Abstract
Objectives
Area postrema syndrome (APS) is one of the core clinical features of neuromyelitis optic spectrum disorder (NMOSD). APS is mostly associated with neuromyelitis optica (NMO) and rarely reported in myelin oligodendrocyte glycoprotein antibody disease. We herein report a case of APS as an initial presentation of double-seropositive aquaporin-4 and myelin oligodendrocyte glycoprotein (MOG) antibodies.
Methods
The patient fulfilled the NMOSD diagnostic criteria. Brain MRI, CSF studies, electrophysiologic test, and serum NMO and MOG antibody testing were performed.
Results
An elderly woman initially presented to a gastroenterology outpatient department with a history of nausea, vomiting, and hiccups for 3 weeks. A detailed medical evaluation, including upper gastrointestinal endoscopy, was performed, which showed normal findings with no improvement with symptomatic therapy. A neurologic examination showed bilateral nystagmus, postural imbalance, and gait ataxia. An MRI examination of the brain showed T2/fluid attenuated inversion recovery hyperintensity in the dorsal medulla involving area postrema. Both anti-NMO and anti-MOG antibodies were found to be positive in serum. She was treated with intravenous methyl prednisolone with complete symptomatic resolution.
Discussion
Double-seropositive APS-onset NMOSD has not been previously reported in literature. An early diagnosis and treatment result in the resolution of APS-related symptoms and prevent further progression of the disease.
PRACTICAL IMPLICATIONS
Area postrema syndrome should be considered in any patient presenting with intractable nausea, vomiting, and hiccups.
Area postrema syndrome (APS) is one of the core clinical features of neuromyelitis optic spectrum disorder (NMOSD), characterized by intractable nausea, vomiting, and hiccups for ≥48 hours.1 The incidence of isolated APS symptoms at onset is 7.1%–10.3% and 9.4%–14.5% during the disease course.1 APS is usually associated with aquaporin-4 (AQP4)-IgG antibody rather than antimyelin oligodendrocyte glycoprotein (MOG) antibody. Double seropositivity in NMOSD has been rarely reported in literature.2,3 We report a rare case of APS as an initial presentation of double-seropositive AQP4 and MOG antibodies.
Case Presentation
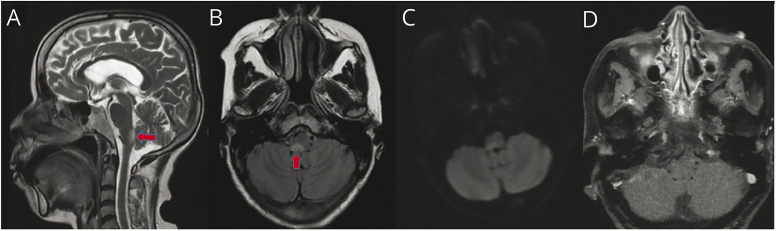
A 60-year-old woman from Eastern India presented to a gastroenterology outpatient department with a history of nausea, vomiting, and hiccups for 20 days. Her food intake was remarkably reduced because of persistent nausea, and vomiting was induced by food. She had 15–20 episodes of vomiting per day with a weight loss of 3 kg. She had no significant medical history. She was started on parenteral proton pump inhibitors and antiemetics but had no relief. A detailed medical evaluation including hematologic, biochemistry, autoimmune workup, ultrasound, and CT abdominal imaging showed normal findings. Upper gastrointestinal endoscopy was also unremarkable. She was then referred to the Neurology department. On examination, she was conscious, well oriented to time, place, and person. She had horizontal nystagmus bilaterally. Her motor and sensory examinations were unremarkable. She had postural instability and truncal and gait ataxia. MRI of the brain was performed, which showed T2/fluid attenuated inversion recovery hyperintensity in dorsal medulla involving area postrema (AP) without diffusion restriction (Figure). MRI orbit and whole spine screening were performed and were unremarkable. Visual evoked potential showed normal bilateral P100 latency. Lumbar puncture with cerebral spinal fluid analysis was normal, with no oligoclonal bands. Antineuromyelitis optica (NMO) and anti-MOG antibodies were sent. Both anti-NMO and anti-MOG antibodies were found to be positive by cell-based indirect immunofluorescence assay. She was managed with intravenous methylprednisolone pulse therapy 1,000 mg/d for 5 days with complete resolution of symptoms.
Figure. MRI of an Elderly Woman With Area Postrema Syndrome.
Sagittal T2-weighted (A) and axial fluid attenuated inversion recovery (B) MR images of the brain showing high signal intensity in the tegmentum of medulla and pontomedullary junction, which represents area postrema (red arrows). A weak high signal at the corresponding site is seen on diffusion-weighted imaging (C). T1 postcontrast image shows lack of contrast enhancement in the same region (D).
Discussion
NMOSD is an immune-mediated CNS demyelinating disease that commonly presents with optic neuritis and transverse myelitis similar to multiple sclerosis (MS).4 Approximately one-third patients present with brainstem syndrome including APS. NMOSD is commonly associated with AQP4 antibody, but discovery of MOG antibodies led to the recognition of a new entity known as myelin oligodendrocyte glycoprotein antibody disease (MOGAD). MOGAD shows a difference from classical NMO in that patients are younger and affect more men, whereas MS shows the distribution of age and gender between classical NMO and MOGAD.
APS is caused by lesions in dorsal medullary tegmentum, known as AP. The area postrema, a reflex emetic center, is a vascular structure consisting of chemosensitive neurons mediating hiccups, fluid balance, and osmoregulation systems. Patients with APS frequently present to a gastroenterologist leading to an extensive workup and misdiagnosis, as seen in our patient. APS is also reported to occur in other neurologic disorders such as anti-glial fibrillary acidic protein encephalomyelitis and Bickerstaff brainstem encephalitis.5
Area postrema is highly rich in AQP4 water channel, so it is usually involved in AQP4 antibody–positive individual. There are only few cases reported in literature showing MOG-positive/AQP4-negative individuals with APS.5,6 APS in MOGAD may be explained by the entry of pathogenic MOG antibody due to the lack of blood-brain barrier at the AP and disruption of emesis circuit.1 Owing to rarity of APS in MOGAD, APS can be of value in differentiating NMO and MOG antibodies. Double-seropositive APS at onset has not been reported in literature yet.
There have been a few reported literature showing double-seropositive (MOG-positive and AQP4-positive) optic neuritis and myelitis.2,3 Double-positive NMOSD is found to have a high relapse rate and residual disability with MRI of the brain showing MS-like brain lesions in two-thirds of patients, while MRI of the spine showing edematous spinal cord from cervical to conus region.2 APS at onset can delay the diagnosis of NMOSD causing further neurologic deficits in the form of optic neuritis, transverse myelitis, or brainstem syndrome.
Acute attacks are managed by methylprednisolone pulse therapy for 3–5 days or plasmapheresis. The pathology of AP usually involves inflammatory reaction rather than demyelination and necrosis, so it is highly responsive to immunomodulatory therapy with complete recovery.7 Long-term immunotherapy must be initiated in double-positive NMOSD with APS at onset because it is associated with further frequent relapses and residual disability. Eculizumab, inebilizumab, and satralizumab should be used as maintenance therapy if available because they are approved for seropositive AQP4+NMOSD. Azathioprine, mycophenolate mofetil, and rituximab are the other effective agents studied. The limitation of this case study is that we have described only the initial presentation and management of the case. A follow-up of the patient is required to understand the course of APS-onset double antibody–positive NMOSD.
APS at onset is a rare presentation of NMOSD. APS is usually associated with AQP4 antibodies. Double-positive NMOSD with APS onset needs appropriate management because it can lead to subsequent attacks and residual neurologic disability.
Acknowledgments
The authors thank Dr Anita Mahadevan, Professor & Head, Neuropathology department, NIMHANS.
Glossary
- AP
area postrema
- APS
area postrema syndrome
- AQP4
aquaporin-4
- MOG
myelin oligodendrocyte glycoprotein
- MOGAD
myelin oligodendrocyte glycoprotein antibody disease
- NMO
neuromyelitis optica
- NMOSD
neuromyelitis optic spectrum disorder
Study Funding
The authors report no targeted funding.
Disclosure
No financial and other conflicts of interest of any of the authors. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Shosha E, Dubey D, Palace J, et al. Area postrema syndrome: frequency, criteria, and severity in AQP4-IgG-positive NMOSD. Neurology. 2018;91:e1642–e1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan Y, Li Y, Fu Y, et al. Autoantibody to MOG suggests two distinct clinical subtypes of NMOSD. Sci China Life Sci. 2016;59(12):1270-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mason MC, Marotta DA, Kesserwani H. Isolated double-positive optic neuritis: a case of aquaporin-4 and myelin oligodendrocyte glycoprotein antibody seropositivity. Cureus. 2021;13(6):e15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou C, Liao L, Sun R, et al. Area postrema syndrome as initial manifestation in neuromyelitis optica spectrum disorder patients: a retrospective study. Revue Neurologique. 2021;177(4):400-406. [DOI] [PubMed] [Google Scholar]
- 6.Hyun J-W, Kwon YN, Kim S-M, et al. Value of area postrema syndrome in differentiating adults with AQP4 vs. MOG antibodies. Front Neurol. 2020;11:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popescu BF, Lennon VA, Parisi JE, et al. Neuromyelitis optica unique area postrema lesions: nausea, vomiting, and pathogenic implications. Neurology. 2011;76(14):1229-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]