Abstract
Background and Objectives
Novel diagnostic techniques and neurologic biomarkers have greatly expanded clinical indications for CSF studies. CSF is most commonly obtained via lumbar puncture (LP). Although it is generally believed that LPs are well tolerated, there is a lack of supportive data for this claim, and patients anticipate LP to be painful. The objective of this study was to prospectively investigate discordance between patient perception and tolerability of LP.
Methods
Adult patients were surveyed before and after LP regarding their perceptions and experience of LP. Physician perceptions were gathered through a web-based survey. Relative risk and Spearman correlation were used to assess the relationship between responses. Paired binomial and paired ordinal responses were compared by McNemar and paired Wilcoxon rank-sum tests.
Results
A total of 178 patients completed the surveys. About half of the patients (58%) reported anxiety pre-LP, at median 3.0 of 10. Physicians overpredicted patients' pre-LP anxiety (median score 5.0, p < 0.001). Experienced pain was significantly less than predicted pain (median scores 0 and 3.0, respectively, p < 0.001). Patients who predicted pain were more likely to report pain from LP (relative risk [RR] 1.3). Predicting pain was also correlated with anxiety before LP (p < 0.001).
Discussion
LP was generally well tolerated. The majority of patients experienced minimal pain. Anticipation of pain was correlated with both feeling anxious and experiencing pain. The results of this study can be used to reassure patients and providers that LP is indeed not as painful as imagined, which may both reduce pre-LP anxiety and improve LP tolerability.
Despite modern neuroimaging and advancements in serum testing, CSF analysis remains critical in the diagnosis of infections, malignancy, and inflammation of the CNS.1,2 In addition, novel diagnostic techniques, liquid biopsy, and the development of CSF biomarkers for neurodegenerative diseases, neuroinflammatory disease, and CNS malignancies have greatly expanded clinical indications for lumbar puncture (LP).3-6 Indeed, consensus guidelines for several neurologic diseases support the use of CSF analyses.7-10 Therapeutically, LP may be useful in removing excess CSF to temporarily relieve symptoms due to increased intracranial pressure.11,12 In addition, select therapeutics may be instilled directly into the thecal sac, although intraventricular methods are generally preferred.13 Anecdotal evidence suggests that patients fear LP and anticipate a long, painful procedure. However, LP is generally well tolerated.3 These discordant perceptions have led to hesitance in performing LPs in both clinical practice and clinical research settings.14-16 As consultants, neurologists recommend CSF collection and commonly face questions regarding tolerability of the procedure.
Answers to such questions are commonly limited to physician experience and recollection and do not take into consideration direct questioning of patients. The current, limited research on the tolerability of LPs presents conflicting results: A study of patients with idiopathic intracranial hypertension who completed diagnostic LPs showed high rates of postprocedural complications, with many patients reporting significant post-LP pain and anxiety.17 In contrast, in memory and aging clinic patient populations, LPs are generally well tolerated with few serious adverse events,14,18 suggesting that there may be differences in LP tolerability in different patient populations. Importantly, in these studies, pre-LP patient opinion was not solicited. We hypothesized that patient pre-LP anxiety may significantly contribute to pain during the procedure. Moreover, we anticipated significant discordance between patient perceptions and physician perceptions of the procedure.
Methods
Patient Surveys
Clinicians were asked to distribute the surveys to patients who were undergoing LP and collect the completed surveys. One pair of surveys was collected per patient to avoid repetitive sampling. A total of 182 patients initiated the surveys from January 2017 to October 2019. Four patients were excluded due to incomplete surveys, and 178 patients completed both pre- and post-LP surveys.
The pre- and post-LP surveys consisted of paired, matched questions regarding patient understanding of indication for LP, previous LP experience, and, if so, how long ago, with multiple-choice answers. The surveys also queried patient anxiety related to LP, predicted (pre-LP) and experienced (post-LP) pain related to LP, and 5 symptoms including headache, nausea, generalized pain, and vision or gait disturbance pre- and post-LP. Symptoms were then rated in a 11-point scale from 0 (none) to 10 (most severe). Absent symptoms were assigned a rating of 0.
Nonsurvey Patient Data
Nonidentifying demographic information was obtained for each patient who completed the survey. This included patients' sex, age range, body mass index (BMI) range, and cancer type if applicable. In addition, we encoded information regarding the procedures including indications (diagnostic, therapeutic, and both), type of performing clinical provider, LP setting (inpatient vs outpatient), location (bedside vs interventional radiology), and procedure success. If patients were taking medications for anxiety or pain before the procedure, the clinicians were asked to record the medication, dose, and schedule, noting whether the patients were taking the medication regularly or just for the procedure.
Physician Survey
A web-based survey link using RedCap was emailed to physicians at Memorial Sloan Kettering Cancer Center and University of Michigan. A total of 300 completed the anonymous survey from May 2019 to June 2019. The specialties surveyed included neurology, neurosurgery, internal medicine, and oncology. The physician survey consisted of multiple-choice questions. The questions addressed: clinical department, sex, age range, years in clinical practice, whether the physician performs LP as part of the clinical practice, how often LPs are ordered, if the physician thinks the LP causes anxiety or pain in patients and rate the assumed anxiety and pain between 0 (none) and 10 (most severe).
Statistical Analyses
Descriptive statistics were used to characterize patients' demographics and disease status. Paired binomial data, including the comparison between pre- and post-LP symptom presence (yes or no) and the comparison between likelihood to repeat LP in the future before and after the LP, were compared using McNemar tests. Paired ordinal data, including the comparison of the severity ratings of symptoms before and after LP, were compared using paired Wilcoxon rank-sum tests. The Pratt method was used to handle ordinal responses with ties. Effects of various pre-LP factors on post-LP responses were quantified by relative risks and Spearman correlations. Subgroup (e.g., oncologic vs nononcologic) heterogeneity was assessed using the χ2 test. The statistical analysis was performed on R (version 4.0.2).
Standard Protocol Approvals, Registrations, and Patient Consents
Prospective surveys were conducted in patients (aged 18–99 years) pre- and post-LP to obtain paired data regarding patient predictive perceptions and actual experience of LP. The study entitled, “Patient and Clinician Based Perspectives of Lumbar Puncture: A Survey Based Study”, was approved by the institutional review board (IRB) at Memorial Sloan Kettering Cancer Center on 12/23/2016 as exempt protocol X16-042. The University of Michigan IRB approved the protocol on January 18, 2018, as exempt protocol HUM00140158. Both IRBs deemed this anonymous survey to pose minimal risk and determined it to be an exempt protocol exemption #2 of the 45 Code of Federal Regulations 46.101.(b).
Data Availability
The anonymized patient data and patient and physician surveys will be shared at the request of any qualified investigator for purposes of replicating procedures and results.
Results
Patient Demographics
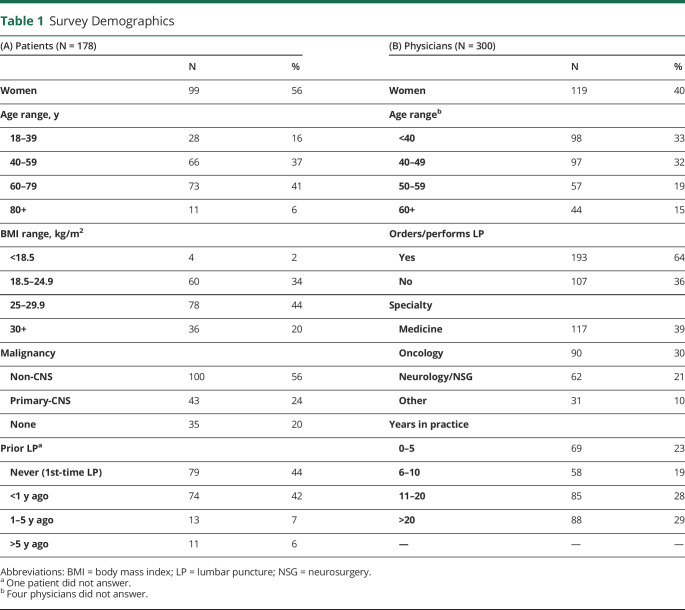
Patient demographics are summarized in Table 1. A total of 178 patients completed both pre-LP and post-LP surveys at Memorial Sloan Kettering Cancer Center (n = 131, 74%) and University of Michigan (n = 47, 26%). About half were women (n = 99, 56%). Age ranged from 18–39 (n = 28, 16%), 40–59 (n = 66, 37%), 60–79 (n = 73, 41%), to ≥80 years (n = 11, 6%). Body mass indices of patients ranged from <18.5 (n = 4, 2%), 18.5–24.9 (n = 60, 34%), 25–29.9 (n = 78, 44%), to ≥30 kg/m2 (n = 36, 20%). The majority of patients carried a diagnosis of malignancy (n = 143, 80%); 43 of these had primary CNS malignancy. Close to half of the patients experienced their first LP as part of this study (n = 79, 44%). The other half had previously undergone LP < 1 year (n = 74, 42%), 1–5 years (n = 13, 7%), or ≥5 years (n = 11, 6%) before the time of completing the survey. One patient could not specify date of prior LP.
Table 1.
Survey Demographics
Physician Demographics
Physician demographics are summarized in Table 1. A total of 300 physicians completed the anonymous web-based survey (40% women) from medical nononcology subspecialties (n = 117, 39%), oncology subspecialties (n = 90, 30%), neurology or neurosurgery (n = 62, 21%), and other subspecialties (n = 31, 10%). The majority of physicians reported that they order or perform LP as a routine part of their clinical practice (n = 193, 64%). Physicians' age ranged from <40 (n = 98, 33%), 40–49 (n = 97, 32%), 50–59 (n = 57, 19%), to ≥60 years (n = 44, 15%). Four physicians did not provide their age range. The physicians' years in practice ranged from ≤5 (n = 69, 23%), 6–10 (n = 58, 19%), 11–20 (n = 85, 28%), to >20 years (n = 88, 29%).
LP Administration
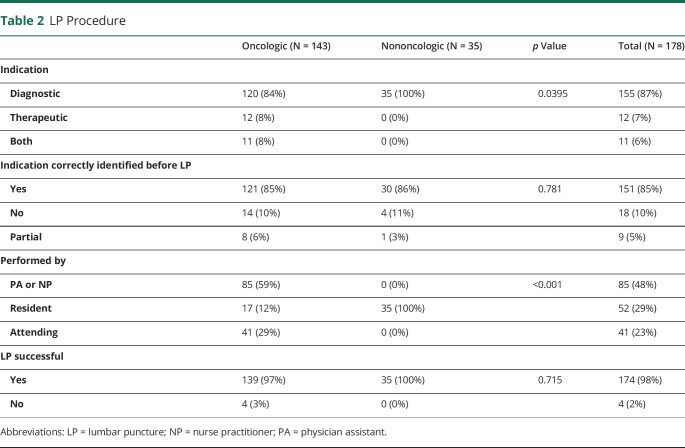
The characteristics of LP procedures are summarized in Table 2. The majority of LPs were performed for diagnostic purpose (n = 155, 87%); the remainder were for therapeutic (n = 12, 7%) or for dual diagnostic and therapeutic (n = 11, 6%) purposes in patients with malignancy. Most patients (n = 151, 85%) correctly identified the indication for LP before the procedure. A small number of patients either incorrectly identified the LP indication (n = 18, 10%) or partially identified the indication such as dual purpose when it was for one indication or vice versa (n = 11, 6%).
Table 2.
LP Procedure
LP was performed by advanced practice providers (n = 85, 48%), resident physicians (n = 52, 29%), or attending physicians (n = 41, 23%). The majority of LPs were performed at the bedside (n = 131, 74%), and about a quarter of the procedures were performed in interventional radiology (n = 47, 26%).
In addition to 15 patients (8%) who take anxiolytics regularly, 10 patients (6%) premedicated with an anxiolytic agent before the LP. Eight patients (4%) took pain medication for the LP before the procedure, and an additional 29 patients (16%) took pain medication regularly. The majority (n = 150, 98%) of LPs were successful.
Pain and Anxiety Related to LP
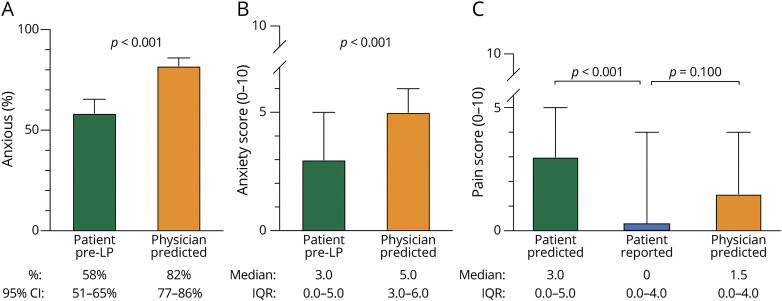
Patients were more anxious before the procedure compared with after the LP (58% vs 35%, p < 0.001), self-rating at a higher score on the 0- to 10-point scale pre-LP at median 3.0 (interquartile range [IQR] 0–5.0) compared with post-LP median 0 (IQR 0–3.0) (p < 0.001). Previous experience with LP did not appreciably alter pre-LP anxiety: pre-LP anxiety frequency was similar between patients with previous LP experience and those who were undergoing the LP for the first time (RR 0.86, 95% CI 0.67–1.11). Physicians overpredicted pre-LP anxiety compared with patient self-report both in frequency (82% vs 58%, p < 0.001) and intensity (median physician predicted score 5.0, IQR 3.0–6.0 vs median patient pre-LP score 3.0, IQR 0–5.0, p < 0.001) (Figure 1). The pain score given to procedural pain from LP did not significantly differ between the 4 BMI ranges (p = 0.1022), or between category of proceduralist (p = 0.0771).
Figure 1. Discordance Between Patient and Physician Perceptions and Patient Experience of Lumbar Puncture.
Physicians overpredicted pre-LP anxiety compared with the patient's answers before the LP, both in (A) frequency (82% vs 58%, p < 0.001) and in (B) intensity out of 0- to 10-point scale (median score 5.0 vs 3.0, p < 0.001). (C) Patients experienced less pain by than predicted on a scale of 0–10 (median 0 vs 3.0, p < 0.001). Notably, 53% of the patients reported LP to be painless. Physician's prediction of procedural pain was comparable to patient's reported pain (1.5 vs 0, p = 0.1). LP = lumbar puncture.
Patients reported significantly less pain than predicted. The post-LP pain score (median 0, IQR 0–4.0) was significantly lower than the pre-LP pain score (median 3.0, IQR 0–5.0) on the 0- to 10-point scale (p < 0.001). In contrast, physicians closely predicted patient-reported procedural pain intensity (physician-predicted score median 1.5, IQR 0–4.0, p = 0.1) (Figure 1).
Patients who predicted pain were 1.3 times more likely to feel pain from LP (RR 1.3, 95% CI 1.0–1.8). There was a positive correlation between predicting pain and experiencing procedural pain (r = 0.23, p = 0.0024) and between predicting pain and feeling anxious before LP (r = 0.52, p < 0.001). Pre-LP anxiety and experiencing procedural pain were less strongly correlated (r = 0.12, p = 0.1) (Figure 2). The procedure setting of the procedure did not significantly affect experienced pain from LP (p = 0.1449). The mean pain score for procedural pain at the bedside was 1.5 (95% CI 0.79–2.19) and under fluoroscopy in interventional radiology was 2.0 (95% CI 1.57–2.46).
Figure 2. Patient Factors Associated With Experiencing LP Pain.
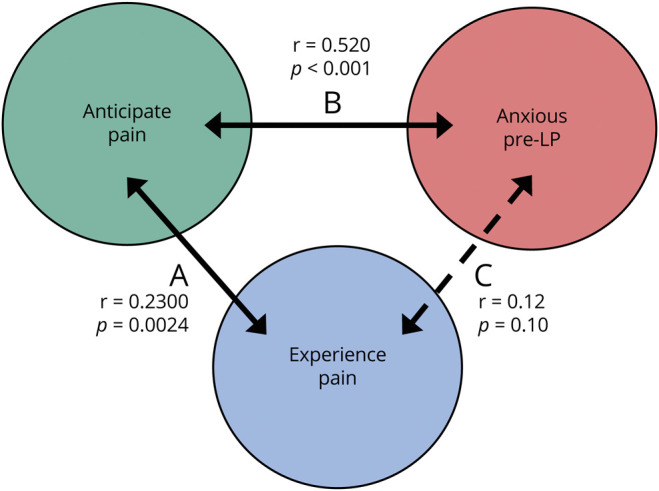
Spearman correlation demonstrates that (A) there is a positive correlation with predicting pain and experiencing pain from LP (r = 0.23, p = 0.0024). (B) Anticipating pain is also positively correlated with feeling anxious before LP (r = 0.52, p < 0.001). (C) Moreover, there may be an association with experiencing procedural pain with feeling anxious before LP (r = 0.12, p = 0.1). LP = lumbar puncture.
Patient Symptoms Pre- and Post-therapeutic LP
All 23 patients undergoing LP for therapeutic purposes carried a cancer diagnosis, and all reported at least 1 of 5 symptoms: gait impairment (n = 12, 52%), headache (n = 12, 52%), generalized pain (n = 11, 48%), visual impairment (n = 7, 30%), or nausea (n = 6, 26%). More than half of those who underwent therapeutic LPs anticipated that the procedure would improve their symptoms (n = 13, 57%), and a third of patients (n = 8, 35%) reported that the LP provided immediate symptom relief. There were no statistically notable differences in the 5 symptoms measured (gait issue, headache, pain, vision trouble, and nausea) either by frequency or by 0–10 scale before and after the LP in the therapeutic LP group.
Would You Undergo LP Again?
Patients were asked whether they would undergo LP again, if recommended by their doctor (Figure 3). Six patients omitted this question and were excluded from analysis. After having undergone the procedure, patient perspectives about LP changed significantly: more patients reported that they were willing to undergo repeat LP after having had the procedure (before LP: n = 114, 66% vs after LP: n = 138, 78%, p < 0.001).
Figure 3. Willingness to Undergo LP Procedure Again.
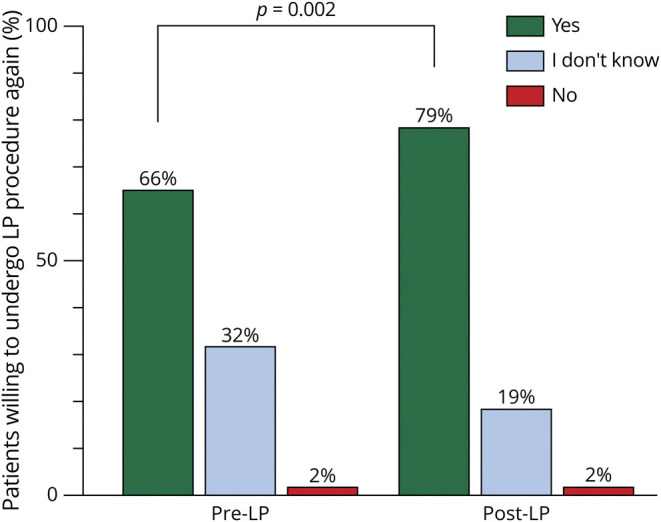
Significantly more patients were agreeable to a repeat LP in the future after having had the LP, compared with the same patients surveyed before the procedure (66% before vs 78% after, p = 0.002). LP = lumbar puncture.
Discussion
In this prospective, multisite, survey-based study, we investigated both patient and physician perceptions of the LP procedure. LPs were generally a well-tolerated procedure, a fact in discordance with patient pre-LP expectations. Patients scored experienced pain to be significantly lower than predicted pain, and less than half of patients actually described the procedure as painful. Patients were more agreeable to future LPs after the procedure than compared to beforehand (79% vs 66% “Yes,” 19% vs 32% “I don't know”), which further suggests that they found the procedure to be acceptable. Two percent of patients answered that they would not want to undergo LP again; this did not change on the pre- and post-LP survey. Across medical specialties, physicians significantly overpredicted patient LP anxiety but accurately rated the procedural pain. We speculate that this overestimation of patient anxiety levels may contribute to delays in ordering a LP. Although the majority of the study population were under care for oncologic diseases, a sizeable minority of nononcologic patients were surveyed for comparison. No differences between these subgroups were identified.
Analysis of CSF for the purposes of tumor sampling or liquid biopsy is rapidly becoming an essential diagnostic tool in the oncologic armamentarium.7,19 In the realm of neuroimmunology, novel CSF biomarkers provide key diagnostic information.20-22 In the setting of degenerative disease, CSF analyses can improve clinical prognostication.23-25 Despite the utility of these studies, there is a reluctance to include CSF collection(s) as part of clinical trial design and/or routine clinical diagnostics: LP is perceived to pose an undue burden on the patient. However, our study suggests that LPs are well tolerated by patients. Importantly, 78% of patients were amenable to a repeat procedure.
Patients who reported higher pre-LP anxiety and who predicted higher pain levels on pre-LP survey subsequently rated higher levels of pain from LP on the post-LP survey. This is in concordance with prior studies that have demonstrated that pre-LP fear and anxiety are risk factors for post-LP complications: patients who are more frightened of the procedure demonstrate up to a sixfold increase in LP complications including pain.3,17,26 Our data emphasize the importance of pre-LP pain and anxiety as a targetable intervention to improve LP tolerability.27 Indeed, previous work in patients with idiopathic intracranial hypertension, patients who felt less informed about the procedure tended to have more post-LP complications.17,28 A nonpharmacologic approach to sooth anxiety, such as music therapy, has shown to reduce LP-related pain and anxiety in a randomized trial.29 Other nonpharmacologic approaches might include improved patient education before the procedure. A pharmacologic intervention might reasonably include the use of anxiolytics. In our study, the number of patients who received anxiolytics before the LP was too small to draw conclusions as to whether this is an effective intervention. This approach is likely most beneficial for patients with exceptionally high pre-LP anxiety.30-32 Our results, similar to prior findings,33 also indicate that a prior history of LPs does not influence patient's pre-LP anxiety levels, and therefore, clinicians should take the time to adequately inform and describe the procedure to all patients, including LP veterans. Of interest, the BMI ranges did not affect the procedural pain significantly. This is likely due to the majority of this study's sample population being patients with cancer and having BMI less than 30 kg/m2. Although patients undergoing diagnostic LP might be expected to experience some degree of post-LP anxiety in anticipation of CSF results, we did not find increased anxiety levels in our patient population post-LP.
Although LPs are generally a diagnostic procedure, they can also be therapeutic and help to treat symptoms of elevated intracranial pressure stemming from hydrocephalus, leptomeningeal disease, and infections such as cryptococcus.17,34,35 In 23 patients who underwent a therapeutic LP in our study, there were no significant differences post-LP symptom ratings. However, the small sample size prohibits definitive conclusions, and further studies are needed to investigate the benefit of therapeutic LPs.
There are several limitations to our study. Our cohort's LP success rate of 98% is significantly higher than the usual bedside LP success rate.36 There are likely several reasons for this discrepancy. In our study, most patients were nonobese and had BMIs <30 kg/m2, and in addition, all patients did not have significantly altered mental status; they needed to complete surveys, both of which are factors that influence LP difficulty. In addition, although most LPs were completed by midlevel providers or resident physicians, all these providers were specialty trained and possessed significant procedural experience: the high level of expertise of the practitioners that likely attributed to the high success rates seen in this study is likely true of many dedicated LP clinic in academic centers or neurology practice. In this study, information regarding existing comorbid mood disorder and/or pain disorders was not collected; however, it would be of interest in future studies to determine whether there are additional risk factors predisposing patients to feeling anxious before LP. Our population's reported anxiolytic and analgesic use (6% and 4%, respectively) is lower than the general neurologic and oncologic populations.37-39 Our study also did not control for needle type (Quincke or Sprotte) or the patient position (sitting vs recumbent), both of which may influence post-LP pain.3,40
LPs are generally well tolerated, and the majority of patients experienced minimal procedural pain. Anticipation of pain is associated with pre-LP anxiety and ultimately procedural tolerability and pain; thus, patient education using evidence-based data to curb the fear of the procedure and pre-LP anxiolytic in highly anxious may improve the tolerability of this essential procedure in neuro-oncology practice and clinical research.
Acknowledgment
The authors thank their patients for generously sharing their time and perspectives to help them improve lumbar punctures for future patients.
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
Y. Umemura, B. Khan, B.Weill, J. Buthorn, A. Skakodub, A.J. Ridder, K.S. Nevel, and Y. Sun report no disclosures relevant to the manuscript. A. Boire reports serving as unpaid member of SAB for Evren Scientific, US Provisional Patent Applications 62/258,044 and 63/052,139, and US Patent 10413522, awarded September 17, 2019, and institutional support NCI Core Grant P30 CA008748. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ Lumbar punctures are less painful than patients predict.
→ Physicians accurately predict patient pain from lumbar puncture but overestimate patient anxiety about the procedure.
→ Patients anxious before lumbar puncture are more likely to experience a higher level of pain with the procedure.
→ Measures to reduce patient anxiety may improve lumbar puncture tolerability.
References
- 1.Wright BL, Lai JT, Sinclair AJ. Cerebrospinal fluid and lumbar puncture: a practical review. J Neurol. 2012;259(8):1530-1545. doi: 10.1007/s00415-012-6413-x. [DOI] [PubMed] [Google Scholar]
- 2.Lee EQ. Neurologic complications in patients with cancer. Continuum (Minneap Minn). 2020;26(6):1629-1645. doi: 10.1212/CON.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 3.Engelborghs S, Niemantsverdriet E, Struyfs H, et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement (Amst). 2017;8:111-126. doi: 10.1016/j.dadm.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nevel KS, Wilcox JA, Robell LJ, Umemura Y. The utility of liquid biopsy in central nervous system malignancies. Curr Oncol Rep. 2018;20(8):60. doi: 10.1007/s11912-018-0706-x. [DOI] [PubMed] [Google Scholar]
- 5.Mattox AK, Yan H, Bettegowda C. The potential of cerebrospinal fluid-based liquid biopsy approaches in CNS tumors. Neuro Oncol. 2019;21(12):1509-1518. doi: 10.1093/neuonc/noz156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Constantinescu R, Mondello S. Cerebrospinal fluid biomarker candidates for parkinsonian disorders. Front Neurol. 2012;3:187. doi: 10.3389/fneur.2012.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boire A, Brandsma D, Brastianos PK, et al. Liquid biopsy in central nervous system metastases: a RANO review and proposals for clinical applications. Neuro Oncol. 2019;21(5):571-584. doi: 10.1093/neuonc/noz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teunissen CE, Petzold A, Bennett JL, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73(22):1914-1922. doi: 10.1212/WNL.0b013e3181c47cc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewczuk P, Riederer P, O'Bryant SE, et al. Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: an update of the consensus of the task force on biological markers in psychiatry of the world federation of societies of biological psychiatry. World J Biol Psychiatry. 2018;19(4):244-328. doi: 10.1080/15622975.2017.1375556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teunissen CE, Tumani H, Bennett JL, et al. Consensus guidelines for CSF and blood biobanking for CNS biomarker studies. Mult Scler Int. 2011;2011:246412. doi: 10.1155/2011/246412. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Wikkelso C, Hellstrom P, Klinge PM, Tans JT, European i NPHMSG. The European iNPH Multicentre Study on the predictive values of resistance to CSF outflow and the CSF Tap Test in patients with idiopathic normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2013;84(5):562-568. doi: 10.1136/jnnp-2012-303314. [DOI] [PubMed] [Google Scholar]
- 12.Rangel-Castilla L, Gopinath S, Robertson CS. Management of intracranial hypertension. Neurol Clin. 2008;26(2):521-541. doi: 10.1016/j.ncl.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nayar G, Ejikeme T, Chongsathidkiet P, et al. Leptomeningeal disease: current diagnostic and therapeutic strategies. Oncotarget. 2017;8(42):73312-73328. doi: 10.18632/oncotarget.20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peskind ER, Riekse R, Quinn JF, et al. Safety and acceptability of the research lumbar puncture. Alzheimer Dis Assoc Disord. 2005;19(4):220-225. doi: 10.1097/01.wad.0000194014.43575.fd. [DOI] [PubMed] [Google Scholar]
- 15.Glimaker M, Johansson B, Bell M, et al. Early lumbar puncture in adult bacterial meningitis: rationale for revised guidelines. Scand J Infect Dis. 2013;45(9):657-663. doi: 10.3109/00365548.2013.799289. [DOI] [PubMed] [Google Scholar]
- 16.Kapoor SG, Ahmed S. Cervical epidural blood patch: a literature review. Pain Med. 2015;16(10):1897-1904. doi: 10.1111/pme.12793. [DOI] [PubMed] [Google Scholar]
- 17.Scotton WJ, Mollan SP, Walters T, et al. Characterising the patient experience of diagnostic lumbar puncture in idiopathic intracranial hypertension: a cross-sectional online survey. BMJ Open. 2018;8(5):e020445. doi: 10.1136/bmjopen-2017-020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duits FH, Martinez-Lage P, Paquet C, et al. Performance and complications of lumbar puncture in memory clinics: results of the multicenter lumbar puncture feasibility study. Alzheimers Dement. 2016;12(2):154-163. doi: 10.1016/j.jalz.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Pentsova EI, Shah RH, Tang J, et al. Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J Clin Oncol. 2016;34(20):2404-2415. doi: 10.1200/JCO.2016.66.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stangel M, Fredrikson S, Meinl E, Petzold A, Stuve O, Tumani H. The utility of cerebrospinal fluid analysis in patients with multiple sclerosis. Nat Rev Neurol. 2013;9(5):267-276. doi: 10.1038/nrneurol.2013.41. [DOI] [PubMed] [Google Scholar]
- 21.Deisenhammer F, Zetterberg H, Fitzner B, Zettl UK. The cerebrospinal fluid in multiple sclerosis. Front Immunol. 2019;10:726. doi: 10.3389/fimmu.2019.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magliozzi R, Cross AH. Can CSF biomarkers predict future MS disease activity and severity? Mult Scler. 2020;26(5):582-590. doi: 10.1177/1352458519871818. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues FB, Byrne LM, McColgan P, et al. Cerebrospinal fluid inflammatory biomarkers reflect clinical severity in Huntington's disease. PLoS One. 2016;11(9):e0163479. doi: 10.1371/journal.pone.0163479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigues FB, Byrne L, McColgan P, et al. Cerebrospinal fluid total tau concentration predicts clinical phenotype in Huntington's disease. J Neurochem. 2016;139(1):22-25. doi: 10.1111/jnc.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desikan RS, McEvoy LK, Thompson WK, et al. Amyloid-beta: associated clinical decline occurs only in the presence of elevated P-tau. Arch Neurol. 2012;69(6):709-713. doi: 10.1001/archneurol.2011.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howell JC, Parker MW, Watts KD, Kollhoff A, Tsvetkova DZ, Hu WT. Research lumbar punctures among African Americans and Caucasians: perception predicts experience. Front Aging Neurosci. 2016;8:296. doi: 10.3389/fnagi.2016.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shpritz DW. Neurodiagnostic studies. Nurs Clin North Am. 1999;34(3):593-606. [PubMed] [Google Scholar]
- 28.Yiangou A, Mitchell J, Markey KA, et al. Therapeutic lumbar puncture for headache in idiopathic intracranial hypertension: minimal gain, is it worth the pain? Cephalalgia. 2019;39(2):245-253. doi: 10.1177/0333102418782192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen TN, Nilsson S, Hellstrom AL, Bengtson A. Music therapy to reduce pain and anxiety in children with cancer undergoing lumbar puncture: a randomized clinical trial. J Pediatr Oncol Nurs. 2010;27(3):146-155. doi: 10.1177/1043454209355983. [DOI] [PubMed] [Google Scholar]
- 30.van Vlymen JM, Sa Rego MM, White PF. Benzodiazepine premedication: can it improve outcome in patients undergoing breast biopsy procedures? Anesthesiology. 1999;90(3):740-747. doi: 10.1097/00000542-199903000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Carroll JK, Cullinan E, Clarke L, Davis NF. The role of anxiolytic premedication in reducing preoperative anxiety. Br J Nurs. 2012;21(8):479-483. doi: 10.12968/bjon.2012.21.8.479. [DOI] [PubMed] [Google Scholar]
- 32.Thomas D, Tipping T, Halifax R, Blogg CE, Hollands MA. Triazolam premedication. A comparison with lorazepam and placebo in gynaecological patients. Anaesth. 1986;41(7):692-697. doi: 10.1111/j.1365-2044.1986.tb12833.x. [DOI] [PubMed] [Google Scholar]
- 33.Tsvetkova DZ, Bergquist SH, Parker MW, et al. Fear and uncertainty do not influence reported willingness to undergo lumbar punctures in a U.S. multi-cultural cohort. Front Aging Neurosci. 2017;9:22. doi: 10.3389/fnagi.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang CC, Perfect JR. Repeated therapeutic lumbar punctures in cryptococcal meningitis - necessity and/or opportunity? Curr Opin Infect Dis. 2016;29(6):539-545. doi: 10.1097/QCO.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 35.DeAngelis LM, Posner JB, Posner JB. Neurologic complications of cancer, In: Contemporary neurology series. 2nd ed. Oxford University Press; 2009:634. [Google Scholar]
- 36.Williams P, Tait G, Wijeratne T. Success rate of elective lumbar puncture at a major Melbourne neurology unit. Surg Neurol Int 2018;9:12. doi: 10.4103/sni.sni_426_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanner AM, Barry JJ. The impact of mood disorders in neurological diseases: should neurologists be concerned? Epilepsy Behav 2003;4(suppl 3):S3-S13. doi: 10.1016/j.yebeh.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 38.Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i45-47. doi: 10.1136/jnnp.2004.060467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unseld M, Zeilinger EL, Fellinger M, et al. Prevalence of pain and its association with symptoms of post-traumatic stress disorder, depression, anxiety and distress in 846 cancer patients: a cross sectional study. Psychooncology. 2021;30(4):504-510. doi: 10.1002/pon.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majd SA, Pourfarzam S, Ghasemi H, Yarmohammadi ME, Davati A, Jaberian M. Evaluation of pre lumbar puncture position on post lumbar puncture headache. J Res Med Sci. 2011;16(3):282-286. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymized patient data and patient and physician surveys will be shared at the request of any qualified investigator for purposes of replicating procedures and results.