| Thick cap fibro atheroma |
25 (73.53) |
| Thrombus | N% |
| Red | 14 (45.16) |
| White | 7 (22.58) |
| Red & White | 10 (32.26) |
3.1. Lipoprotein a levels
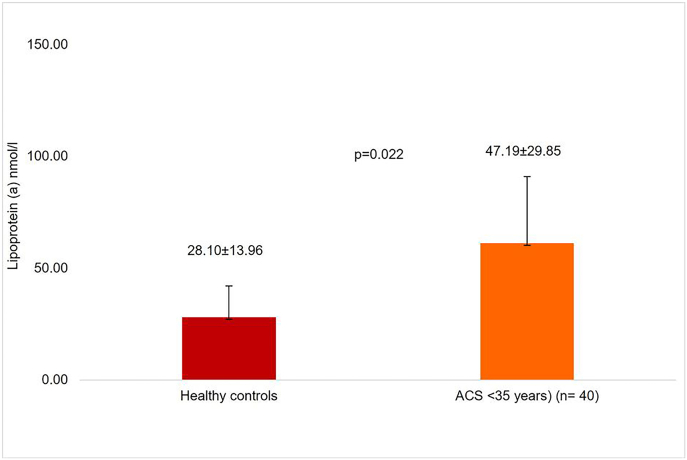
Mean LP(a) levels were 28.10 ± 13.96 nmol/l for healthy controls, and 47.19 ± 29.85 nmol/l for very young (<35 years) ACS patients (p = 0.022) as illustrated in Fig. 3.
Fig. 3.
Lipoprotein (a) levels in healthy controls and very young (<35 years) acute coronary syndrome patients.
3.2. Lipoprotein levels and plaque thickness
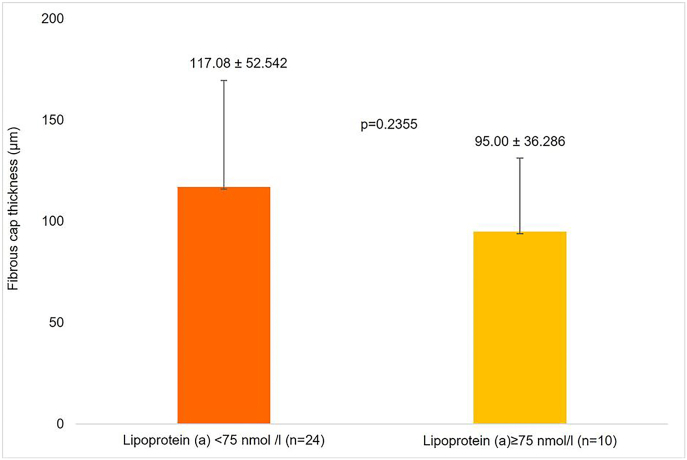
Study population having a fibroatheromaous plaque (n = 34), those with LP(a) levels <75 nmol/l had a mean fibrous cap thickness of 117.08 ± 52.542 μm and those with LP(a) levels ≥75 nmol/l had a mean fibrous cap thickness of 95.00 ± 36.286 μm (p = 0.2355) (Fig. 4).
Fig. 4.
Comparison of fibrous cap thickness atheroma in very young (<35 years) acute coronary syndrome patients with lipoprotein (a) < 75 and ≥ 75 nmol/l.
Six ACS patients had TCFA with lipoprotein (a) < 75 nmol/l, whilst three ACS patients had TCFA with lipoprotein (a) ≥75 nmol/l (Table 3). Correlation of various LP(a) levels was done with OCT plaque characteristics (Table 4).
Table 3.
Thin cap fibro atheroma (TCFA) among total fibroatheroma (n = 34) in very young (<35 years) acute coronary syndrome patients with lipoprotein (a) < 75 and ≥ 75 nmol/l.
| TCFA |
Total | ||
|---|---|---|---|
| Yes | No | ||
| LP(a) < 75 nmol/l | 6 | 18 | 24 |
| LP(a) ≥75 nmol/l | 3 | 7 | 10 |
| Total | 9 | 25 | 34 |
Lp(a): lipoprotein.
p value = 0.7633.
Table 4.
Correlation of Lipoprotein (a) levels with Plaque Rupture, Erosion, and Thrombosis, Intimal dissection.
| Karl-Pearson's correlation coefficient | p-Value | |
|---|---|---|
| Lipoprotein (a) with plaque rupture | −0.046 | 0.779 |
| Lipoprotein (a) with plaque erosion | 0.110 | 0.500 |
| Lipoprotein (a) with calcified nodule | – | – |
| Lipoprotein (a) with dissection | – | – |
| Lipoprotein (a) with thrombus | −0.05 | 0.757 |
| Lipoprotein (a) with cholesterol crystals | 0.125 | 0.440 |
| Lipoprotein (a) with thin cap fibro atheroma (TCFA) | 0.282 | 0.124 |
| Lipoprotein (a) with MLA | −0.008 | 0.962 |
TCFA: Thin-cap fibroatheroma.
3.3. Case 1
A typical case of present study, 35 year diabetic young male, presented with chest pain for 3 h. ECG showed QS with biphasic T wave in precordial leads (V1–V3). Troponin was raised and there was LAD territory hypokinesia on echocardiography. Diagnosis of AWMI was made. Coronary angiography was done, which showed proximal LAD 50%. His LP(a) was 118 nmol/l, OCT imaging showed plaque erosion with overlying white thrombus shown in Fig. 2.
4. Discussion
LP(a) plays a key role as a strong genetic risk factor for premature coronary artery disease and elevated levels are reported in young Indians with malignant coronary artery disease.14 Several earlier studies conducted in Indian populations have corroborated this finding.9,15, 16, 17, 18, 19 Hanif et al20 revealed 29.69 ± 23.50 mg/dl, 43.92 ± 32.69 mg/dl, and 50.15 ± 55.62 mg/dl for healthy controls, elder ACS patients, and young ACS patients, respectively. In line with these findings, Bhattacharjee et al21 reported LP(a) > 30 mg/dl in 41.3% young ACS patients as compared to 22.2% elder ACS patients. Further, Yusuf et al22 have also reported increased LP(a) levels to be associated with increased risk of ACS. A similar trend was observed in our study in ACS patients with age, sex-matched, and apparently healthy controls. LP(a) levels were 28.10 ± 13.96 nmol/l, 61.20 ± 55.88 nmol/l for healthy controls and very young (<35 years) ACS patients (p = 0.022), respectively.
Plaque rupture is the most commonly reported cause for ACS. Thin capped fibroatheroma infiltrated by macrophages and lymphocytes along with scant smooth muscle cells overlying a large necrotic core are known precursors for plaque rupture. Elevated LP(a) levels are a predictor for plaque destabilization and rupture.23
In our study, plaque rupture was the underlying cause for ACS in 67.5% of younger patients. Contrary to our findings, Jia et al have reported erosions to be the commonest feature in younger patients with ACS.24
Elevated LP(a)levels are strongly associated with the development of vulnerable plaques with complex morphology that have a likelihood to rupture. Larger amounts of LP(a) are concentrated in the culprit lesions in patients with ACS than in patients with stable angina.23 We observed higher levels of LP(a) in younger patients when compared to the older patients with ACS (61.20 ± 55.88 nmol/l vs. 47.19 ± 29.85 nmol/l) (Fig. 3).
In our study, patients with LP(a) levels <75 nmol/l had a mean TCFA thickness of 117.08 ± 52.542 μm whilst patients with LP(a) levels ≥75 nmol/l had a mean TCFA thickness of 95.80 ± 36.28 μm (p = 0.235).
Numerically higher values of fibrous cap thickness were seen in patients with lower levels of LP(a). Currently, lipoprotein apheresis is the only reliable means of achieving a substantial reduction of plasma Lp(a); however, its use is limited by its high cost, low capacity and lack of accessibility. Injectable antisense oligonucleotides targeting hepatic LPA RNA have been shown to reduce apo(a) production and apo(a) assembly with apoB, leading to >90% reduction in Lp(a) particle concentrations.25 And this finding may be hypothesis generating that reducing the LP (a) levels by pharmacological means aggressively, might lead to increase fibrous cap thickness, thus stabilizing the plaque. Increase in cap thickness of plaque might lead to lesser vulnerability for plaque rupture, as shown in numerous studies of statin leading to plaque stabilization.26 In present study multiple correlation analysis of OCT images with LP (a) levels was carried out. Lp(a) levels were not significantly correlated to plaque erosion, dissection, TCFA, Cholesterol crystals, red or white thrombus.
LP(a), with its high degree of sequence identity with plasminogen, plays an important role in thrombogenesis.6,10 Our study could not find any significant association of high LP(a) levels with either red or white thrombus as perceived by intra coronary OCT imaging. Considering the thrombogenic potential of high LP(a) levels, authors anticipated more thrombus burden in subset of patient having high levels of LP(a), but no statistically significant difference was observed. This finding may be attributed to study being single centre and recruiting small number of subjects. But still there is a need of multicentre large studies to assess the more thrombogenic milieu in patients having high levels of LP(a).
Coronary artery disease that manifests at a younger age can have devastating consequences for an individual, the family, and society. A strategy involving prevention of cardiovascular disease long before its onset will be more cost-effective than providing interventions at a stage wherein the disease is well established. Along with the conventional assessment of lipids, LP(a) may serve as a marker for risk stratification of cardiovascular disease and in determining the propensity to have an ACS. This can guide the intensification of therapy in patients at high risk.
4.1. Limitations
The present study has a few limitations. Firstly, the study was a prospective, observational study conducted at a single centre with a small sample size. Thus, the findings of this study cannot be generalized. Secondly, patients with ACS undergoing percutaneous coronary intervention using OCT were enrolled, which can be interpreted as selection bias. Thirdly, LP(a)≥75 nmol/L was defined as a cut-off based on the ROC analysis to identify OCT-TCFA. However, its use as a discriminator of OCT-TCFA might be limited as the cut-off level of LP(a) is not very well defined in an Indian population. Extrapolation of cut-off to an Indian population has not been validated. In addition, the use of a different cut-offs might yield different results. Fourthly, the effect of pharmacological therapy before OCT assessment on the prevalence of vulnerable plaque features including OCT-TCFA could not be determined. The high percentage of statin and antiplatelet drugs prescription may obscure the difference in the presence of TCFA and other vulnerable plaque features among the groups. Finally, the long-term clinical impact of the present findings were not investigated. Future studies may investigate the significance of LP(a) levels for the risk stratification of cardiovascular disease and propensity to have an acute coronary event.
5. Conclusion
In the present study of very young ACS patients with age, sex matched and apparently healthy controls, it was revealed that LP(a) levels were independently associated with ACS in very young (<35 years) patients. Plaque rupture was the commonest mechanism of acute coronary syndrome in very young ACS. Patients with high LP(a) levels had lesser thickness of fibrous cap in OCT imaging compared with those having low levels of LP(a).
Sources of support/funding
This original article did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
No acknowledgement.
Contributor Information
Sharad Chandra, Email: sharadaiims@gmail.com.
Saurabh Nagar, Email: saurabhnagar@gmail.com.
Ayush Shukla, Email: ayushgeorgian@gmail.com.
Gaurav Chaudhary, Email: gauravchaudhary@kgmcindia.edu.
Akhil Sharma, Email: akhil.med@gmail.com.
Akshyaya Pradhan, Email: akshyaya33@gmail.com.
Monika Bhandari, Email: drmonikab@gmail.com.
Pravesh Vishwakarma, Email: praveshvishwakarma@kgmcindia.edu.
Rishi Sethi, Email: drrishisethi1@gmail.com.
Varun Shankar Narain, Email: vnarain@yahoo.com.
Sudhanshu Kumar Dwivedi, Email: dr_skdwivedi@rediffmail.com.
References
- 1.Bentzon J.F., Otsuka F., Virmani R., et al. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 2.Guo J., Tian F., Liu H.-b., et al. Thrombosis and morphology of plaque rupture using optical coherence tomography. Chin Med J. 2013;126(6):1092–1095. [PubMed] [Google Scholar]
- 3.Otsuka F., Yasuda S., Noguchi T., et al. Pathology of coronary atherosclerosis and thrombosis. Cardiovasc Diagn Ther. 2016;6(4):396–408. doi: 10.21037/cdt.2016.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regar E., van Soest G., Bruining N., et al. Optical coherence tomography in patients with acute coronary syndrome. Euro Interv. 2010;6:G154–G160. [PubMed] [Google Scholar]
- 5.Sinclair H., Bourantas C., Bagnall A., et al. OCT for the identification of vulnerable plaque in acute coronary syndrome. JACC Cardiovasc Imaging. 2015;8(2):198–209. doi: 10.1016/j.jcmg.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal A., Srivastava S., Velmurugan M. Newer perspectives of coronary artery disease in young. World J Cardiol. 2016;8(12):728–734. doi: 10.4330/wjc.v8.i12.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandkamp M., Funke H., Schulte H., et al. Lipoprotein (a) is an independent risk factor for myocardial infarction at a young age. Clin Chem. 1990;36(1):20–23. [PubMed] [Google Scholar]
- 8.Bostom A.G., Cupples L.A., Jenner J.L., et al. Elevated plasma lipoprotein (a) and coronary heart disease in men aged 55 years and younger: a prospective study. JAMA. 1996;276(7):544–548. doi: 10.1001/jama.1996.03540070040028. [DOI] [PubMed] [Google Scholar]
- 9.Gambhir J., Kaur H., Gambhir D., et al. Lipoprotein (a) as an independent risk factor for coronary artery disease in patients below 40 years of age. Indian Heart J. 2000;52(4):411–415. [PubMed] [Google Scholar]
- 10.Rallidis L.S., Pavlakis G., Foscolou A., et al. High levels of lipoprotein (a) and premature acute coronary syndrome. Atherosclerosis. 2018;269:29–34. doi: 10.1016/j.atherosclerosis.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Paré Guillaume, Çaku M.D. Artuela, Md Matthew McQueen M.D., PhD Sonia S., Anand M.D. PhD enas enas, MD robert clarke, MD michael B. Boffa, PhD marlys koschinsky, PhD xingyu wang, PhD salim Yusuf, MD, PhD on behalf of the INTERHEART investigators circulation. Lipoprotein(a) Levels and the Risk of Myocardial Infarction Among 7 Ethnic Groups. 2019;139:1472–1482. doi: 10.1161/CIRCULATIONAHA.118.034311. [DOI] [PubMed] [Google Scholar]
- 12.Xavier D., Pais P., Devereaux P., et al. Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet. 2008;371(9622):1435–1442. doi: 10.1016/S0140-6736(08)60623-6. [DOI] [PubMed] [Google Scholar]
- 13.Gaubatz J.W., Hoogeveen R.C., Hoffman A.S., et al. Isolation, quantitation, and characterization of a stable complex formed by Lp a binding to triglyceride-rich lipoproteins. J Lipid Res. 2001;42:2058–2068. [PubMed] [Google Scholar]
- 14.Enas E.A., Varkey B., Dharmarajan T., et al. Lipoprotein (a): an underrecognized genetic risk factor for malignant coronary artery disease in young Indians. Indian Heart J. 2019;71(3):184–198. doi: 10.1016/j.ihj.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luthra K., Vasisht S., Chhabra S., et al. LP (a) phenotypes and levels in angiographically proven coronary heart disease patients and controls. Indian J Clin Biochem. 1998;13(1):12–19. doi: 10.1007/BF02873437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasisht S., Gulati R., Srivastava L., et al. Apolipoprotein (a) polymorphism and its association with plasma lipoprotein (a) levels: a north Indian study. Indian Heart J. 2000;52(2):165–170. [PubMed] [Google Scholar]
- 17.Mohan V., Deepa R., Rema M. Lipoprotein-A and coronary artery disease in Indians. J Assoc Phys India. 1998;46(11):979–980. [PubMed] [Google Scholar]
- 18.Geethanjali F., Luthra K., Lingenhel A., et al. Analysis of the apo (a) size polymorphism in Asian Indian populations: association with Lp (a) concentration and coronary heart disease. Atherosclerosis. 2003;169(1):121–130. doi: 10.1016/s0021-9150(03)00143-6. [DOI] [PubMed] [Google Scholar]
- 19.Gupta R., Kastia S., Rastogi S., et al. Lipoprotein (a) in coronary heart disease: a case-control study. Indian Heart J. 2000;52(4):407–410. [PubMed] [Google Scholar]
- 20.Hanif S., Akhtar B., Afzal M.N. Serum Lipoprotein (a) levels in acute coronary syndrome; Comparison of younger and elderly patients with healthy controls. Pakistan J Med Sci. 2019;35(6):1718–1723. doi: 10.12669/pjms.35.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharjee P., Munshi S., Das G., et al. Clinico-investigative assessment and comparison of cardiovascular risk factors in young and elderly patients of acute coronary syndrome. Int J Adv Med. 2014;1(3):180–188. [Google Scholar]
- 22.Yusuf J., Yadav N., Mukhopadhyay S., et al. Relook at lipoprotein (A): independent risk factor of coronary artery disease in North Indian population. Insian Heart J. 2014;66(3):272–279. doi: 10.1016/j.ihj.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Virmani R., Burke A.P., Kolodgie F.D., et al. Pathology of the thin-cap fibroatheroma: a type of vulnerable plaque. J Intervent Cardiol. 2003;16(3):267–272. doi: 10.1034/j.1600-0854.2003.8042.x. [DOI] [PubMed] [Google Scholar]
- 24.Jia H., Abtahian F., Aguirre A.D., et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62(19):1748–1758. doi: 10.1016/j.jacc.2013.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viney N.J., van Capelleveen J.C., Geary R.S., et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
- 26.Ozaki Y., Garcia-Garcia H.M., Beyene S.S., et al. Effect of statin therapy on fibrous cap thickness in coronary plaque on optical coherence tomography―review and meta-analysis. Circ J. 2019;83(7):1480–1488. doi: 10.1253/circj.CJ-18-1376. [DOI] [PubMed] [Google Scholar]