Abstract
Background and Objectives
Transient global amnesia (TGA) is an acute amnestic disorder with unclear pathophysiology. Although considered a benign phenomenon, the possibility of a recurrence is a major concern for the patient. Our objective is to identify the prevalence and risk factors of relapse to help clinicians counsel patients about it.
Methods
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidance, we screened 1,658 studies from MEDLINE, Lilacs, and Embase databases, published from 1985 to April 2021, in English or Spanish. We included 36 observational case-control and cohort studies that included patients with TGA according to the Caplan or Hodges and Warlow diagnostic criteria. We performed a meta-analysis with a random effect model for proportions and calculation of odds ratio (OR) for identified risk factors. Methodological quality was assessed according to the Newcastle-Ottawa scale.
Results
We identified 4,514 TGA cases and 544 recurrence events (12.73%). A follow-up had no effect on its variance. We identified a statistically significant association between recurrence and sexual activity as a trigger, a personal history or current state of migraine and depression (OR 1,481 95% CI [1.0341–2.1222] p = 0.04; OR = 2.0795 95% CI [1.3892–3.1128] p = 0.003; and OR = 4.4871 95% CI [1.890–10.651] p = 0.0288, respectively).
Discussion
The analysis showed that approximately 1 of 8 participants may experience recurrence, with an increased risk in the case of a history or current state of migraine, depression, or sexual intercourse before the event. A personal history of migraine and depression was associated with 2 and 4 times risk, respectively.

Among acute amnestic disorders, transient global amnesia (TGA) remains an enigma in neurologic practice. It consists of an episode of sudden-onset anterograde and, occasionally, retrograde amnesia with a complete resolution of symptoms within 24 hours,1,2 frequently associated with triggers including Valsalva-associated maneuvers.3-5
Usually, patients undergo a brain MRI to rule out other differential diagnoses. When an MRI examination is performed within 48 hours of the episode, a punctate hyperintense hippocampal lesion in diffusion-weighted imaging (DWI) can be seen, typically in the cornus ammonis (CA1) field.6
The pathophysiology persists incompletely clarified. Epilepsy-related activity,7 arterial ischemia,8 migraine-associated cortical-spreading depression,9 and temporal lobe venous congestion due to valvular incompetence and internal jugular reflux10,11 were suggested as possible mechanisms.
Although considered self-limited and benign, TGA is a stressful experience for patients, becoming the possibility of relapse a major concern. Variable recurrence rates have been reported in the literature (between 2.9% and 22.8%),3 but its exact prevalence remains unclear.
Despite the growing evidence, clinicians are faced with heterogeneous data concerning TGA recurrence, making it difficult for patient counseling in daily practice. In this meta-analysis, we retrieved observational case-control and cohort studies, aiming to identify the prevalence and risk-conferring factors for having a new TGA episode and their effect during a follow-up. Furthermore, identifying these factors could help enlighten its pathophysiologic mechanisms.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The protocol of this study was a priori registered in the international prospective register of systematic reviews PROSPERO (registration number PROSPERO 2021 CRD42021249506).
Eligibility Criteria
This systematic review and meta-analysis were conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) recommendations.12 The Population, Intervention/issue of interest, Comparison, Outcome, and Study design method was used.13
The inclusion criteria for study eligibility were as follows: (1) observational studies with case-control design and prospective or retrospective cohorts that assessed recurrence and potential risk factors exposure, (2) studies published after 1985 reporting the application of either the Caplan or Hodges and Warlow diagnostic criteria, and (3) studies published in Spanish or English. The exclusion criteria were as follows: (1) other types of studies: reviews, meta-analysis, single-case studies or case series without a follow-up, conference abstracts, editorials, and commentaries.
Search Strategy
Eligible studies were identified from literature published from 1985 to April 2021 using 3 electronic databases: MEDLINE (through PubMed), Lilacs, and Embase. The search terms used were established by a panel of experts in neurology and epidemiology and included the following strategy: MEDLINE and Lilacs: “Amnesia, Transient Global” [Mesh] OR Global Amnesia Transient [tiab] OR TGA OR TGAs OR Global Transient Amnesia [tiab]” including recurrence as a major term. EMBASE: “Amnesia, Transient Global” OR “Global Amnesia Transient” OR “TGA” OR “TGA” OR “Global Transient Amnesia.”
Reference lists of selected publications were also screened to identify additional articles.
The search was rerun on May 25, 2021, before analysis.
Data Extraction and Synthesis and Risk of Bias
Two reviewers applied eligibility criteria and selected studies for inclusion in the meta-analysis. One screened and the other checked for decisions. Disagreements were resolved by consensus. One of the reviewers performed data extraction and the other reviewer assessed the accuracy of the extracted data.
To include as much of the available data as possible, we identified studies where information about recurrence and risk factors were likely to exist but not explicit. These groups were contacted and invited to participate in the analysis by submitting the data.14,15 During data extraction, we checked regarding the authors, affiliation, and origin of the cohort. To avoid overrepresentation, in cases in which it was unclear whether more than 1 article was extracted from the same cohort, we contacted the authors to confirm this. In that case, only the study with the highest sample size, follow-up time, and methodological quality was included.10,11,16
The following data categories were collected: (1) demographic and personal history, (2) exposure to trigger events, (3) duration and characteristics of the event, (4) ancillary studies (brain MRI, EEG, and jugular doppler ultrasound), and (5) a follow up. All aspects concerning personal history, characteristics of the event, and findings in complementary studies were considered as potential risk factors for recurrence.
Risk factors with explicit and precise information in at least 2 studies were meta-analyzed. Owing to the nature of included studies, we stratified methodological quality through the Newcastle-Ottawa scale (NOS)17 for assessing the quality of nonrandomized studies in meta-analyses and converted it to AHRQ standards.
Statistical Analysis and Assessment of Bias
All data were analyzed using R v4.0.5 (2021-03-31) and the meta and dmetar packages. To determine the pooled prevalence of recurrence of at least 1 new TGA event, a meta-analytic study was conducted with a proportion meta-analysis random-effects model based on the inverse variance method and the Freeman-Tukey double arcsine transformation for variance stability. Heterogeneity estimation was performed through the DerSimonian-Laird estimator for tau2 statistics and tested with Cochran Q test. The Jackson method was applied for the confidence interval (CI) of tau2 calculation. The detection of outliers was performed through the calculation of the Clopper-Pearson CI for individual studies. Studies were defined as outliers when their 95% CI lied outside the 95% CI of the pooled effect. When outliers were detected, the meta-analysis was recalculated with their exclusion. To avoid misinterpretation with other situations such as publication bias, both sets of results (with and without the exclusion of outliers) are shown.
To determine the effect of follow-up time on prevalence, a subgroup analysis was performed and a meta-regression with follow-up time as an independent variable in the prevalence estimation. For the estimation of risk factors for recurrence, multiple independent OR meta-analyses were calculated. This analysis was conducted under a random-effects model with the Mantel-Haenszel method and Hartung-Knapp adjustment for random effects. Sidik-Jonkman estimator and Q-profile method were used for tau2 and its CI, respectively.
We defined a statistical significance level of p < 0.05 (2-sided), and effects and predictions were presented with a 95% CI. We assessed publication bias with a funnel plot and Egger test for asymmetry. Two reviewers independently rated the quality of included studies using the NOS and converted it to AHQR standards.
Data were synthesized for the prevalence of risk factors, the number of participants, and events (recurrence) between exposed and nonexposed participants. We used OR as a measure of association and risk.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Data set, data script, and data dictionary are available in eAppendices 1–3, links.lww.com/CPJ/A345, links.lww.com/CPJ/A346, links.lww.com/CPJ/A347.
Results
Overview of the Included Studies
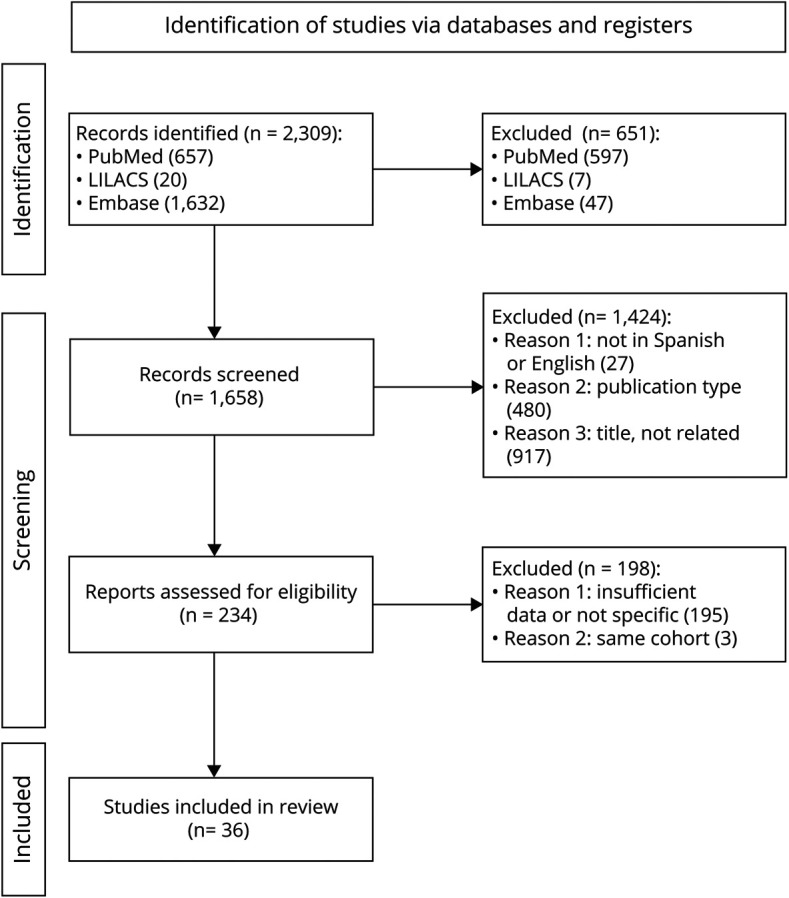
A total of 1,658 studies were identified from databases. 364-11,14-16,18-40,e1,e2 studies were selected covering 4,514 TGA cases and 544 recurrence events. The mean age was 62.67 years, CI 95% (61.78–63.56), and tau2 = 4.719, and the female's proportion was 59.34%, CI 95% (51.30%–66.90% 0.1948), and tau2 = 0.1948. Regarding study design, 14 were case-control9-11,14,16,19,27,28,30,33,35-37,40 and 22, cohort studies.4-8,15,18,20-26,29,31,32,34,38,39,e1,e2 Table 1 summarizes the descriptive features of the studies. eTable 1, links.lww.com/CPJ/A349 shows NOS. Figure 1 presents the PRISMA flow diagram.
Table 1.
Study Characteristics
Figure 1. PRISMA Flowchart.
Recurrence Prevalence
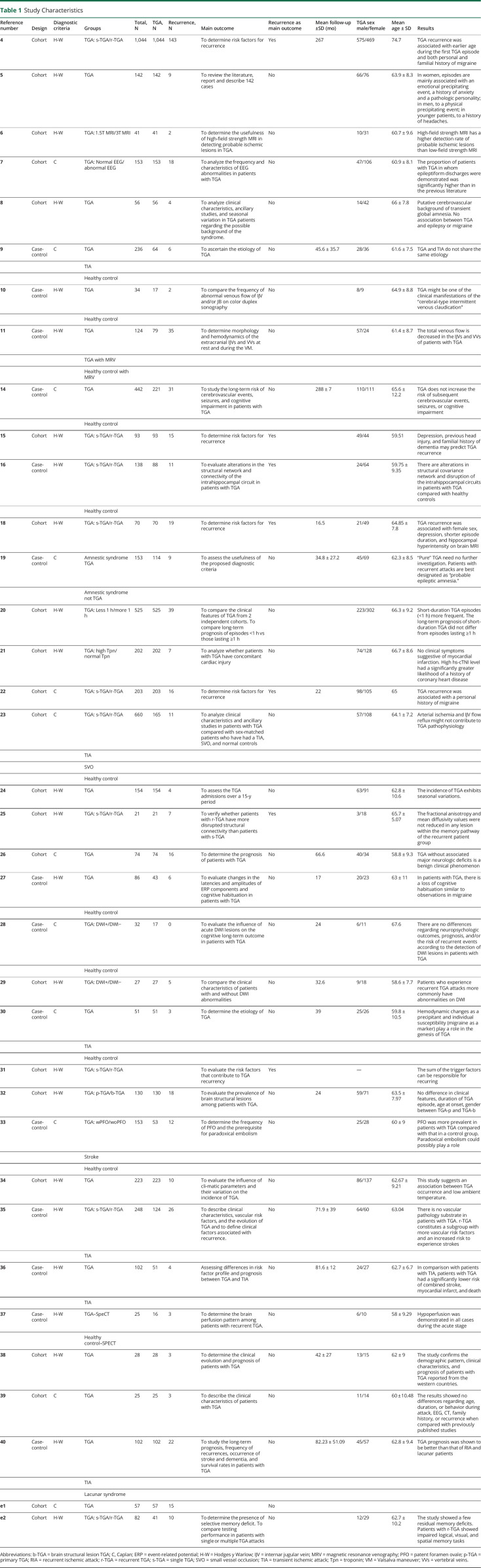
We evaluated 35 studies4-11,14-16,18-30,32-40,e1,e2 reporting the prevalence of recurrence after an episode of TGA. Eight studies were diagnosed as possible outliers (Oliveira, 202018; Romoli, 202020; Eisele, 201921; Han, 201911; Keret, 201624; Moon, 201525; Akkawi, 200534; Fredericks, 1993e1): prevalence after outliers' removal was 11.98% (9.96%–14.15%), tau2 = 0.0032 (0.0009–0.0106), and I2 = 57.6% (34.9%–72.3%).
We found an overall prevalence of 12.37% IC 95% (9.80%–15.17%), tau2 = 0.1008 IC 95% (0.0823–0.1558). Those studies that reported follow-up time were included in a subgroup analysis (n = 14).9,14,18,19,22,26-30,35,36,38,40 Figure 2 represents the recurrence prevalence meta-analysis in a forest plot.
Figure 2. Prevalence of Recurrence Meta-analysis.
The prevalence of recurrence in studies with a follow-up of less than 2 years (n = 5) was 11.95% IC 95% (5.42%–20.36%), in studies with a follow-up between 2 and 4 years (n = 5): 8.70% IC 95% (5.51%–12.45%), and in studies with a follow-up of more than 4 years (n = 4): 18.41% IC 95% (12.80%–24.75%).
The metaregression model with follow-up time as a predictor showed an estimated residual heterogeneity (tau2) of 0.0051. The follow-up time did not have a significant impact (p = 0.7653) on the prevalence estimation. eFigure 1, links.lww.com/CPJ/A348 presents the subgroups analysis and a bubble plot for metaregression.
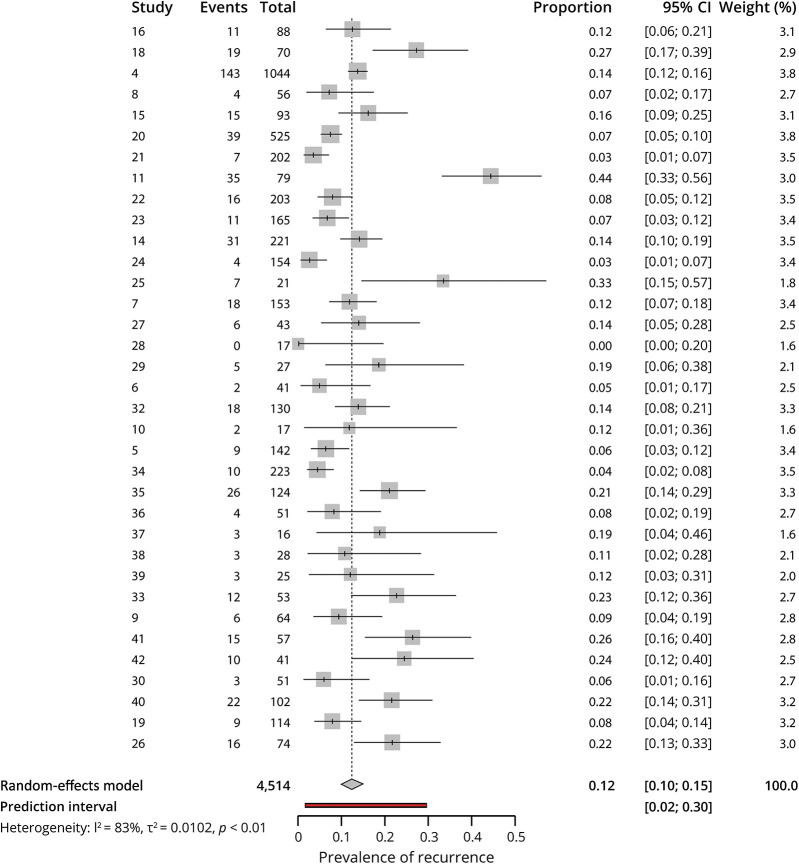
Bias publication was analyzed through the funnel plot (Figure 3), and the Egger test for asymmetry (intercept 1.128 IC 95% [−0.52 to −2.78], p = 0.190) did not indicate the presence of funnel plot asymmetry.
Figure 3. Contour-enhanced Funnel Plot.
Risk Factors for Recurrence
Over the 35 studies, 154,7,10,14-16,18,22,23,25,26,28,29,31,37 reported explicit data concerning possible risk factors for recurrence.
Sex
Eleven of the studies4,10,14-16,18,22,23,25,31,37 presented data to estimate sex as a factor for recurrence. Of 1,030 female patients, 139 experienced a recurrence. Female sex was not a risk factor for TGA recurrence; OR = 0.9536 95% CI (0.6224–1.4609); p = 0.808, with a prediction interval of 95% CI (0.2936–3.0966). Heterogeneity: tau2 = 0.484 CI 95% (0.000–0.998).
Cardiovascular Risk Factors
High blood pressure was present in 426 participants,14,15,18,22,23,25,31,37 with an estimated prevalence of 48.77% 95% CI (45.32%–52.24%), tau2 < 0.0001 95% CI (0–0.006). A total of 13.14% experienced a relapse. Hypertension was not found to be associated with TGA recurrence: OR = 0.9875 95% CI (0.4721–2.0658); p = 0.9691 with a prediction interval of 95% CI (0.1000–9.7522). Heterogeneity: tau2 = 0.7785 CI 95% (0.000–4.522).
The presence of dyslipidemia was reported in 292 participants,14,15,22,23,25,31,37 with a prevalence estimated in 30.74% 95% CI (20.16%–42.40%); tau2 = 0.021 95% CI (0.008–0.135). A total of 11.64% recurred. No association was found with the recurrence of events: OR = 1.181 95% CI (0.930–1.500); p = 0.138 with a prediction interval 95% CI (0.6510–2.1445). Heterogeneity: tau2 = 0.0442.
A history of diabetes was assessed in 52 participants,14,15,18,22,23,37 with an estimated prevalence of 6.65% 95% CI (4.08%–9.73%), tau2 = 0.002 95% CI (0–0.0292). Six participants presented a new event. No association with the recurrence was found, OR = 0.930 95% CI (0.434–1.993); p = 0.817 with a prediction interval of 95% CI (0.188–4.606). Heterogeneity: tau2 = 0.244 95% CI (0–2.462).
A total of 175 particpants14,15,18,22,23 assessed smoking personal history. Prevalence was estimated at 22.54% 95% CI (12.71%–34.18%); tau2 = 0.0195 95% CI (0.0055–0.1701). Only 12% recurred. A meta-analysis showed no risk association: OR = 1.0033 95% CI (0.7860–1.2808); p = 0.9715, prediction interval 95% CI (0.7178–1.4026). Heterogeneity: tau2 = 0.003.
Atrial fibrillation was detected in 22 participants,14,15,22 with an estimated prevalence of 4.21% 95% CI (2.59%–6.17%); tau2 = 0 95% CI (0.0000–0.0012). A total of 6 experienced a recurrence. The meta-analysis showed no significant association: OR 1.1847 95% CI (0.002–609.608); p = 0.918. Heterogeneity: tau2 = 2.9459 95% CI (0.0000; >100.0000).
Medical History of Vascular Events
Personal history of coronary artery disease (CAD) and stroke was present in 4314,15,22,25,31,37 and 52 participants,14,15,18,22,25 respectively. For both expositions, 6 participants experienced a recurrence.
Neither ictus nor CAD had a significant association with TGA recurrence (OR = 0.987 95% CI [0.3229–3.0194]; p = 0.9764, prediction interval 95% CI [0.058–16.771]; heterogeneity: tau2 = 0.630 95% CI [0–14.791]; and OR = 1.289 95% CI [0.497–3.347]; p = 0.523, prediction interval 95% CI [0.161–10.306], heterogeneity: tau2 = 0.423 95% CI [0–5.060], respectively).
Migraine
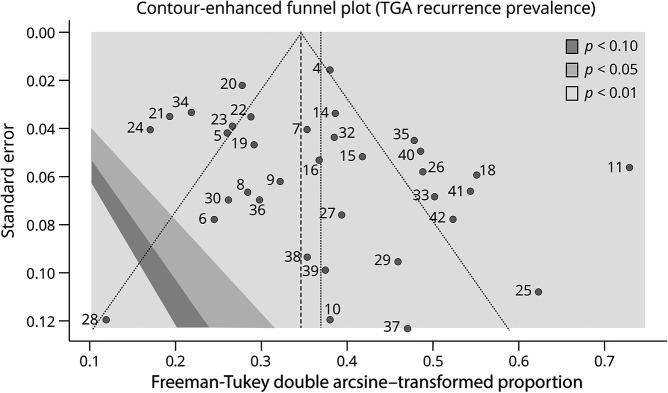
Eight studies assessed a history of migraine in 370 participants.4,14,15,18,22,25,26,37 The estimated prevalence in participants with TGA was 19.81% 95% CI (15.44–24.54) tau2 = 0.0036 (0.0003–0.0318). Altogether, 22.4% (n = 83) experienced a new event. A statistically significant association was found between the presence of migraine and the recurrence of TGA events, OR = 2.0795 95% CI (1.3892–3.1128) p = 0.0036, prediction interval (0.7314–5.9126), Heterogeneity: tau2 = 0.1533 (0.0000–0.7264).
Depression
The estimated prevalence of a history of depression was 22.64% among those with TGA (95% CI [0.164–0.294], heterogeneity: tau2 = 0), reported in only 2 studies.15,18 A total of 16 of 37 exposed participants (43.2%) experienced a recurrence. A statistically significant association with recurrence was found: OR = 4.4871 95%-CI [1.890–10.651] p = 0.0288, and heterogeneity: tau2 = 0.0001.
Triggers
Nine studies4,10,14-16,18,22,25,31 reported at least 1 triggering situation before the event, (prevalence of 47.84%) 95% CI [32.26–63.63], tau2 = 0.0421 with nonsignificant risk of recurrence: OR = 1.1310, 95% CI (0.628–2.036) p = 0.6265, prediction interval (0.149–8.570); heterogeneity: tau2 = 0.563 (0.000–4.431).
A subset of specific triggers reported by at least 2 studies were identified: self-reported stress in 196 individuals,4,14,16,22,31 physical exercise in 156,4,10,14,16,22,25 a shower excessively cold or hot in 63,4,10,14,22,25,31 sexual intercourse before the event in 79,4,14,22,31 a coughing fit in 12, 10,15,22 and vomiting in 11.14,22
There was no association between stress (p = 0.352), exercise (p = 0.963), shower (p = 0.815), vomiting (p = 0.817), and coughing (p = 0.205) as triggers and the recurrence of the event.
Albeit 13 participants of 79 (20.6%) who had sexual intercourse as a trigger experienced a recurrence. An association was found between sexual intercourse as a trigger and recurrence: OR 1.481 95% CI (1.0341–2.1222) p = 0.0401; prediction interval 95% (0.8050–2.7261), heterogeneity: tau2 = 0.007 95% CI (0–0.474).
DWI Lesions
Eight studies4,14,16,18,25,28,29,37 reported typical MRI TGA findings; although not all participants were studied (n = 627), only 6 studies presented data about its association with recurrence. No association was found between the presence of these lesions and the likelihood of a new event. OR 1.7385 95% CI (0.2365–12.7784), p = 0.508; tau2 = 2.939 95% CI (0–31.6675).
EEG (EEG)
In 10 studies,4,7,10,14,16,18,22,29,31,37 a routine EEG was performed on their participants to rule out seizures. In 1 study,25 an EEG was performed only in case a recurrence was present. Six studies4,7,14,18,31,37 (643 participants) reported EEG abnormal findings. It was not related to the occurrence of new events, OR 1.253 95% CI (0.6457–2.4328) p = 0.3579; tau2 = 0.071 CI 95% (0–3.361).
Ultrasound Study
Two studies10,31 evaluated jugular reflux in those with TGA. Finding jugular reflux was not related to TGA recurrence, OR 2.1947 95% CI (0.004–1047.65) p = 0.3522; tau2 = 0.2947.
Other Risk Factors
Some factors were identified only in 1 study.15 Owing to the absence of data, they were not meta-analyzed. The presence of obstructive sleep apnea was reported, but was not associated with recurrence prevalence OR 0.0663 CI 95% (0.0001–34.9115), p = 0.396 nor alcohol abuse history OR 0.0949 95% CI (0.0002–50.815), p = 0.4626. Previous head injury reported in the study was correlated with recurrence OR 18.7 95% CI (4.980–70.209), p ≤ 0.0001.
Another study reported generic Valsalva maneuver as a trigger in 28 participants and was not associated with recurrence (p = 0.926).4 Figure 4 presents a forest plot of significant factors associated with recurrence.
Figure 4. Forest Plot, Significant Odds Ratio (OR).
Discussion
According to our findings, the estimated prevalence of TGA recurrence is 12.37%. A personal history of migraine, depression, or recent sexual activity may increase the risk of a new event.
It is important to mention that recurrence prevalence calculation was made with a considerable degree of heterogeneity between studies. We conjecture that not considering prevalence as a primary outcome and differences in design and follow-up time could be the sources of this heterogeneity.
Only half of the studies reported follow-up time. Although the longer the follow-up is, the higher the probability of a recurrence, a stratified analysis showed that the occurrence of new events did not vary considerably through time (from 11.95% IC 95% [5.42%–20.36%] in studies with follow-up shorter than 2 years to 18.41% IC 95% [12.80%–24.75%] in studies with follow-up longer than 4 years). These results suggest that recurrence events mostly occur within 2 years after the initial event. Furthermore, the regression showed that follow-up time is not a determinant effect in variability of prevalence.
The identification of several outliers showing atypical recurrence prevalence deserves a separate section. Although the reasons for this are conjectures, we will describe some of them. Eisele (2019),21 selected 202 patients from a data set of more than 400 patients with TGA diagnosis; we lacked data concerning the selection process, but that might be the reason for these differences. Studies conducted by Keret24 and Akkawi34 did not specify follow-up time, perhaps it was too short, and therefore, the prevalence was underestimated.
One of the initially described etiologic explanations for TGA is arterial ischemia.8 Consequently, cardiovascular risk factors are those for which the evidence is stronger. Our data and the contribution of other reviews9,23,e3 seem to indicate that recurrence is not linked to the traditional risk factors for ischemic events. Although it is not our intention (nor the design of this study) to explain the TGA physiopathology, these results undoubtedly oppose the arterial ischemic theory.
EEG abnormalities in TGA were previously described,e4 but evidence concerning epilepsy causing TGA is still inconclusive. In studies where abnormal activity was found on EEG (slow temporal waives or epileptiform discharges), clinical and brain MRI findings ruled out the diagnosis of epilepsy.
The fact that internal jugular venous insufficiency (IJVI) is highly prevalent and long-lasting,e5 although TGA recurrence is uncommon, in addition to the absent association between IJVI and relapse, opposes the venous insufficiency theory.
Migraine was previously found to be associated with TGA primarily through a cortical spreading depression mechanism.3,4,15,18,22,e3,e6,e7 One study22 found an association between migraine and TGA recurrence, whereas the prospective cohort in a different study15 and the retrospective cohort in another study18 did not find that association.
In our meta-analysis, we found that a personal history of migraine and a history of depression increased the risk of recurrence by approximately 2 and 4 times, respectively.
Recent experimental evidence supports the association between depression and TGAe8 because it can cause locus coeruleus norepinephrine system overresponse.e9 In addition, experimental models showed that chronic depression could increase vulnerability to hippocampal dysfunction due to ischemia and reduction of LC to CA1 hippocampal projections.e10 It is important to point out that while migraine episodes tend to decrease with age, TGA is more prevalent in older people, indicating that an acute episode of TGA is not a reflection of an episode of acute migraine.
The limitations of our study stems from the evidence about all risk factors, which at times may be scarce. Of 35 studies, only 15 addressed some of them. Personal history and comorbidities were described as reported by the patient or family, but not specified whether it was present during the event or not. Furthermore, data concerning patients with recurrence of 2 or more times, were scarce, impeding a subcohort analysis.
The best-studied factors were those associated with cardiovascular risk. Contrarily, personal history of depression was only described in 2 studies. In consequence, lack of data must be taken into consideration when weighing the conclusions of each analysis.
Studies did not provide information about applied criteria for risk factor diagnosis (including depression and migraine), and did not consistently assess the presence of aura.
This study aims to answer the frequent concern about recurrence. We conclude that approximately 1 in 8 participants may experience a new episode, with an increased risk in case of personal history of migraine, depression, or sexual intercourse before the event. Our work is also a wake-up call for researchers and clinicians to systematically look for these factors in their cohorts.
Acknowledgments
The authors thank Dr. Panegyres and Dr. Rabinstein who provided additional data about patients regarding risk factors for recurrence.
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Caplan LB. Transient global amnesia. Handb Clin Neurol Amsterdam. 1985;1:205-218. [Google Scholar]
- 2.Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry .1990;53(10):834-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc. 2015;90(2):264-272. [DOI] [PubMed] [Google Scholar]
- 4.Morris KA, Rabinstein AA, Young NP. Factors associated with risk of recurrent transient global amnesia. JAMA Neurol. 2020;77(12):1551-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129(pt 7):1640-1658. [DOI] [PubMed] [Google Scholar]
- 6.Lee SY, Kim WJ, Suh SH, Oh SH, Lee KY. Higher lesion detection by 3.0T MRI in patient with transient global amnesia. Yonsei Med J. 2009;50(2):211-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon Y, Yang Y, Jang JW, et al. Left dominance of EEG abnormalities in patients with transient global amnesia. Seizure. 2014;23(10):825-829. [DOI] [PubMed] [Google Scholar]
- 8.Waliszewska-Prosol M, Nowakowska-Kotas M, Bladowska J, Papier P, Budrewicz S, Pokryszko-Dragan A. Transient global amnesia - risk factors and putative background. Neurol India. 2020;68(3):624-629. [DOI] [PubMed] [Google Scholar]
- 9.Zorzon M, Antonutti L, Masè G, Biasutti E, Vitrani B, Cazzato G. Transient global amnesia and transient ischemic attack. Natural history, vascular risk factors, and associated conditions. Stroke. 1995;26(9):1536-1542. [DOI] [PubMed] [Google Scholar]
- 10.Chung CP, Hsu HY, Chao AC, Sheng WY, Soong BW, Hu HH. Transient global amnesia: cerebral venous outflow impairment-insight from the abnormal flow patterns of the internal jugular vein. Ultrasound Med Biol. 2007;33(11):1727-1735. [DOI] [PubMed] [Google Scholar]
- 11.Han K, Hu HH, Chao AC, et al. Transient global amnesia linked to impairment of brain venous drainage: an ultrasound investigation. Front Neurol. 2019;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(7):b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S, eds. Cochrane Handbook For Systematic Reviews Of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. Accessed September 10, 2021. handbook.cochrane.org. [Google Scholar]
- 14.Arena JE, Brown RD, Mandrekar J, Rabinstein AA. Long-term outcome in patients with transient global amnesia: a population-based study. Mayo Clin Proc. 2017;92(3):399-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tynas R, Panegyres PK. Factors determining recurrence in transient global amnesia. BMC Neurol. 2020;20(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DA, Lee S, Kim DW, Lee HJ, Park KM. Effective connectivity alteration according to recurrence in transient global amnesia. Neuroradiology. 2021. 63(9):1441-1449. [DOI] [PubMed] [Google Scholar]
- 17.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if non randomized studies in meta-analyses. Accessed September 10, 2021. ohri.ca/programs/clinical_epidemiology/oxford.htm.
- 18.Oliveira R, Teodoro T, Marques IB. Risk factors predicting recurrence of transient global amnesia. Neurol Sci. 2021;42(5):2039-2043. [DOI] [PubMed] [Google Scholar]
- 19.Hodges JR, Warlow CP. The aetiology of transient global amnesia. A case-control study of 114 cases with prospective follow-up. Brain. 1990;113(pt 3):639-657. [DOI] [PubMed] [Google Scholar]
- 20.Romoli M, Tuna MA, Li L, et al. Time trends, frequency, characteristics and prognosis of short-duration transient global amnesia. Eur J Neurol. 2020;27(5):887-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisele P, Baumann S, Noor L, et al. Interaction between the heart and the brain in transient global amnesia. J Neurol. 2019;266(12):3048-3057. [DOI] [PubMed] [Google Scholar]
- 22.Alessandro L, Calandri IL, Suarez MF, et al. Transient global amnesia: clinical features and prognostic factors suggesting recurrence. Arq Neuropsiquiatr. 2019;77(1):3-9. [DOI] [PubMed] [Google Scholar]
- 23.Himeno T, Kuriyama M, Takemaru M, et al. Vascular risk factors and internal jugular venous flow in transient global amnesia: a study of 165 Japanese patients. J Stroke Cerebrovasc Dis. 2017;26(10):2272-2278. [DOI] [PubMed] [Google Scholar]
- 24.Keret O, Lev N, Shochat T, Steiner I. Seasonal changes in the incidence of transient global amnesia. J Clin Neurol. 2016;12(4):403-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon Y, Moon WJ, Han SH. The structural connectivity of the recurrent transient global amnesia. Acta Neurol Scand. 2016;134(2):160-164. [DOI] [PubMed] [Google Scholar]
- 26.Hinge HH, Jensen TS, Kjaer M, Marquardsen J, de Fine Olivarius B. The prognosis of transient global amnesia. Results of a multicenter study. Arch Neurol. 1986;43(7):673-676. [DOI] [PubMed] [Google Scholar]
- 27.Buhr J, Evers S, Husstedt IW, Frese A. Event related potentials in patients with transient global amnesia: a prospective controlled study. J Neurol Sci. 2013;325(1-2):57-60. [DOI] [PubMed] [Google Scholar]
- 28.Uttner I, Prexl S, Freund W, Unrath A, Bengel D, Huber R. Long-term outcome in transient global amnesia patients with and without focal hyperintensities in the CA1 region of the hippocampus. Eur Neurol. 2012;67(3):155-160. [DOI] [PubMed] [Google Scholar]
- 29.Auyeung M, Tsoi TH, Cheung CM, et al. Association of diffusion weighted imaging abnormalities and recurrence in transient global amnesia. J Clin Neurosci. 2011;18(4):531-534. [DOI] [PubMed] [Google Scholar]
- 30.Melo TP, Ferro JM, Ferro H. Transient global amnesia. A case control study. Brain. 1992;115(pt 1):261-270. [DOI] [PubMed] [Google Scholar]
- 31.Agosti C, Akkawi NM, Borroni B, Padovani A. Recurrency in transient global amnesia: a retrospective study. Eur J Neurol. 2006;13(9):986-989. [DOI] [PubMed] [Google Scholar]
- 32.Agosti C, Borroni B, Akkawi NM, De Maria G, Padovani A. Transient global amnesia and brain lesions: new hints into clinical criteria. Eur J Neurol. 2008;15(9):981-984. [DOI] [PubMed] [Google Scholar]
- 33.Klötzsch C, Sliwka U, Berlit P, Noth J. An increased frequency of patent foramen ovale in patients with transient global amnesia. Analysis of 53 consecutive patients. Arch Neurol. 1996;53(6):504-508. [DOI] [PubMed] [Google Scholar]
- 34.Akkawi NM, Agosti C, Grassi M, et al. Weather conditions and transient global amnesia. A six-year study. J Neurol. 2006;253(2):194-198. [DOI] [PubMed] [Google Scholar]
- 35.Toledo M, Pujadas F, Purroy F, Lara N, Quintana M, Alvarez-Sabin J. Recurrent Transient Global Amnesia, a manifestation of ischemic cerebrovascular disease [in Spanish] [published correction appears in Med Clin (Barc). 2006 Mar 4;126(8):316]. Med Clin (Barc). 2005;125(10):361-365. [DOI] [PubMed] [Google Scholar]
- 36.Pantoni L, Bertini E, Lamassa M, Pracucci G, Inzitari D. Clinical features, risk factors, and prognosis in transient global amnesia: a follow-up study. Eur J Neurol. 2005;12(5):350-356. [DOI] [PubMed] [Google Scholar]
- 37.Lampl Y, Sadeh M, Lorberboym M. Transient global amnesia: not always a benign process. Acta Neurol Scand. 2004;110(2):75-79. [DOI] [PubMed] [Google Scholar]
- 38.Chen ST, Tang LM, Hsu WC, Lee TH, Ro LS, Wu YR. Clinical features, vascular risk factors, and prognosis for transient global amnesia in Chinese patients. J Stroke Cerebrovasc Dis. 1999;8(5):295-299. [DOI] [PubMed] [Google Scholar]
- 39.Pai MC, Yang SS. Transient global amnesia: a retrospective study of 25 patients. Zhonghua Yi Xue Za Zhi (Taipei). 1999;62(3):140-145. [PubMed] [Google Scholar]
- 40.Gandolfo C, Caponnetto C, Conti M, Dagnino N, Del Sette M, Primavera A. Prognosis of transient global amnesia: a long-term follow-up study. Eur Neurol. 1992;32(1):52-57. [DOI] [PubMed] [Google Scholar]
- eReferences e1–e10 are available at: links.lww.com/CPJ/A350.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.
Data set, data script, and data dictionary are available in eAppendices 1–3, links.lww.com/CPJ/A345, links.lww.com/CPJ/A346, links.lww.com/CPJ/A347.