Abstract
Endothelial damage is characteristic of infection with Shiga toxin (Stx)-producing Escherichia coli (STEC). Because Stx-mediated endothelial cell damage at the site of infection may lead to the characteristic hemorrhagic colitis of STEC infection, we compared the effects of Stx1 and Stx2 on primary and transformed human intestinal microvascular endothelial cells (HIMEC) to those on macrovascular endothelial cells from human saphenous vein (HSVEC). Adhesion molecule, interleukin-8 (IL-8), and Stx receptor expression, the effects of cytokine activation and Stx toxins on these responses, and Stx1 and Stx2 binding kinetics and bioactivity were measured. Adhesion molecule and IL-8 expression increased in activated HIMEC, but these responses were blunted in the presence of toxin, especially in the presence of Stx1. In contrast to HSVEC, unstimulated HIMEC constitutively expressed Stx receptor at high levels, bound large amounts of toxin, were highly sensitive to toxin, and were not further sensitized by cytokines. Although the binding capacities of HIMEC for Stx1 and Stx2 were comparable, the binding affinity of Stx1 to HIMEC was 50-fold greater than that of Stx2. Nonetheless, Stx2 was more toxic to HIMEC than an equivalent amount of Stx1. The decreased binding affinity and increased toxicity for HIMEC of Stx2 compared to those of Stx1 may be relevant to the preponderance of Stx2-producing STEC involved in the pathogenesis of hemorrhagic colitis and its systemic complications. The differences between primary and transformed HIMEC in these responses were negligible. We conclude that transformed HIMEC lines could represent a simple physiologically relevant model to study the role of Stx in the pathogenesis of hemorrhagic colitis.
Enteric infection with Shiga toxin (Stx)-producing Escherichia coli (STEC) is associated with bloody diarrhea, often presenting as a characteristic clinical syndrome, hemorrhagic colitis (HC). STEC infections can lead to the development of hemolytic uremic syndrome (HUS) and thrombotic thrombocytopenic purpura (TTP) (10, 16). The pathogenesis of HC, HUS, and TTP is characterized by a thrombotic microangiopathy related to endothelial damage. This damage is believed to be due to circulating bacterial exotoxins (Stxs), endotoxins, and host-derived cytokines (tumor necrosis factor alpha [TNF-α] and interleukin-1β [IL-1β]), which may play a pivotal role by activating endothelial cells (EC) to respond to the toxins (18, 25).
Stx1 and Stx2 inhibit protein synthesis in a variety of EC of human origin (11, 15, 17, 20, 25, 26, 28). These data demonstrate that EC cultures derived from the endothelium of large blood vessels, such as umbilical and saphenous veins, do not constitutively produce large amounts of globotriaosylceramide (Gb3), the Stx receptor glycolipid, and are not very sensitive to toxin unless activated by lipopolysaccharides (LPS) or certain cytokines (15, 17, 20, 26) that induce de novo synthesis of Gb3. It is the increase in surface expression of the Stx receptor that leads to enhanced sensitivity to the toxins. In contrast, human renal microvascular EC (HRMEC) constitutively express maximum amounts of Gb3, are highly sensitive to Stx1, and to fail to respond further after activation by LPS or cytokine (15, 20). These data provide a potential explanation for targeting of glomeruli in HUS. Recently, another group using a different HRMEC line has reported just the opposite, that is, limited sensitivity of resting cells to Stx1 but marked activation following exposure to TNF-α (28). Preliminary reports suggest that responses of cerebral microvascular EC to Stx1 are also enhanced by TNF-α or IL-1β treatment (11, 23).
STEC colonize portions of the large intestine, and by using cultured human intestinal epithelial cell lines that develop tight junctions as an in vitro model, translocation of biologically active Stx has been demonstrated across this epithelial barrier (1). Microvascular EC in the intestine therefore will be the first endothelium to contact translocating toxin. Toxin-mediated EC damage may result in the characteristic bleeding of the HC syndrome associated with some STEC infections and gain access to the circulation to ultimately act on EC at distant sites such as the kidney and the brain.
EC isolated from different organs, and macrovascular and microvascular EC from the same organ, demonstrate considerable phenotypic heterogeneity (21, 22). Recently, microvascular EC isolated from human intestine have become available (4, 8, 9). The purpose of the present studies was to examine EC derived from the human intestine and determine their response to two E. coli-derived Stxs, Stx1 and Stx2. Our goal was to develop an in vitro model to study the pathogenesis of HC due to STEC infection.
(This work was presented in part at the annual meeting of the American Society for Microbiology [1a] and at the 3rd International Symposium and Workshop on Shiga Toxin [Verocytotoxin]-Producing Escherichia coli Infections, June 1997, Baltimore, Md. [abstr. V144/V].)
MATERIALS AND METHODS
Toxin purification, iodination, and assay.
Stx1 was purified from cell lysates of E. coli HB101-H19B, an STEC expressing Stx1 only. Stx2 was obtained from the culture supernatants of E. coli C600 lysogenized with bacteriophage 933W. Both toxins were purified by affinity chromatography on a P1 blood group glycoprotein-Sepharose 4B column, as previously described (5).
Toxins were iodinated by incubating 10 μg of toxin in 0.2 M potassium phosphate buffer, pH 7.5, with 1 mCi of dried 125I-labeled Bolton-Hunter reagent (ICN, Costa Mesa, Calif.) at 0°C with rocking, and the incubation was stopped after 1 h with 20 μl of 1 mM glycine. Iodinated toxins, purified by Sephadex G-25 chromatography, retained full biological activity (as shown by cytotoxicity assay) and had a specific activity between 10,000 and 20,000 cpm/ng of protein.
Cytotoxicity was measured as the inhibition of protein synthesis in toxin-treated cells according to our previously published methods (13). Target cells were grown in 96-well plates at 37°C and incubated for 24 h with serial 10-fold dilutions of Stx1 or Stx2 or medium alone. [3H]leucine (1 μCi/100 μl) was added for 30 min, and the percent inhibition of incorporation of label into trichloroacetic acid-precipitable protein was measured. Cytotoxicity was expressed as the amount of toxin needed to inhibit leucine incorporation by 50% (TI50). In some experiments, primary human intestinal microvascular EC (HIMEC) were incubated for 72 h with 40 μM of d,l-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol · HCl (PDMP), an inhibitor of neutral glycolipid synthesis which reduces the Gb3 content of cells (12).
Binding of 125I-labeled Stx was measured following exposure of cells to labeled toxin for 1 h at 4°C, as previously described (13). Data were subjected to Scatchard analysis to determine the binding affinity and the number of binding sites per cell for each toxin and cell line. To assess movement of bound toxin from the cell surface, antibody rescue experiments were performed. Primary HIMEC monolayers in 96-well plates were treated with Stx1 or Stx2 (100 pg/ml) at 4°C for 1 h and washed, fresh medium at 37°C was added, and the cells were transferred to a 37°C incubator. At times varying from 0 to 120 min following temperature shift, an excess of specific polyclonal Stx1 or Stx2 neutralizing antibody was added. [3H]leucine incorporation by the monolayers was measured after an overnight incubation at 37°C, as described above.
Isolation and maintenance of HIMEC.
HIMEC were isolated from mucosal strips of intestine from surgically resected intestinal specimens from one patient by collagenase treatment and mechanical compression, as previously described (4). A transformed cell line was established from intestinal tissues from a second patient by treatment with the Linker CMVT retroviral construct, which encodes the simian virus 40 large T antigen and neomycin phosphotransferase enzyme (3). The cells were maintained in fibronectin-coated 75-cm2 flasks in MDCB 131 medium (Sigma) supplemented with 20% heat-inactivated fetal bovine serum, 2.5% (vol/vol) penicillin-streptomycin-fungizone, heparin (90 μg/ml) (all from Gibco-BRL), and EC growth factor (50 μg/ml; Boehringer Mannheim). For all assays, cells were grown in fibronectin-coated 96-well microtiter plates and used when confluent.
Isolation and maintenance of HSVEC.
Human saphenous vein macrovascular EC (HSVEC) were isolated by collagenase treatment of discarded saphenous vein segments after coronary bypass operations. Cells were maintained in gelatin-coated 75-cm2 flasks in medium 199 supplemented with 10% fetal bovine serum, penicillin-streptomycin, heparin (50 μg/ml) (all from Gibco-BRL) and retina-derived growth factor extracted from bovine retinas. For all assays, cells were grown in gelatin-coated 96-well microtiter plates and used when confluent.
Measurement of Gb3 content of cells.
Total cellular Gb3 was measured by high-performance liquid chromatography using extracts of one 75-cm2 flask of cells, as described previously (14). Neutral glycolipids were isolated, benzoylated, and analyzed on a pellicular Zipax column (DuPont, Wilmington, Del.) by eluting with a gradient of 2 to 46% dioxane-hexane (46:54, vol/vol) in hexane at a flow rate of 2 ml/min. Eluted peaks were detected by absorption at 230 nm and analyzed with System Gold software (Beckman Instruments, San Ramon, Calif.).
Measurement of markers of cell activation.
Cell adhesion molecules—intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin—were assayed for HIMEC and HSVEC. Triplicate wells in 96-well microtiter plates were pretreated with medium containing different concentrations of LPS, TNF-α, IL-1β, Stx1 or Stx2, or medium alone for 24 h. Adhesion molecules expressed on the cell surface were measured by a modified enzyme-linked immunosorbent assay (ELISA). Cells were washed with phosphate-buffered saline (PBS) and fixed with 100 μl of methanol for 20 min, blocked with 200 μl of 1% gelatin in PBS (blocking solution) for 2 h, and treated with 50 μl of either anti-human VCAM-1 (1/2,000 dilution of 1 mg/ml; Endogen, Woburn, Mass.), anti-human ICAM-1 (1/500 dilution of 40 μg/ml; T Cell Diagnostics, Inc., Cambridge, Mass.), or anti-human E-selectin (1/2,000 dilution of 1 mg/ml; R&D Systems, Abingdon, United Kingdom) per well. As all three antibodies were murine immunoglobulin G (IgG) antibodies, a negative control of mouse IgG was included (DAKO Corp., Carpintiera, Calif.). Bound antibody was detected by the addition of 50 μl of peroxidase-conjugated anti-mouse IgG (1/6,000 dilution of 1 mg/ml; Promega, Madison, Wis.) for 1 h and developed with 100 μl of tetramethyl-benzidine reagent (DAKO) per well for 10 to 30 min, but always for the same time on a single plate. All antibodies were diluted in blocking solution, and the monolayers were washed five times with 200 μl of PBS–0.1% gelatin after each incubation. The reaction was stopped with 100 μl of 1 N HCl, and the A450 was measured on an ELISA plate reader, with wells treated with the negative control antibody considered blanks. Cells were considered to be activated when the A450 per cell in treated wells was significantly higher than in the corresponding medium-only wells. Cell number was determined by hemocytometer counting at a confidence level of 1% when subjected to Student’s t test.
Measurement of IL-8.
IL-8 was measured in culture supernatants with a commercially available kit (Endogen), used according to the manufacturer’s instructions.
RESULTS
Expression of adhesion molecules and IL-8.
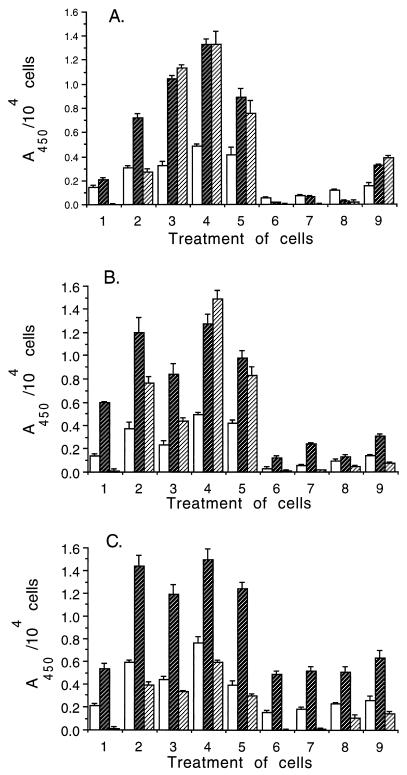
ICAM-1 and VCAM-1 were expressed on the surface of resting cells of all three lines (Fig. 1), and their expression increased significantly (P < 0.001) 24 h following activation by TNF-α, IL-1β, or LPS and did not increase further at 48 h. E-selectin was not detected on resting cells but was expressed at high levels in all three lines following activation and was expressed maximally in the presence of either LPS or the combination of TNF-α and IL-1β. There was no evidence of activation when cells were incubated with Stx1 or Stx2; on the contrary, expression of ICAM-1 and VCAM-1 decreased in toxin-treated primary (Fig. 1A, treatments 6 and 7) and transformed (Fig. 1B, treatments 6 and 7) HIMEC (P < 0.001). Toxin treatment did not significantly affect expression of adhesion molecules on HSVEC (Fig. 1C, treatments 6 and 7). VCAM-1 and ICAM-1 decreased to below resting levels on all cell lines pretreated with TNF-α and/or IL-1β and toxin (Fig. 1, treatments 7 and 8). In the presence of both TNF-α and IL-1β, inhibition of HIMEC adhesion molecule was greater for Stx1 (Fig. 1A and B, treatment 9) in HIMEC (P < 0.01).
FIG. 1.
The expression of adhesion molecules by resting and activated EC was measured by an ELISA. VCAM-1 (open bars), ICAM-1 (dark bars with light hatching), and E-selectin (light bars with dark hatching) are shown. Data represent A450 after subtraction of background absorbance in the presence of the negative control IgG. (A) Primary HIMEC; (B) transformed HIMEC; (C) HSVEC. Cells were exposed to one of the following treatments: 1, medium alone (resting level); 2, TNF-α, 2 ng/ml; 3, IL-1β, 2 ng/ml; 4, TNF-α, 2 ng/ml, plus IL-1β, 2 ng/ml; 5, E. coli O55:B5 LPS, 1 μg/ml; 6, Stx1, 10 ng/ml; 7, Stx2, 10 ng/ml; 8, TNF-α, 2 ng/ml, plus IL-1β, 2 ng/ml, and Stx1, 10 ng/ml; 9, TNF-α, 2 ng/ml, plus IL-1β, 2 ng/ml, and Stx2, 10 ng/ml. Data shown are from one representative experiment of three separate studies and are expressed as the mean changes in A450 of the triplicate measurements of each value (error bars, standard deviations).
IL-8 production was measured in the supernatant medium of the same primary HIMEC monolayers used to measure adhesion molecules. IL-8 levels were greatly increased in TNF-α-treated cells compared to untreated cells (72.82 and 2.93 ng/ml/105 cells, respectively) and were increased to an even greater extent in cells pretreated with TNF-α and IL-1β (180.3 ng/ml/105 cells). Overnight exposure to toxin of cells exposed to TNF-α and IL-1β resulted in a sharp decrease in IL-8 production (40.33 and 59.61 ng/ml/105 cells in the presence of Stx1 and Stx2, respectively).
Receptor glycolipid levels in EC lines.
The total Gb3 content of resting, confluent EC was determined once for each cell line. Gb3 content was similar in the primary and transformed lines (3,962 and 3,428 pmol/mg of cell protein, respectively) and was 10-fold higher than in SVEC (340 pmol/mg of cell protein). In HIMEC, the major neutral glycolipid was Gb3, comprising 67 to 75% of the total neutral glycolipid fraction on a molar basis, whereas in HSVEC Gb3 accounted for less than 10% of the total neutral glycolipids.
Binding of iodinated Stx1 and Stx2 to EC.
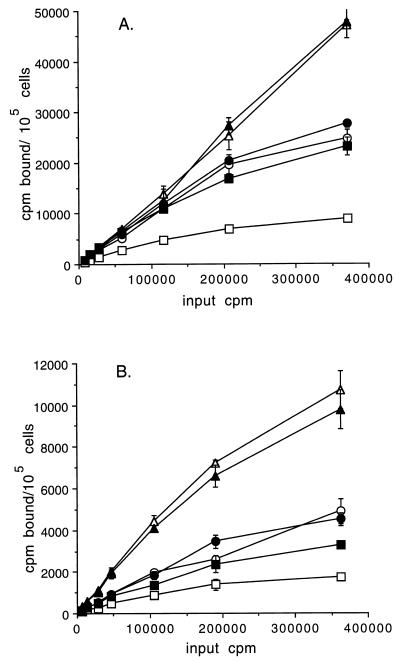
Binding of iodinated Stx1 and Stx2 to EC is shown in Fig. 2. 125I-labeled Stx1 bound to all cells to a much greater extent than did 125I-labeled Stx2. Both toxins bound to unactivated HIMEC at much higher levels than to unactivated HSVEC, and the primary HIMEC bound more Stx1 and Stx2 than the transformed lines. Pretreatment of either HIMEC line with TNF-α or IL-1β failed to increase toxin binding capacity, whereas toxin binding to cytokine-activated HSVEC increased almost to the levels found on HIMEC. Binding capacity and affinity parameters for both toxins and the three cell lines are shown in Table 1. The number of binding sites per cell was approximately the same for each toxin for a given cell line, although the binding capacity of HIMEC was 10-fold greater for both toxins compared to those of HSVEC. The binding affinity of Stx1 was 50-fold greater than the binding affinity of Stx2 for both HIMEC lines and for HSVEC.
FIG. 2.
Binding of iodinated Stx1 (A) and Stx2 (B) to resting or cytokine-activated EC lines. Data are expressed as the means ± 1 standard deviations (error bars) of triplicate data points of one representative experiment of three separate studies. Symbols: open triangles, primary HIMEC; solid triangles, primary HIMEC activated with TNF-α–IL-1β (each at 2 ng/ml); open circles, transformed HIMEC; solid circles, transformed HIMEC activated with TNF-α–IL-1β (each at 2 ng/ml); open squares, HSVEC; solid squares, HSVEC activated with TNF-α–IL-1β (each at 2 ng/ml).
TABLE 1.
Binding parameters of Stx to untreated ECa
| Cell line (toxin) | Binding affinity (M−1) | No. of binding sites/cell |
|---|---|---|
| Primary HIMEC (Stx1) | (2.4 ± 0.8) × 108 A, D | (7.7 ± 2.0) × 105 F, H |
| Primary HIMEC (Stx2) | (7.1 ± 1.3) × 106 A, E | (6.9 ± 1.8) × 105 F, H |
| Transformed HIMEC (Stx1) | (1.9 ± 0.5) × 108 B, D | (6.5 ± 2.3) × 105 F, H |
| Transformed HIMEC (Stx2) | (7.4 ± 2.1) × 106 B, E | (6.8 ± 1.4) × 105 F, H |
| HSVEC (Stx1) | (1.7 ± 0.7) × 108 C, D | (2.9 ± 0.8) × 104 G, H |
| HSVEC (Stx2) | (8.5 ± 2.4) × 106 C, E | (2.2 ± 0.9) × 104 G, H |
Letters next to values correspond to statistical significance for comparisons as follows: A and B, P < 0.007; C, P < 0.02; D, P > 0.04; E, P > 0.7; F, P > 0.8; G, P > 0.3; H, P < 0.001.
Sensitivity of cells to Stx.
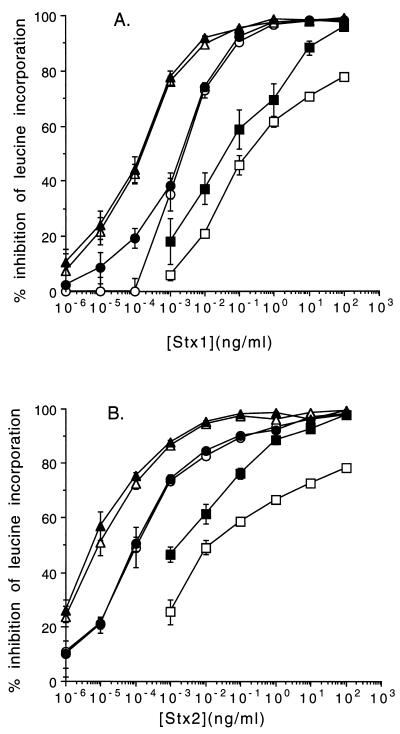
Both primary and transformed HIMEC were highly sensitive to both Stx1 and Stx2 (Fig. 3). Both confluent and subconfluent (data not shown) HIMEC monolayers were equally toxin sensitive. A consistent finding was that both cell lines were more sensitive to Stx2 than to Stx1. Primary cells were more sensitive to both toxins than the transformed cells; thus, the TI50 for Stx1 was 10−4 and 10−5 ng/ml for Stx2 in primary cells compared to 10−3 ng/ml for Stx1 and 10−4 ng/ml for Stx2 in transformed HIMEC. Both cell lines appeared to be fully sensitized, and activation by incubating with TNF-α (2 ng/ml)–IL-1β (2 ng/ml) overnight did not increase the cytotoxicity response of HIMEC to either toxin.
FIG. 3.
Sensitivity of EC to Stx1 (A) and Stx2 (B) following overnight exposure to the toxins. Toxicity is measured as the percent inhibition of incorporation of leucine into protein. Data are expressed as the means ± 1 standard deviations (error bars) of triplicate data points of one representative experiment of three separate studies. Symbols: open triangles, primary HIMEC; solid triangles, primary HIMEC treated with TNF-α (2 ng/ml); open circles, transformed HIMEC; solid circles, transformed HIMEC treated with TNF-α (2 ng/ml); open squares, HSVEC; solid squares, HSVEC treated with TNF-α (2 ng/ml).
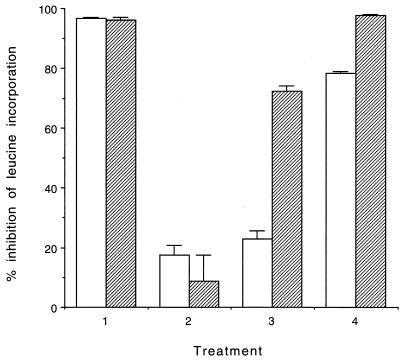
Pretreatment of primary HIMEC over 3 days with 40 μM PDMP, an inhibitor of Gb3 synthesis, neither was cytotoxic nor reduced basal leucine incorporation into protein (data not shown). However, this treatment rendered the cells resistant to Stx1 (Fig. 4). Inclusion of TNF-α (5 ng/ml) with PDMP for the final 2 days of the incubation period did not alter the response to the toxin. However, if TNF-α was added following removal of PDMP the cells recovered their sensitivity more rapidly than did cells in the presence of medium alone (Fig. 4). In contrast, HSVEC were relatively resistant to both toxins (TI50, approximately 10−1 ng/ml for both), but their sensitivity increased significantly following cytokine pretreatment.
FIG. 4.
Effect of TNF-α on recovery of sensitivity to Stx1 of primary HIMEC treated with PDMP. Cells were treated with medium or 40 μM PDMP for 3 days, and then PDMP was removed and the cells were allowed to recover for 0, 2, or 4 days in the absence (open bars) or presence (hatched bars) of TNF-α (5 ng/ml) for the final 2 days of incubation. Stx1 was added at a final concentration of 100 pg/ml to each well for the final 24 h of incubation, and [3H]leucine incorporation was compared to that in similarly treated wells without the addition of toxin. Data are expressed as the means of triplicate data points of a representative experiment of two separate studies (error bars, standard deviations). Treatments: 1, medium for 3 days with or without TNF-α for the final 2 days; 2, PDMP for 1 day followed by PDMP with or without TNF-α for an additional 2 days; 3, PDMP for 3 days followed by medium or TNF-α for an additional 2 days; 4, PDMP for 3 days followed by medium for 2 days and then medium with or without TNF-α for an additional 2 days.
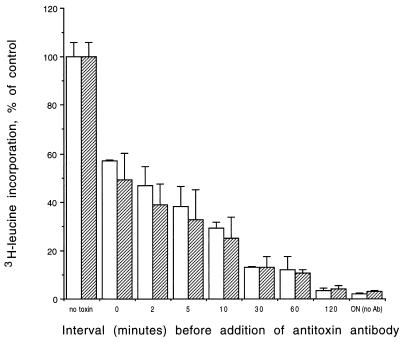
Antibody rescue of toxin bound to HIMEC at 4°C is shown in Fig. 5. The addition of an excess of antibody at time zero after washing and warming cells to 37°C protected the cells; however, there was still considerable cytotoxicity, indicating rapid uptake of toxin. There was no significant difference between Stx1 and Stx2 in the time course of antibody rescue. The ability of the added antibody to reduce toxicity decreased as a function of time following toxin removal, and after 120 min cytotoxicity was the same in cells with and without added antibody.
FIG. 5.
Antibody rescue of primary HIMEC exposed to Stx1 (open bars) or Stx2 (hatched bars) at 4°C. Cells were then warmed to 37°C, and at various times afterward, an excess of antibody was added. Percent cell survival was determined by comparing leucine incorporation by treated HIMEC to that by untreated cells (no toxin). Cells exposed to toxin and incubated overnight without antibody [DN (no Ab)] are included to demonstrate maximum cytotoxicity. Data are expressed as the means of triplicate measurements of each data point from one experiment (error bars, standard deviations).
DISCUSSION
These studies were undertaken to determine if microvascular EC of intestinal origin were sensitive to Stxs and could serve as a model to study the pathogenesis of STEC-related bloody diarrhea. The intestinal capillary network is the first EC target to be encountered as small quantities of Stx translocate across the intestinal epithelial cell layer. The exquisite sensitivity of HIMEC in vitro to Stx1 and Stx2 is consistent with a role in the pathogenesis of HC. These EC are also the most likely gateway to the systemic circulation for Stx to reach the kidney and brain in the pathogenesis of HUS and TTP, and toxin-mediated local EC damage could facilitate dissemination to the more distal targets.
Both primary and transformed HIMEC lines expressed EC adhesion markers and responded to cytokines and LPS with increased surface expression. HIMEC constitutively produced the inflammatory chemokine IL-8 (2), and this too was stimulated nearly 27-fold when cells were treated with TNF-α. Stx1 increases inflammatory cytokine production by human macrophages (24), and a cytokine-mediated burst in IL-8 could be relevant to recruitment of neutrophils to the lamina propria of the intestine in HC, thereby explaining the elevated levels of IL-8 in serum of patients with diarrhea-associated HUS (6).
Inflammatory cytokines and LPS did not increase expression of the Stx receptor glycolipid, Gb3, or increase binding of toxin, as they do in macrovascular EC (15, 17, 20, 21, 27). The two HIMEC lines constitutively produced threefold-greater levels of Gb3 than did Vero cells, the most toxin-sensitive cell line we have previously studied in our laboratory (14). Interestingly, Stx1 bound to all cell lines to a much greater extent than Stx2, although the number of receptors was the same for both toxins. This difference is presumably a consequence of the higher binding affinity of Stx1 for the receptor. However, despite this, HIMEC were more sensitive to inhibition of protein synthesis by Stx2 than Stx1. This differential effect of the two toxins was not noted in HSVEC. While the mechanism is uncertain, we have no evidence that this is due to a difference in the rate at which the toxins are internalized following binding to their cellular receptor, since we found no difference in the time course for specific antibody neutralization of toxin bound to the cell surface at 4°C. These findings may be clinically important, since epidemiological data suggest that HUS is more likely to follow infection by Stx2-producing organisms than following infection by STEC producing Stx1 only (7). If less Stx2 is needed to damage the intestinal endothelium and if the binding affinity of Stx2 is also lower in vivo than that of Stx1, more toxin may be able to access the bloodstream to reach the kidneys and the brain.
The characteristics of the intestinal mucosal microvascular EC reported here (high constitutive production of Gb3, exquisite sensitivity to toxin [more so to Stx2 than Stx1], and no upregulation by LPS or cytokines) are in agreement with those originally reported for HRMEC (18, 19, 21). Others, however, have reported that a homogeneous preparation of HRMEC does not produce high levels of Gb3 and that TNF-α increases the sensitivity to Stx1 (27, 28). The difference in the results may be due to conditions of study, such as cell density, since subconfluent cells may be more sensitive to Stx than confluent cells, or may be related to cell-cell interactions in nonhomogeneous cell lines. In the present study of HIMEC, the lines were highly homogeneous and were not contaminated by other cell types (4) and there were no density-dependent differences in response to either toxin. The HIMEC lines also responded to inflammatory cytokines and LPS by increasing expression of adhesion molecules, and when we first depleted cellular Gb3 by blocking the biosynthetic pathway with PDMP we were also able to demonstrate upregulation of Gb3 by TNF-α during the recovery phase. We remain cautious in generalizing from these results until more HIMEC derived from more individuals can be studied.
Finally, we found only minor differences between the primary and transformed HIMEC lines, for example, a greater induction of cell adhesion markers by IL-1β than TNF-α in primary cells, with the reverse in transformed cells, and a somewhat greater sensitivity of primary cells to the two toxins. However, the two lines were obtained from different donors, and donor variability in the response to Stx is a known property of EC lines (17, 20). More important, Gb3 was fully expressed in the two lines, and while both were highly sensitive to both toxins, they were more sensitive to Stx2 than to Stx1 even though Stx1 bound to a much greater extent to both cell lines. Microvascular cells are in vivo targets for Stx and thus represent a more biologically relevant investigative target than macrovascular cells. A major problem is that primary HIMEC are difficult to grow. The overall similarity in response to Stx1 and Stx2, cytokines, and LPS between the primary and transformed HIMEC make the latter an attractive model for studying toxin-mediated pathophysiological changes in vitro. This will allow us to address many unanswered questions relative to the role of toxin in the pathogenesis of STEC-related thrombotic microangiopathy.
ACKNOWLEDGMENTS
This work was supported by the following grants from the National Institutes of Health, Bethesda, Md.: AI-16242 and DK-07329 (G.T.K.), AI-39067 (D.A.), P 30 DK-34928 for the Center for Gastroenterology Research on Absorptive and Secretory Processes, DK-30399, DK-50984 (C.F.), and DK-02417 (D.G.B.).
REFERENCES
- 1.Acheson D W K, Moore R, De Breucker S, Lincicome L, Jacewicz M, Skutelsky E, Keusch G T. Translocation of Shiga toxin across polarized intestinal cells in tissue culture. Infect Immun. 1996;64:3294–3300. doi: 10.1128/iai.64.8.3294-3300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Acheson D, Jacewicz M, Lincicome L, Bielinski D, Binion D, West G, Fiocchi C, Keusch G. Abstracts of the 97th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1997. Shiga toxin action on human intestinal microvascular endothelial cells (HIMEC), abstr. B-160; p. 56. [Google Scholar]
- 2.Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992;307:97–101. doi: 10.1016/0014-5793(92)80909-z. [DOI] [PubMed] [Google Scholar]
- 3.Binion D G, Orosz D E, Kugasthan S, Miller K A, Fu S, Wilson K T, Ziats N P, Emancipator S E, Jacobberger J W, Keusch G T, Fiocchi C. Generation and characterization of immortalized human intestinal microvascular endothelial cell lines (HIMEC-CMVT) Gastroenterology. 1997;112:A936. . (Abstract.) [Google Scholar]
- 4.Binion D G, West G A, Ina K, Ziats N P, Emancipator S N, Fiocchi C. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology. 1997;112:1895–1907. doi: 10.1053/gast.1997.v112.pm9178682. [DOI] [PubMed] [Google Scholar]
- 5.Donohue-Rolfe A, Acheson D W K, Kane A V, Keusch G T. Purification of Shiga and Shiga-like toxins I and II by receptor analog affinity chromatography with immobilized P1 glycoprotein and the production of cross-reactive monoclonal antibodies. Infect Immun. 1989;57:3888–3893. doi: 10.1128/iai.57.12.3888-3893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzpatrick M M, Shah V, Trompeter R S, Dillon M J, Barratt T M. Interleukin-8 and polymorphoneutrophil leukocyte activation in hemolytic uremic syndrome of childhood. Kidney Int. 1992;42:951–956. doi: 10.1038/ki.1992.372. [DOI] [PubMed] [Google Scholar]
- 7.Griffin P M, Ostroff S M, Tauxe R V, Greene K D, Wells J G, Lewis J H, Blake P A. Illnesses associated with Escherichia coli O157:H7 infections. A broad clinical spectrum. Ann Intern Med. 1988;108:705–712. doi: 10.7326/0003-4819-109-9-705. [DOI] [PubMed] [Google Scholar]
- 8.Haraldsen G, Rugtveit J, Kvale D, Scholz T, Muller W A, Hovig T, Brandtzaeg P. Isolation and long term culture of human intestinal microvascular endothelial cells. Gut. 1995;37:225–234. doi: 10.1136/gut.37.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haraldsen G, Kvale D, Lien B, Farstad I N, Brandtzaeg P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human intestinal microvascular endothelial cells. J Immunol. 1996;156:2558–2565. [PubMed] [Google Scholar]
- 10.Hofmann S L. Southwestern internal medicine conference: Shiga-like toxins in hemolytic-uremic syndrome and thrombotic thrombocytopenic purpura. Am J Med Sci. 1993;306:398–406. doi: 10.1097/00000441-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Hutchison J, Stanimirovic D, Shapiro A, Armstrong G. Verotoxin causes cytotoxicity in human cerebral endothelial cells. In: Kaper J B, O’Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C: American Society for Microbiology; 1997. pp. 323–328. [Google Scholar]
- 12.Inokuchi J, Radin N S. Preparation of the active isomer of 1-phenyl-2-decanoylamino-3-morpholino-propanol, an inhibitor of murine glucocerebroside synthetase. J Lipid Res. 1987;28:565–571. [PubMed] [Google Scholar]
- 13.Jacewicz M, Feldman H A, Donohue-Rolfe A, Keusch G T. Pathogenesis of Shigella diarrhea. XIV. Analysis of Shiga toxin receptors on cloned HeLa cells. J Infect Dis. 1989;159:881–889. doi: 10.1093/infdis/159.5.881. [DOI] [PubMed] [Google Scholar]
- 14.Jacewicz M S, Mobassaleh M, Gross S K, Balasubramanian K A, Daniel P F, Raghavan S, McCluer R H, Keusch G T. Pathogenesis of Shigella diarrhea. XVII. A mammalian cell membrane glycolipid, Gb3, is required but not sufficient to confer sensitivity to Shiga toxin. J Infect Dis. 1994;169:538–546. doi: 10.1093/infdis/169.3.538. [DOI] [PubMed] [Google Scholar]
- 15.Kaye S A, Louise C B, Boyd B, Lingwood C A, Obrig T G. Shiga toxin-associated hemolytic uremic syndrome: interleukin-1β enhancement of Shiga toxin cytotoxicity toward human vascular endothelial cells in vitro. Infect Immun. 1993;61:3886–3891. doi: 10.1128/iai.61.9.3886-3891.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keusch G T, Acheson D W K. Thrombotic thrombocytopenic purpura associated with Shiga toxins. Semin Hematol. 1997;34:106–116. [PubMed] [Google Scholar]
- 17.Keusch G T, Acheson D W K, Aaldering L, Erban J, Jacewicz M. Comparison of the effect of Shiga-like toxin 1 on cytokine- and butyrate-treated human umbilical and saphenous vein endothelial cells. J Infect Dis. 1996;173:1164–1170. doi: 10.1093/infdis/173.5.1164. [DOI] [PubMed] [Google Scholar]
- 18.Louise C B, Obrig T G. Human renal microvascular endothelial cells as a potential target in the development of the hemolytic uremic syndrome as related to fibrinolysis factor expression. Microvascular Res. 1994;47:377–387. doi: 10.1006/mvre.1994.1030. [DOI] [PubMed] [Google Scholar]
- 19.Louise C B, Obrig T G. Specific interaction of Escherichia coli O157:H7-derived Shiga-like toxin II with human renal endothelial cells. J Infect Dis. 1995;172:1397–1401. doi: 10.1093/infdis/172.5.1397. [DOI] [PubMed] [Google Scholar]
- 20.Obrig T G, Louise C B, Lingwood C A, Boyd B, Barley-Maloney L, Daniel T O. Endothelial heterogeneity in Shiga toxin receptors and responses. J Biol Chem. 1993;268:15484–15488. [PubMed] [Google Scholar]
- 21.Page C, Rose M, Yacoub M, Pigott R. Antigenic heterogeneity of vascular endothelium. Am J Pathol. 1992;141:673–683. [PMC free article] [PubMed] [Google Scholar]
- 22.Petzelbauer P, Bender J R, Wilson J, Pober J S. Heterogeneity of dermal microvascular endothelial cell antigen expression and cytokine responsiveness in situ and in cell culture. J Immunol. 1993;151:5062–5072. [PubMed] [Google Scholar]
- 23.Ramegowda B, Fonseca O G, Samuel J E, Tesh V L. Shiga toxin 1 (Stx1) interaction with human brain microvascular endothelial cells: cytokines as sensitizing agents. In: Kaper J B, O’Brien A D, editors. Program and abstracts of the 3rd International Symposium and Workshop on Shiga Toxin (Verocytotoxin)-Producing Escherichia coli Infections. Washington, D.C: American Society for Microbiology; 1997. p. 71. [Google Scholar]
- 24.Ramegowda B, Tesh V L. Differentiation-associated toxin receptor modulation, cytokine production, and sensitivity to Shiga-like toxins in human monocytes and monocytic cell lines. Infect Immun. 1996;64:1173–1180. doi: 10.1128/iai.64.4.1173-1180.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson S E, Karmali M A, Becker L E, Smith C R. The histopathology of the hemolytic uremic syndrome associated with verocytotoxin-producing Escherichia coli infections. Hum Pathol. 1990;19:1102–1108. doi: 10.1016/s0046-8177(88)80093-5. [DOI] [PubMed] [Google Scholar]
- 26.Tesh V L, Samuel J E, Perera L P, Sharefkin J B, O’Brien A D. Evaluation of the role of Shiga and Shiga-like toxins in mediating direct damage to human vascular endothelial cells. J Infect Dis. 1991;164:344–352. doi: 10.1093/infdis/164.2.344. [DOI] [PubMed] [Google Scholar]
- 27.Van de Kar N C, Monnens L A, Van Hinsbergh V W. Tumor necrosis factor and interleukin 1 induce expression of the glycolipid verotoxin receptor in human endothelial cells. Implications for the pathogenesis of the haemolytic uraemic syndrome. Behring Inst Mitt. 1993;92:202–209. [PubMed] [Google Scholar]
- 28.van Setten P A, van Hinsbergh V W, van der Velden T J, van der Kar N C, Vermeer M, Mahan J D, Assmann K J, van den Heuvel L P, Monnens L. Effects of TNF alpha on verocytotoxin cytotoxicity in purified human glomerular microvascular endothelial cells. Kidney Int. 1997;51:1245–1256. doi: 10.1038/ki.1997.170. [DOI] [PubMed] [Google Scholar]