Abstract
Purpose of Review
Paroxysmal nonepileptic events (PNEEs) are a heterogenous group of time-limited events, characterized by changes in motor or behavioral activity beginning abruptly and ending in a short time. Owing to their manifestation, these conditions can clinically simulate seizures.
Recent Findings
These episodes belong to different categories, including syncopal events, psychiatric disorders, and movement disorders. PNEEs are a common cause of diagnostic mistakes and families' concerns, and the risk of useless and sometimes even injurious treatment is considerable. The high frequency of these manifestations in clinical practice makes PNEEs a diagnostic challenge for clinicians.
Summary
This review is focused on the distinctive clinical findings and treatment of PNEEs. Illustrative video recordings of the PNEEs and a video collection as a support tool for differential diagnosis are provided.

Paroxysmal nonepileptic events (PNEEs) represent a complex condition that affects all age groups. Due to their presentation with various signs and symptoms, including fainting, loss of consciousness, headache, vomiting, dizziness, irregular breathing, and emotional and psychiatric problems, PNEEs are often misdiagnosed as epilepsy. In children, physiologic and organic disorders are the most common diseases that can mimic seizures,1 while in adults, these conditions are represented by psychogenic seizures and cardiac events. A variable percentage of patients consistently referred to epilepsy centers presents with nonepileptic events. Nearly 25% of pediatric patients admitted to epilepsy clinics and monitoring units do not have epilepsy. A few are further referred for video-electroencephalographic (VEEG) monitoring to rule out seizures, but among them, up to 43% are diagnosed with PNEEs.2 Thus, these manifestations are frequently observed in the clinical practice but are usually presented to the family pediatrician or the emergency department, which is a common cause of diagnostic error and family concern.3 Early and correct diagnosis is then fundamental to avoid unnecessary treatment and to initiate the right treatment. Although the presentation of PNEEs has been detailed in the literature, there is little information on differential diagnosis. Despite accurate clinical history is crucial for a correct diagnosis, video recording is often mandatory. Although instrumental analysis, as EEG recording, is essential for the differential diagnosis between epileptic and nonepileptic episodes, it has important limitations: a normal interictal EEG does not exclude the diagnosis of epileptic seizures and some patients may have epileptiform interictal activity without being epileptic.4 This review aims to highlight the most important diagnostic clues to distinguish PNEEs from epilepsy and to provide a relevant number of video recordings that can help clinicians in the differential diagnosis.
Syncopes and Breath-Holding Attacks
Syncope is the paroxysmal event most commonly misdiagnosed as an epileptic seizure.5 Clinical presentation, a detailed history, and an event description are crucial for the diagnosis. After syncope diagnosis, it is important to investigate the cause, first excluding the most dangerous conditions associated with the highest mortality, such as cardiovascular disease. PNEEs due to alterations of the cardiovascular system may derive from different pathophysiologic processes and be associated with different precipitating factors. Indeed, although all syncopal attacks are characterized by decreased cerebral blood flow with decreased oxygenation of the cerebral cortex, in some cases, this leads to a loss of consciousness and postural tone, whereas in others, this leads to an increase in tone (decortication/opisthotonus), probably due to corticoreticular inhibition.6 Furthermore, these pathophysiologic processes may manifest differently depending on age-related triggers. In adults, the most PNEEs manifest on a cardiovascular basis as situational and orthostatic reflex syncope, whereas in children, they manifest as breath-holding spells.7 The latter is usually determined by prolonged apnea or an exaggerated Valsalva maneuver and a resulting increase in intrathoracic pressure with a reduction in venous return and cardiac output during episodes of anger and frustration (e.g., cyanotic breath-holding attacks or excessive bradycardia/asystole on a vasovagal basis after injury.)8
Vasovagal syncope (VVS) or neurocardiogenic syncope is a transient loss of consciousness with spontaneous and rapid recovery.9 A detailed history usually identifies the most frequent triggers as prolonged standing, dehydration, abnormal posture, and emotional stress.10 Early symptoms include blurred vision, tinnitus, and dizziness. Autonomic symptoms such as pallor, flushing, sweating, heat flash, nausea, and gastrointestinal symptoms may occur. Visual hallucinations are observed in both conditions, but in the case of epileptic seizures, these are usually stereotyped. Stiffening and convulsive movements are reported in about 50% of syncopal episodes but can be differentiated from tonic and tonic-clonic seizures for their shorter duration, triggers, accompanying symptoms, and recovery time. The convulsive movements are typically not rhythmic. Recovery time of less than 1 minute is typical of syncope, whereas confusion lasting more than 10 minutes is suggestive of an epileptic seizure.11
Tongue biting can occur in both conditions; however, in the case of syncope, it is usually localized to the tip of the tongue, while lateral tongue biting is more suggestive of seizure. A family history of sudden death, drowning, and cardiovascular diseases are key points of cardiac syncope. Interictal EEG has no diagnostic or prognostic value for syncope. However, the EEG may show the typical findings of cerebral hypoperfusion. Initially, there is a slowing of background rhythms, usually in the theta range, maximal in the anterior brain regions, followed by an increase in amplitude with a further decrease in activity in the delta range. However, no paroxysmal epileptiform discharges are usually recorded. The tilt table test, on the other hand, is indicated to confirm a suspected diagnosis of reflex syncope or when evidence of the patient's clinical susceptibility to reflex syncope is clinically relevant.12
Reflex anoxic or asystolic syncope usually begins during infancy and either remits in preschool-age or develops into VVS.13 Alternative names are breath-holding and reflex asystolic syncope.14 Reflex syncope typically occurs after a specific trigger, such as a painful stimulus that activates the sympathetic or parasympathetic nervous system (vasovagal pathogenesis) to induce either hypotension (vasodepressor) or asystole/bradycardia (cardioinhibitory) or both (mixed). Decerebrate rigidity may simulate a tonic seizure especially if followed by involuntary muscle contractions as flexor spasms. However, the duration of the episode of a few seconds, and a fast recovery time, is more suggestive of reflex anoxic syncope. If reflex anoxic syncopes are frequent, atropine or a pacemaker can be taken into consideration. There is a rare condition where an anoxic seizure can precipitate a secondary convulsive seizure: the anoxic-epileptic seizure. The 2 phases of the event can be differentiated by a careful history.
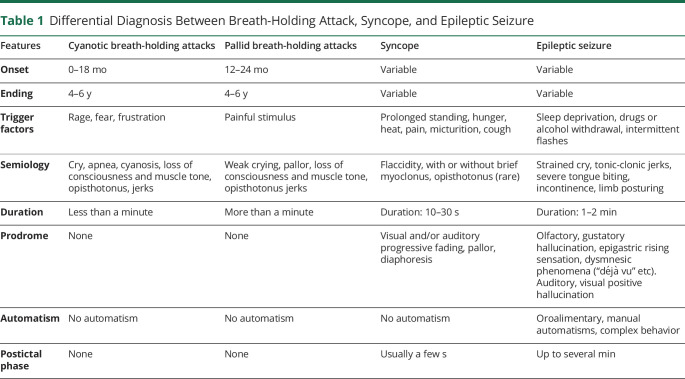
The breath-holding spell is a brief, involuntary cessation of breathing that occurs in healthy infants between 6 and 48 months of age, triggered by anger, frustration, fear, or injury (Video 1). The episodes may progress to respiratory arrest, cyanosis, and syncope. Infants may recover and inhale at this time or develop syncope with transient loss of consciousness. The physiopathology of the cyanotic variant of breath-holding seizures is likely multifactorial.15 Various factors believed to be responsible for the development of these crises include hyperventilation and the Valsalva maneuver, which cause increased intrathoracic pressure, decreased venous return, and decreased cardiac output, resulting in decreased cerebral perfusion and oxygenation. The episodes are extremely frightening to observe but have benign consequences. The episodes occur more frequently in the case of iron-deficiency anemia and can be mistaken for respiratory failure. Their distinction is not determinant except in the very rare situation where reflex anoxic seizures are so common that treatment is required.16 Differential diagnosis among breath-holding attack, syncope, and epileptic seizure is presented in Table 1.
Table 1.
Differential Diagnosis Between Breath-Holding Attack, Syncope, and Epileptic Seizure
Cyanotic breath-holding attack.Download Supplementary Video 1 (14.2MB, mp4) via http://dx.doi.org/10.1212/069580_Video_1
Movement Disorders
Tics are involuntary, sudden, rapid, repetitive movements (motor tics) or sounds (vocal tics). They are usually preceded by a feeling of urgency, discomfort, and a compulsion to perform the tic itself17 (Video 2). Most tic disorders are genetic or of unknown cause and usually occur in childhood18; therefore, secondary causes of tics that occur in adulthood must be ruled out. Tics are divided phenomenologically into motor and vocal tics. Motor tics are again divided into simple and complex. Simple motor tics involve a single muscle group and include eye blinking, head twitching, or shoulder twitching. Tics can be misdiagnosed as myoclonic seizures, yet there are some distinguishing features: myoclonic twitches typically do not recur in the same body part, the tic can be suppressed (to some degree), and myoclonic seizures are often triggered by sleep deprivation, fatigue, and alcohol consumption.19
Tic.Download Supplementary Video 2 (1.7MB, mp4) via http://dx.doi.org/10.1212/069580_Video_2
Stereotypies (or mannerisms) are repetitive movements, or sounds that can be simple, as thumb sucking, or teeth clenching, or complex (rocking and hand flapping or waving).20 Age at onset is typically between 2 and 5 years, and they are often elicited by excitement. Stereotypies are defined primary, in normal developing children, or secondary, when associated with autism, intellectual disability, and other conditions, such as Rett syndrome (Video 3). Stereotypies can be differentiated from epilepsy automatisms because of their distinctive movements and preserved attention and responsiveness, yet videos of the events can help support the diagnosis.21
Stereotypies.Download Supplementary Video 3 (3MB, mp4) via http://dx.doi.org/10.1212/069580_Video_3
Paroxysmal dyskinesias (PDs) are a group of rare hyperkinetic disorders characterized mainly by sudden abnormal involuntary movements with episodic onset manifesting as dystonia, athetosis, chorea, choreoathetosis, and ballism. PDs can be classified according to their triggers as (1) paroxysmal kinesigenic dyskinesias (PKD), (2) paroxysmal nonkinesigenic dyskinesias (PNKD), and (3) paroxysmal exertion-induced dyskinesias.22
PKD is a hyperkinetic movement disorder, usually lasting less than 1 minute, characterized by recurrent, involuntary bouts of abnormal movements such as choreoathetosis, ballism, athetosis, or dystonia.23 It is usually triggered by a sudden normal movement involving the whole body, including standing up or stepping out of a car. Patients report sometimes an ‟aura-like” sensation preceding the abnormal movement described as a ‟rushing” sensation through the body or a sensation of stiffness or numbness. Sporadic or familial (autosomal dominant inherited) mutation of the PRRT2 gene has been identified as the cause of the disease.24 PKD may coexist with epilepsy as part of the infantile convulsion and choreoathetosis syndrome. The seizures may mimic frontal lobe seizures, but the age at onset in middle childhood and adolescence, duration of less than 1 minute, preserved consciousness during the seizure, and overall sudden voluntary movements as triggers should support the diagnosis.25 PKD responds dramatically to low-dose carbamazepine and may remit after the third decade of life.
PNKD is a hyperkinetic movement disorder characterized by a mixture of dystonia and choreoathetosis lasting from a few minutes to several hours, although both shorter and longer durations up to days have been reported.26 The attacks, usually beginning in infancy or early adolescence, are typically not triggered by sudden movements but by emotional stress, alcohol, or coffee. Approximately 60% of patients affected by PNKD exhibit mutations in the PNKD gene usually transmitted in an autosomal dominant fashion. Although rare, symptomatic forms of PNKD have also been reported, mainly associated with multiple sclerosis.27 Preserved consciousness and specific triggers should avoid misdiagnosis with epilepsy.
Benign paroxysmal tonic upgaze occurs in early childhood and is characterized by episodes of persistent or intermittent conjugate upward deviation of the eyes that may last hours or days28 (Video 4). Other clinical features include saccades with downward squinting, apparently preserved horizontal eye movements, and a frequent association with accompanying episodic ataxia (EA) or clumsiness.29 The attacks occur more frequently in intercurrent illness and are relieved by sleep. In a significant proportion of individuals, they can be followed by intellectual disability disabilities or other neurologic symptoms.30
Paroxysmal tonic upgaze.Download Supplementary Video 4 (3.7MB, mp4) via http://dx.doi.org/10.1212/069580_Video_4
Hyperekplexia is a rare neurologic disorder characterized by an overactive startle response (eye blinking or body spasms) to tactile or auditory stimuli.31 During seizures, patients exhibit massive rigidity of the trunk, limbs, and in some cases, the respiratory system with laryngospasm, which can lead to a life-threatening condition. Other symptoms include twitching and clenched fists. The onset may be in utero, but it usually occurs after birth or in childhood. Hyperekplexia is usually inherited as an autosomal dominant trait, but autosomal recessive or, rarely, X-linked inheritance has also been reported, including GLRA1, GPHN, GLRB, and other genes mostly associated with impairment of the inhibitory glycinergic pathway in the nervous system.32 The startle response can be a rapid jerk or series of jerks that can simulate a myoclonic, tonic, or tonic-clonic seizure, and VEEG is helpful to aid in the diagnosis.
EAs are rare autosomal dominant channelopathies that present as sporadic attacks of imbalance and lack of coordination with or without myokymia (Video 5). Two main categories of EAs are known: EA type 1 (EA1) and 2 (EA2). EA1 is associated with mutations in potassium ion channel gene KCNA1, and its onset is typically in middle childhood, with episodes occurring throughout life. EA1 is characterized by brief episodes of cerebellar ataxia lasting seconds or minutes, associated with myokymia usually localized in eyelids or fingers, especially when they are extended. Approximately 10% of people with EA1 have epileptic seizures. Differential diagnosis can be based on the presence of myokymia and specific cerebellar involvement.
Episodic ataxia.Download Supplementary Video 5 (4.1MB, mp4) via http://dx.doi.org/10.1212/069580_Video_5
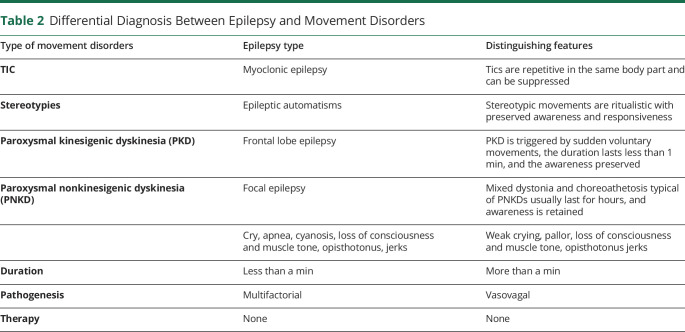
EA2 is caused by loss-of-function mutations of the calcium ion channel gene CACNA1A and is characterized by recurrent episodes of cerebellar ataxia triggered by physical and emotional stress; seizures last longer than in EA1, even up to several hours. Gait and upper limb ataxia can be associated with nystagmus and dizziness, and the attacks may last minutes to hours. Because the seizures are prolonged, it should be possible to record them on home videos, which should facilitate differential diagnosis with epilepsy. Acetazolamide and 4-aminopyridine, a potassium channel blocker, can be very effective treatments. Differential diagnosis between epilepsy and movement disorders is presented in Table 2.
Table 2.
Differential Diagnosis Between Epilepsy and Movement Disorders
Behavioral, Psychological, and Psychiatric Disorders
Among the manifestations that are most frequently misdiagnosed with seizures and whose pathophysiologic bases and related triggers have not been fully understood, there are behavioral alterations of psychological origin. To this category belong self-gratification, psychogenic nonepileptic seizures, hallucinations, or panic attacks. Evaluating the clinical history is crucial to recognize any psychiatric features or specific favoring factors.33
Self-gratification is a self-stimulating behavior that can occur from childhood onward, more frequently in preschool girls. Rhythmic movements of hip flexion and adduction associated with blank look, flushing, and sometimes followed by sleepiness may be mistaken for epileptic infantile spasms. The blank look, occasionally associated with straining and head-turning, may be confused with focal dyscognitive seizures. The relatively high frequency of these episodes and their occurrences in specific situations, such as when bored or seated, lend this behavior to home video recording (HVR). One of the most important diagnostic clues is that the child may be stopped during gratification if distracted and often shows anger and annoyance when interrupted and no postictal state occurs.34
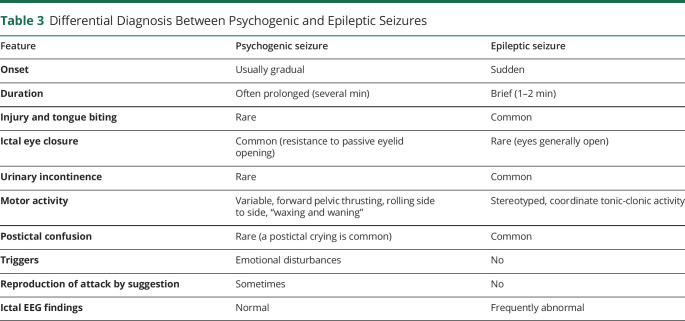
Psychogenic nonepileptic seizures (PNES) have no electrophysiologic correlation or clinical evidence for epileptic seizures. Approximately 20% of patients presenting to physicians with seizure-like events receive this diagnosis.35 Despite psychogenic factors that can facilitate the onset of PNES, psychological mechanisms underlying nonepileptic seizures are poorly understood, and there is a lack of well-established, evidence-based treatments.36 The seizure-like events may have motor features or dyscognitive features, but they are often characterized by a gradual onset and a rapid postictal reorientation compared with seizures. Motor features that differentiate PNES from seizures include a predominance of proximal or truncal movements, a “waxing-and-waning” pattern of motion with typical pelvic thrusting, variable rate, the direction of jerking, side-to-side head-shaking, crying during or after the event with lack of cyanosis, and closed eyelid during the event with resistance to passive eyelid opening.37 According to DSM-V, PNES belong to somatic symptom disorders in the case of maladaptive thoughts, feelings, and behaviors associated with somatic symptoms with or without a diagnosed medical condition. A prompt diagnosis of PNES is essential, given the potential side effects of antiseizure drugs and potentially invasive procedures sometimes adopted for treating seizures as intubation. Moreover, misdiagnosis with epilepsy also delays appropriate psychological treatment. VEEG monitoring and targeted psychological assessment contribute to establishing the correct diagnosis and treatment. Differential diagnosis between psychogenic and epileptic seizures is presented in Table 3.
Table 3.
Differential Diagnosis Between Psychogenic and Epileptic Seizures
Migraine
Migraine with aura (MAs) is a highly prevalent disorder affecting 8% of the general population.38 Visual aura symptoms are by far the most common representing nearly 99% of MAs. Paresthesia and language disturbances occur in 36% and 10% of auras, respectively.39 The visual aura of migraine can take a variety of forms, but it typically regards one visual field and contains positive phenomena, such as flashes, arcs of lights (fortification spectra), or flames, and negative phenomena, such as scotoma with blanking out or graying of the visual field. Differential diagnosis between MA and epilepsy is common and challenging because the conditions often coexist as comorbidity disorder. Visual phenomena of occipital seizures, however, are more likely to be colored and can include a variety of different shapes including diamonds, squares, circles, and lines. Migraine attacks might also be associated with more complex sensory illusions or perceptions as the feeling that a body part or parts have grown dramatically or that everything in the environment is louder. These feelings may be misdiagnosed as a seizure, but they are more likely to be a feature of ‟Alice in Wonderland” syndrome, considered an aura of migraine variant.40
Benign paroxysmal torticollis is an unusual movement disorder, considered a migraine variant of early childhood. Attacks of retrolateral torticollis may last minutes to hours. Autonomic symptoms, such as pallor and vomiting, or distress may be associated. Ataxia has also been reported in older children. Migraine can occur in later childhood of affected individuals. Rare cases have been associated with mutations of the CACNA1A gene (Video 6). Such cases are more likely to be found in children with benign paroxysmal torticollis if accompanied by family histories of familial hemiplegic migraine (HM), EA, or paroxysmal tonic upgaze.e1
Torticollis.Download Supplementary Video 6 (2.6MB, mp4) via http://dx.doi.org/10.1212/069580_Video_6
HM is a rare disorder characterized by migraine attacks preceded by an aura with unilateral weakness always associated with at least another type of aura, such as scintillating scotoma, visual field defect, numbness, paresthesia, or aphasia. Symptoms usually last 20–30 minutes; however, it may take hours to resolve. HM can be classified as familial and sporadic depending on the pattern of transmission.
Sporadic HM is referred to patients with no affected family members. Conversely, familial HM (FHM) is transmitted in an autosomal dominant pattern. CACNA1A, ATP1A2, and SCN1A are the most common causes of FHM. In addition to FHM, ATP1A2 and SCN1A genes have been associated with several types of epilepsies, including Dravet syndrome, making the differential diagnosis with epilepsy even more difficult. Sometimes, HM may be confused with postictal Todd paralysis. Nevertheless, duration of motor symptoms, presence of headache and absence of limb jerking, head-turning, or loss of consciousness usually help to determine the correct diagnosis.
Other Paroxysmal Nonepileptic Disorders
Benign myoclonus of infancy and shuddering attacks are both benign variants of normal behavior in children. The age at onset of these attacks is typically around 4 months, but they can persist up to the age of 6–7 years, with a relapsing-remitting course. Attacks, usually self-limiting, can be very frequent and normally last a few seconds. They can be triggered by feeding or head movements. During these events, children present brief bilateral jerks, rapid movements of the head, shoulders, and trunk, sometimes followed by a modification in facial expression, and the flexion of the upper limbs. After these episodes, children return to their previous activity without loss of consciousness during the event in contrast to myoclonic seizures (as in the syndrome of myoclonic epilepsy in infancy), which represent the most common differential diagnosis. Shuddering attacks are also known as benign nonepileptic spasms. A video recording of episodes is the most useful diagnostic tool, and treatment is unnecessary.e2
Jitteriness is a common movement disorder of the neonatal period, generally transient, self-limiting, and benign. Jitteriness, however, may also occur as a result of pathologic conditions such as hypoglycemia, hypocalcemia, hypoxic-ischemic encephalopathy, intracranial hemorrhage, sepsis, hypothermia, hyperthyroidism, and drug withdrawal reaction. The most important feature of this movement is a recurrent tremor of the extremities which increases in intensity with stimuli and stops with slight flexion of the extremities. It can be distinguished from epileptic seizures because it is triggered when the infant is unwrapped, stimulated, or during crying, but it is suppressed when the infant is wrapped or the affected limb is gently grabbed. Moreover, movements and autonomic changes (hypertension, apnea) do not accompany jitteriness. The presence of these changes may suggest epileptic episodes.e3
Sandifer syndrome is an uncommon association of paroxysmal dystonic movement with gastroesophageal reflux disease (GERD) (1% of children with GERD),e4 which should be considered in the differential diagnosis of infants and children presenting with nonepileptic posturing and dystonic movements (Video 7). Sandifer syndrome usually occurs in childhood or early childhood and clinically manifested as a sudden onset of transient (1–3 minutes) spasmodic torsional dystonia with arching of the back and opisthotonic posturing in patients with GERD or hiatal hernia.e5 The pathogenesis remains unknown, and movements associated with this disorder can challenge clinicians because the presentation can mimic seizures or infantile spasms. However, the arching of the back and its occurrence during or after feeding are key features that distinguish this disorder from epileptic seizures. Early treatment of gastroesophagal reflux may result in the resolution of symptoms.e6
Sandifer syndrome.Download Supplementary Video 7 (1.4MB, mp4) via http://dx.doi.org/10.1212/069580_Video_7
Nonepileptic head drops may arise during childhood and may simulate epileptic spasms or atonic seizures. This disorder is defined as an abrupt loss of muscle tone of the neck, causing the head drop (Video 8). Head drops can be more or less intense and are typically accompanied by crying. These involuntary movements usually occur several times per day, up to a 100 episodes, characteristically in a cluster and potentially resulting in head bobbing. Nonepileptic head drops begin at the age of 3–6 months, and they usually resolve within the first year of life. Infants develop normally. The EEG recording can be misleading because the EEG artifact induced by the head drops might be considered as an ictal paroxysmal event.e7
Head drop.Download Supplementary Video 8 (1MB, mp4) via http://dx.doi.org/10.1212/069580_Video_8
Spasmus nutans is characterized by the triad of asymmetric and pendular nystagmus, head nodding or head tilt, and torticollis. This disorder is typically observed in infants with onset between 4 and 12 months of age, and its cause is still unknown. This normally resolves spontaneously with time, but neuroimaging is usually required to exclude structural brain abnormalities. Indeed, a substantial proportion of patients with spasmus nutans-like nystagmus may have underlying ocular, intracranial, or systemic abnormalities that may require further evaluation and management. In particular, spasmus nutan symptoms may be associated with arachnoid cyst, optic nerve hypoplasia, diencephalic syndrome, subacute necrotizing encephalopathy, and intracranial tumors.e8
Paroxysmal extreme pain disorder (PEPD) is a rare autosomal dominant disorder associated with mutations in the Nav1.7 voltage-gated sodium channel gene (SCN9A), characterized by extremely painful paroxysms. Symptoms begin during the neonatal period and can be life-long. Gain-of-function mutations in the SCN9A gene result in abnormal pain transmission, whereas loss-of-function mutations commonly result in insensitivity to pain. PEPD manifests with 4 types of painful episodes: (1) birth crisis: babies usually appear red and stiff at birth; (2) rectal crisis: triggered by defecation in infants and young children and by a variety of emotional factors in older children and adults; (3) ocular crisis: it may be provoked by the cold wind, but more frequently it is spontaneous; and (4) mandibular crisis, which is often triggered by eating and yawning. The distinctive feature of this condition is paroxysmal pain attacks described as extreme, burning, or stabbing in the rectal, ocular, and mandibular areas, accompanied by autonomic manifestations such as skin flushing, lacrimation, and rhinorrhea.e9 Episodes are most frequent during early childhood, and bradycardia or asystole may occur, resulting in syncope and a nonepileptic anoxic seizure with tonic posturing. The events can be confused with tonic seizures, but a normal EEG and the identification of the typical triggering factors followed by the confirmation with the genetic analysis lead to a correct diagnosis. PEPD has been described in less than 500 patients,e10 but the diagnosis is very important due to the severity of the symptoms, which can be very debilitating and can benefit from carbamazepine treatment.e9
Discussion
This review highlights the importance of knowledge about PNEEs, given their wide variability of presentation and frequent incidence within the pediatric population. Moreover, accurate knowledge of these manifestations may avoid unnecessary interventions and misdiagnosis of epilepsy, which would result in exposing the patient not only to useless therapies but also to potentially serious side effects. The clinical presentation is often crucial for the differential diagnosis in epilepsy, and accurate history combined with the evaluation of HVRs could be sufficient and thus avoid unnecessary investigations to distinguish epilepsy from PNEEs. For this reason in the era of smartphone devices that are ubiquitous in society, there is growing interest in the role of HVRs in the diagnosis of epilepsy. Several studies have highlighted their noninferiority to VEEG monitoring in epilepsy diagnosis,e11 and there are already many examples of smartphone applications assisting clinicians in decision making and optimal management of patients with epilepsy.e12 HVRs indeed offer the possibility of detecting at any time semiological aspects that VEEG might miss due to its dependence on hospitalization time.
To increase their clinical value for a general neurologist or nonneurologist, it is essential to define generally accepted quality standards for HVRs.e13 Practical recommendations on the quality standard of HVRs and safety assessment consist of demonstrating interactivity with the patient,1,2 recording the episode from the beginning,3 and providing good lighting and visibility of the face as well as the rest of the body without obstruction. Regarding safety standards, time recording of no more than 2–3 minutes, creation of a safe environment, and strict observation of a patient's autonomic changes should not be ignored.e14 Further studies should be initiated to create a global video gallery to identify and classify the key points to distinguish a seizure from PNEEs. Indeed, a more informed and widely shared approach to PNES could facilitate the development of practical recommendations to ensure appropriate safety and quality standards of seizure HVRs to allow accurate diagnosis. The creation of an online platform accessible to clinicians while respecting data protection and privacy should be the focus of future research efforts in this area, given the increasing role of telemedicine, particularly in the wake of the recent 2019 coronavirus pandemic.
Supplementary Material
Acknowledgments
The authors developed this work within the framework of the DINOGMI Department of Excellence of MIUR 2018–2022 (legge 232 del 2016). We thank Dr. Anna Capurro, Istituto Gaslini, Genova, for providing mother tongue revisions of the revised manuscript.
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
I. Lagorio and L. Brunelli report no disclosures relevant to the manuscript. P. Striano reports fees for participation in review activities such as data monitoring boards, statistical analysis, endpoint committees, and the like by Zogenyx, GW pharma, and Proveca. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ Paroxysmal nonepileptic events occur frequently in the pediatric population and are often misdiagnosed because of their clinical heterogeneity.
→ Home-made videos are becoming increasingly essential to a correct diagnosis, although a detailed clinical picture and accurate medical history are mandatory.
→ Increasing awareness is important for a prompt diagnostic framing and for avoiding unnecessary procedures and potentially serious side effects derived from inappropriate/unneeded treatments.
→ The use of diagnostic tools such as EEG must be guided by specific clinical suspicions.
→ Growing awareness on this topic, together with a specific video collection widely shared among physicians, can reduce the risk for diagnostic mistakes.
References
- 1.Kotagal P, Costa M, Wyllie E, Wolgamuth B. Paroxysmal nonepileptic events in children and adolescents. Pediatrics. 2002;110(4):e46. [DOI] [PubMed] [Google Scholar]
- 2.Bye AM, Kok DJ, Ferenschild FT, Vles JS. Paroxysmal non-epileptic events in children: a retrospective study over a period of 10 years. J Paediatr Child Health. 2000;36(3):244-248. [DOI] [PubMed] [Google Scholar]
- 3.Oto MM. The misdiagnosis of epilepsy: appraising risks and managing uncertainty. Seizure. 2017;44:143-146. [DOI] [PubMed] [Google Scholar]
- 4.Xiang X, Fang J, Guo Y. Differential diagnosis between epileptic seizures and psychogenic nonepileptic seizures based on semiology. Acta Epileptol. 2019;1(1):6. [Google Scholar]
- 5.Patel PR, Quinn JV. Syncope: a review of emergency department management and disposition. Clin Exp Emerg Med. 2015;2(2):67-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sotero de Menezes MA. Paroxysmal non-epileptic events. J Pediatr. 2002;78:73-88. [DOI] [PubMed] [Google Scholar]
- 7.Chen-Scarabelli C, Scarabelli TM. Neurocardiogenic syncope. Br Med J. 2004;329:336-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yilmaz U, Doksoz O, Celik T, Akinci G, Mese T, Sevim Yilmaz T. The value of neurologic and cardiologic assessment in breath holding spells. Pak J Med Sci. 2014;30(1):59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aksu T, Davila A, Gupta D. The “heart brain” and neuromodulation for vasovagal syncope. Auton Neurosci. 2021. Dec;236:102892. [DOI] [PubMed] [Google Scholar]
- 10.Ruzieh M, Ammari Z, Dasa O, Karim S, Grubb B. Role of closed loop stimulation pacing (CLS) in vasovagal syncope. Pacing Clin Electrophysiol. 2017;40(11):1302-1307. [DOI] [PubMed] [Google Scholar]
- 11.Stephenson JB. Syncopes and other paroxysmal events. Handb Clin Neurol. 2013;112:861-866. [DOI] [PubMed] [Google Scholar]
- 12.Petkar S, Cooper P, Fitzpatrick AP. How to avoid a misdiagnosis in patients presenting with transient loss of consciousness. Postgrad Med J. 2006;82(972):630-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30(21):2631-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephenson JB. Anoxic seizures: self-terminating syncopes. Epileptic Disord. 2001;3(1):3-6. [PubMed] [Google Scholar]
- 15.Evans OB. Breath-holding spells. Pediatr Ann. 1997;26(7):410-414. [DOI] [PubMed] [Google Scholar]
- 16.Goldman RD. Breath-holding spells in infants. Can Fam Physician. 2015;61(2):149-150. [PMC free article] [PubMed] [Google Scholar]
- 17.Black KJ, Kim S, Schlaggar BL, Greene DJ. The new tics study: a novel approach to pathophysiology and cause of tic disorders. J Psychiatr Brain Sci. 2020;5:e200012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jankovic J. Tourette's syndrome. N Engl J Med. 2001;345(16):1184-1192. [DOI] [PubMed] [Google Scholar]
- 19.Chouinard S, Ford B. Adult onset tic disorders. J Neurol Neurosurg Psychiatry. 2000;68(6):738-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer HS. Motor stereotypies. Semin Pediatr Neurol. 2009;16(2):77-81. [DOI] [PubMed] [Google Scholar]
- 21.Freeman RD, Soltanifar A, Baer S, Freeman R. Stereotypic movement disorder: easily missed. Dev Med Child Neurol. 2010;52(8):733-738. [DOI] [PubMed] [Google Scholar]
- 22.Unterberger I, Trinka E. Therapeutic advances in neurological disorders diagnosis and treatment of paroxysmal dyskinesias revisited. Ther Adv Neurol Disord. 2008;1(2):4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruno MK, Hallett M, Gwinn-Hardy K, et al. Clinical evaluation of idiopathic paroxysmal kinesigenic dyskinesia: new diagnostic criteria. Neurology. 2004;63(12):2280-2287. [DOI] [PubMed] [Google Scholar]
- 24.Chen WJ, Lin Y, Xiong ZQ, et al. Exome sequencing identifies truncating mutations in PRRT2 that cause paroxysmal kinesigenic dyskinesia. Nat Genet. 2011;43(12):1252-1255. [DOI] [PubMed] [Google Scholar]
- 25.Zhou JQ, Zhou LM, Fang ZY, et al. Analyzing clinical and electrophysiological characteristics of Paroxysmal Dyskinesia. J Res Med Sci. 2011;16(1):110-114. [PMC free article] [PubMed] [Google Scholar]
- 26.Spacey S, Adams P. Familial paroxysmal nonkinesigenic dyskinesia. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews. University of Washington; 2004-2019. [PubMed] [Google Scholar]
- 27.Machado C, Amorim JM, Rodrigues M, Cerqueira J, Lourenço E, Pinho J. Paroxysmal dystonia as a manifestation of multiple sclerosis. Neurologist. 2015;19(5):132-134. [DOI] [PubMed] [Google Scholar]
- 28.International League against Epilepsy, Lispi ML, Vigevano F. Epileptic disorders. Epileptic Disord. 2002;3:203-206. [Google Scholar]
- 29.Lispi ML, Vigevano F. Benign paroxysmal tonic upgaze of childhood with ataxia. Epileptic Disord. 2002;3(4):203-206. [PubMed] [Google Scholar]
- 30.Hayman M, Harvey AS, Hopkins IJ, Kornberg AJ, Coleman LT, Shield LK. Paroxysmal tonic upgaze: a reappraisal of outcome. Ann Neurol. 1998;43(4):514-520. [DOI] [PubMed] [Google Scholar]
- 31.Tijssen MA, Rees MI. Hyperekplexia. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews. University of Washington; 2007-2019. [Google Scholar]
- 32.Lee Y, Kim NY, Hong S, et al. Familiar hyperekplexia, a potential cause of cautious gait: a new Korean case and a systematic review of phenotypes. J Mov Disord. 2017;10(1):53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beach PEJ. How do you differentiate pseudoseizures from real seizures? Evidence-Based Practice. Evid Based Pract. 2014;17(11):1-2. [Google Scholar]
- 34.Couper RT, Huynh H. Female masturbation masquerading as abdominal pain. J Paediatr Child Health. 2002;38(2):199-200. [DOI] [PubMed] [Google Scholar]
- 35.Kotsopoulos IA, De Krom MC, Kessels FG, et al. The diagnosis of epileptic and non-epileptic seizures. Epilepsy Res. 2003;57(1):59-67. [DOI] [PubMed] [Google Scholar]
- 36.Brown RJ, Reuber M. Psychological and psychiatric aspects of psychogenic non-epileptic seizures (PNES): a systematic review. Clin Psychol Rev. 2016;45:157-182. [DOI] [PubMed] [Google Scholar]
- 37.Doss RC, LaFrance WC. Psychogenic non-epileptic seizures. Epileptic Disord. 2016;18(4):337-343. [DOI] [PubMed] [Google Scholar]
- 38.Russell MB, Rasmussen BK, Thorvaldsen P, Olesen J. Prevalence and sex distribution of different forms of migraine. Ugeskr Laeger. 1996;158(10):1369-1372. [PubMed] [Google Scholar]
- 39.Viana M, Sances G, Linde M, et al. Clinical features of migraine aura: results from a prospective diary-aided study. Cephalalgia. 2017;37(10):979-989. [DOI] [PubMed] [Google Scholar]
- 40.Ilik F, Ilik K. Alice in Wonderland syndrome as aura of migraine. Neurocase. 2014;20(4):474-475. eReferences e1–e14 are available at links.lww.com/CPJ/A343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cyanotic breath-holding attack.Download Supplementary Video 1 (14.2MB, mp4) via http://dx.doi.org/10.1212/069580_Video_1
Tic.Download Supplementary Video 2 (1.7MB, mp4) via http://dx.doi.org/10.1212/069580_Video_2
Stereotypies.Download Supplementary Video 3 (3MB, mp4) via http://dx.doi.org/10.1212/069580_Video_3
Paroxysmal tonic upgaze.Download Supplementary Video 4 (3.7MB, mp4) via http://dx.doi.org/10.1212/069580_Video_4
Episodic ataxia.Download Supplementary Video 5 (4.1MB, mp4) via http://dx.doi.org/10.1212/069580_Video_5
Torticollis.Download Supplementary Video 6 (2.6MB, mp4) via http://dx.doi.org/10.1212/069580_Video_6
Sandifer syndrome.Download Supplementary Video 7 (1.4MB, mp4) via http://dx.doi.org/10.1212/069580_Video_7
Head drop.Download Supplementary Video 8 (1MB, mp4) via http://dx.doi.org/10.1212/069580_Video_8