Abstract
We have investigated the effect of the in vivo administration of recombinant transforming growth factor β (rTGF-β) on the pathogenic mechanisms involved in Salmonella typhimurium experimental infection in mice. The protective response elicited by macrophages was induced by rTGF-β1 by 2 days after experimental infection, as demonstrated by an increased NO production, while the humoral protective effect began with cytokine mRNA expression 2 days after the challenge and continued after 5 days with cytokine release and lymphocyte activation. We demonstrated that all mice who received rTGF-β1 survived 7 days after infection. The number of bacteria recovered in the spleens and in the livers of rTGF-β1-treated mice 2 and 5 days after infection was significantly smaller than that found in the same organs after phosphate-buffered saline (PBS) inoculation. Furthermore, 2 and 5 days after infection, splenic macrophages from rTGF-β1-treated mice showed a greater NO production than did those from PBS-treated mice. The effect of rTGF-β1 on S. typhimurium infection in mice was correlated with the expression of cell costimulatory CD28 molecules. Five days after S. typhimurium infection, the percentage of CD28+-expressing T cells in splenic lymphocytes from rTGF-β1-treated mice increased with respect to that from control mice. Gamma interferon (IFN-γ) mRNA was present in a greater amount in spleen cells from rTGF-β1-treated mice after 2 days, although the intensity of the band decreased 5 days after the challenge. A similar pattern was obtained with the mRNAs for interleukin-1α (IL-1α), IL-6, TGF-β, and inducible nitric oxide synthase, which showed greater expression in cells obtained from rTGF-β1-treated and S. typhimurium-infected mice 2 days after challenge. The treatment with rTGF-β1 induced an increase in IL-1α and IFN-γ release in the supernatant of splenocyte cultures 5 days after the experimental infection with S. typhimurium. Moreover, we demonstrated that 5 days after infection, the IFN-γ titer was significantly greater in the sera of rTGF-β-treated mice than in those of PBS-treated mice. Also, hsp60 showed greater expression 2 days after the challenge in splenocytes from rTGF-β1-treated mice. The role played by proinflammatory and immunoregulatory cytokines and by CD28 is discussed.
Transforming growth factor β (TGF-β) is a 24-kDa protein produced by different cell types, including B and T lymphocytes and activated macrophages (2, 23, 24), and is a multifunctional cytokine capable of a variety of immunological effects. These include suppression of lymphocyte responses to antigens and mitogens (24, 33), modulation of the production and effects of monocyte proinflammatory cytokines (11), and modulation of monocyte expression of surface immunoregulatory molecules such as HLA-DR determinants (14) and receptors for the Fc fragment of immunoglobulins (FcγRIII and CD16) (42). TGF-β inhibits both gamma interferon (IFN-γ) (19) and interleukin-2 (IL-2) expression in T-cell responses (15), although there are no studies showing inhibition of IL-2 expression in primary T cells (1, 40). TGF-β suppresses IL-2-dependent T-cell proliferation through inhibition of IL-2-dependent phosphorylation of proteins that are important in T-cell cycle progression (1). TGF-β plays an important role in the progression of infections (as demonstrated by several studies) such as those due to Mycobacterium avium (5), Staphylococcus aureus (26), Leishmania amazonensis (5), Leishmania brazilensis (4, 5), Trypanosoma cruzi (34), and Toxoplasma gondii (22). In recent studies, TGF-β was shown to play a beneficial role in acquired resistance against Listeria monocytogenes infections (28) and during Candida albicans infections (35). In experimental infection by Salmonella typhimurium in mice, endogenous cytokines play important roles in host resistance correlated to the development of Th1 and Th2 cell functions (21).
Since TGF-β is associated with both immunoregulation and control of macrophage activities, in this study we have investigated the effect of the in vivo administration of recombinant TGF-β1 (rTGF-β1) on some cellular and molecular mechanisms involved in the inflammatory and immune response to S. typhimurium experimental infection in mice. Even though the gastrointestinal tract is considered to be the natural route of infection by Salmonella spp., we used intraperitoneal (i.p.) challenge, since it is the most commonly used route in establishing an experimental infection.
MATERIALS AND METHODS
Mice.
BALB/c mice weighing 20 to 25 g were obtained from Nossan (Corezzana, Milan, Italy). These animals were maintained in a controlled room (20 ± 2°C with automatic 12-h cycles of lighting) and had free access to water. A group of 50 mice were each treated with 0.5 μg of rTGF-β1 (A. F. Schnetzdeller, Tübingen, Germany) per ml by i.p. inoculation. A control group of 50 mice were each inoculated with 0.01 M phosphate-buffered saline (PBS) (pH 7.4).
Microorganism.
The microorganism used was S. typhimurium 74 NCTC grown in nutrient broth (Difco Laboratories, Detroit, Mich.).
Experimental infection and CFU enumeration.
To establish the experimental infection, mice were inoculated i.p. with PBS or rTGF-β1 2 h before being infected with a sublethal dose of S. typhimurium (4 × 105 CFU/mouse). At 2 to 5 days after infection, a group of three mice were killed by cervical dislocation, their spleens and livers were aseptically removed and homogenized in 2 ml of PBS, and serial dilutions in sterile PBS were plated on nutrient agar. CFU were counted after an overnight incubation.
Protection experiments.
Protection against experimental infection was evaluated in two groups of 10 mice each. The control mice were injected i.p. with PBS 2 h before being infected with 1 50% lethal dose (LD50) of S. typhimurium 74 NCTC (8 × 105 CFU/mouse) that had been prepared from log-phase cultures, resuspended in sterile PBS, and administered i.p. The other 10 mice were treated i.p. with rTGF-β1 (0.5 μg/mouse) 2 h before infection with being infected with 1 LD50 of S. typhimurium. The survival of rTGF-β1-treated and untreated animals was observed during the next 7 days.
Cell preparation and culture.
Spleens were aseptically removed from the mice and minced, and the released cells were washed three times in RPMI 1640 (Labtek Labs., Eurobio, Paris, France). Erythrocytes were lysed with 0.17 M NH4Cl (9), and the splenic cells were washed twice with RPMI 1640. Mononuclear cells were isolated by centrifugation on an MSL (mileu de separations des lymphocytes; Labtek Labs., Eurobio) at 600 × g for 30 min at room temperature. The isolated cells were suspended in RPMI 1640 supplemented with 10% fetal calf serum and antibiotics and incubated for 1 h under 5% CO2 at 37°C in plastic culture flasks. The adherent cells were cultured overnight in RPMI 1640 with 10% fetal calf serum. Cell viability was evaluated by the trypan blue exclusion test. At least 96% of the cells thus obtained were monocytes as determined with a FACS analyzer (Becton Dickinson, Mountain View, Calif.) with monoclonal antibody CD14 (Boehringer, Mannheim, Germany). Nonadherent cells (lymphocytes) were harvested, washed, and resuspended at 3 × 106 cells/ml. Flow cytometry analysis of stained cells with monoclonal antibody CD3 (Boehringer) demonstrated that more than 94% of the isolated cells were lymphocytes.
Nitrite determination.
The nitrite concentration in 24-h culture supernatants obtained from macrophages (107 cells) isolated from mice which had received or not received rTGF-β1 2 h before experimental infection was measured by a standard Griess reaction and compared to that in supernatants obtained from macrophages of mice given PBS only (control). Briefly, 0.1 ml of supernatant was mixed with 0.1 ml of Griess reagent (0.5% sulfanilamide and 0.05% N-1-naphthylenedimine hydrochloride in 2.5% H3PO4) and incubated for 10 min at room temperature, and the absorbance of the mixture at 570 nm was read with a spectrophotometer. The data represent the means and standard deviations of triplicate determinations and are expressed as micromoles of NO2−/107 cells (10).
Cytokine release in vitro.
Splenic monocytes and lymphocytes (3 × 106 cells/ml) from rTGF-β1-treated mice were harvested at 2 and 5 days after infection with 4 × 105 cells of S. typhimurium and subjected to one cycle of in vitro restimulation with Escherichia coli O128:B12 lipopolysaccharide (Sigma Chemical Co., St. Louis, Mo.) (1 μg/ml). Control experiments were carried out with untreated mice. After 24 h, the cell viability was checked and the culture supernatants were collected and stored at −20°C until assayed for IL-1α and tumor necrosis factor alpha (TNF-α) production. The incubation time was prolonged to 48 h to determine IFN-γ release by splenic lymphocytes. All measurements were carried out with monoclonal antibodies. Cytokine production was measured by immunoenzymatic methods (Intertest Mouse IL-1α enzyme-linked immunosorbent assay [ELISA] kit, Intertest Mouse TNF-α ELISA kit, and Intertest Mouse IFN-γ ELISA kit [Genzyme, Cambridge, Mass.]).
IFN-γ and TNF-α release in vivo.
Mice were inoculated i.p. with 0.5 μg of rTGF-β1 per ml or with PBS 2 h before infection with S. typhimurium. IFN-γ and TNF-α titers were determined in pooled sera 2 and 5 days after infection by using immunoenzymatic methods (see above). Control experiments were carried out with PBS-injected and infected mice.
RNA isolation and cDNA preparation.
Mice were inoculated i.p. with 0.5 μg of rTGF-β1 or PBS 2 h before infection with S. typhimurium. Cells were recovered 2 and 5 days after infection, and total RNA was extracted from spleen cells (107) obtained from treated and untreated mice by the method of Chomczynski and Sacchi (12). The RNA pellet was resuspended in 75% ethanol, precipitated, vacuum dried, and dissolved in 15 μl of RNase-free water. A 1-μg portion of oligo(dT) (Promega Biotec, Madison, Wis.) was added to the suspension, and the mixture was heated at 65°C for 5 min. After being cooled on ice, the mixture was incubated for 2 h at 42°C with 14 μl of 20 mM dithiothreitol (Sigma Chemical Co.); 1 mM (each) dATP, dGTP, dCTP, and dTTP; 35 U of RNasin (Promega); and 525 U of Moloney murine leukemia virus reverse transcriptase (Promega) in reverse transcription buffer.
PCR procedure.
Primer pair sequences were designed on the basis of published gene sequences as indicated in Table 1. The primer sequences were complementary to sequences in the exons or spanner exon-exon junctions and thus were RNA specific. A 2-μl volume of cDNA prepared as described above was amplified in the presence of 500 nM (final concentration) 5′ and 3′ primers; 200 μM (each) dATP, dGTP, dCTP, dTTP; and 1.25 U of Taq DNA polymerase (Promega) in a final volume of 50 μl of Taq DNA polymerase 10× buffer (Promega). The PCR was performed in a Perkin-Elmer thermal cycler for 30 cycles as follows: 1 min of denaturation at 94°C, 2 min of annealing at 60°C, and 3 min of extension at 72°C. The reaction product was visualized by electrophoresis with 25 μl of the reaction mixture at 100 V in a 1.5% agarose gel containing ethidium bromide (1 μg/ml). The gels were then examined on a UV light box and photographed. A 1-μg portion of BglI- and HinfI-digested pBR328 DNA (Boehringer) was run in parallel as a molecular size marker (providing bands at 2,176, 1,766, 1,230, 1,033, 653, 517, 453, 394, 298, 234, 220, and 154 bp).
TABLE 1.
Primer sequences used for PCR-assisted mRNA amplification
| Target gene | Oligonucleotide sequence |
|---|---|
| TNF-α | 5′TTCTGTCTACTGAACTTCGGGGTGATCGGTCC3′ 5′GTATGAGATAGCAAATCGGCTGACGGTGTGGG3′ |
| IFN-γ | 5′TGCATCTTGGCTTTGCAGCTCTTCCTCATGGC3′ 5′TGGACCTGTGGGTTGTTGACCTCAAACTTGGC3′ |
| IL-1α | 5′AAGATGTCCAACTTCACCTTCAAGGAGAGCCG3′ 5′AGGTCGGTCTCACTACCTGTGATGAGTTTTGG3′ |
| IL-4 | 5′ATGGGTCTCAACCCCCAGCTAGT3′ 5′GCTCTTTAGGCTTTCCAGGAAGTC3′ |
| IL-10 | 5′CTGGAAGACCAAGGTGTCTAC3′ 5′GAGCTGCTGCAGGAATGATGA3′ |
| IL-6 | 5′ATGAAGTTCCTCTCTGCAAGAGACT3′ 5′CACTAGGTTTGCCGAGTAGATCTC3′ |
| TGF-β | 5′TGGACCGCAACAACGCCATCTATGAGAAAACC3′ 5′TGGAGCTGAAGCAATAGTTGGTATCCAGGGCT3′ |
| iNOS | 5′CCCTTCCGAAGTTTCTGGCAGCAGC3′ 5′GGCTGTCAGAGCCTCGTGGCTTTGG3′ |
| β-Actin | 5′GTGGGCCGCTCTAGGCACCAA3′ 5′CTCTTTGATGTCACGCACGATTTC3′ |
Cytofluorometric analysis of CD28 expression.
Single- and double-immunolabeling procedures were performed by standard techniques. Briefly, splenic lymphocytes (106 cells) were suspended in 100 μl of RPMI 1640. Double staining was performed by pairing 1 μg of fluorescein isothiocyanate-conjugated anti-CD3 with 1 μg of phycoerythrin-conjugated anti-CD28 monoclonal antibody (Pharmingen, San Diego, Calif.). After incubation at 0°C for 30 min, the cells were washed with RPMI 1640 and analyzed by flow cytometry (FACS IV; Becton Dickinson) (26).
Immunoblot analysis for hsp60.
Splenic lymphocytes were washed three times in 0.01 M PBS (pH 7.2). Cell pellets were suspended at 107 cells/ml in lysis buffer (50 mM Tris-HCl, Nonidet P-40, 1% sodium dodecyl sulfate [SDS], 1 μM leupeptin, 100 μM phenylmethylsulfonyl fluoride, 1 μM pepstatin A, 100 μM EDTA [pH 8.0]) for 30 min at 4°C. The insoluble debris were removed by centrifugation at 18,600 × g for 10 min at 4°C. The protein concentration was determined by the method of Lowry et al. (27). Protein extracts were resuspended in sample buffer (0.5 M Tris-HCl, 10% glycerol, 2% SDS, 2% 2-mercaptoethanol, 0.05% bromophenol blue) and boiled for 3 min at 100°C. The proteins were separated by SDS-polyacrylamide gel electrophoresis for 1 h at 20 mA. Proteins separated by SDS-polyacrylamide gel electrophoresis were transferred to nitrocellulose filters (Bio-Rad Laboratories, Hercules, Calif.) by electroblotting for 30 min to 1 h at 100 V with cooling. Labeled antigen bands were detected with an immunoblot assay kit (Bio-Rad Laboratories). The filters were blocked with 3% gelatin in Tris-buffered saline (TBS) (20 mM Tris, 500 mM NaCl [pH 7.5]), washed in TBS containing 0.05% Tween 20 (T-TBS), incubated with an anti-hsp60 monoclonal antibody (1:1,000) (Biotechnologies Corp., Stress-Gen, Victoria, Canada) for 1 h at room temperature, and then washed three times in T-TBS. The blots were incubated with a 1:3,000 dilution of peroxidase-labeled anti-mouse IgG for 1 h at room temperature and washed in two changes of T-TBS and then once for 5 min in TBS. The reaction was stopped by washing the nitrocellulose blots in water. Low-molecular-weight protein standards were used as a reference (Pharmacia Biotech, Freiburg, Germany). In all cases, nonspecific binding of antibodies to nitrocellulose was prevented by blocking of the membranes with 1% bovine serum albumin in PBS for 2 h at room temperature.
The monoclonal antibody used was anti-hsp60, referenced in the literature as clone LK-1, which is specific for an epitope located between amino acids 383 and 447 of the human hsp 60-kDa sequence. (28).
Statistics.
All experiments were carried out in triplicate; the results are expressed, unless otherwise indicated, as the mean ± standard deviation. Comparison between tests was made by Student’s t test, with statistical significance considered to be P < 0.01.
RESULTS
Effect of rTGF-β1 administration on survival of infected mice.
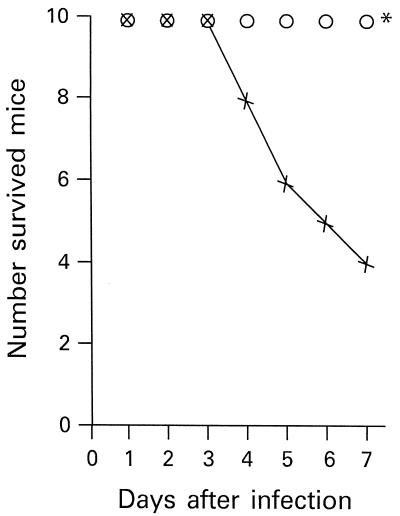
We evaluated the effect of rTGF-β1 on the survival of mice infected i.p. with 1 LD50 of S. typhimurium (8 × 105 cells). The results are shown in Fig. 1. Mice that had received 0.5 μg of rTGF-β1 showed an increase in survival after the experimental infection with S. typhimurium compared with control mice, which were injected with PBS. All mice that had received rTGF-β1 2 h before the S. typhimurium infection were still alive after 7 days. After the same time, only 4 of 10 PBS-treated mice had survived. All the mice that were alive 7 days after infection had survived after 30 days.
FIG. 1.
Effect of rTGF-β1 administration on the survival of mice infected with 1 LD50 of S. typhimurium (8 × 105 CFU/mouse). Ten mice were injected i.p. with PBS 2 h before infection with S. typhimurium (×), and 10 mice were injected i.p. with 0.5 μg of rTGF-β1 2 h before infection with S. typhimurium (○). ∗, P < 0.01 with respect to infected, nontreated mice.
Effect of rTGF-β1 administration on bacterial invasion of the host.
The effect of rTGF-β1 on host tissue bacterial invasion after a sublethal infection with S. typhimurium (4 × 105 cells) was evaluated. The results are reported in Table 2. Mice were treated i.p. with 0.5 μg of rTGF-β1 per ml or with PBS 2 h before infection. The numbers of bacterial cells in the spleens and in the livers were determined 2 and 5 days after S. typhimurium infection. The number of bacteria recovered in the spleens and the livers of rTGF-β1-treated mice 2 days after infection was significantly smaller than that found in the same organs of PBS-inoculated mice (P < 0.01). A similar result was observed 5 days after infection.
TABLE 2.
Effect of rTGF-β1 administration on host S. typhimurium invasion
| Treatmenta | Organ | Bacterial
count (log CFU/organ)b after:
|
|
|---|---|---|---|
| 2 days | 5 days | ||
| PBS only | Spleen | 8.8 ± 1.4 | 22.0 ± 5 |
| Liver | 9.3 ± 1.4 | 20.5 ± 4 | |
| rTGF-β1 | Spleen | 1.6 ± 0.4c | 15.0 ± 4c |
| Liver | 1.4 ± 0.3c | 13.0 ± 3c | |
Mice were injected i.p. with PBS or rTGF-β1 (0.5 μg/mouse) 2 h before infection with 4 × 105 CFU of S. typhimurium.
Each result is expressed as the mean ± standard deviation for a group of three mice.
Significantly different from the value for PBS-treated mice (P < 0.01).
NO production by macrophages in vitro.
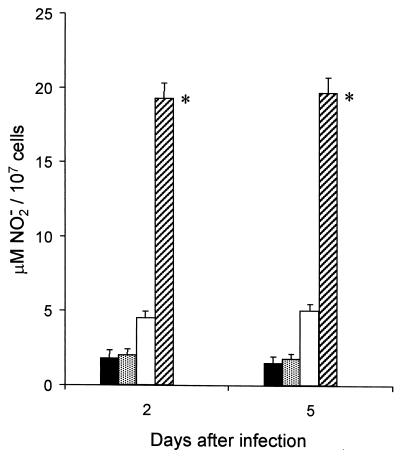
To verify whether mouse macrophages were able to kill S. typhimurium cells, we studied NO release by macrophages isolated from mice that had received or not received rTGF-β1 2 h before experimental infection. Splenic adherent macrophages from mice treated with PBS or with rTGF-β1 and then infected with a sublethal dose of S. typhimurium (4 × 105 cells) were compared for their relative ability to produce NO in vitro upon an overnight incubation in the absence of activating stimuli. The results are shown in Fig. 2. Two days after infection, splenic macrophages from rTGF-β1-treated mice showed NO production greater than that observed in macrophages from PBS-treated mice. In particular, macrophages from rTGF-β1-injected and infected mice were able to release NO at 18 ± 4 μM whereas macrophages from PBS-treated and infected mice produced NO at 4.8 ± 2 μM and macrophages from PBS-only-injected mice released NO at 2 ± 1 μM. Five days after infection, macrophages from rTGF-β1-treated mice also produced more NO (20 ± 4 μM) than did those from PBS-injected mice. No further increase in NO production was observed when cultures from rTGF-β1-treated and infected mice were treated with IFN-γ as the activating stimulus (data not shown).
FIG. 2.
Nitric oxide production by splenic macrophages from mice treated with PBS or 0.5 μg of rTGF-β1 2 h before sublethal infection with S. typhimurium (4 × 105 CFU/mouse). Symbols: ■, PBS-only-injected mice; , TGF-β1-only-injected mice; □, PBS-injected, infected mice; ▨, rTGF-β1-injected (0.5 μg), infected mice. Each result represents the mean of three determinations, and the bars indicate standard deviations. ∗, P < 0.01 with respect to PBS-injected, infected mice.
Effect of rTGF-β1 on CD28 expression in mice infected with S. typhimurium.
Costimulatory molecules are thought to be critical in eliciting cellular proliferation and cytokine production upon immunologic stimulation. We therefore attempted to determine if the effect of rTGF-β1 on sublethal S. typhimurium infection in mice was correlated with the expression of the T-cell costimulatory CD28 molecules. Single and double immunolabeling with anti-CD28 and anti-CD3 monoclonal antibodies revealed that the percentages of CD28-expressing T cells in splenic lymphocytes which were treated with rTGF-β1 and infected and those in control mice were similar 2 days after experimental infection (35% ± 6% and 33% ± 6%, respectively). Five days after S. typhimurium infection, the percentage of CD28+-expressing T cells in splenic lymphocytes from rTGF-β1-treated mice increased with respect to the percentage for control mice (P < 0.01). Furthermore, the percentage of CD3-expressing cells in splenic lymphocytes from rTGF-β1-injected mice increased significantly compared to that for PBS-treated mice (P < 0.01). The results are shown in Table 3.
TABLE 3.
Expression of CD28 in mice treated with rTGF-β1 and infected with S. typhimurium
| Treatmenta | CD28 expression
(%)b after:
|
|||
|---|---|---|---|---|
| 2
days
|
5 days
|
|||
| CD3+ | CD3+ CD28+ | CD3+ | CD3+ CD28+ | |
| PBS only | 38 ± 6 | 33 ± 6 | 25 ± 5 | 20 ± 5 |
| rTGF-β1 | 39 ± 6 | 35 ± 6 | 41c ± 6 | 31c ± 5 |
Mice were injected i.p. with PBS or rTGF-β (0.5 μg/mouse) 2 h before infection with 4 × 105 cells of S. typhimurium.
Each result is expressed as the mean ± standard deviation for a group of three mice.
Significantly different from the value for PBS-treated mice (P < 0.01).
Cytokine release in vitro from cells of infected and uninfected rTGF-β1-treated mice.
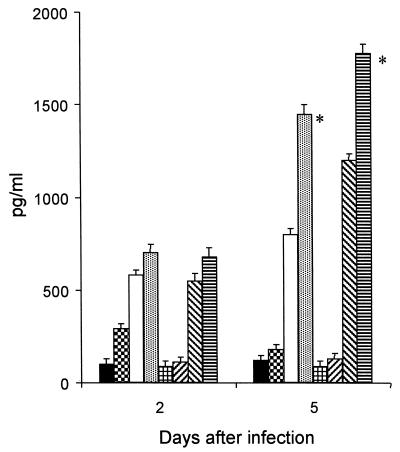
To explore the involvement of TGF-β1 in the immune response to S. typhimurium, we analyzed IL-1α, TNF-α, and IFN-γ production in vitro by using splenic monocytes or lymphocytes obtained from rTGF-β1-treated and untreated mice infected with S. typhimurium. Cells were harvested 2 and 5 days after treatment and subjected to one cycle of in vitro restimulation with lipopolysaccharide (1 μg/ml) for 24 and 48 h. As shown in Fig. 3, the amount of IL-1α in the supernatant of splenic monocytes from rTGF-β1-treated mice 2 days after infection did not increase significantly with respect to that found in monocytes from PBS-injected mice. In contrast, 5 days after infection, the amount of IL-1α released by splenic lymphocytes from rTGF-β1-treated animals (1,400 ± 37 pg/ml) increased significantly compared to the amount released by lymphocytes from PBS-injected mice (800 ± 28 pg/ml) (P < 0.01). As shown in Fig. 3, IFN-γ release followed a similar pattern, with a greater amount being found in the supernatant of rTGF-β1-treated mice 5 days after infection (1,800 ± 42 pg/ml). TNF-α release was not significant after 2 and 5 days after infection in rTGF-β1-treated mice or in PBS-injected mice (data not shown).
FIG. 3.
Effect of rTGF-β1 administration on IL-1α (■, , □, and ) and IFN-γ ( , ▨, ▧, and ▤) release in the supernatants of monocytes from S. typhimurium-infected mice (4 × 105 CFU/mouse). Results for PBS-only-treated mice (■ and ), rTGF-β1-only-treated mice ( and ▨), PBS-treated and infected mice (□ and ▧), and rTGF-β1-treated and infected mice ( and ▤) are shown. Each result represents the mean of three determinations, and the bars indicate standard deviations. ∗, P < 0.01 with respect to PBS-injected, infected mice.
Effect of TGF-β1 on endogenous IFN-γ and TNF-α induced by a sublethal infection with S. typhimurium.
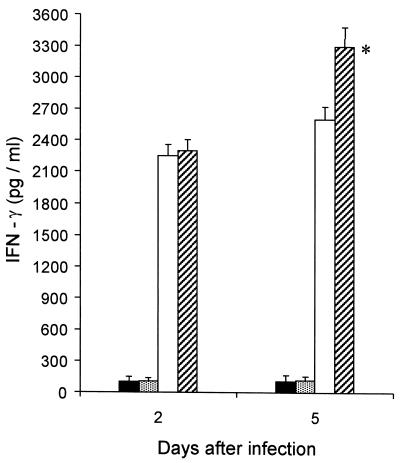
At 2 and 5 days after mice were infected with 4 × 105 CFU of S. typhimurium, the levels of IFN-γ and TNF-α in the bloodstream were monitored. In a previous study by Nakane et al. (28), the titers of endogenous IFN-γ and TNF-α in sera peaked 2 to 3 days after the sublethal infection. In our study, we demonstrated that 2 days after infection the amount of IFN-γ in the serum of rTGF-β1-treated mice (2,250 ± 45 pg/ml) and in the serum of PBS-injected mice (2,220 ± 45 pg/ml) was not significantly different even if it was increased with respect to that in control mice. As shown in Fig. 4, 5 days after infection the IFN-γ titer was significantly greater in the serum of rTGF-β1-treated mice (3,261 ± 57 pg/ml) than in the serum of PBS-treated mice (2,591 ± 51 pg/ml). No endogenous TNF-α was observed in the bloodstream of rTGF-β1- or PBS-treated mice (data not shown).
FIG. 4.
Effect of rTGF-β1 administration on the amount of IFN-γ in the serum of S. typhimurium-infected mice (4 × 105 CFU/mouse). Symbols: ■, PBS-only-treated mice; , rTGF-β1-only-treated mice; □, PBS treated, infected mice; ▨, rTGF-β1-treated, infected mice. Each result represents the mean of three determinations, and the bars indicate standard deviations. ∗, P < 0.01 with respect to PBS injected, infected mice.
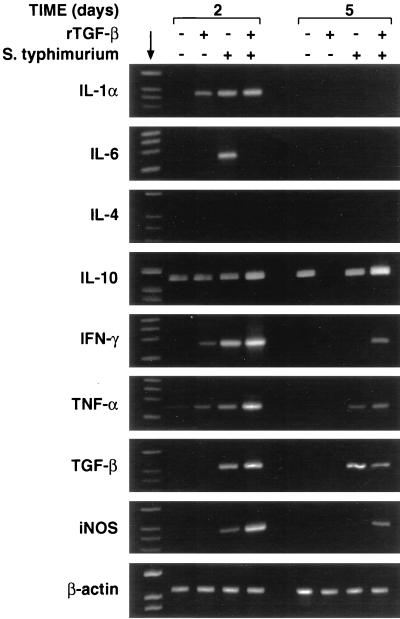
FIG. 5.
RT-PCR analysis of cytokine mRNA expression in spleens of mice 2 and 5 days after S. typhimurium infection. Mice were inoculated i.p. with 0.5 μg of rTGF-β1 or PBS 2 h before infection with S. typhimurium, and cells were recovered 2 and 5 days after infection. Total RNA was isolated from 107 splenocytes obtained from treated and untreated mice and RT-PCR amplified with specific primer pairs. The reaction products were run on 1.5% agarose gels in the presence of appropriate molecular size markers (providing bands at 2,176, 1,766, 1,230, 1,033, 653, 517, 453, 394, 298, 234, 220, and 154 bp) (arrow); β-actin was the positive transcription control.
Detection of cytokine and iNOS mRNA.
Cytokine mRNA analysis of spleen cells from rTGF-β1-treated and untreated mice after S. typhimurium infection was performed by reverse transcriptase PCR (RT-PCR) and focused on tissue from mice at 2 and 5 days after treatment. At our limits of detection, mRNAs for IL-1α, IL-6, IFN-γ, IL-4, TGF-β, and inducible nitric oxide synthase (iNOS) were not expressed in spleen cells from untreated mice and mRNAs for IL-10 and TNF-α were barely expressed. The pattern of cytokine mRNA expression in splenocytes from rTGF-β1-treated and untreated mice, 2 and 5 days after a sublethal infection with S. typhimurium, is shown in Fig. 5.
By RT-PCR analysis, IFN-γ mRNA was detected in spleen cells from PBS-injected mice after 2 days, although the band was weak and had disappeared entirely by 5 days after the challenge. In rTGF-β1-treated mice, IFN-γ mRNA was present in a greater amount than in untreated mice after 2 days, although the intensity of the band had decreased by 5 days after the challenge. A similar pattern was obtained with the mRNAs for IL-1α, IL-6, TGF-β, and iNOS, which showed their greater expression in cells obtained from rTGF-β1-treated and S. typhimurium-infected mice 2 days after challenge. IL-4 mRNA was absent both 2 and 5 days after the different treatments.
hsp appearance in splenocytes from rTGF-β1-treated mice after S. typhimurium infection.
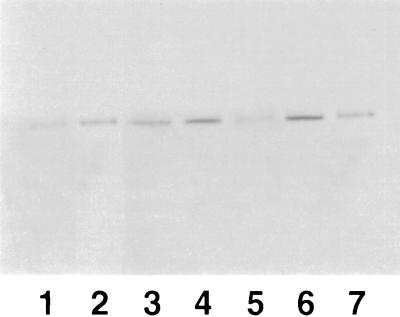
The presence of hsp60 was checked in murine splenocytes from rTGF-β1-treated animals by using a monoclonal antibody to hsp60 (clone LK-1). The number of cells that we used (107) was not enough to show the presence of constitutive hsp in splenocytes from untreated control mice by immunoblotting. Two days after infection, rTGF-β1-treated mice showed greater expression of hsp60 than did PBS-injected mice. In contrast, 5 days after infection, higher expression of hsp60 was found in splenocytes from PBS-injected mice but the expression was lower in splenocytes from rTGF-β1-treated mice. Results of a representative experiment are shown in Fig. 6.
FIG. 6.
Time course of hsp expression (estimated by immunoblotting) in splenic lymphocytes from rTGF-β1-treated mice. Lanes: 1, control mice; 2, rTGF-β1-only-treated mice 2 days after injection; 3, PBS-treated mice 2 days after infection; 4, rTGF-β1-treated mice 2 days after infection; 5, rTGF-β1-only-treated mice 5 days after injection; 6, PBS-treated mice 5 days after infection; 7, rTGF-β1-treated mice 5 days after infection.
DISCUSSION
The role of TGF-β in immune responses might involve complicated mechanisms; it is considered to play a key role in the regulation of infection with certain intracellular microorganisms. It modulates the production of cytokines and the cell response to cytokine stimulation. TGF-β plays an important role in the progression of Mycobacterium avium infection (41); its production in vivo is considered to be a virulence mechanism in protozoan infections. Early treatment in vivo with rTGF-β was demonstrated to induce a protective effect against experimental infection with L. monocytogenes in mice (28). Furthermore, in mice infected with virulent C. albicans, the administration of rTGF-β delays progression of the disease (35).
The resistance to infections is related to the development of a Th1-Th2 response. The preferential development of CD4+ or CD8+ Th1 or Th2 cells is under the control of several mechanisms. The early presence of Th1 or Th2 cytokines influences the outcome of the type of the cells that will develop. IL-4 plays an important role in stimulating Th2 response, whereas IFN-γ and TGF-β favor the development of the Th1 response (20, 36). The treatment with TGF-β could be protective either by promoting the generation of Th1 cells or by regulating the synthesis and release of proinflammatory cytokines.
Our studies demonstrate that after 2 days, treatment with rTGF-β1 before experimental infection with S. typhimurium induces an increase in the mRNA expression of cytokines that promote the development of a Th1 response. This effect tends to decline 5 days after the infection. The cytokine pattern is related to the activation of both specific and nonspecific mechanisms of host defense; in fact, we observed that rTGF-β1 administration affected the activation of macrophages (as demonstrated by NO production), the expression of molecules involved in the immune response (CD28), and the induction of cellular stress (as shown by hsp60 expression). Treatment with rTGF-β1 2 h before the experimental infection led to the survival of all infected mice, in comparison with the mortality rate of 4 to 5 of 10 observed in PBS-injected mice which received 8 × 105 CFU of S. typhimurium. This survival rate is related to a remarkably smaller number of bacteria in the spleens and livers of rTGF-β1-treated mice compared to control mice. The smaller number of CFU in these organs is related to the higher NO production as demonstrated in vitro by macrophages obtained from rTGF-β1-treated mice. Macrophage NO release is suppressed by TGF-β (15), and inhibition of NO production in vivo is associated with impaired Th1-cell development (18) and effector function (17, 37). Under our experimental conditions, S. typhimurium-infected mice show enhanced NO production, which further increases in rTGF-β1-treated mice. While in vitro the production of TGF-β is associated with an inhibition of NO production (15), under our experimental conditions the in vivo treatment does not have the same effect, probably because of the presence of the other regulatory cytokines. The amounts of IL-1α and IFN-γ increase remarkably in vivo during rTGF-β1 administration, as shown by their mRNA expression and release by cell culture. It is of great interest that IL-1 and IFN-γ are cytokines that contribute to an enhanced NO response (17). Our findings demonstrate that 2 days after infection, administration of rTGF-β1 induces greater expression of IFN-γ and TNF-α mRNA, which directly or indirectly also promote the activation of macrophages.
Because the costimulatory molecules can decisively influence the evolution of a protective response and because intracellular pathogens downregulate the expression of specific costimulating molecules, we became interested in analyzing the expression of CD28 in CD3+ cells in infected mice and in rTGF-β1-treated and infected mice. The administration of rTGF-β1 induces an upregulation of CD28 expression 5 days after infection. Under our experimental conditions, the upregulation of the costimulatory molecule (CD28) is preceded at 2 days after S. typhimurium infection by a remarkable upregulation of IFN-γ mRNA expression. The T-cell costimulatory molecule CD28 plays a critical role in T-cell activation and cytokine production. CD28 is present on resting T cells and is therefore likely to predominate in initial costimulatory activity. The findings in this report provide insight into the mechanism by which TGF-β regulates immune responses through the network of cytokines and costimulatory molecules.
Recombinant TGF-β1 treatment greatly influences the proinflammatory and immunoregulatory cytokine pattern 2 days after infection, while only IFN-γ release is still enhanced at later times. IFN-γ was detected after 2 and 5 days in the bloodstream in a larger amount in rTGF-β1-treated mice infected with S. typhimurium than in untreated, infected mice. TNF-α was never found in the bloodstream at 2 and 5 days after infection. Greater IFN-γ, TNF-α, and IL-10 mRNA expression was always seen in the spleens of TGF-β1-treated mice after S. typhimurium infection than in those of PBS-injected mice. IL-6 mRNA expression was barely evident in PBS-treated, infected mice but showed a good rate of induction in rTGF-β1-injected mice. IL-6 has a wide variety of activities; IL-6 pretreatment enhances resistance against infection (6, 38), although this has not been found consistently (8). IL-4 mRNA was never detected under the same experimental conditions. TGF-β mRNA showed good expression in the spleens of infected mice compared to control animals. The administration of rTGF-β1 further increases this upregulation during the first 2 days of infection. Endogenous TGF-β expression is also upregulated by TGF-β priming, which is consistent with TGF-β autoinduction in other cell types (39). The functional pattern of the cytokines expressed and released in the spleens of infected animals either treated or not treated with rTGF-β1 is related to a Th1 response. Our findings demonstrate a cytokine pattern related to activation of CD8+ effector production as revealed by splenic mRNA analysis. The influence of certain cytokines on the development of naive CD4+ T cells to express Th1 and Th2 cytokines is well recognized for the dominant effects of IL-12 and IL-4, respectively (31). More recently, Th1- and Th2-like cytokine patterns have also been demonstrated by CD8+ T cells upon antigen priming in the presence of IL-12 or IL-4 (13, 32, 43) and upon TGF-β priming. CD8+ cells primed by TGF-β are typified by a distinct cytokine profile of IL-10 and TGF-β production (30). TGF-β growth response occurs predominantly among CD8+ T cells of naive CD44low CD45RBhigh phenotype, which usually accounts for more than 85% of splenic CD8+ T cells of young, conventionally maintained BALB/c or C57BL/6 mice (25).
In our study TGF-β1 is associated with a faster appearance (2 days) of hsp60 in splenic cells and with a faster disappearance (5 days) in untreated and rTGF-treated mice, respectively. The lower level of hsp60 5 days after infection in rTGF-β1 treated mice (compared to untreated mice) may depend on a decrease of the bacterial load in the spleen or on a direct effect of TGF-β on the recovery of cellular activities. Rich et al. (29) suggest that there is a TGF-β-dependent protection of CD8+ T cells from apoptosis.
In conclusion, the greater resistance to infection with S. typhimurium in rTGF-β1-treated mice is associated under our experimental conditions with different mechanisms, both nonspecific (NO production and hsp) and specific (costimulatory molecules activation and release of cytokines), in the protective response. The widespread distribution of TGF-β (and its receptors) suggests that it plays an important role in these mechanisms, whose final result depends on several variables (TGF-β concentration and time of production, cell type, and degree of differentiation).
REFERENCES
- 1.Ahuja S S, Paliogianni F, Hidehiro Y, Balow J E, Boumpas D T. Effect of transforming growth factor-β on early and late activation events in human T cells. J Immunol. 1993;150:3109–3118. [PubMed] [Google Scholar]
- 2.Assoian R K, Fleurdelys B E, Stevenson H C, Miller P J, Madres D K, Raines E W, Ross R, Sporn M B. Expression and secretion of type β transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA. 1987;84:6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes D F, Mistry S D, Cooper C L, Pirmez C, Rea T H, Modlin R L. Compartmentalization of a CD4+T lymphocyte subpopulation in tuberculous pleuritis. J Immunol. 1989;142:1114–1119. [PubMed] [Google Scholar]
- 4.Barral A, Barral-Netto M, Young E C, Brownell E, Twardzik D R, Reed S G. Transforming factor-β as a virulence mechanism for Leishmania braziliensis. Proc Natl Acad Sci USA. 1993;90:3442–3446. doi: 10.1073/pnas.90.8.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barral-Netto M, Barral A, Brownell C E, Skeiky Y A W, Ellingsworth L R, Twardzik D R, Reed S G. Transforming growth factor-β in leishmanial infection: a parasite escape mechanism. Science. 1992;257:545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- 6.Barton B E, Jackson J V. Protective role of interleukin-6 in the lipopolysaccharide-galactosamine septic shock model. Infect Immun. 1993;61:1496–1499. doi: 10.1128/iai.61.4.1496-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boog E R, de Graeff-Meeder E R, Lucassen M A, van der Zee R, Voorhorst-Ogink M M, van Kooten P J, Geuze H J, Van Eden W. Two monoclonal antibodies generated against human HSP60 show reactivity with synovial membranes of patients with juvenile chronic arthritis. J Exp Med. 1992;175:1805–1810. doi: 10.1084/jem.175.6.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucklin S E, Silverstein R, Morrison D C. An interleukin-6-induced acute-phase response does not confer protection against lipopolysaccharide lethality. Infect Immun. 1993;61:3184–3189. doi: 10.1128/iai.61.8.3184-3189.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buttke T M. Inhibition of lymphocyte proliferation by free fatty acids. Immunology. 1984;53:5–42. [PMC free article] [PubMed] [Google Scholar]
- 10.Cenci E, Romani L, Mencacci A, Spaccapelo R, Schiaffella E, Puccetti P, Bistoni F. Interleukin-4 and interleukin-10 inhibit nitric oxide-dependent macrophage killing of Candida albicans. Eur J Immunol. 1993;23:1034–1038. doi: 10.1002/eji.1830230508. [DOI] [PubMed] [Google Scholar]
- 11.Chantry D, Turner M, Abney E, Feldman M. Modulation of cytokine production by TGF-β. J Immunol. 1989;142:4295–4300. [PubMed] [Google Scholar]
- 12.Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 13.Croft M, Carter L, Swain S, Dutton R W. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czariecki C W, Chiu H H, Wong G H W, McCabe S M, Palladino M A. Transforming growth factor-β1 modulates the expression of class II histocompatibility antigens on human cells. J Immunol. 1988;140:4217–4223. [PubMed] [Google Scholar]
- 15.D’Angeac A D, Dornand J, Emonds J A, Garcia-Sanz A X, Erard F. Transforming growth factor-β1down-regulates interleukin-2 production and up-regulates interleukin-2 receptor expression in a thymoma cell line. J Cell Physiol. 1991;147:460–469. doi: 10.1002/jcp.1041470312. [DOI] [PubMed] [Google Scholar]
- 16.Denis M, Ghadirian E. Transforming growth factor-β1 (TGF-β1) plays a detrimental role in the progression of experimental Mycobacterium aviuminfection: in vivo and in vitro evidence. Microb Pathog. 1991;11:367–372. doi: 10.1016/0882-4010(91)90022-3. [DOI] [PubMed] [Google Scholar]
- 17.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 18.Ellner J J. Suppressor cells in tuberculosis. J Immunol. 1978;121:2573–2579. [PubMed] [Google Scholar]
- 19.Espevik T, Figari I S, Shalaby M R, Lackides G A, Lewis G D, Shepard M, Palladino M A. Inhibition of cytokine production by cyclosporin A and transforming growth factor β. J Exp Med. 1987;166:571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gajewski T F, Fitch F W. Anti-proliferative effect of IFN-γ in immune regulation. I. IFN-γ inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988;140:4245–4252. [PubMed] [Google Scholar]
- 21.Galdiero F, Cipollaro de L’Ero G, Benedetto N, Galdiero M, Tufano M A. Release of cytokines induced by Salmonella typhimuriumporins. Infect Immun. 1993;61:155–161. doi: 10.1128/iai.61.1.155-161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter C A, Bermudez L, Beernink H, Waegell W, Remington J S. Transforming growth factor-β inhibits interleukin-12-induced production of interferon-γ by natural killer cells: a role for transforming growth factor-β in the regulation of T cell-independent resistance to Toxoplasma gondii. Eur J Immunol. 1995;25:994–1000. doi: 10.1002/eji.1830250420. [DOI] [PubMed] [Google Scholar]
- 23.Kehrl J H, Roberts A B, Wakefield L M, Jakowlew S, Sporn M B, Fauci A S. Transforming growth factor-β is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986;137:3855–3860. [PubMed] [Google Scholar]
- 24.Kehrl J H, Wakefield L M, Roberts A B, Jakowlew S, Alverez-Mon M, Derynck R, Sporn M B, Fauci A S. Production of transforming growth factor-β by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163:1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H M, Rich S. Costimulation of T cell proliferation by transforming growth factor-β1. J Immunol. 1991;147:1127–1133. [PubMed] [Google Scholar]
- 26.Lowrance J H, O’Sullivan F X, Caver T E, Waegell W, Gresham H D. Spontaneous elaboration of transforming growth factor β suppresses host defence against bacterial infection in autoimmune MRL/lpr mice. J Exp Med. 1994;180:1693–1703. doi: 10.1084/jem.180.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 28.Nakane A, Asano M, Sasaki S, Nishikawa S, Miura T, Kohanawa M, Minagawa T. Transforming growth factor β is protective in host resistance against Listeria monocytogenesinfection in mice. Infect Immun. 1996;64:3901–3904. doi: 10.1128/iai.64.9.3901-3904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rich S, Seelig M, Lee H M, Lin J. Transforming growth factor β1 costimulated growth and regulatory function of staphylococcal enterotoxin B-responsive CD8+T cells. J Immunol. 1995;155:609–618. [PubMed] [Google Scholar]
- 30.Salgame P, Abrams J S, Clayberger C, Goldstein H, Convit J, Modlin R L, Bloom B R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254:279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 31.Seder R A, Paul W E. Acquisition of lymphokine producing phenotype by CD4+T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 32.Seder R A, Boulay J L, Finkelman F, Barbier S, Ben-Sasson S Z, Le Gros G, Paul W E. CD8+T cells can be primed in vitro to produce IL-4. J Immunol. 1992;148:1652–1656. [PubMed] [Google Scholar]
- 33.Shalaby M R, Amman A J. Suppression of immune cell function in vitro by recombinant human transforming growth factor β. Cell Immunol. 1988;112:343–350. doi: 10.1016/0008-8749(88)90303-6. [DOI] [PubMed] [Google Scholar]
- 34.Silva J S, Twardizik D R, Reed S G. Regulation of Trypanosoma cruzi infections in vitro and in vivoby transforming growth factor-β (TGF-β) J Exp Med. 1991;172:1774–1777. doi: 10.1084/jem.174.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spaccapelo R, Romani L, Tonnetti L, Cenci E, Mencacci A, Del Sero G, Tognellini R, Reed S G, Puccetti P, Bistoni F. TGF-β1 is important in determining the in vivo patterns of susceptibility or resistance in mice infected with Candida albicans. J Immunol. 1995;155:1349–1360. [PubMed] [Google Scholar]
- 36.Swain S L, Bradley L M, Croft M, Tonkonogy S, Atkins G, Weinberg A D, Duncan D D, Hedrick S M, Dutton R W, Huston G. Helper-cell subset phenotype, function and the role of lymphokines in regulating their development. Immunol Rev. 1991;123:115–144. doi: 10.1111/j.1600-065x.1991.tb00608.x. [DOI] [PubMed] [Google Scholar]
- 37.Toossi Z, Lapurga J P, Ondash R J, Sedor J R, Ellner J J. Expression of functional interleukin-2 receptors by peripheral blood monocytes from patients with active pulmonary tuberculosis. J Clin Invest. 1990;85:1777–1784. doi: 10.1172/JCI114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van der Meer J W M, Helle M, Aarden A. Comparison of the effects of recombinant interleukin-6 and recombinant interleukin-1 on non- specific resistance to infection. Eur J Immunol. 1989;19:413–416. doi: 10.1002/eji.1830190229. [DOI] [PubMed] [Google Scholar]
- 39.Van Obberghen-Schilling E, Roche N S, Flanders K C, Sporn M, Roberts A. Transforming growth factor β1 positively regulates its own expression in normal and transformed cells. J Biol Chem. 1988;263:7741–7746. [PubMed] [Google Scholar]
- 40.Wahl S M, Hunt D A, Wakefield L M, McCartney-Francis N, Wahl L M, Roberts A B, Sporn M B. Transforming growth factor type β induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci USA. 1987;84:5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wahl S M, Hunt D A, Wong H L, Dougherty S, McCartney Francis N, Wahl L M, Ellingsworth L, Schmidt J A, Hall G, Roberts A B, Sporn M B. Transforming growth factor-β is a potent immunosuppressive agent which inhibits interleukin-1 dependent lymphocyte proliferation. J Immunol. 1988;140:3026–3032. [PubMed] [Google Scholar]
- 42.Welch G R, Wong M L, Wahl S M. Selective induction of FcγRIII on human monocytes by TGF-β. J Immunol. 1990;144:3444–3448. [PubMed] [Google Scholar]
- 43.Wild E M T, Garcia-Sanz J A, Le Gros G. Switch of CD8 T cells to noncytolytic CD8− CD4−cells that make Th2 cytokines and help B cells. Science. 1993;260:1802–1805. doi: 10.1126/science.8511588. [DOI] [PubMed] [Google Scholar]