To the Editor:
The extrinsic tenase complex consists of the serine protease coagulation factor VII(a) [FVII(a)] and its cellular receptor tissue factor (TF). In a previous issue of Blood, Le Gall et al reported that binding of FVII(a) to cellular TF induces rapid and prolonged enhancement of the barrier function of epithelial monolayers in vitro. They showed that the binding of FVII(a) to epithelial TF results in cleavage and activation of matriptase, a membrane-anchored serine protease. In turn, matriptase cleaves and activates protease-activated receptor 2 (PAR2), which triggers transmembrane signaling and reduces epithelial permeability.1
Le Gall et al hypothesized that activation of matriptase and PAR2 by the TF/FVII(a) complex could have evolved as a physiological response to external stimuli. Here, we present data with developmentally relevant human samples indicating that coagulation FVII(a) present in human amniotic fluid may interact with TF exposed on the fetal epidermis, thereby regulating epithelial skin barrier function.
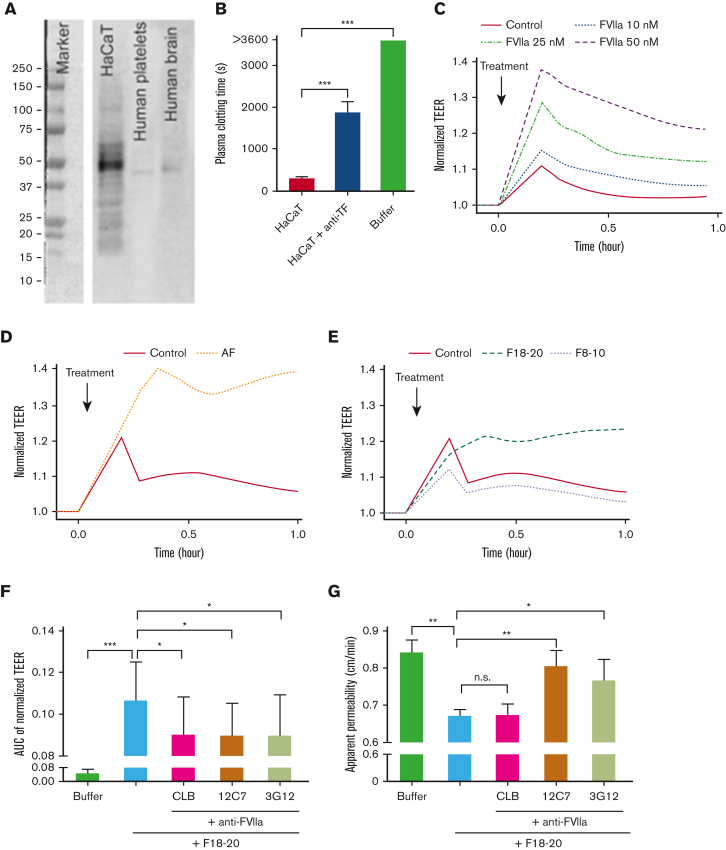
Le Gall et al used a human keratinocyte cell line, HaCaT cells, for their in vitro studies and showed that HaCaT cells expose TF and that the binding of FVII(a) thereby reduces the epithelial permeability. Our results confirm that HaCaT cells produce TF (Figure 1A), expose TF (Figure 1B), and that the addition of FVII(a) to HaCaT cells reduces the epithelial permeability (Figure 1C, supplemental Figure 2A, methods are provided in the supplement).
Figure 1.
Activated factor VII in amniotic fluid induces an increase in barrier function of TF exposing immortalized human keratinocytes. (A) HaCaT lysate was blotted for TF. Lysates from human platelets and brain were used as negative and positive controls, respectively. (B) Citrate-anticoagulated human plasma was added to HaCaT cells, and clotting time was monitored after recalcification in the absence and presence of a TF antibody (clone HTF-1). (C) TEER of HaCaT cells was measured after addition of human factor VIIa (FVIIa) to HaCaT cells. (D) AF and (E) Sepharose 2B-size exclusion chromatography (SEC2B) separated AF fractions 8 to 10, and 18 to 20 were added to HaCaT cells before measuring TEER. (F) The effect of AF SEC2B fractions 18 to 20 on TEER was measured in the absence and presence of 3 different monoclonal anti-FVII antibodies (CLB, 12C7, and 3G12), which block different specific epitopes of FVII(a). (G) A lucifer yellow rejection assay was performed with AF SEC2B fractions 18 to 20 in the absence and presence of anti-FVII antibodies. All measurements were performed in triplicate. Buffer was used as control. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; AF, amniotic fluid; AUC, area under the curve; TEER, transepithelial electrical resistance; n.s., nonsignificant.
We found that FVII(a) is present in human amniotic fluid (supplemental Figure 1A). The addition of amniotic fluid to HaCaT cells induced an increase in transepithelial electrical resistance (Figure 1D, supplemental Figure 2B), which reflects reduced epithelial membrane permeability. As human amniotic fluid also contains FVII(a), which is associated with TF-exposing extracellular vesicles (EVs),2 we separated soluble (free, unbound) FVII(a) from EV-associated FVII(a) by Sepharose 2B size exclusion chromatography.3 As expected, the bulk of (soluble) FVII(a) was detectable in Sepharose 2B size exclusion chromatography, fractions 18 to 20 (supplemental Figure 1B). When these fractions were pooled and added to HaCaT cells, an increase in transepithelial electrical resistance was observed (Figure 1E) that was partially inhibited by antibodies against FVII(a), which block different specific epitopes of FVII(a)4, 5, 6 (Figure 1F). The observation that FVII(a) in amniotic fluid is likely to reduce the epithelial membrane permeability was confirmed in a luciferase yellow rejection assay (Figure 1G). Taken together, our findings indicate that FVII(a) in human amniotic fluid can bind to TF exposed on epithelial cells in vitro, thereby reducing the epithelial membrane permeability.
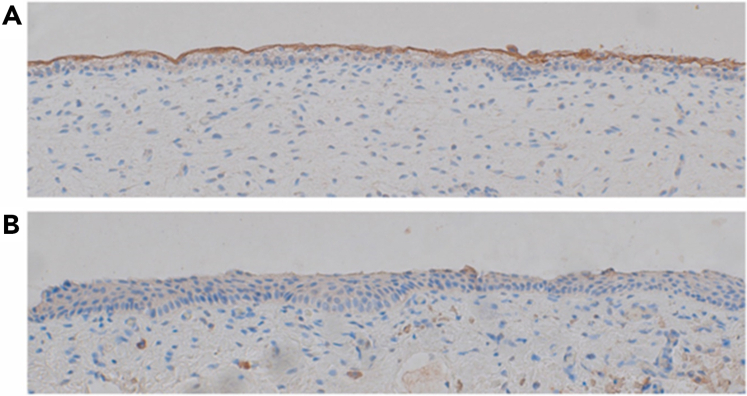
The obvious question is, when human amniotic-fluid-FVII(a) can change the epithelial membrane permeability in vitro, can FVII(a) also affect the epithelial membrane permeability in vivo? Although this is impossible to study directly, the presence of TF on fetal skin would be a prerequisite for such an interaction to take place. Therefore, we determined the presence of TF immunohistochemically in periumbilical skin samples from 6 fetuses (median gestational age: 19 weeks; range: 15-22). TF was abundantly present in the outermost layer of the uncornified fetal epidermis in all samples, which is consistent with previous findings,7,8 whereas no TF was detectable in lower epidermal cell layers (Figure 2A and supplemental Figure 3A-E). The uncornified foreskin of a 4-year-old boy stained negative for TF (Figure 2B). As the fetal epidermis permanently contacts amniotic fluid in utero, and based on our results, we hypothesize that the FVII(a) present in human amniotic fluid may bind to TF present on the fetal epidermis, and this interaction may be involved in the regulation of epithelial membrane permeability by potentially activating downstream proteases and PAR2 signaling.
Figure 2.
Epidermal TF expression. Immunohistochemical staining for TF in (A) a periumbilical skin sample of a fetus at gestational week 15 and (B) the foreskin of a 4-year-old boy. Pictures were taken at original 20× magnification.
Camerer et al reported that fusion of the surface ectoderm over caudal and hindbrain neuropores in midgestation requires PAR1 and PAR2 signaling in mice, but the PAR activating proteases remained to be defined.9 FVII(a) in amniotic fluid may drive PAR2 signaling in this context and may play a role in neural tube closure.
Limitations of our studies with human material are that the in vivo role of these mechanisms remain to be investigated. Moreover, doors are left open for further questions that may arise. Most importantly, it remains to be investigated whether amniotic-fluid-FVII(a)–mediated increase in epithelial barrier function depends on matriptase and PAR2 activation.
Body surfaces and cavities are lined with epithelial cells. Tight junctions, adherence junctions, and desmosomes connect epithelial cells and control the paracellular permeability of epithelia.10 These epithelial junction proteins function as dynamic structures that allow epithelial cells to respond to external stimuli by modifying the permeability of the epithelial barrier. Specific extracellular proteases, such as the serine protease FVII(a), target these epithelial junction proteins and thereby modify epithelial function. Our results indicate that such interactions may occur in vivo. If so, our findings suggest a specific FVII(a)-mediated pathway of epithelial barrier permeability regulation during ontogenesis. This pathway may be relevant to improve treatment of preterm newborns, for example, by adding FVII(a) to skin emollients to improve the immature skin barrier function of preterm newborns to reduce complications such as skin infection, hypothermia, and dehydration.11
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
The authors thank Peter Altevogt (Tumor Immunology Programme, German Cancer Research Center, Heidelberg, Germany) for amniotic fluid collection at the University of Heidelberg; the Center for Reproductive Medicine (Amsterdam UMC, location University of Amsterdam, Amsterdam, the Netherlands) for providing HaCaT cells; Klaudia Schossleitner (Department of Dermatology, Medical University Vienna, Vienna, Austria) for assistance with maintaining and differentiation culturing of HaCaT cells; and Judith de Vos for the assistance with using the apparatus in the department of Biomedical Engineering and Physics, Amsterdam UMC, location University of Amsterdam, Amsterdam, the Netherlands.
Y.H. was supported by a scholarship from the China Scholarship Council. J.T. was supported by an unrestricted travel grant from the International Society on Thrombosis and Haemostasis.
Contribution: Y.H., R.N., and J.T. designed experiments; Y.H. and A.S. performed experiments; Y.H., J.T., R.N., and A.S. analyzed data; J.T. and A.S. acquired samples; R.N. and J.T. supervised the study; Y.H., J.T., C.A., A.S., R.J.B., and R.N. drafted the manuscript; C.A., A.S., R.J.B., W.R., and C.H. provided administrative, technical, or material support; and all authors took part in reviewing and editing the entire manuscript and approved the final version of the manuscript.
Footnotes
Data sharing requests may be submitted via email to the corresponding author: johannes.thaler@meduniwien.ac.at.
The full-text version of this article contains a data supplement.
Supplementary data
References
- 1.Le Gall SM, Szabo R, Lee M, et al. Matriptase activation connects tissue factor-dependent coagulation initiation to epithelial proteolysis and signaling. Blood. 2016;127(25):3260–3269. doi: 10.1182/blood-2015-11-683110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu Y, Repa A, Lisman T, et al. Extracellular vesicles from amniotic fluid, milk, saliva, and urine expose complexes of tissue factor and activated factor VII. J Thromb Haemost. [published online ahead of print 24 June 2022] [DOI] [PMC free article] [PubMed]
- 3.Boing AN, van der Pol E, Grootemaat AE, Coumans FA, Sturk A, Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothmeier AS, Liu E, Chakrabarty S, et al. Identification of the integrin-binding site on coagulation factor VIIa required for proangiogenic PAR2 signaling. Blood. 2018;131(6):674–685. doi: 10.1182/blood-2017-02-768218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamikubo Y, Mendolicchio GL, Zampolli A, et al. Selective factor VIII activation by the tissue factor-factor VIIa-factor Xa complex. Blood. 2017;130(14):1661–1670. doi: 10.1182/blood-2017-02-767079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berckmans RJ, Sturk A, van Tienen LM, Schaap MC, Nieuwland R. Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood. 2011;117(11):3172–3180. doi: 10.1182/blood-2010-06-290460. [DOI] [PubMed] [Google Scholar]
- 7.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134(5):1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 8.Luther T, Flossel C, Mackman N, et al. Tissue factor expression during human and mouse development. Am J Pathol. 1996;149(1):101–113. [PMC free article] [PubMed] [Google Scholar]
- 9.Camerer E, Barker A, Duong DN, et al. Local protease signaling contributes to neural tube closure in the mouse embryo. Dev Cell. 2010;18(1):25–38. doi: 10.1016/j.devcel.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17(9):564–580. doi: 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]
- 11.Telofski LS, Morello AP, 3rd, Mack Correa MC, Stamatas GN. The infant skin barrier: can we preserve, protect, and enhance the barrier? Dermatol Res Pract. 2012;2012 doi: 10.1155/2012/198789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.