Abstract
Introduction:
Accumulating evidence links hearing loss to impaired cognitive performance and increased risk for dementia. Hearing loss can lead to deafferentation-induced atrophy of frontotemporal brain regions and dysregulation of cognitive control networks from increased listening effort. Hearing loss is also associated with reduced social engagement, loneliness, and depression, which are independently associated with poor cognitive function.
Areas Covered:
We summarize the evidence and postulated mechanisms linking hearing loss to dementia in older adults and synthesize the available literature demonstrating beneficial effects of hearing remediation on brain structure and function.
Expert Opinion:
Further research is needed to evaluate whether treatment of hearing loss may reduce risk of cognitive decline and improve neural consequences of hearing loss. Studies may investigate the pathologic mechanisms linking these late-life disorders and identify individuals vulnerable to dementia, and future clinical trials may evaluate whether hearing treatment may reduce risk for dementia.
Keywords: cognitive decline, dementia, hearing loss, hearing aids, modifiable risk factor
Plain Language Summary
Evidence suggests that hearing loss is associated with impaired cognitive performance, increased risk for dementia, and poor brain health in older adults. Fortunately, preliminary studies have shown positive effects of hearing treatment on cognitive outcomes and reversal of adverse neural effects of hearing loss. In the following, we review the available evidence providing support for hearing loss as a modifiable risk factor for dementia. However, there are important limitations to such findings and further research is needed to definitively evaluate whether hearing treatment may protect cognitive and brain health in older adults. If future studies demonstrate that hearing treatment has beneficial effects on cognitive and neural outcomes, such results may take advantage of the recent widespread efforts to improve access to hearing treatment in the United States.
1. INTRODUCTION
Historically considered a benign component of aging, hearing loss (HL) in recent years has been associated with cognitive decline, dementia, and poor brain health in older adults. The 2020 Lancet Commission on dementia estimated that HL is the single largest potentially modifiable risk factor for dementia, accounting for 8% of the population attributable risk for dementia [1]. As HL also is linked to depression, frailty, physical inactivity, and other risk factors for dementia, the impact of HL on cognitive health may even be underestimated. Unfortunately, many older adults with HL go without treatment due to issues related to accessibility, affordability [2], and stigma around HL and aging. High prevalence of HL among older adults (>50% of Americans over 60 years old with HL [3]) coupled with low treatment levels [4] has resulted in widespread efforts to increase availability of HL treatment [2,5,6], including introduction and bipartisan passage of the Over-the-Counter Hearing Aid Act of 2017 in the United States [7].
In concert with accumulating data linking HL to poor brain health, evidence has begun to emerge of specific neural and behavioral mechanisms linking peripheral HL to cognitive dysfunction [8–10]. Preliminary studies have also provided evidence that treatment of HL also may reverse specific maladaptive neuroplastic changes associated with chronic HL. These data provide hope that novel therapeutic targets can be identified and strategies to optimize brain health in the setting of HL can be developed, however a critical synthesis of the relevant literature is needed given divergent findings and methodological complexity among studies of HL and neural functioning. In this review, we summarize the evidence and postulated mechanisms linking HL to dementia in older adults, synthesize the available literature demonstrating beneficial effects of hearing remediation on brain structure and function and propose future research directions to better understand the relationship between HL and dementia.
2. PHYSIOLOGY AND PATHOPHYSIOLOGY OF HEARING LOSS
The ability to hear depends on the precise encoding of sound into a neural signal by the peripheral auditory system followed by decoding of the signal into meaning by the brain. When used in the context of this review and unless stated otherwise, ‘hearing loss’ refers to impairments of the peripheral auditory system (cochlea) that affect the precise peripheral encoding of sound. Audiometry is the most common method used to assess hearing ability, and audiometric measures reflect the sensitivity of the peripheral auditory system to detect pure tones. Importantly, detection of pure tones does not substantively depend on higher-order cortical processing [11], meaning that audiometry can be reliably performed in adults with early dementia [12]. Age-related HL is the most common form of HL observed in adults and reflects progressive, irreversible damage to cells within the cochlea. The cochlea is particularly susceptible to damage over time given that most of the inner ear is post-mitotic (and hence incapable of regeneration), with risk factors for HL being age, race, sex, and noise exposure. Animal models of age-related HL as well as postmortem human temporal bone specimens from older adults demonstrate loss of sensory inner and outer hair cells, damage to the stria vascularis, and loss of cochlear nerve fibers [13]. The end result of accumulated damage to the cochlea (‘sensorineural HL’) is impaired encoding of sound and transmission of an impoverished and degraded auditory signal to the brain [14].
3. PERIPHERAL VERSUS CENTRAL AUDITORY FUNCTION AND DEMENTIA
Higher order assessments of hearing that involve speech, speech-in-noise, or other measures of central auditory function (e.g., dichotic digits) depend on both peripheral encoding of sound as well as extensive central cortical and subcortical pathways for auditory processing [15]. These latter assessments of hearing are more indicative of listening in real-world settings and reflect the combined functional output of both the peripheral and central auditory systems. This review paper focuses only on the role of peripheral hearing and cognitive impairment given that the implications of associations between peripheral versus central auditory function with cognitive function are very different. In the former case, there may be potential mechanistic pathways (detailed later in this review) through which peripheral hearing contributes to cognitive impairment and which may be modifiable. In the latter case, central auditory measures may be acting as a biomarker or surrogate for the same cortical processes underlying dementia. However, unraveling the specific contribution of central (versus peripheral) HL to dementia is complicated, since coexisting peripheral deficits may confound measurements of downstream central auditory processing dysfunction [15].
In the case of central auditory dysfunction, one must consider the reverse pathway (i.e., cognitive decline causing reduced hearing capacity). While AD-specific risk factors such as APOE ε4 allele status have not been associated with peripheral HL [16], not have neuropathologic changes associated with AD been found in peripheral auditory pathways [17–20], the neural and behavioral manifestations of central HL can be difficult to distinguish from that of dementia [21]. For example, neurodegenerative processes implicated in the development of AD can degrade auditory brain function in temporal and parietal areas, which can itself damage central hearing function and affect speech-in-noise perception [15]. Likewise, the relative death of studies linking speech-in-noise perception to cognitive function further limits our understanding of the complex dynamic between central auditory function and cognition, both of which draw in large part on the same cortical resources. One large (N=82,039) study from the UK Biobank highlighted the important role of speech-in-noise perception, a measure of central HL, as a predictor of incident dementia [22]. However, as speech-in-noise performance can be a biomarker for cognitive impairment itself, it is difficult to distinguish between the pathophysiological processes underlying central auditory dysfunction and dementia.
4. HEARING LOSS AS A RISK FACTOR FOR DEMENTIA
HL has been linked consistently to accelerated cognitive decline [23] and increased risk of incident dementia [1] in population-based observational studies of older adults. Against the benefit of large sample sizes in these studies are unavoidable limitations in the measurements of hearing and cognition. Many population-based longitudinal studies evaluate cognition only using clinical diagnosis of dementia or global cognitive screening tools, as opposed to comprehensive neuropsychological assessments with rigorous diagnostic evaluations and strict biomarker support. Such analyses often do not speak to the specific dementia subtype, and ‘Dementia’ as referenced in this section refers to all-cause clinical diagnosis of dementia.
Moreover, available studies often enroll participants who are cognitively normal without pre-existing cognitive impairment and do not include adequate methods to assess for dementia status upon study dropout, which may underestimate the dementia conversion rates attributable to HL on follow-up. Lastly, while many cognitive assessment procedures most utilized in these studies are administered with only verbal (vs. written) instructions, neurocognitive testing in such studies generally occurs in a quiet room involving face-to-face communication between the research participant and examiner. Evidence suggests that HL is still associated with worse cognitive performance even when excluding tests that only rely on auditory stimuli (as opposed to written stimuli) [24,25]. Despite these limitations, the risk imposed by HL on cognitive performance in older adults is a replicated finding that compels research on pathophysiologic mechanisms as well as interventions to mitigate this potential risk.
To cite the strongest studies linking HL to cognitive decline, one meta-analysis of nine prospective cohort studies (mean follow-up 10.4 years) found that HL was associated with accelerated rates of cognitive decline across multiple cognitive domains [23]. In one analysis of N=639 older adults enrolled in the Baltimore Longitudinal Study on Aging who were dementia-free at baseline, those with mild HL (vs. normal hearing) had 1.89 times the risk of developing dementia over 12 years; the estimated risk for individuals with moderate HL (vs. normal hearing) was 3.00 [26]. In another analysis of N=1,889 participants from the Health, Aging and Body Composition Study, moderate-severe HL was associated with 1.55 times the risk of dementia over 9 years. Overall, HL has been estimated to increase risk for dementia by 94% [27], with risk increasing as HL severity increases [28,29].
5. MECHANISMS LINKING HEARING LOSS TO DEMENTIA
Recent research has begun to examine the postulated neural and behavioral pathways linking HL to cognitive dysfunction in older adults. Knowledge of the pathologic mechanisms may identify older adults with HL who may benefit from early interventions and facilitate the development of rationally designed therapeutics to augment hearing treatment in order improve cognitive outcomes. There is accumulating evidence that the development of dementia is related to complex interactions between neuropathological processes and environmental or social factors [1]. We hypothesize that the neural and behavioral mechanisms reviewed below are not specific to a certain neuropathology, as there is limited evidence that HL induces neuropathologic changes associated with dementia (e.g., β-Amyloid, Tau, vascular pathology). Rather, chronic HL through such mechanisms may lower available cognitive reserve, thereby increasing an individual’s susceptibility to functional impairment secondary to existing neuropathological damage.
However, before assuming that compensatory neural changes initiated by chronic HL are in a causal pathway leading to dementia, one must consider that common causes may underly both age-related changes in cognition and hearing [30]. Chronic inflammation, vascular pathology [31], and oxidative stress [32], contribute to both age-related cochlear degeneration and the pathogenesis of neurodegenerative diseases such as Alzheimer’s disease (AD) [33]. Central nervous system–wide structural and functional decline related to normative aging also may result in the development of this frequent comorbidity, leading some to suggest that perhaps HL is not causally related to dementia [34].
5.1. Behavioral Mechanisms
Chronic HL can impair communication and lead to social isolation, loneliness [35], and depression [36], which are risk factors for dementia [37,38]. Loneliness has been associated with over double the risk of AD diagnosis [39], and links between HL and worse episodic memory performance have been suggested to be partially mediated by loneliness and social isolation [40]. One prospective study found that individuals with either HL or depressive symptoms alone had faster cognitive declines over a 10-year follow-up, yet those with both HL and depressive symptoms presented the greatest risk of dementia [41]. One may hypothesize that social factors and late-life depression may represent an intermediate step in the progression of some older adults from HL to dementia [42].
Moreover, older adults with HL have been found to have decreased physical functioning[43], slowed gait speed [44], and greater risk of frailty [45], possibly mediated through effects of HL on cognitive load and reduced awareness of environmental sound cues leading to poorer balance and decreased activity. Fortunately, hearing treatment has been associated with improved gait speed [46] and reduced risk of falls [47]. Lastly, tinnitus (perception of sound without a corresponding external acoustic stimulus) is highly comorbid with HL and can tax available cognitive reserve to direct attentional resources towards salient information and away from the phantom sound [48]. Tinnitus has consistently been associated with worse cognitive performance [49,50], including one case-control study that found an 68% increased risk of developing dementia before the age of 65 [50].
5.2. Neural Mechanisms
5.2.A. Auditory Deprivation Hypothesis
Neuroimaging studies of HL have demonstrated compensatory and neuroplastic changes associated with degraded auditory input that provide plausible pathways by which chronic HL may be linked to cognitive dysfunction [30]. Impoverished auditory signals from the impaired cochlea decrease input to the primary and secondary auditory cortex [51,52] and chronic deafferentation can lead to downstream atrophy of auditory and cognitive control networks (e.g., primary auditory cortex, prefrontal cortex, and anterior cingulate cortex). Cross-sectional structural neuroimaging studies have observed an association between worse hearing ability and smaller frontotemporal brain volumes [53,54]. One longitudinal epidemiological study found that greater audiometric HL was prospectively and independently associated with accelerated volume declines in whole brain and lateral temporal lobe (superior, middle, and inferior temporal gyri) [52]. While the literature is somewhat mixed [34], studies have in general found regional frontotemporal atrophy (vs. widespread central nervous system degeneration), which argues for the auditory deprivation hypothesis and against the common-cause mechanism of age-related changes in structure/function. It is intuitive to hypothesize that such deafferentation-induced atrophy adversely affects cognitive performance in other domains subserved by these frontotemporal regions (e.g., executive functioning/episodic memory) thereby increasing risk for dementia [10]. However, one important caveat is that the available evidence evaluating such neural effects of HL primarily concern the links between HL and cognitive dysfunction, as opposed to dementia diagnosis.
5.2.B. Effortful Listening (or Information Degradation) Hypothesis
HL can result in poor fidelity and distorted encoding of complex sounds (e.g., speech) by the cochlea, leading to increased listening effort [55]. Increased listening effort can tax the cognitive control network (CCN), diminish the resources available for higher level cognitive processing, and impair cognitive reserve [56]. The effect of poor peripheral encoding of sound is demonstrated by studies under conditions where the auditory signal is degraded, and greater engagement of frontal brain system and CCN structures are required for auditory processing to the detriment of other cognitive processes (e.g., working memory) [55,57]. For example, functional MRI studies using background noise, distorted speech, or incongruent audio-visual tasks demonstrate compensatory CCN recruitment during listening tasks in individuals with HL versus controls [58,59]. Aberrant frontotemporal activity among individuals with HL can even be observed in passive listening tasks with clear speech [60] and at rest [61]. One may speculate that in the short term, HL may lead to compensatory activation of the CCN to support speech and cognitive processing, but excessive glutamate excitotoxicity over time may lead to maladaptive remodeling and dysregulation of cognitive networks [62].
5.3. Overlapping Neural and Behavioral Mechanisms
While the causative neural and behavioral pathways reviewed above are presented separately, one must consider the substantial overlap between such mechanisms (Figure 1). For example, tinnitus can impair an individual’s ability to switch attention away from the perceived sound, resulting in dysregulation of CCN structures beyond the specific effects of HL on listening effort. Consistent with this hypothesis, individuals with tinnitus have been found to have altered baseline connectivity in the CCN and attenuated activation of the middle frontal gyrus during a cognitive control task [48]. Likewise, social isolation and loneliness may affect the same neural pathways that are implicated in the causal links between HL and dementia. Higher loneliness scores have been associated with smaller amygdala, hippocampal, and para-hippocampal volumes [63], and can be predicted by connectivity of prefrontal, limbic, and temporal networks (i.e., pathways involved in cognitive functioning) [64]. Lastly, HL is associated with increased risk of depression in older adults [36], which can itself affect CCN structures [65] and executive functioning [66], thereby exacerbating the adverse neural effects of HL. Adverse effects of clinical depression on motivation and processing speed can exacerbate impairments in functional hearing and listening effort, thereby accelerating the process of cognitive decline [67].
Figure 1:
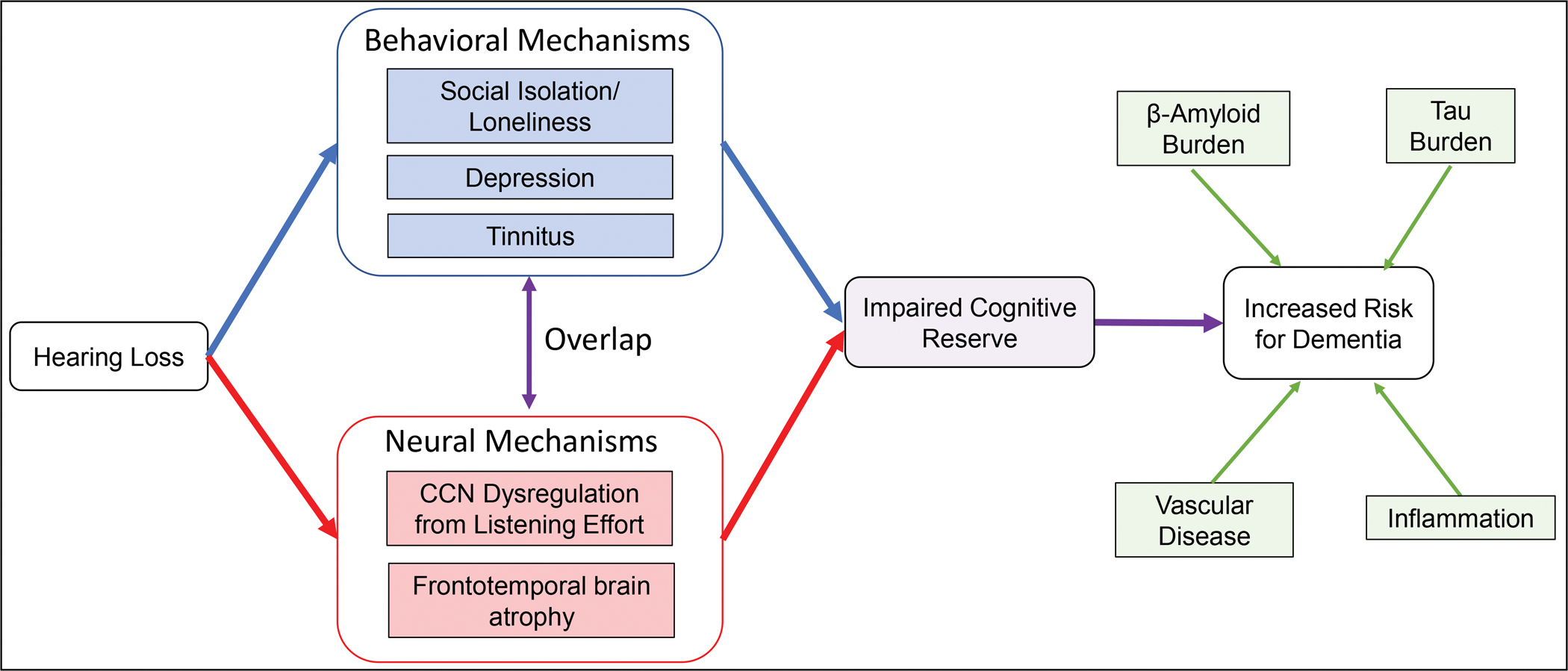
Neural and behavioral consequences of hearing loss on a pathway leading to dementia.
Hearing loss, through the behavioral and neural mechanisms reviewed, can impose a constant load on an individual’s available cognitive reserve. Such effects can deplete available cognitive resources that would otherwise be available to help mitigate and act as a ‘buffer’ against neuropathological contributors to dementia (e.g., emerging AD pathology or vascular disease) resulting in an earlier manifestation of cognitive and functional impairment.
One may hypothesize that such behavioral and neural effects of HL may increase risk for dementia through decreasing cognitive reserve, rather than directly effecting neuropathological biomarkers associated with dementia (Figure 1). For example, as social participation and engagement in leisure activities have been associated with increased cognitive reserve [68], it is possible that loneliness and depression secondary to HL may impair cognitive reserve and increase vulnerability to neuropathological contributors to dementia (e.g., emerging AD pathology or vascular disease). Likewise, deafferentation-induced frontotemporal atrophy and CCN dysregulation can deplete cortical resources that would otherwise be available to help mitigate and act as a ‘buffer’ against such neuropathological changes. According to such a model, HL imposes a constant load on an individual’s cognitive reserve, and clinical symptoms from existing brain pathology may manifest earlier as cognitive and functional impairment.
6. COGNITIVE EFFECTS OF HEARING TREATMENT
Accumulating evidence suggests that hearing treatment may improve cognitive outcomes in older adults, though there are important limitations to such findings. One important caveat to existing data is that many studies utilize cognitive functioning (vs. dementia) as a primary outcome, which hinders our ability to clearly interpret the effects of hearing treatment on risk for dementia. Likewise, many are small case-control studies that evaluate the effects of hearing treatment on cognitive functioning by comparing older adults with HL to normal hearing healthy controls. Most available observational studies examining cognitive status before and after hearing treatment only evaluate cognitively normal participants, which limits generalizability of such findings to older adults with pre-existing cognitive impairment. Lastly, studies of hearing aid use are often plagued by inaccurate reporting, as few use objective measures of when hearing aids are in place, turned on, and actually being used.
The strongest data demonstrating positive effects of hearing aids on cognition arise from large naturalistic studies that evaluate multiple domains of cognitive functioning before and after hearing treatment. Observational studies have found that hearing aids are associated with improved memory, global cognition, and executive functioning [69,70]. One systematic review evaluating the effects of hearing aids on cognition revealed mixed results but found a promising effect of hearing aids on executive functioning [71]. In the only available study evaluating individuals with cognitive impairment, participants with MCI and HL who used hearing aids were at a significantly lower risk of dementia diagnosis, but hearing aid use among those with pre-existing dementia was not associated with improved scores on the clinical dementia rating scale [72]. Some randomized controlled trials demonstrate evidence that hearing aids may improve cognitive outcomes among cognitively intact older adults [73,74], but interpretation of such findings are limited by small sample sizes and mixed results [75].
When HL is severe to profound, hearing aids may not adequately provide clarity of speech and cochlear implants (prosthetic devices that convert acoustic sound to electrical signals that stimulate the cochlear nerve) have become the gold standard for treatment. Observational studies have observed improvement in memory, executive functioning, processing speed, and global cognition at 6–24 months post-implantation [76,77]. Older adults with worse preoperative cognitive scores appear to benefit the most post-implantation [76,78], however no available study evaluates the effects of cochlear implantation on dementia risk.
7. NEURAL EFFECTS OF HEARING TREATMENT
In the past few years, studies have begun to examine how hearing aids and cochlear implants may normalize adverse neural changes associated with chronic HL. Many limitations pertain to the existing literature, including small sample sizes, inadequate control groups, and available studies specifically evaluate brain regions important for auditory and sensory integration regions rather than those relevant to cognitive functioning. As such, it is unclear whether restoration of normative cortical allocation patterns with hearing treatment supports cognitive processing or is associated with reduced dementia risk. However, intriguing findings of relatively rapid neural changes with hearing treatment have been reported despite the insidious development of HL over years.
Older adults with HL can demonstrate cross-modal reorganization, a process by which cortices associated with deficient sensory modalities (e.g., auditory cortex) are recruited by other intact senses (e.g. visual cortex) for neural processing [79]. Such cortical reorganization is associated with worse auditory speech perception [80], and effective multimodal integration (e.g., auditory and visual information) has been shown to aid language and speech comprehension [81]. After one year of hearing aid use, Pereira-Jorge et al. [82] found increased functional MRI activation in auditory and language cortices during auditory tasks, which correlated with improved speech recognition scores. Hearing aid use was associated with deactivation of visual association areas and increased cortical thickness in multimodal integration regions (e.g., superior temporal gyrus and angular gyrus). Following six months of hearing aid use, Glick et al. [83] observed a reversal in cross-modal organization of auditory cortex for visual processing, which coincided with gains in speech perception and improved cognitive performance across several domains.
The neural effects of cochlear implants are less often studied due to the limitations of MRI use in cochlear implant patients, though functional near-infrared spectroscopy is an emerging technology compatible with cochlear implant devices. Chen et al. [84] observed decreased intramodal- and increased cross-modal functional connectivity between visual and auditory cortices, which correlated with speech recognition outcomes among post-lingually deaf cochlear implant users. Other analyses have also observed adaptive cross-modal reorganization of the visual cortex for auditory processes coinciding with improved performance in speech recognition following cochlear implantation [85].
8. EXPERT OPINION
While preliminary studies reveal promising effects of hearing treatment on cognitive outcomes and neural consequences of chronic HL, further research is needed to evaluate whether HL is a modifiable risk factor for dementia. Rigorously designed clinical trials and observational studies are needed to determine whether hearing treatment is effective for the prevention of cognitive decline and for the treatment of current cognitive impairment. A National Institute on Aging-funded Aging and Cognitive Health Evaluation in Elders (ACHIEVE) study is a large ongoing randomized controlled trial that will definitively test if a best practices hearing intervention vs. a health education control intervention reduces cognitive decline (primary outcome), incident dementia, and structural brain atrophy among older adults with HL (NCT03243422) [86].
In this clinical trial, N=977 cognitively intact older adults with HL have been randomized to a hearing intervention (hearing aids, education/counseling) or a successful aging control intervention (individual sessions with a health educator covering healthy aging topics) and followed semi-annually for 3 years. Results from the full trial are not yet published and will become available in 2023. However, initial short-term data of N=40 participants in the ACHIEVE pilot study (NCT02412254) have been published and reveal improved perceived hearing handicap and memory following 6 months of the hearing intervention [75].
Future studies may consider adapting the ACHIEVE trial design to a population with MCI to evaluate whether a hearing intervention (vs. education control) may delay conversion to dementia. While it is unlikely that hearing treatment affects underlying neuropathological contributors to dementia, one may hypothesize that treatment may increase or maintain cognitive reserve and delay clinical symptoms attributable to existing brain pathology associated with dementia. As self-reported hearing aid use can often be overestimated compared to datalogging [87], objective documentation of daily hearing aid use is a critical design element in these trials, especially when evaluating a population with cognitive impairment. Fortunately, modern hearing aids can objectively track usage with built-in data logging features.
As existing studies in HL often assess cognition using global cognitive screening tools or clinical diagnosis of dementia, it is currently unknown which subtype of dementia is most linked to HL. Understanding the links between HL and dementia subtype (e.g., AD, vascular dementia, frontotemporal dementia) may help identify interventions to augment hearing treatment based on an understanding of the underlying pathophysiology. Longitudinal studies incorporating rigorous criteria for classification of cognitive status and diagnosis with longer follow-up are needed. Classification and stratification of dementia pathologies in such studies may include both clinical and biomarker support. Likewise, studies may evaluate whether augmentation of hearing treatment with proposed treatments for cognitive impairment (e.g., cholinesterase inhibitors, strict blood pressure control) or behavioral symptoms of dementia may be associated with greater effect sizes on cognitive performance as well as in other functional domains.
In addition to pursuing clinical trials leveraging current standard of care treatments for HL, mechanistic studies should be conducted to further evaluate the hypothesized pathways by which HL is causally linked to cognitive decline and dementia. Further understanding the mechanisms linking these common disorders of later-life may facilitate identification and dissemination of interventions to augment hearing treatment capable of protecting brain and cognitive health during aging. Longitudinal multimodal neuroimaging studies incorporating comprehensive assessments of cognitive, hearing, and psychiatric functioning in a population with and without HL would help elucidate the model proposed in Figure 1. At the very least, studies evaluating the consequences of HL or hearing treatment on cognitive outcomes should incorporate psychiatric assessments and social phenotyping in order to measure the moderating effects of these variables.
In concert with these mechanistic studies, efforts should be undertaken to understand the individual characteristics of older adults with HL who are at greatest risk for dementia. As social isolation and loneliness are independently associated with poor cognitive outcomes, it is possible that hearing-impaired older adults who are lonely with limited social engagement may be more vulnerable to dementia. Likewise, the presence of depression may synergistically combine with HL through the pathways reviewed above to create a phenotype at ultra-high risk for cognitive problems. While most studies of HL define exposed individuals based solely on the loudness thresholds at which a sound can be heard (i.e., gold-standard pure tone audiometry), considering the pattern of auditory thresholds and speech understanding may better identify individuals vulnerable to dementia. Functional assessments of speech discrimination in quiet (e.g., speech recognition threshold or word recognition score) and in noise (speech-in-noise tests) may be more indicative of hearing ability in real-world settings hearing functioning and reflect the combined functional output of both the peripheral and central auditory systems. However, as central auditory measures can act as a biomarker for the same cortical processes underlying dementia, such studies must be careful to not conflate the measurements of peripheral and central hearing when interpreting the effects of HL on cognitive functioning.
Therefore, large prospective longitudinal studies enriched for individuals at risk for dementia are needed to further understand the links between HL and risk for dementia. Such studies may consider measuring an individual’s social context, hearing profile, and psychiatric functioning to allow for identification of specific patient-related factors that particularly increase risk for dementia. Such studies must carefully adjust for relevant covariates such as cerebrovascular disease, socio-economic status, and education. Better understanding these characteristics may identify those vulnerable to dementia in order to target them with treatments based on the mechanisms involved in the pathogenesis of cognitive decline specific for each individual. For example, interventions such as ‘smart’ assistive hearing technologies, as opposed to conventional hearing aids, may better address individuals with poor speech-in-noise perception who are at risk for dementia.
Such information eventually may allow targeting the neural mediators and behavioral outputs as reviewed above to maximize functionality. Given the significant interactive effects between social, psychiatric, and cognitive functioning in HL, one may speculate that hearing treatment may be necessary but not sufficient to recondition cognitive and brain functioning among older adults with chronic HL. For example, relying on adaptive neuroplastic changes in the CCN induced by repetitive cognitive exercises, cognitive remediation interventions may maximize the effects of hearing aids on executive functioning, memory, and processing speed [88]. Other strategies to optimize hearing aid effects on cognitive and neural outcomes may include a psychosocial intervention to address loneliness [89] or implementation of standard treatments for clinical depression. Treatment with antidepressant medications has been associated with attenuated responses in the hippocampus and medial prefrontal cortex [90], and adaptive changes in regions of the prefrontal cortex have also been reported following completion of evidence-based psychotherapies such as cognitive behavioral therapy [91]. Overall, there is a need to further examine the use of multimodal and integrated interventions that target both audiological and extra-audiological variables (e.g., communication education, psychosocial skills, depression treatment), in order to optimize hearing treatment effects on cognitive outcomes [92,93]. Likewise, research is needed to understand how HL may impact access to or ability to engage in such treatments (e.g., psychotherapy or psychosocial interventions).
Given the high prevalence of HL, its association with cognitive decline, and the dire consequences of dementia for older adults, it is imperative to elucidate the pathologic mechanisms involved and facilitate access to treatment for vulnerable individuals. As hearing aids can be expensive and difficult to obtain, the 2021 Update of the National Plan to Address Alzheimer’s Disease now calls for increased access to hearing aids in order to promote brain health and reduce risk of dementia [94]. More broadly, there is a need for research to consider the role of declining sensory afferents beyond hearing (e.g., vision, olfaction, and proprioception) on cognitive functioning. If future studies demonstrate that treatment of sensory loss may have positive effects on cognitive and brain health in older adults, such results may facilitate education efforts and increase availability of sensory treatments for older adults.
Article highlights.
Historically considered a benign component of aging, hearing loss in recent years has been associated with cognitive decline, dementia, and poor brain health in older adults
Hearing loss can have adverse effects on brain structure and function and can lead to loneliness and late-life depression
Accumulating evidence suggests that hearing treatment may improve cognitive outcomes in older adults, though there are important limitations to such findings
Preliminary studies also provide evidence that hearing treatment may reverse specific neuroplastic changes associated with chronic hearing loss
Further research is needed to determine whether hearing treatment is effective for the prevention and/or treatment of current cognitive decline
Given the potential benefits of hearing treatment on cognitive and neural outcomes, there is an urgent need to provide increased access to hearing care
Funding
This paper was funded by the National Institute of Mental Health (NIMH – T32)
Footnotes
Declaration of interest
FR Lin receives financial support from the Scientific Advisory Board for Fondation Pour L’Audition and Frequency Therapeutics. FR Lin is also the Director of a public health research center funded in part by a philanthropic donation from Cochlear Ltd to the Johns Hopkins Bloomberg School of Public Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Contributor Information
Katharine K. Brewster, Columbia University Vagelos College of Physicians and Surgeons, Department of Psychiatry, New York State Psychiatric Institute, 1051 Riverside Drive, New York, NY 10032
Jennifer A. Deal, Johns Hopkins Bloomberg School of Public Health, Department of Epidemiology, Johns Hopkins University, Center on Aging and Health, Johns Hopkins University School of Medicine, Department of Otolaryngology
Frank R. Lin, Johns Hopkins Bloomberg School of Public Health, Department of Epidemiology, Johns Hopkins University School of Medicine, Department of Otolaryngology
Bret R. Rutherford, Columbia University Vagelos College of Physicians and Surgeons, Department of Psychiatry, New York State Psychiatric Institute
REFERENCES
- 1. Livingston G, Huntley J, Sommerlad A et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet, 396(10248), 413–446 (2020). Based upon a growing body of evidence, the 2020 Lancet Commission on Dementia Prevention, Intervention, and Care supported hearing loss as the number one potentially modifiable risk factor for dementia.
- 2.Chadha S, Cieza A, Krug E. Global hearing health: future directions. Bull World Health Organ, 96(3), 146 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin FR, Niparko JK, Ferrucci L. Hearing loss prevalence in the United States. Arch Intern Med, 171(20), 1851–1852 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chien W, Lin FR. Prevalence of hearing aid use among older adults in the United States. Arch Intern Med, 172(3), 292–293 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.President’s Council of Advisors on Science and Technology. Aging America & Hearing Loss: Imperative of Improved Hearing Technologies. Washington, DC: Executive Office of the President of the United States; September 2015. (Ed.^(Eds) [Google Scholar]
- 6.National Academies of Sciences EMH, Medicine Division BDGDSLCT. Hearing health care for adults : priorities for improving access and affordability. (Ed.^(Eds) (2016) [PubMed]
- 7.S.670, Over-the-Counter Hearing Aid Act of 2017. https://www.congress.gov/bill/115th-congress/senate-bill/670.).
- 8.Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev, 23(Pt B), 154–166 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Lin FR, Albert M. Hearing loss and dementia - who is listening? Aging Ment Health, 18(6), 671–673 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutherford BR, Brewster K, Golub JS, Kim AH, Roose SP. Sensation and Psychiatry: Linking Age-Related Hearing Loss to Late-Life Depression and Cognitive Decline. Am J Psychiatry, 175(3), 215–224 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickles JO. An introduction to the physiology of hearing (Brill, Leiden, 2013). [Google Scholar]
- 12.McClannahan KS, Chiu YF, Sommers MS, Peelle JE. Test-Retest Reliability of Audiometric Assessment in Individuals With Mild Dementia. JAMA Otolaryngol Head Neck Surg, 147(5), 442–449 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu NC, Friedman RA. Age-related hearing loss: Unraveling the pieces. Laryngoscope Investig Otolaryngol, 3(2), 68–72 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peelle JE, Wingfield A. The Neural Consequences of Age-Related Hearing Loss. Trends Neurosci, 39(7), 486–497 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson JCS, Marshall CR, Weil RS, Bamiou DE, Hardy CJD, Warren JD. Hearing and dementia: from ears to brain. Brain, 144(2), 391–401 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mener DJ, Betz J, Yaffe K et al. Apolipoprotein E Allele and Hearing Thresholds in Older Adults. Am J Alzheimers Dis Other Demen, 31(1), 34–39 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinha UK, Hollen KM, Rodriguez R, Miller CA. Auditory system degeneration in Alzheimer’s disease. Neurology, 43(4), 779–785 (1993). [DOI] [PubMed] [Google Scholar]
- 18.Baloyannis SJ, Mauroudis I, Manolides SL, Manolides LS. Synaptic alterations in the medial geniculate bodies and the inferior colliculi in Alzheimer’s disease: a Golgi and electron microscope study. Acta Otolaryngol, 129(4), 416–418 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Parker T, Cash DM, Lane C et al. Pure tone audiometry and cerebral pathology in healthy older adults. J Neurol Neurosurg Psychiatry, 91(2), 172–176 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarant JZ, Harris DC, Busby PA et al. No Influence of Age-Related Hearing Loss on Brain Amyloid-beta. J Alzheimers Dis, 85(1), 359–367 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardy CJ, Marshall CR, Golden HL et al. Hearing and dementia. J Neurol, 263(11), 2339–2354 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevenson JS, Clifton L, Kuzma E, Littlejohns TJ. Speech-in-noise hearing impairment is associated with an increased risk of incident dementia in 82,039 UK Biobank participants. Alzheimers Dement, 18(3), 445–456 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of Age-Related Hearing Loss With Cognitive Function, Cognitive Impairment, and Dementia: A Systematic Review and Meta-analysis. JAMA Otolaryngol Head Neck Surg, 144(2), 115–126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deal JA, Sharrett AR, Albert MS et al. Hearing impairment and cognitive decline: a pilot study conducted within the atherosclerosis risk in communities neurocognitive study. Am J Epidemiol, 181(9), 680–690 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deal JA, Gross AL, Sharrett AR et al. Hearing impairment and missing cognitive test scores in a population-based study of older adults: The Atherosclerosis Risk in Communities neurocognitive study. Alzheimers Dement, 17(10), 1725–1734 (2021). [DOI] [PubMed] [Google Scholar]
- 26. Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol, 68(2), 214–220 (2011). In this prospective study of N=639 individuals older adults enrolled in the Baltimore Longitudinal Study on Aging, individuals with hearing loss had 1.89 times the risk of developing dementia over a median of 12 years of follow-up. Similarly, the estimated risk for individuals with moderate hearing loss was 3.00.
- 27.Livingston G, Sommerlad A, Orgeta V et al. Dementia prevention, intervention, and care. Lancet, 390(10113), 2673–2734 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Gallacher J, Ilubaera V, Ben-Shlomo Y et al. Auditory threshold, phonologic demand, and incident dementia. Neurology, 79(15), 1583–1590 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Deal JA, Betz J, Yaffe K et al. Hearing Impairment and Incident Dementia and Cognitive Decline in Older Adults: The Health ABC Study. J Gerontol A Biol Sci Med Sci, 72(5), 703–709 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Griffiths TD, Lad M, Kumar S et al. How Can Hearing Loss Cause Dementia? Neuron, 108(3), 401–412 (2020). This review paper proposes putative neural mechanisms by which hearing loss is linked to dementia, including common pathology in the cochlea and brain, deterioration of neural resources due to an impoverished environment, and diminished availability of cognitive resources occupied in support of effortful listening. They also suggest avenues for future research at the molecular, neuronal, and systems levels to understand the effects of hearing interventions on dementia.
- 31.Picciotti P, Torsello A, Wolf FI, Paludetti G, Gaetani E, Pola R. Age-dependent modifications of expression level of VEGF and its receptors in the inner ear. Exp Gerontol, 39(8), 1253–1258 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Fetoni AR, Picciotti PM, Paludetti G, Troiani D. Pathogenesis of presbycusis in animal models: a review. Exp Gerontol, 46(6), 413–425 (2011). [DOI] [PubMed] [Google Scholar]
- 33.van Agtmaal MJM, Houben A, Pouwer F, Stehouwer CDA, Schram MT. Association of Microvascular Dysfunction With Late-Life Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry, 74(7), 729–739 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckert MA, Vaden KI Jr., Dubno JR. Age-Related Hearing Loss Associations With Changes in Brain Morphology. Trends Hear, 23, 2331216519857267 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sung YK, Li L, Blake C, Betz J, Lin FR. Association of Hearing Loss and Loneliness in Older Adults. J Aging Health, 28(6), 979–994 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Brewster KK, Ciarleglio A, Brown PJ et al. Age-Related Hearing Loss and Its Association with Depression in Later Life. Am J Geriatr Psychiatry, 26(7), 788–796 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akhter-Khan SC, Tao Q, Ang TFA et al. Associations of loneliness with risk of Alzheimer’s disease dementia in the Framingham Heart Study. Alzheimers Dement, 17(10), 1619–1627 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol, 7(6), 323–331 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson RS, Krueger KR, Arnold SE et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry, 64(2), 234–240 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Maharani A, Pendleton N, Leroi I. Hearing Impairment, Loneliness, Social Isolation, and Cognitive Function: Longitudinal Analysis Using English Longitudinal Study on Ageing. Am J Geriatr Psychiatry, 27(12), 1348–1356 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Powell DS, Brenowitz WD, Yaffe K et al. Examining the Combined Estimated Effects of Hearing Loss and Depressive Symptoms on Risk of Cognitive Decline and Incident Dementia. J Gerontol B Psychol Sci Soc Sci, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brewster KK, Hu MC, Zilcha-Mano S et al. Age-Related Hearing Loss, Late-Life Depression, and Risk for Incident Dementia in Older Adults. J Gerontol A Biol Sci Med Sci, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez-Amezcua P, Powell D, Kuo PL et al. Association of Age-Related Hearing Impairment With Physical Functioning Among Community-Dwelling Older Adults in the US. JAMA Netw Open, 4(6), e2113742 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakurai R, Suzuki H, Ogawa S, Takahashi M, Fujiwara Y. Hearing loss and increased gait variability among older adults. Gait Posture, 87, 54–58 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Tian R, Almeida OP, Jayakody DMP, Ford AH. Association between hearing loss and frailty: a systematic review and meta-analysis. BMC Geriatr, 21(1), 333 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weaver TS, Shayman CS, Hullar TE. The Effect of Hearing Aids and Cochlear Implants on Balance During Gait. Otol Neurotol, 38(9), 1327–1332 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Mahmoudi E, Basu T, Langa K et al. Can Hearing Aids Delay Time to Diagnosis of Dementia, Depression, or Falls in Older Adults? J Am Geriatr Soc, 67(11), 2362–2369 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Trevis KJ, Tailby C, Grayden DB, McLachlan NM, Jackson GD, Wilson SJ. Identification of a Neurocognitive Mechanism Underpinning Awareness of Chronic Tinnitus. Sci Rep, 7(1), 15220 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brueggemann P, Neff PKA, Meyer M, Riemer N, Rose M, Mazurek B. On the relationship between tinnitus distress, cognitive performance and aging. Prog Brain Res, 262, 263–285 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Cheng YF, Xirasagar S, Yang TH, Wu CS, Kao YW, Lin HC. Risk of early-onset dementia among persons with tinnitus: a retrospective case-control study. Sci Rep, 11(1), 13399 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slade K, Plack CJ, Nuttall HE. The Effects of Age-Related Hearing Loss on the Brain and Cognitive Function. Trends Neurosci, 43(10), 810–821 (2020). [DOI] [PubMed] [Google Scholar]
- 52. Lin FR, Ferrucci L, An Y et al. Association of hearing impairment with brain volume changes in older adults. Neuroimage, 90, 84–92 (2014). In the neuroimaging substudy of the Baltimore Longitudinal Study on Aging, greater audiometric HL was prospectively and independently associated with accelerated volume declines in whole brain and lateral temporal lobe (superior, middle, and inferior temporal gyri) after a mean 6.4-year follow-up (N=126).
- 53.Yang M, Chen HJ, Liu B et al. Brain structural and functional alterations in patients with unilateral hearing loss. Hear Res, 316, 37–43 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Qian ZJ, Chang PD, Moonis G, Lalwani AK. A novel method of quantifying brain atrophy associated with age-related hearing loss. Neuroimage Clin, 16, 205–209 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pichora-Fuller MK, Kramer SE, Eckert MA et al. Hearing Impairment and Cognitive Energy: The Framework for Understanding Effortful Listening (FUEL). Ear Hear, 37 Suppl 1, 5S–27S (2016). [DOI] [PubMed] [Google Scholar]
- 56.Lemke U, Besser J. Cognitive Load and Listening Effort: Concepts and Age-Related Considerations. Ear Hear, 37 Suppl 1, 77S–84S (2016). [DOI] [PubMed] [Google Scholar]
- 57.Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol Aging, 24(3), 761–766 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erb J, Obleser J. Upregulation of cognitive control networks in older adults’ speech comprehension. Front Syst Neurosci, 7, 116 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wild CJ, Yusuf A, Wilson DE, Peelle JE, Davis MH, Johnsrude IS. Effortful listening: the processing of degraded speech depends critically on attention. J Neurosci, 32(40), 14010–14021 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Husain FT, Pajor NM, Smith JF et al. Discrimination task reveals differences in neural bases of tinnitus and hearing impairment. PLoS One, 6(10), e26639 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen YC, Chen H, Jiang L et al. Presbycusis Disrupts Spontaneous Activity Revealed by Resting-State Functional MRI. Front Behav Neurosci, 12, 44 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Belkhiria C, Vergara RC, San Martin S et al. Cingulate Cortex Atrophy Is Associated With Hearing Loss in Presbycusis With Cochlear Amplifier Dysfunction. Front Aging Neurosci, 11, 97 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duzel S, Drewelies J, Gerstorf D et al. Structural Brain Correlates of Loneliness among Older Adults. Sci Rep, 9(1), 13569 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng C, Wang L, Li T, Xu P. Connectome-based individualized prediction of loneliness. Soc Cogn Affect Neurosci, 14(4), 353–365 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Respino M, Hoptman MJ, Victoria LW et al. Cognitive Control Network Homogeneity and Executive Functions in Late-Life Depression. Biol Psychiatry Cogn Neurosci Neuroimaging, 5(2), 213–221 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alexopoulos GS. Mechanisms and treatment of late-life depression. Transl Psychiatry, 9(1), 188 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brewster KK, Golub JS, Rutherford BR. Neural circuits and behavioral pathways linking hearing loss to affective dysregulation in older adults. Nature Aging, 1(5), 422–429 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petkus AJ, Gomez ME. The importance of social support, engagement in leisure activities, and cognitive reserve in older adulthood. Int Psychogeriatr, 33(5), 433–435 (2021). [DOI] [PubMed] [Google Scholar]
- 69.Castiglione A, Benatti A, Velardita C et al. Aging, Cognitive Decline and Hearing Loss: Effects of Auditory Rehabilitation and Training with Hearing Aids and Cochlear Implants on Cognitive Function and Depression among Older Adults. Audiol Neurootol, 21 Suppl 1, 21–28 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Sarant J, Harris D, Busby P et al. The Effect of Hearing Aid Use on Cognition in Older Adults: Can We Delay Decline or Even Improve Cognitive Function? J Clin Med, 9(1) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sanders ME, Kant E, Smit AL, Stegeman I. The effect of hearing aids on cognitive function: A systematic review. PLoS One, 16(12), e0261207 (2021). This systematic review analyzed 17 longitudinal studies measuring the effects of cognitive function among individuals without baseline dementia. They found mixed results but suggested that the most beneficial impact of hearing aids was in the domain of executive functioning.
- 72.Bucholc M, McClean PL, Bauermeister S et al. Association of the use of hearing aids with the conversion from mild cognitive impairment to dementia and progression of dementia: A longitudinal retrospective study. Alzheimers Dement (N Y), 7(1), e12122 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karawani H, Jenkins K, Anderson S. Restoration of sensory input may improve cognitive and neural function. Neuropsychologia, 114, 203–213 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brewster KK, Pavlicova M, Stein A et al. A pilot randomized controlled trial of hearing aids to improve mood and cognition in older adults. Int J Geriatr Psychiatry, 35(8), 842–850 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deal JA, Albert MS, Arnold M et al. A randomized feasibility pilot trial of hearing treatment for reducing cognitive decline: Results from the Aging and Cognitive Health Evaluation in Elders Pilot Study. Alzheimers Dement (N Y), 3(3), 410–415 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Volter C, Gotze L, Dazert S, Falkenstein M, Thomas JP. Can cochlear implantation improve neurocognition in the aging population? Clin Interv Aging, 13, 701–712 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohta Y, Imai T, Maekawa Y et al. The effect of cochlear implants on cognitive function in older adults: A prospective, longitudinal 2-year follow-up study. Auris Nasus Larynx, (2021). [DOI] [PubMed] [Google Scholar]
- 78.Mosnier I, Bebear JP, Marx M et al. Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngol Head Neck Surg, 141(5), 442–450 (2015). [DOI] [PubMed] [Google Scholar]
- 79.Cardon G, Sharma A. Somatosensory Cross-Modal Reorganization in Adults With Age-Related, Early-Stage Hearing Loss. Front Hum Neurosci, 12, 172 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Campbell J, Sharma A. Cross-modal re-organization in adults with early stage hearing loss. PLoS One, 9(2), e90594 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Calvert GA, Campbell R, Brammer MJ. Evidence from functional magnetic resonance imaging of crossmodal binding in the human heteromodal cortex. Curr Biol, 10(11), 649–657 (2000). [DOI] [PubMed] [Google Scholar]
- 82.Pereira-Jorge MR, Andrade KC, Palhano-Fontes FX et al. Anatomical and Functional MRI Changes after One Year of Auditory Rehabilitation with Hearing Aids. Neural Plast, 2018, 9303674 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Glick HA, Sharma A. Cortical Neuroplasticity and Cognitive Function in Early-Stage, Mild-Moderate Hearing Loss: Evidence of Neurocognitive Benefit From Hearing Aid Use. Front Neurosci, 14, 93 (2020). In this pilot study, individuals with mild-moderate hearing loss demonstrated a reversal in cross-modal reorganization of auditory cortex for visual processing on cortical visual evoked potentials following 6-months of hearing aid treatment, which coincided gains in speech perception and cognitive performance.
- 84.Chen LC, Puschmann S, Debener S. Increased cross-modal functional connectivity in cochlear implant users. Sci Rep, 7(1), 10043 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen LC, Sandmann P, Thorne JD, Bleichner MG, Debener S. Cross-Modal Functional Reorganization of Visual and Auditory Cortex in Adult Cochlear Implant Users Identified with fNIRS. Neural Plast, 2016, 4382656 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.https://clinicaltrials.gov/ct2/show/NCT03243422. Aging and Cognitive Health Evaluation in Elders (ACHIEVE). NCT03243422.). [Google Scholar]
- 87.Solheim J, Hickson L. Hearing aid use in the elderly as measured by datalogging and self-report. Int J Audiol, 56(7), 472–479 (2017). [DOI] [PubMed] [Google Scholar]
- 88.Morimoto SS, Altizer RA, Gunning FM et al. Targeting Cognitive Control Deficits With Neuroplasticity-Based Computerized Cognitive Remediation in Patients With Geriatric Major Depression: A Randomized, Double-Blind, Controlled Trial. Am J Geriatr Psychiatry, 28(9), 971–980 (2020). [DOI] [PubMed] [Google Scholar]
- 89.Warringa LTL, Henke CE, Pronk M, Kramer SE, Stam M. Relationships Between Coping Behaviors and Social Loneliness in Adults With Self-reported Hearing Problems. Ear Hear, 41(4), 1040–1050 (2020). [DOI] [PubMed] [Google Scholar]
- 90.Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry, 59(9), 816–820 (2006). [DOI] [PubMed] [Google Scholar]
- 91.Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R. Neural correlates of emotional processing in depression: changes with cognitive behavioral therapy and predictors of treatment response. J Psychiatr Res, 45(5), 577–587 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Giallini I, Nicastri M, Inguscio BMS et al. Effects of the “Active Communication Education” Program on Hearing-Related Quality of Life in a Group of Italian Older Adults Cochlear Implant Users. Front Psychol, 13, 827684 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rivera S, Marcotti A, Fuente A, Fuentes-Lopez E, Hickson L. Adaptation of the “active communication education” programme into Spanish for older adults with hearing loss. Int J Audiol, 59(9), 719–725 (2020). [DOI] [PubMed] [Google Scholar]
- 94.National Plan to Address Alzheimer’s Disease: 2021 Update. Action 6.B.2. https://aspe.hhs.gov/reports/national-plan-2021-update.).


