Abstract
Background
Vascular endothelial growth factor inhibitors (VEGFIs) are effective anticancer agents which often induce hypertension. VEGFI-induced hypertension is sodium-sensitive in animal studies. Therefore, the efficacy of dietary sodium restriction (DSR) to prevent VEGFI-induced hypertension in cancer patients was studied.
Methods
Cancer patients with VEGFI-induced hypertension (day mean >135/85 mmHg or a rise in systolic and/or diastolic BP ≥ 20 mmHg) were treated with DSR (aiming at <4 g salt/day). The primary endpoint was the difference in daytime mean arterial blood pressure (MAP) increase between the treatment cycle with and without DSR.
Results
During the first VEGFI treatment cycle without DSR, mean daytime MAP increased from 95 to 110 mmHg. During the subsequent treatment cycle with DSR, mean daytime MAP increased from 94 to 102 mmHg. Therefore, DSR attenuated the increase in mean daytime MAP by 7 mmHg (95% CI 1.3–12.0, P = 0.009). DSR prevented the rise in the endothelin-1/renin ratio that normally accompanies VEGFI-induced hypertension (P = 0.020) and prevented the onset of proteinuria: 0.15 (0.10–0.25) g/24 h with DSR versus 0.19 (0.11–0.32) g/24 h without DSR; P = 0.005.
Discussion
DSR significantly attenuated VEGFI induced BP rise and proteinuria and thus is an effective non-pharmacological intervention.
Subject terms: Targeted therapies, Risk factors
Introduction
Vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFIs) impair the formation of new blood vessels (neo-angiogenesis) required for growth and metastatic spread of malignant tumours. VEGFIs such as cabozantinib, lenvatinib, pazopanib, regorafenib, sorafenib and sunitinib have become a part of regular cancer treatment, and have been shown to improve clinical outcomes in renal cell carcinoma, hepatocellular carcinoma, gastrointestinal stromal tumours, various neuro-endocrine tumours and thyroid cancer [1]. Given that VEGFIs do not selectively inhibit neo-angiogenesis in tumours but also affect the cardiovascular system, it is not surprising that these agents induce cardiovascular side effects. Hypertension is the most frequently observed cardiovascular side effect and occurs in 25–87% of VEGFI-treated patients [2, 3].
In rats, VEGFI-induced hypertension has been demonstrated to be salt-sensitive [4, 5]. High salt intake results in sodium accumulation in the skin, which stimulates skin lymphangiogenesis via activation of the mononuclear phagocyte system cell-derived VEGF-C-VEGF type 3 receptor signalling pathway [6–8]. Inappropriate lymphangiogenesis impairs electrolyte washout, thereby leading to salt accumulation and hypertension [8, 9].
Nephropathy characterised by proteinuria is another well-known side effect of VEGFI [10–12]. These side-effects resemble the characteristics of preeclampsia, a severe complication of pregnancy caused by insufficient angiogenesis of the placenta [13, 14]. This is not surprising, because preeclampsia is characterised by the placental release of soluble fms-like tyrosine kinase-1 (sFlt-1), a soluble VEGF-inactivating receptor, which induces VEGF suppression. Both VEGFI and preeclampsia are accompanied by a rise in the potent vasoconstrictor endothelin-1 (ET-1), a reduction in renin–angiotensin–aldosterone system (RAAS) activity, and an imbalance of the cyclo-oxygenase (COX) products prostacyclin or prostaglandin I2 (PGI2)) and thromboxane A2 (TXA2) [13–18].
Hypertension and/or proteinuria are an often dose-limiting side effect of VEGFI, necessitating either the prescription of antihypertensive drugs or a dose reduction, treatment interruption or early termination of VEGFI [2, 19].
Given these observations, dietary sodium restriction (DSR) could constitute an easy to perform and effective intervention to prevent or treat VEGFI-induced hypertension. To address this hypothesis, we studied the effects of DSR on the rise in BP in patients with solid tumours treated with standard of care cabozantinib, lenvatinib, pazopanib, regorafenib, sorafenib or sunitinib [https://www.ema.europa.eu/en/medicines]. Secondly, we investigated to what extent VEGFI affected the development of proteinuria and studied the changes in ET-1, renin, aldosterone, prostacyclin (PGI2) and thromboxane (TXA2) to gain better insights into its pathogenesis.
Methods
We conducted a prospective, single-centre, open-label, intervention study at the Erasmus MC Cancer Institute Rotterdam, the Netherlands. The study was approved by the Medical Ethics Committee from the Erasmus University Medical Center (MEC-2018-155) and complies with the Declaration of Helsinki. The study was registered at the Dutch trial registry (NTR7556).
Patients
Patients aged ≥18 years were eligible if they received on-label treatment with cabozantinib, lenvatinib, pazopanib, sorafenib (continuous dosing), regorafenib (3 weeks on, 1 week off), or sunitinib (continuous dosing or 4 weeks on, 2 weeks off). Patients were included before VEGFI treatment was started. Exclusion criteria were use of a diuretic or mineralocorticoid receptor antagonist at baseline to minimise the risk of hyponatremia or weight loss of ≥10% in the past six months indicating undernutrition [20]. All patients provided their written informed consent prior to study inclusion.
Study design including primary and secondary objectives
The primary objective was to investigate whether DSR could prevent or diminish VEGFI-induced hypertension. Since the BP rise in subsequent treatment cycles is usually of similar magnitude or larger, the BP change in the treatment period with DSR was compared with the treatment period before DSR [14, 21].
Patients who were normotensive (<135/85 mmHg day average) at baseline and developed VEGFI-induced hypertension (day mean >135/85 mmHg) or patients who experienced a significant and clinically relevant increase in BP (increase in systolic or diastolic blood pressure ≥20 mmHg) or patients who required start or increase in antihypertensive drug treatment due to systolic BP (SBP) repeatedly >170 mmHg during the first treatment cycle were selected to undergo the DSR intervention. BP was measured as day mean 24 h ambulatory blood pressure monitoring (ABPM).
The primary outcome was the difference in mean arterial pressure (MAP) between the VEGFI treatment cycle with and the treatment cycle without DSR. We chose MAP as reflection of both systolic and diastolic blood pressure.
Secondary outcomes included differences in proteinuria as a marker of nephropathy, measured by 24 h urinary protein excretion Proteinuria rather than albuminuria was chosen based on the common terminology criteria for adverse events (CTCAE) used in clinical oncological practice and Furthermore, we compared clinical and biochemical parameters between patients developing a clinically relevant increase in BP (intervention group) and those who did not develop a clinically relevant BP rise and thus finished the study after the second visit (non-intervention group), in particular differences in levels of ET-1, renin, aldosterone, PGI2 and TXA2.
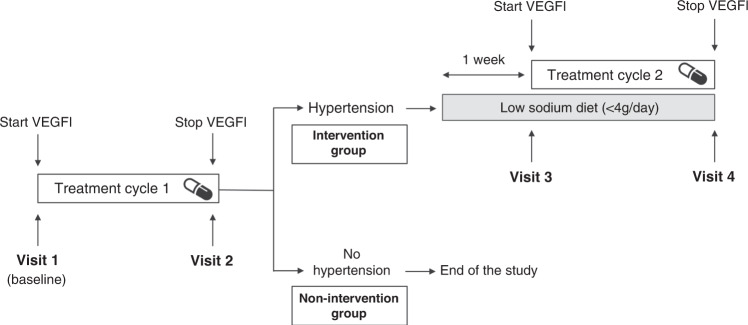
The DSR was started 1 week prior to the planned second VEGFI treatment cycle to allow normalisation of the BP and to apply DSR during the entire treatment cycle [21]. This meant that, for sunitinib, the 4 weeks on, 2 weeks off treatment cycle was maintained. For regorafenib, the standard rest period of one week was extended by five days. For continuously applied cabozantinib, lenvatinib, pazopanib, sorafenib or sunitinib, the second treatment cycle was postponed for 1–1.5 weeks to allow normalisation of BP and initiation of DSR (Fig. 1).
Fig. 1. Study design.
Measurements at time points: visit 1 (baseline): body weight, 24 h ABPM (or home measurement); blood: creatinine, sodium, potassium, aldosterone, renin, endothelin (ET-1); visit 2 (stop VEGFI), visit 3 (start VEGFI + DSR), visit 4 (stop VEGFI + DSR): body weight, 24 h ABPM (or home measurement); 24 h urine: sodium, potassium, protein, creatinine; blood: creatinine, sodium. potassium, renin, aldosterone, ET-1; visit 2 and visit 4: trough drug level used VEGF inhibitor. VEGFI-induced hypertension was defined as day MAP >135/85 mmHg or an increase of ≥20 mmHg in systolic and/or diastolic blood pressure.
Due to the coronavirus disease 2019 (COVID-19) pandemic, from May 2020 onwards, home BP measurements were allowed as replacement for 24 h ABPM according to European Society of Hypertension practice guidelines and recommendations for patients using VEGFI, as long as all measurements were performed using the same method (i.e., either per patient all 24 h ABPM or all home BP measurements) [21, 22].
Patients were referred to a dietician to be informed about DSR that consisted of a dietary intake of maximal 4 g or 70 mmol sodium per day for 4 (in case of regorafenib) or 5 (all others) weeks as performed previously [23].
In addition to dietary counselling, patients received salt-free bread for the whole intervention period. To increase the adherence to DSR, patients were contacted by the dietician after one and three weeks.
If a severe and consistent (at least three occasions at home measurement) BP occurred despite using DSR (SBP > 150 or diastolic blood pressure (DBP) > 95 mmHg), antihypertensive medication was prescribed according to a specified study scheme consisting of amlodipine 5 or 10 mg once daily as first choice. If a patient was already using a calcium channel blocker, doxazosin 4 or 8 mg once daily could be used.
Measurements
Clinical parameters (body weight, 24 h ABPM daytime and overall mean of SBP and DBP) and blood samples to determine creatinine, sodium, potassium, ET-1, renin, and aldosterone were collected at four time points: visit 1 (baseline, before VEGFI treatment was started), visit 2 (after 4 weeks of treatment and 3 weeks for regorafenib), visit 3 (1–1.5 weeks after the first VEGFI treatment cycle) and visit 4 (after 4 weeks of treatment and 3 weeks for regorafenib of the second treatment cycle) (Fig. 1). In addition, 24 h urine samples (for creatinine, sodium, potassium, protein) were collected at visits 2, 3, and 4. Using the oscillometric SpaceLabs 90207 monitor (SpaceLabs Healthcare, Issaquah, WA, USA), 24-h ABPM was recorded with the device attached to the non-dominant arm. Patients were instructed to relax their arms during the measurement and to write down their activities in a diary. BP was measured at 20 min interval during daytime (16 out 24 h) and 30-min interval during nighttime (8 out of 24 h). Day average was chosen to allow removal of 24 h ABPM at night if patients considered this too inconvenient. Measurements were included if >70% of the 24-h measurements were successful.
At visit 1, which coincided with the start of VEGFI treatment when information about the treatment and the current study was provided, asking for 24-h urine collection was considered too demanding for the patients
Blood samples to determine VEGFI were collected at visit 2 and visit 4 (Fig. 1). All study measurements were combined with regular visits and blood sampling for clinical care. Urinary creatinine, sodium, potassium and protein were determined at the Department of Clinical Chemistry of the Erasmus MC.
PGI2 and TXA2 were measured by their stable metabolites 6-keto-PGF1α and TXB2, respectively. Plasma levels of ET-1 (R&D systems. Mineapolis, USA), PGI2 (via stable metabolite 6-keto-PGF1α kit ADI-900-004, Enzo Life Sciences), TXA2 (via stable metabolite TXB2 kit ADI-900-004, Enzo Life Sciences) were determined using a chemiluminescent enzyme-linked immunosorbent assay (ELISA). Plasma renin was measured using an immunoradiometric assay (Cisbio, Saclay, France), and plasma aldosterone was measured by radioimmunoassay (Demeditec, Kiel, Germany), according to the manufacturer’s instructions. All samples were determined at the Laboratory sector Pharmacology, Vascular and Metabolic diseases of the Erasmus MC.
Levels of sunitinib were determined at the laboratory of Translational Pharmacology of Erasmus MC Cancer Institute using a validated ultra-performance liquid chromatography (UPLC)–tandem mass spectrometry (UPLC-MS/MS) method [24]. Other drug levels were measured at different laboratories but not reported due to low numbers.
Statistical analysis
The primary outcome was the difference in the VEGFI-induced rise in MAP between the treatment cycle with and without the DSR. Each patient was his/her own control. Assuming a decrease in blood pressure rise of 10 mmHg, considering a power of 80%, a one-sided alpha of 5% and a standard deviation (SD) of 15 mmHg based on previous studies [14], 16 patients were required. A one-sided alpha was chosen because we did not want to expose more patients to the DSR than necessary and we were certain that salt restriction would not lead to a rise in blood pressure. All main endpoints were analysed according to the intention-to-treat principle. Baseline characteristics were described with descriptive statistics. Results are presented as mean ± SD for normally distributed data, and median and interquartile range (IQR) for non-normally distributed data. The primary outcome was analysed by a one-sided paired t test. The secondary outcomes were analysed using a paired t test or in case of a non-normal distribution using the Wilcoxon signed-rank test. For correlation analysis, the Pearson r correlation coefficient and the Spearman’s rank correlation coefficient were used in case of normally and non-normally distributed data, respectively.
Data were analysed using SPSS Statistics (IBM, version 25.0). P values <0.05 were considered statistically significant.
Results
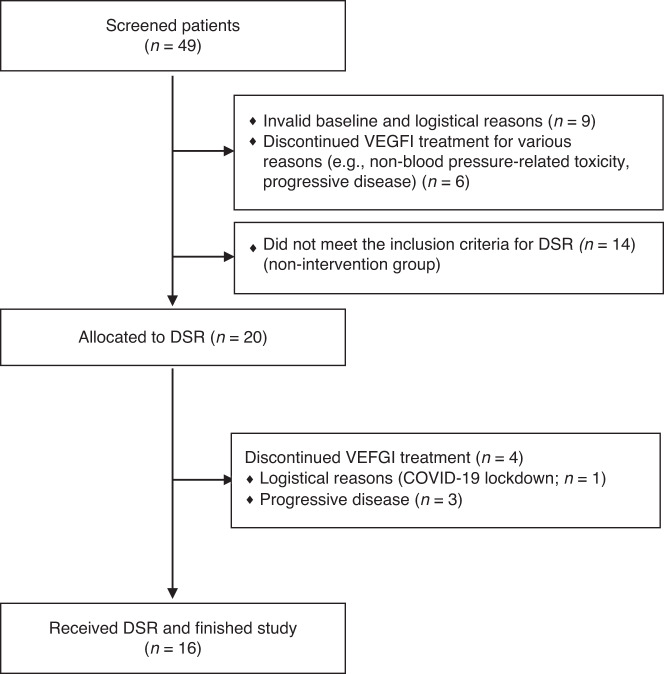
Patients were recruited between October 2018 and August 2021. Forty-nine patients were screened of whom 29 did not meet the inclusion criteria; 15 patients discontinued VEGFI during their first treatment cycle and 14 patients did not develop hypertension according to the inclusion criteria (Fig. 2).
Fig. 2. Flow diagram screening showing included and excluded patients.
DSR dietary sodium restriction.
Twenty patients were eligible for the intervention based on their blood pressure rise. Four patients were not available for follow-up due to interruption of medication for reasons such as non-blood pressure related toxicity or progressive disease. Therefore, 16 patients (“intervention group”; n = 16) were evaluable for this study. To study whether we could define risk factors for VEGFI-induced hypertension, we also studied patients who did not develop such VEGFI-induced hypertension (“non-intervention group”; n = 14).
Patient characteristics are summarised in Table 1; more detailed information on the VEGFI regimen is given in Supplementary Table 1. In the intervention group, mean age was 65.4 ± 8.8 years, and 69% were men. Three patients (19%) had a history of hypertension and were taking antihypertensive drugs. Before initiation of VEGFI treatment, mean SBP and DBP were 129 ± 18 and 78 ± 7 mmHg, respectively (24 h ABPM, n = 12; home measurements, n = 4). In the non-intervention group, mean age was 65.1 ± 11.3 years, 68% were men, and mean SBP and DBP were 115 ± 9 and 69 ± 8 mmHg, respectively (two-side P = 0.010 and 0.004, compared to the intervention group). Two patients in the non-intervention group (14%) were taking antihypertensive treatment at the start of VEGFI treatment.
Table 1.
Baseline characteristics of study participants and patients not eligible for intervention.
| Intervention group (n = 16) | Non-intervention group (n = 14) | |
|---|---|---|
| Men | 11 (69%) | 12 (86%) |
| Age, years | 65.4 ± 8.8 | 65.1 ± 11.3 |
| Hypertension | 3 (19%) | 2 (14%) |
| Angiotensin-converting enzyme inhibitor | 1 (6%) | 1 (7%) |
| Calcium channel blocker | 1 (6%) | 1 (7%) |
| β-Blocker | 1 (6%) | – |
| Body mass index (kg/m2) | 25.5 ± 3.9 | 26.4 ± 3.7 |
| eGFR (ml/min, 1.73 m2) mean ± SD | 74.6 ± 18.6 | 70 ± 24.3 |
| Ambulatory 24 h daytime BP or home measurements average | ||
| Mean arterial pressure (mmHg) | 95 ± 10.6 | 84 ± 8.3 |
| Systolic blood pressure (mmHg) | 129.3 ± 17.7 | 114 ± 9.3 |
| Diastolic blood pressure (mmHg) | 78.1 ± 7.1 | 69.4 ± 7.8 |
| Proteinuria (qualitative measurement) | ||
| Yes | 3 (19%) | 3 (21%) |
| No | 11(68%) | 5 (36%) |
| Not available | 2 (13%) | 6 (43%) |
| Type of treatment | ||
| Cabozantinib | 4 (25%) | 1 (7%) |
| Lenvatinib | 1 (6%) | 2 (14%) |
| Pazopanib | 1 (6%) | 1 (7%) |
| Regorafenib | 4 (25%) | 2 (14%) |
| Sorafenib | 1 (6%) | 2 (14%) |
| Sunitinib | 5 (31%) | 6 (43%) |
| Cancer, diagnosis | ||
| GIST | 2 (13%) | |
| HCC | 4 (25%) | 3 (21%) |
| RCC | 7 (44%) | 7 (50%) |
| Thyroid carcinoma | 2 (13%) | 3 (21%) |
Data are presented as n (%) and mean ± SD.
BP blood pressure, DBP diastolic blood pressure, eGFR estimated glomerular filtration rate, GIST gastrointestinal stromal tumour, HCC hepatocellular carcinoma, pNET pancreas neuroendocrine tumour, RCC renal cell carcinoma, SBP systolic blood pressure.
Primary outcome: effect of DSR on blood pressure rise
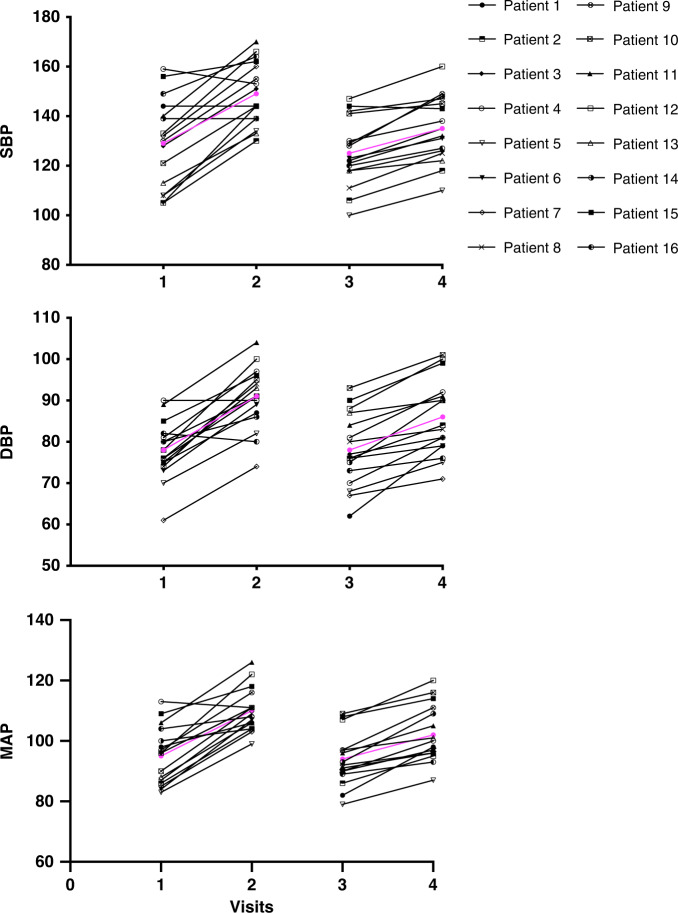
In the intervention group, at visit 1 before start of VEGFI, the daytime MAP was 95 ± 10 mmHg, which increased by 15 ± 8 mmHg at visit 2 (stop VEGFI) (P < 0.001). At visit 3 (start VEGFI + DSR), daytime MAP was 94 ± 9 mmHg that increased by 8 ± 4 mmHg to a daytime MAP of 102 mmHg at visit 4 (end of VEGFI + DSR) (P < 0.001). Thus, DSR significantly reduced the VEGFI-induced rise in MAP by 7 mmHg (95% CI 1.3–12.0; P = 0.009); Fig. 3).
Fig. 3. Effect of dietary salt restriction on blood pressure rise.
Systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP) before and after treatment with the VEGF inhibitor without and with dietary sodium restriction (DSR). Visits: 1: baseline; 2: end of treatment cycle 1; 3: baseline treatment cycle with dietary sodium restriction; 4: end treatment cycle with DSE. In purple, mean BP values. Patients 14 and 16 received amlodipine during the first treatment cycle because of a rise in SBP > 170 mmHg, which could be discontinued during DSR. Patients 1, 4, 11 and 15 received amlodipine or doxazosin during the first treatment cycle and continued during second treatment cycle.
In 12 patients in whom DSR was the only intervention to control the VEGFI-induced hypertension, the rise in daytime MAP was 18 ± 6 mmHg at visit 2 versus 8 ± 5 mmHg at visit 4 indicating that DSR successfully reduced the VEGFI-induced MAP rise by 10 mmHg. In 6 patients (37.5%), SBP increased to ≥170 mm Hg during the first VEGFI treatment cycle and escape medication was started. This high SBP concerned 1 patient already taking antihypertensive medication before VEGFI treatment, and 5 patients without antihypertensive medication. In 2 of these 6 patients, the added antihypertensive treatment could be discontinued during the stop week, and DSR was effective on its own in preventing VEGFI-induced hypertension until the end of the study period. In 1 of these 2 patients a dose reduction of the VEGFI was required due to mucositis. To illustrate the effect of the DSR, these patients are described in detail in the Supplementary Data. In the non-intervention group, daytime MAP at visit 1 was 84 ± 7 mmHg, which increased by 10 ± 5 mmHg at visit 2 (P = 0.010 vs. intervention group). Baseline MAP did not correlate with the rise in MAP (Supplementary Fig. 1).
Urine sodium and protein excretion
Urine sodium excretion decreased from 94 (77–135) (median (IQR)) mmol/24 h at visit 2 to 32 (24–49) mmol/24 h at visit 4 corresponding to 4.7 g salt intake (difference 62 (53–86) mmol/L, P < 0.001; Table 2). The decrease in urine sodium excretion confirmed adherence to DSR in all patients. The difference in urine sodium excretion between visits 2 and 4 did not correlate with the difference in rise in MAP between the two treatment cycles (r = −0.2, P = 0.5).
Table 2.
Plasma concentrations of endothelin-1 (ET-1), renin, aldosterone, Thromboxane B2 (TXB2) and 6-keto-prostaglandin F1α (PGF1α) before and at the end of VEGFI treatment without (treatment cycle 1) or with concomitant dietary sodium restriction (treatment cycle 2) in patients experiencing VEGFI-induced hypertension (intervention group).
| Treatment cycle 1 (VEGFI) | Treatment cycle 2 (VEGFI + dietary sodium restriction) | |||||||
|---|---|---|---|---|---|---|---|---|
| Start (visit 1) | End (visit 2) | P | Start (visit 3) | End (visit 4) | P | P visit 4 vs. visit 2 | P Δvisit 4−visit 3 vs. Δvisit 2−visit 1 | |
| Plasma | ||||||||
| ET-1, pg/ml | 2.1 (1.3–2.9) | 2.1 (1.7–3.6) | 0.63 | 1.7 (1.2–3.8) | 2.1 (1.4–3.3) | 0.30 | 0.21 | 0.95 |
| Renin, pg/ml | 14.4 (6.8–26.1) | 9.9 (5.9–30.7) | 0.76 | 23.3 (11.8–53.3) | 21.9 (13.5–51.6) | 0.60 | 0.13 | 0.98 |
| ET-1/renin ratio | 0.29 ± 0.1 | 0.39 ± 0.1 | 0.52 | 0.26 ± 0.1 | 0.17 ± 0.06 | 0.47 | 0.020 | 0.68 |
| Aldosterone, pg/ml | 259 (172–460) | 204 (168–420) | 0.56 | 332 (199–420) | 332 (226–612) | 0.45 | 0.018 | 0.45 |
| TXB2, pg/ml | 1351 (834–4062) | 1304 (567–2457) | 0.94 | 1740 (1175–2757) | 1744 (1082–3788) | 0.99 | 0.23 | 0.56 |
| 6-keto-PGF1α, pg/ml | 251 (182–414) | 585 (274–1098) | 0.025 | 355 (209–399) | 316 (252–620) | 0.32 | 0.056 | 0.055 |
| 24-h urine | ||||||||
| Sodium | 94 (77–135) | 44 (32–69) | 32 (24–49) | <0.001 | NA | |||
| Protein | 0.19 (0.11–0.32) | 0.16 (0.11–0.31) | 0.15 (0.10–0.25) | 0.0053 | NA | |||
Data are presented as median (IQR). P value indicates comparison between start and end of the same treatment cycle. P Δ indicates comparison of the within-cycle differences between treatment cycles 1 and 2.
VEGFI vascular endothelial growth factor inhibitor.
Urine sodium in the non-intervention group at visit 2 (129 (79–163) mmol/24 h) was not significantly different from that in the intervention group. Proteinuria in the intervention group was 0.19 (0.11–0.32) g/24 h at visit 2 and was significantly lower at visit 4 at the end of the DSR (0.15 (0.10–0.25) g/24 h (P = 0.005)) (Table 2). Two patients had proteinuria CTCAE grade 2 and 3 at visit 2, which was grade 1 and grade 2, respectively, at visit 4 (Supplementary Table 2). We were unable to make a direct comparison between the change in proteinuria in the treatment cycle with versus without DSR, given the lack of 24 h urine samples at visit 1. Proteinuria in the non-intervention group at visit 2 (0.12 (0.09–0.97) g/24 h) was comparable to that in the intervention group.
Effects on endothelin-1, renin, aldosterone and prostanoids
Plasma levels of ET-1, TXB2 and aldosterone did not change significantly during the treatment cycle without DSR (Table 2). Results in the non-intervention group were comparable with the exception that in this group ET-1 rose significantly (P = 0.022; Supplementary Table 3). A tendency towards a decrease in plasma renin was observed at visit 2 compared to visit 1. After start of the DSR (visit 3), renin levels were higher, as expected, and the slight decrease during VEGFI treatment was less in the treatment cycle with DSR, although this difference was not significant (visit 4). Given our previous observation that VEGFI increases circulating ET-1 and decreases renin, we also calculated the ET-1/renin ratio [14]. This ratio increased in both the intervention and non-intervention group, although this was significant only in the latter (P = 0.017). The ET-1/renin ratio was lower at visit 4 than at visit 2 (P = 0.020). Also, the difference in the ET-1/renin ratio demonstrated a trend towards a positive correlation with the rise in MAP in the intervention group (Spearman correlation R = 0.498, P = 0.072; Fig. 4).
Fig. 4. Correlation changes in endothelin (ET-1)/renin ratio and daytime mean arterial pressure (MAP).
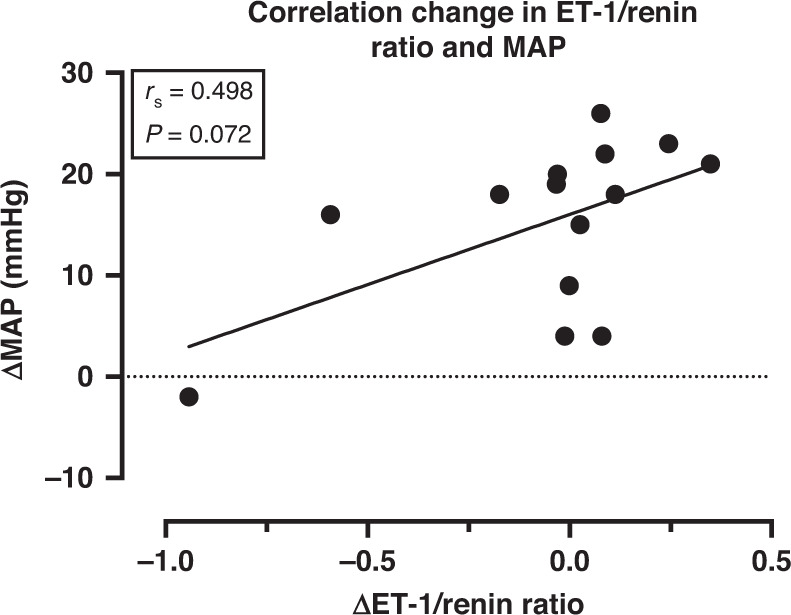
Correlation coefficient calculated using Spearman rank-order correlation.
As expected, DSR upregulated aldosterone in the intervention group (P = 0.018 for the difference between visit 2 and 4), which was accompanied by a non-significant rise in renin (P = 0.13). VEGFI treatment without DSR increased plasma 6-keto-PGF1α (P = 0.025), while it decreased during DSR although this difference was not significant (P = 0.056).
VEGFI levels
To verify that the preventive effects of DSR on VEGFI-induced hypertension was not mediated by a reduction in plasma VEGFI concentrations, sunitinib trough levels were measured without (visit 2) and with DSR (visit 4) (n = 5) (Supplementary Fig. 2). Variation in drug levels was high especially at visit 4, but there was no indication that reduction in MAP rise at visit 4 was due to a reduction in VEGFI levels. Although similar data were obtained for regorafenib, sorafenib and cabozantinib, no formal statistical analyses were possible due to low numbers of patients per VEGFI (data not shown).
Treatment safety
During DSR, 2 patients with antihypertensive treatment developed dizziness, which disappeared after adjustment of antihypertensive treatment. There were no other related serious adverse events during the intervention period with DSR. Five patients continued DSR voluntarily after the end of the study.
Discussion
Hypertension is the most frequently observed side effect of VEGFI. This study demonstrates that the daytime MAP in patients receiving VEGFI treatment is significantly lower by application of DSR. To the best of our knowledge, this is the first prospective study investigating—and proving—the effect of DSR on VEGFI-induced hypertension. Thus, DSR appears an effective strategy to prevent VEGFI-induced hypertension. In 12 out of the 16 patients who developed hypertension during the first treatment cycle, blood pressure could be significantly reduced using DSR only, whereas this routinely would have been managed using antihypertensive drugs. Moreover, DSR was well tolerated, as indicated by the voluntary continuation of DSR by five of the patients. These promising results warrant prospective studies to address the long-term effects and tolerability of DSR, and to evaluate whether it could replace antihypertensive drugs in the treatment of VEGFI-induced hypertension.
The observed antihypertensive effect is in line with the previously demonstrated salt sensitivity of sunitinib-induced hypertension in preclinical studies [25]. Proteinuria during VEGFI treatment was lower at the end of the treatment cycle with DSR than at the end of the treatment cycle without, comparable with the difference between high and low salt diet in rats [4]. However, since we did not collect urine samples before the first VEGFI treatment cycle, it cannot be concluded that DSR directly prevented or reduced VEGFI-induced proteinuria.
To assess whether we could predict a clinically relevant BP rise to select eligible patients at start of VEGFI treatment, we also studied patients not fulfilling the eligibility criteria for intervention (non-intervention group). It should be noted that their BP rise was more modest than the patients eligible to undergo the intervention, but not absent. Differences in sodium excretion at visit 2 corresponding to pre-existent salt intake and differences in baseline MAP were not correlated to the rise in MAP during VEGFI (Supplementary Fig. 1). Therefore, most likely, the rise in BP is determined by a combination of factors, including the type of VEGFI.
The pathophysiology of the salt sensitivity of VEGFI-induced hypertension is currently not fully understood. ET-1 is not only an important factor in VEGFI-induced hypertension [13, 14], but may also contribute to salt sensitivity: high salt leads to higher ET-1 levels, and the vasoconstrictive responses to ET-1 are increased in a high-salt environment [26, 27]. Salt accumulation in the skin, due to inappropriate lymphangiogenesis, is a proposed mechanism underlying salt-sensitive hypertension [6–8]. A recent pilot study confirmed the hypothesis that salt accumulation is increased during treatment with VEGFI [28]. This might be caused by effects on lymphangiogenesis dependent on macrophage-derived VEGF-C acting on VEGF-3 receptors [29]. VEGFI target this pathway, and thus diminished lymphangiogenesis could occur during VEGFI treatment [9]. ET-1is believed to play a role in lymphangiogenesis although its exact role is still unknown [30, 31]. Since ET-1 is released abluminally, it is still possible that local rises in ET-1 (outside the circulation) were higher in patients who developed a higher BP, so that ET-1 plasma levels are not representative for the involvement of ET-1 in salt sensitivity [32]. Clearly, a rise in ET-1 is a uniform phenomenon in VEGFI-treated patients. Yet, its final effects on BP seems to vary, among others because of its salt-modulating and RAAS-suppressing properties [33].
Another player that could upregulate VEGF-C is COX-2-dependent thromboxane (TP) receptor signalling [34, 35], which is either stimulated by the natural TP receptor agonist TXA2, or excessive levels of PGI2. Since COX-1 predominantly generates TXA2, while COX-2 predominantly generates PGI2, a unifying concept might be that the elevated PGI2 levels observed after VEGFI in this and an earlier study [18, 36] are needed to allow normal lymphangiogenesis. This could also be translated to the related condition preeclampsia. Preeclampsia, like VEGFI treatment, resembles a state of VEGF suppression [15]. In this condition ET-1 levels are also upregulated, renin is lowered, and the PGI2/TXA2 balance is disregulated [17, 37, 38]. It is now well-established that acetyl salicylic acid (ASA) reduces the risk of preeclampsia although its mechanism of action is still not fully understood [17, 39]. Given the low (COX-1-selective) doses that are usually applied, it might rely on blockade of COX-1 rather than COX-2. Nevertheless, in rats ASA prevented sunitinib-induced hypertension at a low (COX-1-selective) dose, while the prevention of proteinuria required a high (COX-1- and COX-2-blocking) dose. The high dose also prevented the rise in PGI2 that was observed after VEGFI [18, 36]. Our patient study now confirms this PGI2 rise, which is prevented by DSR. Future studies are required to establish the precise contribution of COX-1 and COX-2 and the role of PGI2 in VEGFI-induced hypertension and preeclampsia, and the interaction with salt intake. Initial studies applying DSR to treat preeclampsia were inconclusive. However, a recent study demonstrated that women with a lower dietary sodium intake had a lower risk of developing preeclampsia, in line with our study design of applying salt restriction in a period without VEGFI instead of starting during VEGFI-induced hypertension [40, 41]. DSR and ASA are both mild interventions that deserve further exploration in clinical practice.
RAAS activation is unlikely to be the initiator of VEGFI-induced hypertension [42]. We earlier described a patient developing VEGFI induced hypertension after bilateral adrenalectomy, ruling out a major role for aldosterone [42]. Moreover, plasma renin concentrations usually decrease during VEGFI treatment [14], most likely representing the normal physiological response to a rise in BP. Given the modest changes in ET-1 and renin, we calculated the ET-1/renin ratio as a more powerful tool to evaluate these changes. This ratio increased after VEGFI treatment prior to DSR (although significant only in the non-intervention group), and was diminished in the intervention group after DSR. The latter corresponds with the concept that a lower rise in BP induces a smaller drop in renin.
An important strength of the current study is the prospective design. Although the field of cardio-oncology is expanding rapidly, prospective studies and intervention studies in particular are scarce. The study combines a clinically relevant primary research question with biochemical parameters further elucidating the pathophysiology of VEGFI-induced hypertension. The number of patients treated (16) may seem low, but this was exactly the number we determined a priori in our sample size calculation, and in our estimations we also took into account that around 1/3 would be eligible (protocol published Dutch trial register NTR7556). We included until this number of patients undergoing the intervention was reached. Our power calculation was based on a clinically relevant decrease in blood pressure rise to minimise the number of patients required to undergo the potentially burdensome intervention.
Some limitations should be mentioned. Firstly, this was not a randomised controlled trial (RCT). However, our design was pragmatic aiming to limit the number of patients needed to be screened and undergo the intervention due to the potential burden of 24 h ABPMs and the intervention. Furthermore, we were afraid that patients in the control group of a RCT might limit salt intake based on the information in the patient leaflet as we know from other trials, making the contrast less large. Since blood pressure rise is comparable in subsequent cycles when no intervention has been started this enables comparison within one patient without the risk of “regression to the mean” [14, 21]. However, changes in antihypertensive drug regime and changes in VEGFI dosing made interpretation of the exact magnitude of the effect on blood pressure rise difficult. Now we have shown the efficacy and safety in this proof-of-concept study that a larger randomised controlled trial is possible. Secondly, patients with different VEGFI were included. However, the mechanism of VEGFI-induced hypertension is generalisable to all VEGFIs. Also, patients with hypertension and on antihypertensive treatment at baseline were included. Even though this can be considered a bias to adequately assesses the VEGFI-induced blood pressure rise, this represents a real-life representation of patients for whom VEGFI treatment are prescribed. To limit the number of additional measurements at start of the study (and start of the VEGFI treatment for the patient), we did not collect 24 h urine sodium measurement at baseline and therefore no difference between visit 2 and visit 1 could be calculated. Possibly, the three patients with sodium levels of <70 mmol/24 h at visit 2 already started DSR after reading about a potential benefit in the patient leaflet. However, they still developed a blood pressure rise that made them eligible to undergo the intervention and DSR lowered sodium excretion even further. Lastly, due to the COVID-19 pandemic, BP results of the final four included patients undergoing the intervention were obtained from home measurements rather than 24 h ABPM assessments. Nevertheless, since these four patients used a validated BP monitor and a standardised form for recording BP we consider the data just as reliable and reproducible. Since the same type of measurements was used for each individual patient for all time points, we expect the effect on the studied differences to be minimal.
In conclusion, our study shows that DSR is an effective intervention to prevent VEGFI-induced hypertension which can prevent the need to reduce the VEGFI dose or to prescribe antihypertensive drugs. DSR therefore should be considered a relevant—and cheap and easy to perform—intervention in case of VEGF-induced hypertension. Although we did not formally assess quality of life during DSR, based on personal feedback from the participating patients, the intervention appeared well-tolerated. This is supported by the high adherence to DSR during the study and the fact that more than 30% of patients chose to continue DSR after the study period. Effective DSR requires the use of salt-free bread, clear instructions and follow-up by a dietitian [23]. In the current study, we chose a strict limit of dietary salt intake of 4 grams. A follow-up RCT should confirm our findings and define which patients benefit most from dietary salt restriction, what is the best time point to start DSR, and how strict the DSR needs to be to optimise efficacy and tolerability as long as VEGFI-treatment is effective.
Supplementary information
Acknowledgements
We thank Frank Geurts for help with the analyses and collecting the urine samples.
Author contributions
LvD helped design the study, included patients, helped in sample collection, performed the analyses, interpreted the results and wrote the first draft of the manuscript. WJV helped design the study, developed the sodium restricted diet and monitoring plan, helped in sample collection and analyses and reviewed the manuscript. DCHvD helped in sample collection, analyses, writing and reviewing the manuscript. KMMC and SLWK helped in analyses and reviewing the manuscript. AvE-dM helped in applying and monitoring the sodium restricted diet and reviewing the manuscript. IMG, DMB and SB helped in data acquirement, analyses and reviewing the manuscript. EO-dH helped in design of the statistical plan and helped in interpreting the results and reviewed the manuscript. FALME included helped in data acquisition, interpreting the results and writing and reviewing the manuscript. EJH helped design the study and reviewed the manuscript. AHJD and RHJM helped design the study and in interpretation of the results and helped in writing and reviewing the manuscript. JV had the original research idea, received funding, designed the study, performed the analyses and helped writing and reviewing the manuscript. All authors approved the final version and agreed to be accountable for all aspects of the work.
Funding
This work was funded by Stichting De Merel, The Hague, the Netherlands. The funders had no role in study design, data collection and analysis, or preparation of the manuscript.
Data availability
Data will be made available upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Medical Ethics Committee from the Erasmus University Medical Center (MEC-2018-155) and complies with the Declaration of Helsinki. The study was registered at the Dutch trial registry (NTR7556).
Consent for publication
No individual person’s data are given.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Wesley J. Visser, Daan C. H. van Dorst.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-02036-6.
References
- 1.Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15:385–403. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 2.Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375:1457–67. doi: 10.1056/NEJMra1100265. [DOI] [PubMed] [Google Scholar]
- 3.van Dorst DCH, Dobbin SJH, Neves KB, Herrmann J, Herrmann SM, Versmissen J, et al. Hypertension and prohypertensive antineoplastic therapies in cancer patients. Circ Res. 2021;128:1040–61. doi: 10.1161/CIRCRESAHA.121.318051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lankhorst S, Baelde HF, Clahsen-van Groningen MC, Smedts FM, Danser AH, van den Meiracker AH. Effect of high salt diet on blood pressure and renal damage during vascular endothelial growth factor inhibition with sunitinib. Nephrol Dial Transplant. 2016;31:914–21. doi: 10.1093/ndt/gfv410. [DOI] [PubMed] [Google Scholar]
- 5.Gu JW, Manning RD, Jr., Young E, Shparago M, Sartin B, Bailey AP. Vascular endothelial growth factor receptor inhibitor enhances dietary salt-induced hypertension in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R142–8. doi: 10.1152/ajpregu.90972.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–52. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 7.Selvarajah V, Mäki-Petäjä KM, Pedro L, Bruggraber SFA, Burling K, Goodhart AK, et al. Novel mechanism for buffering dietary salt in humans: effects of salt loading on skin sodium, vascular endothelial growth factor C, and blood pressure. Hypertension. 2017;70:930–7. doi: 10.1161/HYPERTENSIONAHA.117.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlsen TV, Nikpey E, Han J, Reikvam T, Rakova N, Castorena-Gonzalez JA, et al. High-salt diet causes expansion of the lymphatic network and increased lymph flow in skin and muscle of rats. Arterioscler Thromb Vasc Biol. 2018;38:2054–64. doi: 10.1161/ATVBAHA.118.311149. [DOI] [PubMed] [Google Scholar]
- 9.Wiig H, Schröder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest. 2013;123:2803–15. doi: 10.1172/JCI60113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Totzeck M, Mincu RI, Mrotzek S, Schadendorf D, Rassaf T. Cardiovascular diseases in patients receiving small molecules with anti-vascular endothelial growth factor activity: a meta-analysis of approximately 29,000 cancer patients. Eur J Prev Cardiol. 2018;25:482–94. doi: 10.1177/2047487318755193. [DOI] [PubMed] [Google Scholar]
- 11.Versmissen J, Mirabito Colafella KM, Koolen SLW, Danser AHJ. Vascular cardio-oncology: vascular endothelial growth factor inhibitors and hypertension. Cardiovasc Res. 2019;115:904–14. doi: 10.1093/cvr/cvz022. [DOI] [PubMed] [Google Scholar]
- 12.Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: systematic review and meta-analysis. Acta Oncol. 2009;48:9–17. doi: 10.1080/02841860802314720. [DOI] [PubMed] [Google Scholar]
- 13.Kappers MH, Smedts FM, Horn T, Van Esch JH, Sleijfer S, Leijten F, et al. The vascular endothelial growth factor receptor inhibitor sunitinib causes a preeclampsia-like syndrome with activation of the endothelin system. Hypertension. 2011;58:295–302. doi: 10.1161/HYPERTENSIONAHA.111.173559. [DOI] [PubMed] [Google Scholar]
- 14.Kappers MH, Van Esch JHM, Sluiter W, Sleijfer S, Danser AHJ, Van den Meiracker AHJ. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension. 2010;56:675–81. doi: 10.1161/HYPERTENSIONAHA.109.149690. [DOI] [PubMed] [Google Scholar]
- 15.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 16.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Investig. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirabito Colafella KM, Neuman RI, Visser W, Danser AHJ, Versmissen J. Aspirin for the prevention and treatment of pre-eclampsia: a matter of COX-1 and/or COX-2 inhibition? Basic Clin Pharmacol Toxicol. 2020;127:132–41. doi: 10.1111/bcpt.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirabito Colafella KM, Neves KB, Montezano AC, Garrelds IM, Van Veghel R, De, et al. Selective ETA versus dual ETA/B receptor blockade for the prevention of sunitinib-induced hypertension and albuminuria in WKY rats. Cardiovasc Res. 2019;116:1779–90. doi: 10.1093/cvr/cvz260. [DOI] [PubMed] [Google Scholar]
- 19.de Jesus-Gonzalez N, Robinson E, Moslehi J, Humphreys BD. Management of antiangiogenic therapy-induced hypertension. Hypertension. 2012;60:607–15. doi: 10.1161/HYPERTENSIONAHA.112.196774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bossi P, Delrio P, Mascheroni A, Zanetti M. The spectrum of malnutrition/cachexia/sarcopenia in oncology according to different cancer types and settings: a narrative review. Nutrients. 2021;13:1980. [DOI] [PMC free article] [PubMed]
- 21.Azizi M, Chedid A, Oudard S. Home blood-pressure monitoring in patients receiving sunitinib. N Engl J Med. 2008;358:95–7. doi: 10.1056/NEJMc072330. [DOI] [PubMed] [Google Scholar]
- 22.Parati G, Stergiou G, O’Brien E, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359–66. doi: 10.1097/HJH.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 23.Bovée DM, Visser WJ, Middel I, De Mik-van Egmond A, Greupink R, Masereeuw R, et al. A randomized trial of distal diuretics versus dietary sodium restriction for hypertension in chronic kidney disease. J Am Soc Nephrol. 2020;31:650–62. doi: 10.1681/ASN.2019090905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kloth JS, Klümpen HF, Yu H, Eechoute K, Samer CF, Kam BLR, et al. Predictive value of CYP3A and ABCB1 phenotyping probes for the pharmacokinetics of sunitinib: the ClearSun study. Clin Pharmacokinet. 2014;53:261–9. doi: 10.1007/s40262-013-0111-4. [DOI] [PubMed] [Google Scholar]
- 25.Lankhorst S, Kappers MHW, Van Esch JHM, Smedts FMM, Sleijfer S, Mathijssen RHJ, et al. Treatment of hypertension and renal injury induced by the angiogenesis inhibitor sunitinib: preclinical study. Hypertension. 2014;64:1282–9. doi: 10.1161/HYPERTENSIONAHA.114.04187. [DOI] [PubMed] [Google Scholar]
- 26.Khalil RA. Modulators of the vascular endothelin receptor in blood pressure regulation and hypertension. Curr Mol Pharmacol. 2011;4:176–86. doi: 10.2174/1874467211104030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Ren Physiol. 2001;281:F144–150. doi: 10.1152/ajprenal.2001.281.1.F144. [DOI] [PubMed] [Google Scholar]
- 28.Markó L, Dörr A, Linz P, Van den Meiracker AH, Garrelds IM, Kuehne T, et al. Effect of sunitinib treatment on skin sodium accumulation in patients with renal cancer: a pilot study. Hypertension. 2022;79:e103–e105. doi: 10.1161/HYPERTENSIONAHA.122.19079. [DOI] [PubMed] [Google Scholar]
- 29.Dahlmann A, Linz P, Zucker I, Haag V, Jantsch J, Dienemann T, et al. Reduction of tissue Na(+) accumulation after renal transplantation. Kidney Int Rep. 2021;6:2338–47. doi: 10.1016/j.ekir.2021.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lankhorst S, et al. Salt sensitivity of angiogenesis inhibition-induced blood pressure rise: role of interstitial sodium accumulation? Hypertension. 2017;69:919–26. doi: 10.1161/HYPERTENSIONAHA.116.08565. [DOI] [PubMed] [Google Scholar]
- 31.Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev. 2011;91:1–77. doi: 10.1152/physrev.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner OF, Christ G, Wojta J, Vierhapper H, Parzer S, Nowotny PJ, et al. Polar secretion of endothelin-1 by cultured endothelial cells. J Biol Chem. 1992;267:16066–8. [PubMed] [Google Scholar]
- 33.Verdonk K, Saleh L, Lankhorst S, Smilde JEI, Van Ingen MM, Garrelds IM, et al. Association studies suggest a key role for endothelin-1 in the pathogenesis of preeclampsia and the accompanying renin-angiotensin-aldosterone system suppression. Hypertension. 2015;65:1316–23. doi: 10.1161/HYPERTENSIONAHA.115.05267. [DOI] [PubMed] [Google Scholar]
- 34.Matsuda H, Ito Y, Hosono K, Tsuru S, Inoue T, Nakamoto S, et al. Roles of thromboxane receptor signaling in enhancement of lipopolysaccharide-induced lymphangiogenesis and lymphatic drainage function in diaphragm. Arterioscler Thromb Vasc Biol. 2021;41:1390–407. doi: 10.1161/ATVBAHA.120.315507. [DOI] [PubMed] [Google Scholar]
- 35.Kashiwagi S, Hosono K, Suzuki T, Takeda A, Uchinuma E, Majima M. Role of COX-2 in lymphangiogenesis and restoration of lymphatic flow in secondary lymphedema. Lab Invest. 2011;91:1314–25. doi: 10.1038/labinvest.2011.84. [DOI] [PubMed] [Google Scholar]
- 36.Mirabito Colafella KM, Van Dorst DCH, Neuman RI, Van Doorn L, Neves KB, Montezano AC, et al. Differential effects of cyclo-oxygenase 1 and 2 inhibition on angiogenesis inhibitor-induced hypertension and kidney damage. Clin Sci. 2022;13:675–94. doi: 10.1042/CS20220182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verdonk K, Visser W, Van den Meiracker AH, Danser AHJ. Are aldosterone levels inappropriately low in preeclampsia? Hypertension. 2013;62:e39. doi: 10.1161/HYPERTENSIONAHA.113.02006. [DOI] [PubMed] [Google Scholar]
- 38.Verdonk K, Visser W, Van Den Meiracker AH, Danser AH. The renin-angiotensin-aldosterone system in pre-eclampsia: the delicate balance between good and bad. Clin Sci. 2014;126:537–44. doi: 10.1042/CS20130455. [DOI] [PubMed] [Google Scholar]
- 39.Roberge S, Giguére Y, Villa P, Nicolaides K, Vanio M, Forest JC, et al. Early administration of low-dose aspirin for the prevention of severe and mild preeclampsia: a systematic review and meta-analysis. Am J Perinatol. 2012;29:551–6. doi: 10.1055/s-0032-1310527. [DOI] [PubMed] [Google Scholar]
- 40.Birukov A, Andersen LB, Herse F, Rakova N, Kitlen G, Kyhl HB, et al. Aldosterone, salt, and potassium intakes as predictors of pregnancy outcome, including preeclampsia. Hypertension. 2019;74:391–8. doi: 10.1161/HYPERTENSIONAHA.119.12924. [DOI] [PubMed] [Google Scholar]
- 41.Moutquin JM, Garner PR, Burrows RF, Rey E, Helewa ME, Lange IR, et al. Report of the Canadian Hypertension Society Consensus Conference: 2. Nonpharmacologic management and prevention of hypertensive disorders in pregnancy. CMAJ. 1997;157:907–19. [PMC free article] [PubMed] [Google Scholar]
- 42.Versmissen J, Van Doorn L, Mirabito Colafella KM, Mathijssen RH, Danser AHJ. Sunitinib-induced blood pressure rise does not involve aldosterone: observations in a patient after bilateral adrenalectomy. J Hypertens. 2018;36:2279–80. doi: 10.1097/HJH.0000000000001894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon reasonable request.