Abstract
Background and Objectives
Specialty palliative care (PC) may benefit patients with dementia by aligning treatment with goals and relieving symptoms. We aimed to compare demographics and processes and outcomes of PC for inpatients with dementia with those with systemic illnesses or cancer.
Methods
This multicenter cohort study included standardized data for hospitalized patients with a primary diagnosis of dementia, systemic illnesses (cardiovascular, pulmonary, hepatic, or renal disease), or cancer among the 98 PC teams submitting data to the Palliative Care Quality Network from 2013 to 2019.
Results
Of 155,356 patients, 4.5% (n = 6,925) had a primary diagnosis of dementia, 32.5% (n = 50,501) systemic illness, and 29.2% (n = 45,386) cancer. Patients with dementia were older (mean 85.5 years, 95% confidence interval [CI] 85.3–85.6) than those with systemic illnesses (mean 73.2, 95% CI 73.0–73.3) or cancer (mean 66.6, 95% CI 66.4–66.7; p < 0.0001). Patients with dementia were more likely to receive a PC consult within 24 hours of admission (52.3% vs systemic illnesses 37.4%; cancer 45.3%; p < 0.0001), more likely to be bed-bound (vs systemic illnesses odds ratio (OR) 2.23, 95% CI 2.09–2.39, p < 0.0001; vs cancer OR 3.45, 95% CI 3.21–3.72, p < 0.0001), and more likely to be discharged alive (vs systemic illnesses OR 2.22, 95% CI 2.03–2.43, p < 0.0001; vs cancer OR 1.51, 95% CI 1.36–1.67, p < 0.0001). Advance care planning/goals of care (GOC) was the primary reason for consultation for all groups. Few patients overall had advance directives or Physician Orders for Life-Sustaining Treatment before consultation. At the time of referral and at discharge, patients with dementia were more likely to have a code status of do not resuscitate/do not intubate (DNR/DNI) (62.6% and 81.0% vs 38.7 and 64.2% for patients with systemic illnesses and 33.4% and 60.5% for patients with cancer; p < 0.0001). Among the minority of patients with dementia that could self-report, moderate-to-severe symptoms were uncommon (pain 6.4%, anxiety 5.8%, nausea 0.4%, and dyspnea 3.5%).
Discussion
Inpatients with a primary diagnosis of dementia receiving PC consultation were older and more functionally impaired than those with other illnesses. They were more likely to have a code status of DNR/DNI at discharge. Few reported distressing symptoms. These results highlight the need for routine clarification of GOC for patients with dementia.

Over 5 million US adults have dementia, a number projected to more than double by 2060 because of population aging.1,2 People with dementia have twice as many hospital stays per year as other older people and are at a higher risk of dying during hospitalization.3,4 Those with advanced dementia are frequently hospitalized, even when comfort is the main goal of care.5,6
Prior studies have shown that specialty palliative care (PC) may benefit patients with dementia by aligning care with goals and alleviating burdensome symptoms.7-9 Despite high numbers of hospitalized patients with dementia in the United States, little is known about their characteristics, PC needs, and interactions with PC specialists. Single-center US studies have examined the PC needs of hospitalized patients with neurologic diseases, including dementia.10,11 A recent multicenter Australian study of patients admitted to inpatient PC services found that patients with dementia had higher rates of functional impairment but less self-rated distress than those with other diseases.12 The aim of this multicenter cohort study was to compare characteristics and PC needs of hospitalized patients with dementia in the United States who received PC consultation to those with cancer and systemic illnesses who received PC consultation, to guide efforts to improve care for hospitalized patients with dementia and inform future research.
Methods
Study Population
The Palliative Care Quality Network (PCQN) comprises specialty PC teams from across the United States that collect patient-level standardized data on processes of care and treatment outcomes to improve the quality of care.13 As of December 2019, there were 98 PC teams in the PCQN from a diverse group of hospitals across 15 states. Inpatient PC teams within the PCQN work in hospitals that vary in size (mean hospital size = 348 beds, median = 286 beds, range 26–1120) and status (not for profit = 68.9%, academic = 14.8%, public = 14.8%, for profit = 0.0%, and other = 1.6%). About half of these PC teams were interdisciplinary in nature (51.1%), consisting of a physician (98.8%), nurse/registered nurse (92.2%), social worker (87.1%), and chaplain (69.9%). Many team members were credentialled (physician: 94.7%, registered nurse: 65.3%, nurse practitioner: 58.0%, social workers: 33.3, and chaplain: 18.6%).
Data Set
The PCQN data set has been described in detail in previous reports.13,14 Teams in the PCQN collect a standardized set of 23 data elements for all patients referred for inpatient specialty PC, and the study population thus includes all these inpatients. Patient characteristics at the time of referral include age, sex, referral location (e.g., medical/surgical unit, critical care, and telemetry/step down), and the primary condition that led to the PC consult and assigned by the PC team. This categorization is intended to focus on the broad categories of diseases and conditions that help PC teams understand which patient populations they are caring for. It is distinct from the reason for hospitalization, which could be for issues such as aspiration pneumonia, pulmonary embolus, or altered mental status and is not collected as a part of the PCQN data set. Processes of care include time to PC consultation, number of family meetings held, number of visits by the PC team, and reason(s) given for the consultation. Initial PC team assessments include the presence of advance care planning (ACP) documentation including either a Physician's Orders for Life-Sustaining Treatment (POLST) form or a non-POLST advance directive (AD) such as a living will; patient report of their symptoms (pain, dyspnea, nausea, and anxiety) as none, mild, moderate or severe; and functional status, which is assessed using the Palliative Performance Scale (PPS) (assessed using a 0%–100% measure of functional status, with higher scores reflecting greater function).15 PPS was assessed at the initial visit by the PC team based on the current status of the patient. Treatment outcomes are also documented, which include patient code status at the time of consult and discharge (full code, do not resuscitate/do not intubate [DNR/DNI], or partial code), ACP documentation, discharge location, and services arranged after discharge.
For this analysis, inpatients with a primary diagnosis of dementia were compared with 2 groups: (1) inpatients with a primary diagnosis of cancer (who generate the most PC consultations nationwide)13 and (2) inpatients with a primary diagnosis of a systemic illness (defined as a cardiac, pulmonary, hepatic, or renal disease). Many cardiac, pulmonary, hepatic, and renal diseases cause gradual decline over years, punctuated by hospitalizations; while this illness trajectory differs from that of dementia (prolonged dwindling until death), it was the best comparator among the disease categories represented in the PCQN.16
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the University of California San Francisco Institutional Review Board (No. 16-18596). Medical record numbers are excluded from the data set, and only aggregated data are reported, minimizing the risk of identifying individual patients. Patient consent was not required or obtained because the study represents an ongoing quality improvement project.
Procedure
Deidentified data for this retrospective cohort study were extracted on February 18, 2020, and include information for 162,749 inpatients who received their first PC consultation between January 1, 2013, and December 31, 2019. The PCQN data are collected in the course of clinical care and submitted by PC providers onto the PCQN's secure online database.
Statistical Analysis
Continuous variables were analyzed using means (95% confidence intervals [CIs]) and medians (with range). Frequencies were calculated for categorical variables. We used χ2 tests to examine bivariate associations between categorical variables and analysis of variance to examine associations between categorical and continuous variables. A McNemar-Bowker test was undertaken to examine change in code status from first consultation to discharge. We used mixed-effects logistic regression models to study the association between our 2 dependent variables: inpatients with dementia compared with those with systemic illnesses and inpatients with dementia compared with those with cancer. For each analysis, age, sex, and referral location were included as a fixed effect, and PC team was modeled as a random effect to account for intrateam correlation of patient measures.
There was no adjustment or imputation for missing data. Analyses were performed only for patients for whom data were available for each specific data element, resulting in different n values for each analysis. An alpha of ≤0.05 was used to determine statistical significance. Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL) for Mac (version 27) was used to conduct all analyses.
Data Availability
As a collaborative project, the PCQN has protocols to ensure fair and equitable access to data. Qualified investigators interested in accessing anonymized PCQN data should contact the corresponding author.
Results
Patient Characteristics
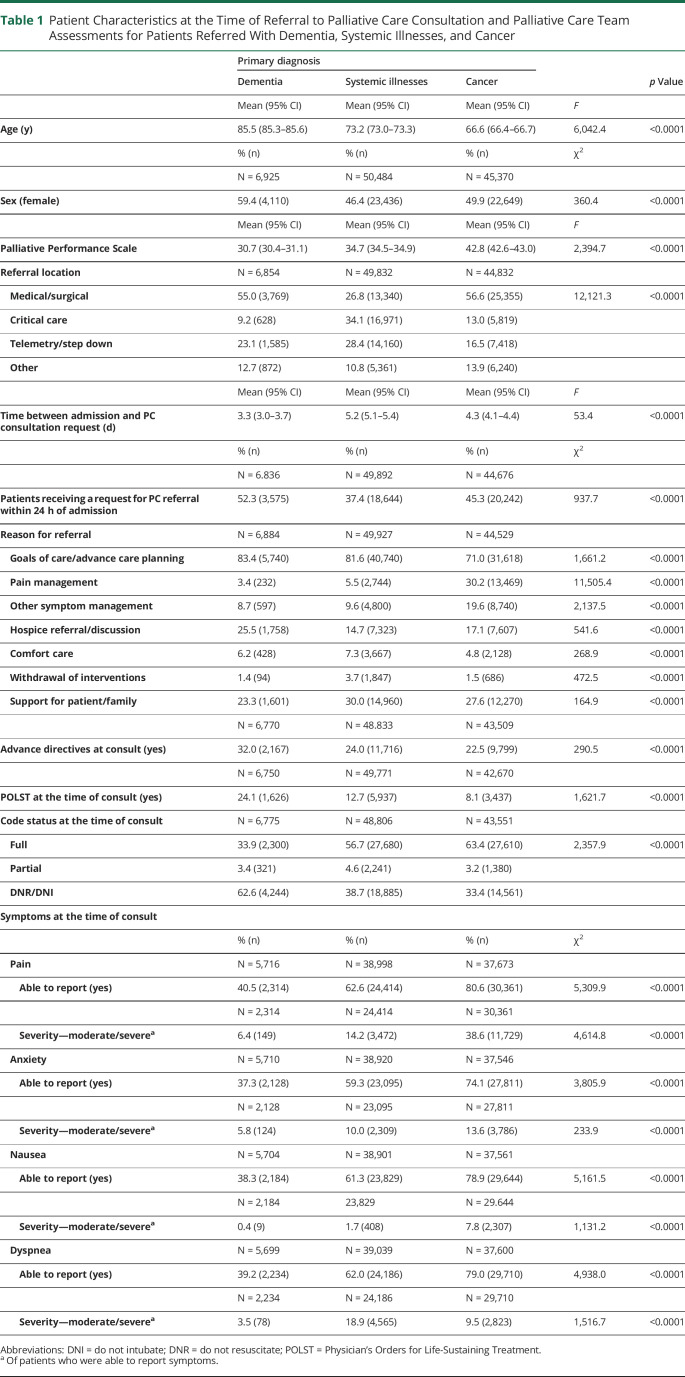
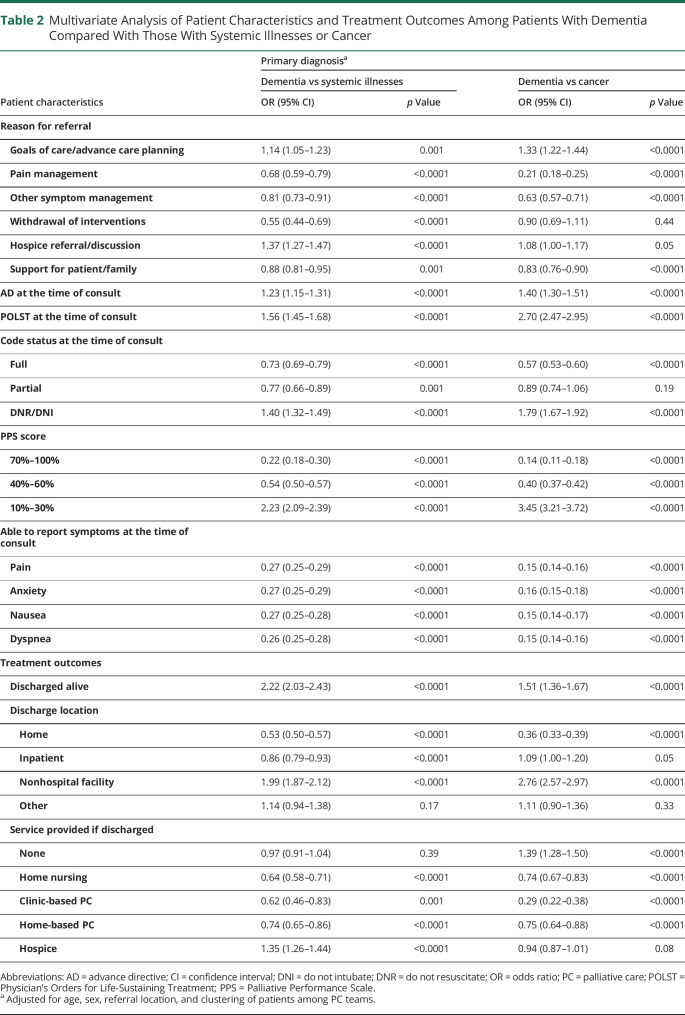
Primary diagnosis was documented for 155,356 inpatients receiving PC consultations during the study period, of whom 4.5% (n = 6,925) had a diagnosis of dementia, 32.5% (n = 50,501) had a diagnosis in 1 of the systemic illness categories (cardiovascular [14.2%, n = 22,024], pulmonary [11.6%, n = 18,077], hepatic [3.6%, n = 5,645], or renal disease [3.1%, n = 4,755]), and 29.2% (n = 45,386) had a diagnosis of cancer (Table 1). Patients with dementia were older (85.5 years; 95% CI: 85.3, 85.6, p < 0.0001) than those with systemic illnesses (73.2; 95% CI: 73.0, 73.3) or cancer (66.6 years; 95% CI: 66.4, 66.7; p < 0.0001) and more likely to be female (59.4% vs 46.4%; 49.9%; p < 0.0001). After adjustment for patient age, sex, referral location, and PC team (Table 2), inpatients with dementia were more likely to have a lower functional status with a PPS score of 10–30% (meaning they were bed-bound) than those with systemic illnesses (odds ratio [OR] = 2.23, 95% CI: 2.09–2.39; p < 0.0001) or cancer (OR = 3.45, 95% CI: 3.21–3.72; p < 0.0001).
Table 1.
Patient Characteristics at the Time of Referral to Palliative Care Consultation and Palliative Care Team Assessments for Patients Referred With Dementia, Systemic Illnesses, and Cancer
Table 2.
Multivariate Analysis of Patient Characteristics and Treatment Outcomes Among Patients With Dementia Compared With Those With Systemic Illnesses or Cancer
Most inpatients with dementia (55.0%) and cancer (56.6%) were referred to PC from medical/surgical units, whereas those with systemic illnesses were most often referred from critical care units (34.1%; p < 0.0001) (Table 1). The mean time between admission and PC consultation request was 3.3 days for patients with dementia (95% CI 3.0–3.7), 5.2 days for patients with systemic illnesses (95% CI 5.1–5.4), and 4.3 days for patients with cancer (95% CI 4.1–4.4; p < 0.0001). Inpatients with dementia (52.3%) were more likely to be referred to PC within 24 hours of admission than those with systemic illnesses (37.4%) or cancer (45.3%; p < 0.0001).
Goals of care (GOC) discussion/ACP was the most common reason for PC consultation in all patient groups: 83.4% of inpatients with dementia, 81.6% of inpatients with systemic illnesses, and 71.0% of inpatients with cancer. The second most common reason for referral was hospice referral/discussion among patients with dementia (25.5%), support for the patient/family among patients with systemic illnesses (30.0%), and pain management among patients with cancer (30.2%) (Table 1). After adjustment for patient age, sex, referral location, and PC team, patients with dementia were more likely to be referred to PC for ACP/GOC compared with those with systemic illnesses (OR = 1.14, 95% CI: 1.05–1.23; p = 0.001) or cancer (OR = 1.33, 95% CI: 1.22–1.44; p < 0.0001) (Table 2).
Thirty-two percent of inpatients with dementia had completed ADs by the time of PC consultation, and 24.1% had completed POLSTs, compared with 24.0% AD and 12.7% POLST completion among those with systemic illnesses and 22.5% AD and 8.1% POLST completion among those with cancer (Table 1). Over half (62.6%) of inpatients with dementia had a code status of DNR/DNI at the time of referral compared with 37.7% of those with systemic illnesses (38.7%) and 33.4% of those with cancer (p < 0.0001). After adjustment for patient age, sex, referral location, and PC team, inpatients with dementia were more likely to have an AD documented at the time of referral than those with systemic illnesses (OR = 1.23, 95% CI: 1.15–1.31; p < 0.0001) or cancer (OR = 1.40, 95% CI: 1.30–1.51; p < 0.0001), more likely to have a POLST documented at the time of referral (vs systemic illnesses OR = 1.56, 95% CI: 1.45–1.68, p < 0.0001; vs cancer OR = 2.70, 95% CI 2.47–2.95, p < 0.0001), and more likely to have a code status of DNR/DNI at the time of referral (vs systemic illnesses OR = 1.40, 95% CI: 1.32–1.49, p < 0.0001; vs cancer OR = 1.79, 95% CI: 1.67–1.92, p < 0.0001) (Table 2).
Most inpatients with dementia were unable to report symptoms of pain (59.5%), anxiety (62.7%), nausea (61.7%), or dyspnea (60.8%), whereas most inpatients with systemic illnesses and cancer were able to report their symptoms (Tables 1 and 2). Among those with dementia, self-reported moderate-to-severe symptoms were uncommon (moderate/severe pain 6.4%, anxiety 5.8%, nausea 0.4%, and dyspnea 3.5%) (Table 1).
Processes of Care and Outcomes
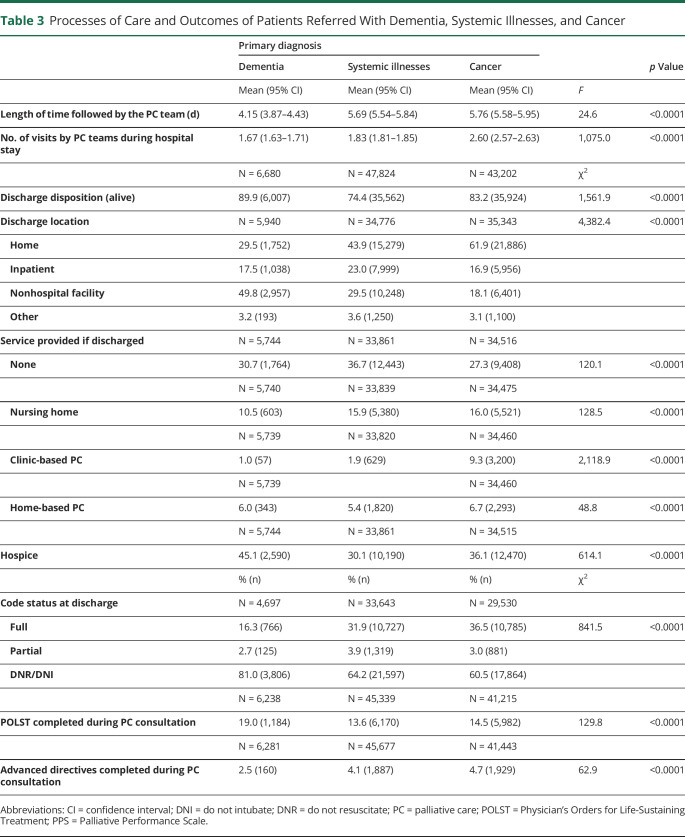
Table 3 shows PC processes of care and outcomes after PC referral. PC teams followed inpatients with dementia for a mean duration of 4.15 days (95% CI 3.87–4.43) and visited patients a mean of 1.67 times, compared with 5.69 days and 1.83 visits for inpatients with systemic illnesses (95% CI 5.54–5.84) and 5.76 days and 2.60 visits for inpatients with cancer (95% CI 5.58–5.95; p < 0.0001). Most patients across all groups were discharged alive (89.9% of patients with dementia, 74.4% of patients with systemic illnesses, and 83.2% of patients with cancer; p < 0.0001). After adjustment for age, sex, referral location, and PC team (Table 2), more patients with dementia were discharged alive than those with systemic illnesses (OR = 2.22, 95% CI: 2.03–2.43; p < 0.0001) or cancer (OR = 1.51, 95% CI: 1.36–1.67; p < 0.0001). Among survivors, patients with dementia were less likely to be discharged home than the other groups (vs systemic illnesses OR = 0.53, 95% CI: 0.50–0.57, p < 0.0001; vs cancer OR 0.36, 95% CI: 0.33–0.39, p < 0.0001). They were more likely to be discharged to hospice compared with patients with systemic illnesses (OR = 1.35, 95% CI: 1.26–1.44; p < 0.0001) and were more likely to be discharged with no additional services provided compared with patients with cancer (OR = 1.39, 95% CI: 1.28–1.50; p < 0.0001). They were less likely than both other groups to be discharged with clinic-based PC (vs systemic illnesses OR 0.62, 95% CI: 0.46–0.83, p = 0.001; vs cancer OR 0.29, 95% CI: 0.22–0.38, p < 0.0001) or home-based PC (vs systemic illnesses OR 0.74, 95% CI: 0.65–0.86, p < 0.0001; vs cancer OR 0.75, 95% CI: 0.64–0.88, p < 0.0001).
Table 3.
Processes of Care and Outcomes of Patients Referred With Dementia, Systemic Illnesses, and Cancer
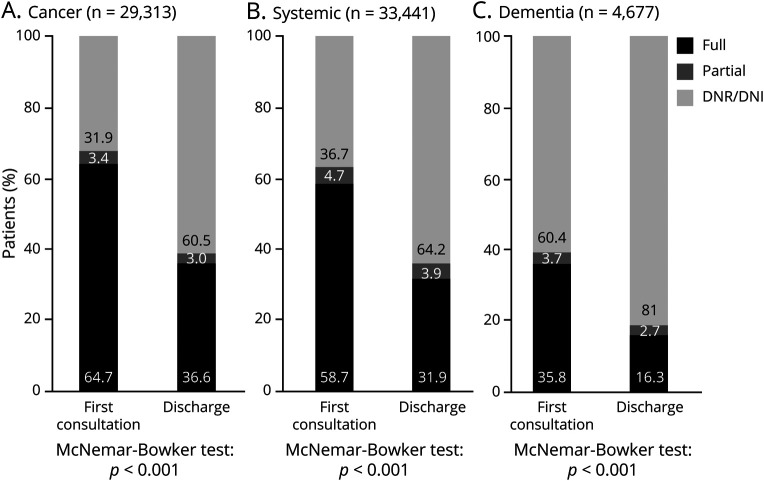
At discharge, more inpatients with dementia and a majority of inpatients from all 3 groups had a code status of DNR/DNI (81.0% of patients with dementia, 64.2% of patients with systemic illnesses, and 60.5% of patients with cancer; p < 0.0001) (Table 1), which represented for all groups a significant change in the code status that was documented at the time of initial consultation (Figure).
Figure. Code Status at Initial Consult Compared With Code Status at Discharge for Patients Referred With Cancer (A), Systemic Illnesses (B), and Dementia (C).
DNI = do not intubate; DNR = do not resuscitate.
Discussion
This large, multicenter cohort study compared the characteristics, processes of care, and outcomes of PC consultation for hospitalized patients with dementia with those with systemic illnesses and cancer. Although inpatients across the 3 illness groups received PC consultation most often for ACP/GOC discussions, we found substantial variation between groups in the level of functional impairment, preconsultation ACP, preconsultation and discharge code status, end-of-life care planning, and the need for symptom management. These results suggest that the focus of PC consultations for many inpatients with dementia differs from those of the comparator groups. These differences highlight opportunities to improve care.
Hospitalized patients with dementia seen by specialty PC teams in our sample were less acutely ill than those with systemic illnesses or cancer, with lower rates of in-hospital mortality and fewer consultations from the critical care setting. However, they were older and more functionally impaired at baseline as measured by the PPS. They had higher rates of DNR/DNI status at the time of consult and at discharge, suggesting a decision to limit some forms of life-prolonging therapy. Patients with dementia were also more likely to be seen by PC earlier in the hospitalization and to receive a PC consultation for GOC/ACP. These findings suggest that clinicians readily identified PC needs around GOC for these patients and requested assistance from PC providers to address those needs. Once consulted, PC teams had fewer visits with patients with dementia compared with those with cancer or systemic illnesses and followed patients for less time. These findings suggest that PC's role in clarifying GOC—largely with surrogates, as patients were much less likely to be able to communicate symptoms, let alone preferences—was less complex than for patients in the other illness groups. It is possible that previous, undocumented discussions around GOC/ACP for patients with dementia simplified these in-hospital discussions; alternatively, it is possible that GOC/ACP discussions for these patients were inherently less complicated than for the other 2 groups. Understanding the nature of these discussions may provide guidance for addressing specific care planning issues in the outpatient setting by neurologists or primary care physicians before hospitalization and potentially avoid some of these hospitalizations.
Patients with dementia and their designated decision-makers have been shown to choose less aggressive care as the disease progresses, including choosing not to be hospitalized and enrolling in hospice services.6,17 However, they are at risk of receiving unwanted interventions, including hospitalization, in part because these interventions are the default in acute care settings if care preferences are unknown.18-20 ACP may help patients avoid undesired interventions and is associated with positive outcomes at the end of life for patients with dementia and their caregivers.20,21 In our sample, less than one-third of inpatients with dementia receiving PC consultation had ADs and less than one-quarter had POLSTs, although the majority had a code status of DNR/DNI at the time of PC consultation and 81% had DNR/DNI status at the time of discharge. This finding of low rates of ACP documentation among inpatients with dementia receiving specialty PC consultation is consistent with prior reports and suggests a need to increase ACP documentation for these patients in the outpatient setting.22 Because ACP documentation is only one outcome of ACP itself, it is likely that the rate of ACP documentation does not completely capture how many patients in this data set had had prior discussions about ACP or GOC. However, we also found that inpatients with dementia, who had higher rates of ACP documentation than those in other illness groups, were more likely to receive PC consults for ACP/GOC discussions. This finding may suggest a need to further refine standard AD strategies to address the needs of hospitalized patients with dementia. Because the diseases causing dementia tend to cause a slow functional decline until death, inpatients with dementia may be offered various invasive tests or procedures that could prolong life at the expense of comfort and that are not covered by standard questions (code status, etc.) on AD forms.
Another finding in our study was the pattern of early and brief PC consultation for inpatients with dementia with far lower prevalence of moderate-to-severe symptoms compared with other illness groups, suggesting that the PC provided to these patients was more focused. Routine, targeted PC consultation for people with dementia in the outpatient and inpatient setting may help address GOC earlier in the course of illness and avoid unwanted hospitalizations. Because the demand for PC increases, another model has been proposed that distinguishes primary PC (skills that all clinicians should have) from specialist PC (provided by a typically interdisciplinary team of PC specialists).23,24 In this model, the primary team delivers the majority of PC25,26 and calls upon PC consultants as needed to assist with complex issues such as refractory symptoms, difficult family meetings, conflicting GOC, end-of-life transitions, and arranging hospice care.24 Our findings suggest that primary teams, with some additional training, may be able to address many of the PC needs of these patients with dementia.
Finally, we found that symptom management was a reason for PC consultation for only 1 in 7 inpatients with dementia in this cohort. Most inpatients with dementia in our sample were unable to report their symptoms. Pain and other distressing symptoms often go underrecognized and undertreated in dementia, partly because of cognitive barriers to symptom communication.27 This finding emphasizes the need to use nonverbal symptom assessment tools when caring for patients with dementia. It is likely that most PC teams were using such tools; however, the PCQN database records only symptom assessments made by patients.
Our study had several limitations, many of which have been described previously.14,28,29 First, the PCQN data set is limited to elements that are useful for ongoing clinical care, quality reporting, and quality improvement and data are collected by interdisciplinary PC teams prospectively during patient care. This approach allows for aggregation of data and comparison across PC teams but prevents finer distinctions such as differentiation between dementia types, stages of dementia, precise reasons for hospitalization, or distinction between chronic and acute illnesses. Nonetheless, the large number of teams that participate and the very large sample size allow us to draw important conclusions. Second, although inpatients with systemic illnesses were chosen as a comparison group for inpatients with dementia because of the possibility of a shared illness trajectory, our study reveals differences between these groups in age, illness acuity, and code status at the time of the consult, suggesting that the systemic illnesses group was too heterogeneous to represent a single illness trajectory comparable to dementia; this heterogeneity was in part addressed through multivariate analysis. The fact that all inpatients in our sample received specialty PC consultation means that their physicians identified PC needs, and thus they provide helpful context for understanding the needs of people with dementia. Finally, we are aware that because of the large sample size in this study, statistically significant results are not necessarily clinically meaningful. We attempted to highlight only clinically meaningful findings.
The PCQN data set, representing numerous PC consultations across multiple hospitals and regions in the United States, offers a unique picture of the characteristics and PC needs of hospitalized patients with dementia. Our study highlights strengths and gaps in care for inpatients with dementia that can be used by neurologists, PC providers, and other non-PC clinicians to identify issues amenable to system-level solutions in both the inpatient and outpatient settings. In particular, our study showed the need to clarify GOC for people with dementia, most of whom had a DNR/DNI code status at initial PC consultation and even more at discharge. Our findings suggest that primary inpatient teams with additional training may be able to clarify GOC on their own or with focused assistance of PC teams. We also found that most inpatients with dementia are unable to report symptoms and thus routine assessments that do not rely on patient report are essential. To better understand the needs of people with dementia and improve care, future qualitative and quantitative research ought to (1) identify prehospital factors leading to hospitalization among inpatients with dementia; (2) assess reasons for specialist PC involvement in inpatient ACP/GOC discussions and the issues that are clarified; and (3) compare hospitalized patients with dementia who receive PC consultation with those who do not.
TAKE-HOME POINTS
→ Among hospitalized patients receiving PC consultation by members of the 98-PC teams that submitted data to the PCQN from 2013 to 2019, those with dementia listed as the condition that led to the PC consultation were older and more functionally impaired than those with systemic illnesses (cardiovascular, pulmonary, hepatic, or renal disease) or those with cancer.
→ Less than half of hospitalized patients with dementia receiving PC consultation could report their symptoms (40.5% able to report their pain; 37.3% anxiety; 38.3% nausea; 39.2% dyspnea); few reported moderate-to-severe symptoms (pain 6.4%; anxiety 5.8%; nausea 0.4%; dyspnea 3.5%). This finding highlights the need for inpatient providers to use nonverbal symptom assessment tools when caring for patients with dementia.
→ Inpatients with dementia receiving PC consultation were more likely than those in the other groups to have a code status of DNR/DNI at discharge.
→ These results underline the need for routine clarification and documentation of goals of care and advance care planning for patients with dementia.
Appendix. Authors
Study Funding
This research was supported by grants from the Stupski Foundation (grant #3942), the UniHealth Foundation (grant #3623), the Archstone Foundation (grant #19-01-06), and the California HealthCare Foundation (grant #19625).
Disclosure
The authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer's disease and related dementias in the United States (2015-2060) in adults aged ≥65 years. Alzheimers Dement. 2019;15(1):17-24. doi: 10.1016/j.jalz.2018.06.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80(19):1778-1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beydoun MA, Beydoun HA, Gamaldo AA, et al. Nationwide inpatient prevalence, predictors, and outcomes of Alzheimer's disease among older adults in the United States, 2002-2012. J Alzheimers Dis. 2015;48(2):361-375. doi: 10.3233/JAD-150228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.2020 Alzheimer's disease facts and figures. Alzheimers Dement. 2020;16(3):391-460. doi: 10.1002/alz.12068. [DOI] [Google Scholar]
- 5.Mitchell SL, Palmer JA, Volandes AE, Hanson LC, Habtemariam D, Shaffer ML. Level of care preferences among nursing home residents with advanced dementia. J Pain Symptom Manage. 2017;54(3):340-345. doi: 10.1016/j.jpainsymman.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529-1538. doi: 10.1056/NEJMoa0902234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manfredi PL, Morrison RS, Morris J, Goldhirsch SL, Carter JM, Meier DE. Palliative care consultations: how do they impact the care of hospitalized patients? J Pain Symptom Manage. 2000;20(3):166-173. doi: 10.1016/S0885-3924(00)00163-9. [DOI] [PubMed] [Google Scholar]
- 8.Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL. Quality of end-of-life care provided to patients with different serious illnesses. JAMA Intern Med. 2016;176(8):1095-1102. doi: 10.1001/jamainternmed.2016.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miranda R, Bunn F, Lynch J, Van den Block L, Goodman C. Palliative care for people with dementia living at home: a systematic review of interventions. Palliat Med. 2019;33(7):726-742. doi: 10.1177/0269216319847092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chahine LM, Malik B, Davis M. Palliative care needs of patients with neurologic or neurosurgical conditions. Eur J Neurol. 2008;15(12):1265-1272. doi: 10.1111/j.1468-1331.2008.02319.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Kline D, Aerts S, et al. Inpatient palliative care for neurological disorders: lessons from a large retrospective series. J Palliat Med. 2017;20(1):59-64. doi: 10.1089/jpm.2016.0240. [DOI] [PubMed] [Google Scholar]
- 12.Ding J, Johnson CE, Lee YC (Olivia), Gazey A, Cook A. Characteristics of people with dementia vs other conditions on admission to inpatient palliative care. J Am Geriatr Soc. 2020;68(8):1825-1833. doi: 10.1111/jgs.16458. [DOI] [PubMed] [Google Scholar]
- 13.Pantilat SZ, Marks AK, Bischoff KE, Bragg AR, O'Riordan DL. The palliative care quality network: improving the quality of caring. J Palliat Med. 2017;20(8):862-868. doi: 10.1089/jpm.2016.0514. [DOI] [PubMed] [Google Scholar]
- 14.Bischoff K, O'Riordan DL, Marks AK, Sudore R, Pantilat SZ. Care planning for inpatients referred for palliative care consultation. JAMA Intern Med. 2018;178(1):48-54. doi: 10.1001/jamainternmed.2017.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative performance scale (PPS): a new tool. J Palliat Care. 1996;12(1):5-11. [PubMed] [Google Scholar]
- 16.Murray SA, Kendall M, Boyd K, Sheikh A. Illness trajectories and palliative care. BMJ. 2005;330(7498):1007-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell SL, Shaffer ML, Cohen S, Hanson LC, Habtemariam D, Volandes AE. An advance care planning video decision support tool for nursing home residents with advanced dementia: a cluster randomized clinical trial. JAMA Intern Med. 2018;178(7):961-969. doi: 10.1001/jamainternmed.2018.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365(13):1212-1221. doi: 10.1056/NEJMsa1100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Givens JL, Kiely DK, Carey K, Mitchell SL. Healthcare proxies of nursing home residents with advanced dementia: decisions they confront and their satisfaction with decision-making. J Am Geriatr Soc. 2009;57(7):1149-1155. doi: 10.1111/j.1532-5415.2009.02304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Givens JL, Selby K, Goldfeld KS, Mitchell SL. Hospital transfers of nursing home residents with advanced dementia. J Am Geriatr Soc. 2012;60(5):905-909. doi: 10.1111/j.1532-5415.2012.03919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon J, Karagiannidou M, Knapp M. The effectiveness of advance care planning in improving end-of-life outcomes for people with dementia and their carers: a systematic review and critical discussion. J Pain Symptom Manage. 2018;55(1):132-150.e1. doi: 10.1016/j.jpainsymman.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell SL, Kiely DK, Hamel MB. Dying with advanced dementia in the nursing home. Arch Intern Med. 2004;164(3):321-326. doi: 10.1001/archinte.164.3.321. [DOI] [PubMed] [Google Scholar]
- 23.Ferrell BR, Twaddle ML, Melnick A, Meier DE. National consensus project clinical practice guidelines for quality palliative care guidelines, 4th edition. J Palliat Med. 2018;21(12):1684-1689. doi: 10.1089/jpm.2018.0431. [DOI] [PubMed] [Google Scholar]
- 24.Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. doi: 10.1056/NEJMp1215620. [DOI] [PubMed] [Google Scholar]
- 25.Creutzfeldt C, Engelberg R, Healey L, et al. Palliative care needs in the neuro-ICU. Crit Care Med. 2015;43(8):1677-1684. doi: 10.1097/CCM.0000000000001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holloway RG, Ladwig S, Robb J, Kelly A, Nielsen E, Quill TE. Palliative care consultations in hospitalized stroke patients. J Palliat Med. 2010;13(4):407-412. doi: 10.1089/jpm.2009.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brecher DB, West TL. Underrecognition and undertreatment of pain and behavioral symptoms in end-stage dementia. Am J Hosp Palliat Care. 2016;33(3):276-280. doi: 10.1177/1049909114559069. [DOI] [PubMed] [Google Scholar]
- 28.Grubbs V, O'Riordan D, Pantilat S. Characteristics and outcomes of in-hospital palliative care consultation among patients with renal disease versus other serious illnesses. Clin J Am Soc Nephrol. 2017;12(7):1085-1089. doi: 10.2215/CJN.12231116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor BL, O'Riordan DL, Pantilat SZ, Creutzfeldt CJ. Inpatients with neurologic disease referred for palliative care consultation. Neurology. 2019;92(17):e1975-e1981. doi: 10.1212/WNL.0000000000007364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As a collaborative project, the PCQN has protocols to ensure fair and equitable access to data. Qualified investigators interested in accessing anonymized PCQN data should contact the corresponding author.