Abstract
Cyclophosphamide is associated with chemotherapy-related ovarian failure (CROF) in breast cancer survivors, however little is known about predicting individual risks. We sought to identify genetic alleles as biomarkers for risk of CROF after cyclophosphamide treatment. 115 premenopausal women with newly diagnosed breast cancer were genotyped for single nucleotide polymorphisms (SNPs) in genes involved in cyclophosphamide activation (CYP3A4 and CYP2C19) and detoxification (GSTP1 and GSTA1). Patients prospectively completed menstrual diaries. With median follow up of 808 days, 28% experienced CROF. Survivors homozygous for the GSTA1 minor allele had lower hazards for developing CROF (HR 0.22 [95% CI 0.05–0.94], p=0.04), while survivors homozygous for the CYP2C19 minor allele had higher hazards for developing CROF (HR 4.5 [95% CI 1.5–13.4], p=0.007) compared to patients with at least one major allele. In separate multivariable models adjusting for age and tamoxifen use, the associations were attenuated (GSTA1 HR 0.24 [95% CI 0.06–1.0], p=0.05; CYP2C19 HR 2.5 [0.8–7.6], p=0.11). CYP3A4 and GSTP1 SNPs were not significantly related to CROF. In younger breast cancer survivors undergoing cyclophosphamide-based chemotherapy, genetic variation in CYP2C19 and GSTA1 may be associated CROF.
Keywords: Breast cancer, ovarian failure, GSTA1, CYP2C19, CYP3A4, GSTP1
Introduction
Over 3.5 million American women are breast cancer survivors(Siegel et al., 20190. In 2019, approximately 270,000 women will be diagnosed with invasive breast cancer in the United States (Siegel et al., 2019); fifteen percent will be younger than 45 (Anon, 2016). Most of these patients undergo cyclophosphamide-based chemotherapy, incurring damage to the finite pool of ovarian follicles (Byrne et al., 1992, Bines et al., 1996, Goodwin et al., 1999). Consequently, young breast cancer survivors are at higher risk for premature ovarian aging and ovarian failure (Su et al., 2010, Anderson and Cameron, 2011).
Ovarian function has significant implications for young breast cancer survivors, including cancer prognosis, choice of adjuvant therapy, need for contraception, fertility potential, and menopausal concerns (Ganz et al., 2000, Partridge et al., 2004, Early Breast Cancer Trialists’ Collaborative Group 2005, Su et al., 2010). Age, pre-chemotherapy ovarian reserve, and chemotherapy regimen are known predictors of ovarian function after chemotherapy (Petrek et al., 2006, Walshe et al., 2006, Anderson and Cameron, 2011). However, little else is known about risk factors for chemotherapy-related ovarian failure (CROF).
Cyclophosphamide is a common chemotherapy in breast cancer treatment. It intercalates DNA in a non-cell cycle specific manner, potentially damaging both growing and non-growing ovarian follicles (Warne et al., 1973). Cyclophosphamide requires activation by several cytochrome P450 enzymes. P450 enzymes activate cyclophosphamide through hydroxylation to the cytotoxic form 4-hydroxycyclophosphamide (Figure 1) (Scripture et al., 2005). This cytotoxic form is then inactivated by glutathione S-transferase enzymes through glutathione conjugation (Choi et al., 2006). There is known genetic variation in these enzymes, many of which are associated with variable protein expression (de Morais et al., 1994, Kuehl et al., 2001, Lang et al., 2001, Takada et al., 2004). These functional single nucleotide polymorphisms (SNPs) in drug metabolizing enzymes have been associated with cancer-related outcomes, such as recurrence and survival (Sweeney et al., 2000b, Ambrosone et al., 2001, Sweeney et al., 2003, DeMichele et al., 2007, Gor et al., 2010).
Figure 1:
Simplified schematic of cyclophosphamide activation and detoxification. Cyclophosphamide is activated by hepatic cytochrome P-450 enzymes, including CYP3A4 and CYP2C19. The active drug, 4-hydroxycyclophosphamide, is detoxified via various pathways. One pathway is via conjugation with glutathione by glutathione S-transferase enzymes.
We previously demonstrated an association between the CYP3A4*1B SNP and CROF in a subset of younger women within a cohort of breast cancer survivors(Su et al., 2010). Our objective is to test the association between CROF and CYP3A4*1B and three new candidate drug metabolizing enzyme SNPs in a new cohort of young breast cancer patients to serve as potential biomarkers for predicting CROF. We hypothesize that SNPs predicted to increase cyclophosphamide activation or decrease cyclophosphamide detoxification are associated with increased risk of ovarian failure following cyclophosphamide-based chemotherapy (Table 1).
Table 1:
Drug metabolizing enzyme single nucleotide polymorphisms and hypothesized effect on chemotherapy-related ovarian failure
| Gene | rs number | Minor allele | Effect of minor allele on enzyme expression | Hypothesized effect on CROF risk |
|---|---|---|---|---|
| CYP2C19 | rs4244285 | A | Decrease ((de Morais et al., 1994, Sweeney et al., 2000a, Takada et al., 2004, Sharda et al., 2008)) | Decrease |
| CYP3A4 | rs2740574 | G | Increase (Amirimani et al., 2003, DeMichele et al., 2007) | Increase |
| GSTA1 | rs4715332 | C | Decrease (Sweeney et al., 2003) | Increase |
| GSTP1 | rs1695 | G | Decrease (Sweeney et al., 2000a, Sharda et al., 2008) | Increase |
Materials and Methods
Study Population
This is a prospective cohort study of 115 premenopausal women with newly diagnosed breast cancer (stages I-III), who subsequently underwent cyclophosphamide-based chemotherapy. Participants were identified by systematic medical record screening of all new breast cancer patients from breast clinics at the University of California, San Diego and the University of Pennsylvania between 2009 and 2012. Inclusion criteria were premenopausal at time of initiation of chemotherapy (defined as menstruating in the previous 12 months), planned treatment with cyclophosphamide-based chemotherapy, and the presence of a uterus and at least one ovary. Exclusion criteria were prior malignancy and chemotherapy.
Data Collection
Participants were recruited from academic breast programs at the University of California, San Diego and the University of Pennsylvania. They were enrolled at cancer diagnosis prior to the start of chemotherapy and followed longitudinally for up to 5 years. All participants received cyclophosphamide according to body surface area (cumulative dose 2400 mg/m2). At enrollment, participants provided peripheral blood specimens, from which the buffy coat and DNA were extracted for genotyping. Participants prospectively recorded daily menstrual diaries and completed questionnaires every three to six months. All participants provided written informed consent. The institutional review boards at the University of California, San Diego and the University of Pennsylvania approved the study. This study is registered as a national clinical trial (NCT01197456).
SNP Selection
We chose four candidate SNPs based on their published associations with cancer outcomes or ovarian function after cyclophosphamide exposure(Aithal et al., 2000, Sweeney et al., 2003) (Table 1). Candidate SNPs also needed sufficient representation of major and minor alleles in our population for power considerations. We hypothesized that women who were homozygous for the minor alleles in CYP3A4*1B, GSTA1, and GSTP1 would experience shorter time to ovarian failure, while women homozygous for the minor allele of CYP2C19 variant would experience longer time, (Dirven et al., 1994a, Amirimani et al., 1999, Amirimani et al., 2003, Sweeney et al., 2003, Timm et al., 2005, Zhong et al., 2006, Pinto et al., 2009)
Sample Preparation and Genotyping
Participant DNA was extracted from buffy coat specimens using the Qiagen QIAamp DNA Blood Kit (Qiagen,Valencia, CA) and quantitated using Invitrogen Pico Green dsDNA quantitation kit (Invitrogen, Paisley, U.K.). Participants were genotyped for the four SNPs using the Taqman OpenArray System.
Data Analysis
Stata (release 11; Stata Corp.; College Station, Tx) software was used for analysis. We selected “time to CROF” as our primary endpoint to account for different lengths of follow-up time for each subject. We defined CROF as ≥ 12 months of amenorrhea after the end of chemotherapy; this definition parallels the final menstrual period in the definition of menopause (Harlow et al., 2012). Participants who underwent CROF contributed the time to the analysis from end date of chemotherapy to the start date of 12 months of amenorrhea. For example, a patient who completed chemotherapy on January 1, 2009 and recorded a last menstrual period of February 1, 2009 with no subsequent periods for the remaining 3 years of follow up would contribute 31 days to the analysis, despite significantly longer follow up in the study. Participants who did not undergo CROF contributed all of the time from end date of chemotherapy until their last follow up or until the date when they were censored. Causes for censorship included GnRH agonist use, bilateral oophorectomy, hysterectomy, withdrawal from the study, cancer metastasis, and death.
We generated Kaplan-Meier curves and compared time to CROF between SNP variants using log rank tests. Explanatory Cox proportional hazard regression models tested SNP associations with time to CROF after controlling for potential confounders. We adjusted for both age and tamoxifen exposure, two variables known to affect menstrual pattern (Sunderland and Osborne, 1991, Jordan, 2003, Petrek et al., 2006, Dolleman et al., 2014). A priori sample size calculations estimated 80% power to detect relative risks of 1.7–2.6. For this study, a p-value <0.05 in the adjusted model was considered to be significant.
Results
Table 2 depicts baseline characteristics. Seventy-three participants were enrolled at the University of Pennsylvania, and 42 participants were enrolled at the University of California, San Diego. The cohort median age (range) was 39.7 (20.8–46.1) at end of chemotherapy. The median total follow-up time was 808 days, with a range of 125 to 2,119 days. Accounting for CROF and censoring events, the median time contributed to the analysis was 277 days (range 1 to 1884 days). Thirty-two (28%) participants experienced CROF. Twenty-five of these women reported no further menses after the end of chemotherapy; while they were followed for a median of 1,063 days in the study (range 599 to 2,119), their CROF event occurred immediately and contributed one day to the analysis.
Table 2:
Cohort Characteristics and unadjusted hazard ratios (HR) for chemotherapy-related ovarian failure (N=115)
| Cohort characteristic | N (%) | Unadjusted HR (95% CI) |
|---|---|---|
| Median age at chemotherapy (range), years | 39.7 (20.8–46.1) | 1.2 (1.1–1.3) |
| Race Caucasian African American Other |
79 (68.7) 15 (13.0) 21 (18.3) |
ref 1.3 (0.51–3.6) 1.2 (0.47–2.9) |
| Income > $60,000 ≤ $60,000 Declined to report |
65 (58.6) 33 (29.7) 13 (11.7) |
ref 0.56 (0.22–1.4) 1.02 (0.35–3.0) |
| Education High school College Masters/doctorate |
19 (16.5) 61 (53.0) 32 (27.8) |
ref 0.69 (0.28–1.7) 0.72 (0.27–1.9) |
| Smoker | 8 (7.0) | 0.89 (0.21–3.7) |
| Cancer Type Ductal Lobular Mixed |
107 (93.0) 3 (2.6) 4 (3.5) |
ref 1.3 (0.17–9.2) 1.1 (0.15–8.1) |
| ER or PR+ | 75 (65.2) | 1.6 (0.71–3.6) |
| Cancer stage 1 2 3 |
26 (22.6) 58 (50.4) 26 (22.6) |
ref 0.57 (0.25–1.3) 0.96 (0.37–2.5) |
| Her2 neu+ | 24 (20.9) | 1.1 (0.47–2.5) |
| Chemotherapy regimen Adriamycin, cyclophosphamide ± paclitaxel Docetaxel, cyclophosphamide |
85 (73.9) 30 (26.1) |
ref 1.1 (0.49–2.3) |
| Tamoxifen | 57 (51.8) | 1.8 (0.86–3.9) |
| Radiation | 82 (73.2) | 1.3 (0.57–3.1) |
Due to missing data, not all variables sum to 115 participants
Regarding baseline characteristics, only younger age was associated with longer time to CROF (Table 2). Cancer characteristics such as cancer type, ER/PR status, cancer stage, Her2neu status, chemotherapy regimen, tamoxifen exposure, and radiation were not associated with time to CROF.
Genotype frequencies for the four SNPs are depicted in Table 3. The distributions were consistent with reported reference SNP frequencies (Timm et al., 2005). There were no genotype failures for CYP3A4, GSTA1 and GSTP1. For CYP2C19, the genotype failure rate was 12%. The rate of CROF did not differ between those who were successfully genotyped and those who were unable to be genotyped for CYP2C19. Total follow up time also did not differ by genotype.
Table 3:
Unadjusted hazard ratios (HR) of chemotherapy-related ovarian failure by genotype
| Genotype (SNP ID) | Genotypes | Genotype frequencies n (%) (N=115) | Unadjusted HR (95% CI) |
|---|---|---|---|
|
CYP2C191
(rs4244285) |
AA GG/AG |
5 (4.3) 96 (83.5) |
4.5 (1.5–13.4) ref |
|
CYP3A4 (rs2740574) |
GG AG |
102 (88.7) 13 (11.3) |
0.89 (0.3–2.6) ref |
|
GSTA1 (rs4715332) |
CC AA/AC |
26 (22.6) 89 (77.4) |
0.22 (0.05–0.9) ref |
|
GSTP1 (rs1695) |
GG AA/AG |
15 (13.0) 100 (87.0) |
0.68 (0.21–2.2) ref |
Genotype failure occurred in 14 participants (12%)
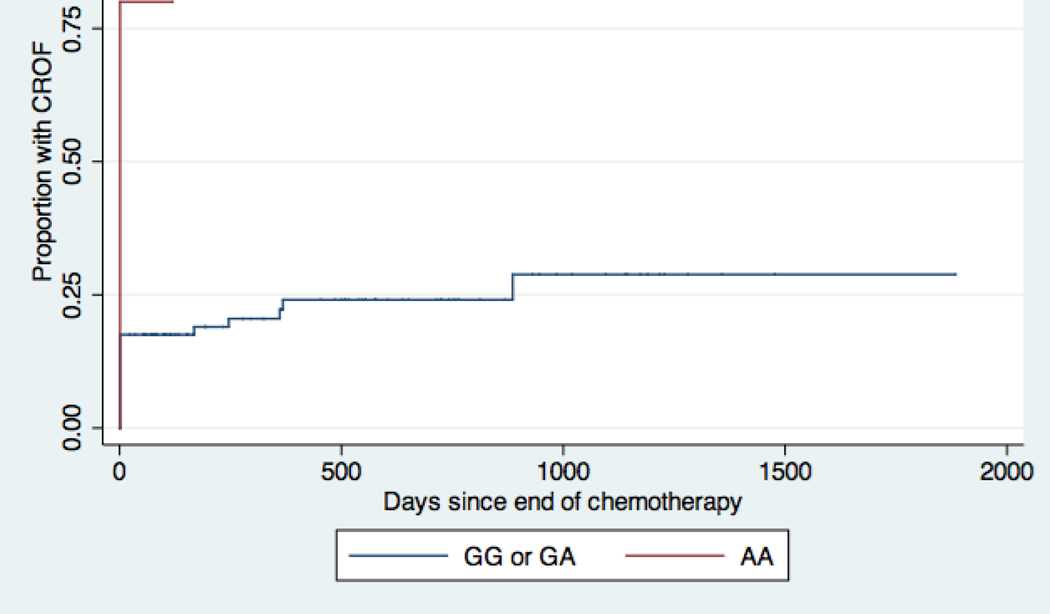
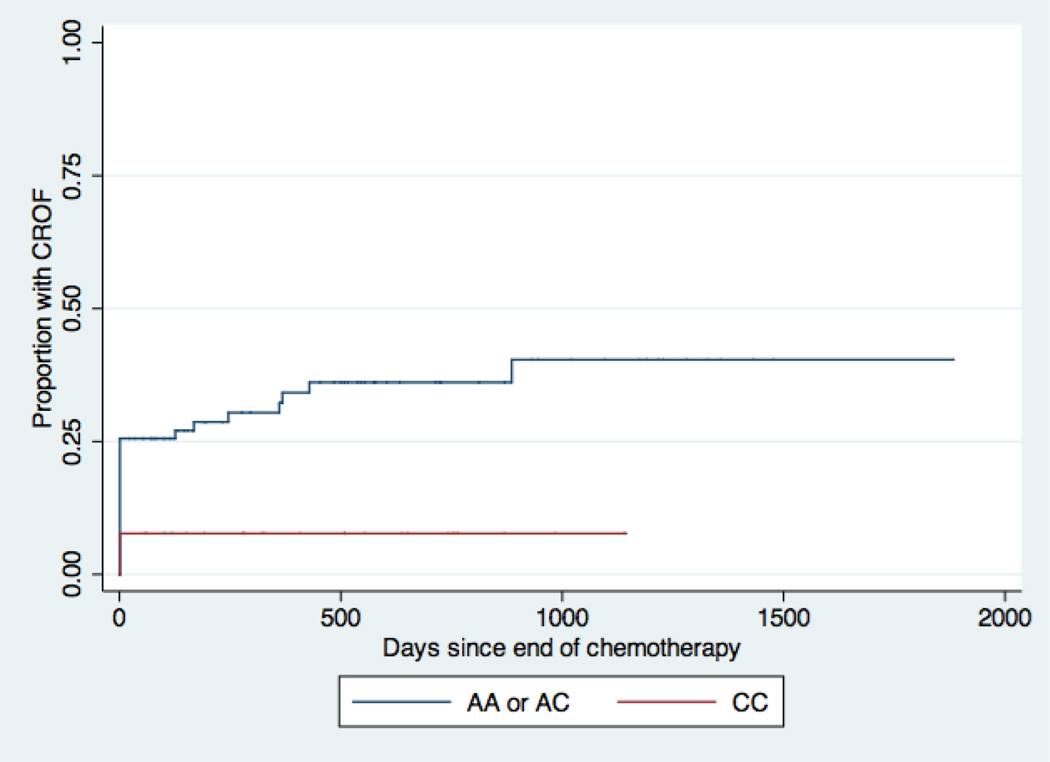
Compared with participants who carried at least one major allele for CYP2C19, participants who were homozygous for the minor allele had a significantly shorter time to ovarian failure with a hazard ratio of 4.5 (95% CI 1.5–13.4) in the unadjusted model (Table 3, Figure 2A). Survivors who were homozygous for the minor allele of GSTA1 had a longer time to ovarian failure compared to survivors who had at least one major allele of GSTA1 (HR 0.22 [95% CI 0.05–0.9]) in the unadjusted model (Table 3, Figure 2B). The CYP3A4 and GSTP1 SNPs were not significantly associated with time to CROF.
Figure 2A:
Kaplan-Meier Curves for chemotherapy-related ovarian failure (CROF) by CYP2C19 genotype. Patients homozygous for the minor allele, A, have decreased activity of CYP2C19 and would be predicted to have decreased activation of cyclophosphamide compared to patients with at least one major allele, G (See also Table 1) (de Morais et al., 1994, Sweeney et al., 2000a, Takada et al., 2004, Sharda et al., 2008). Every step up represents a CROF event. Patients homozygous for the minor allele A actually had higher hazards for developing CROF.
Figure 2B:
Kaplan-Meier Curves for chemotherapy-related ovarian failure (CROF) by GSTA1 genotype. Patients homozygous for the minor allele, C, have decreased activity of GSTA1 and would be predicted to have decreased clearance of cyclophosphamide compared to patients with at least one major allele, A (See also Table 1) (Sweeney et al., 2003). Every step up represents a CROF event. Patients homozygous for the minor allele C actually had lower hazards for developing CROF.
After adjusting for age and tamoxifen exposure, the association between GSTA1 (HR 0.24 [95% CI 0.06–1.0]) and CYP2C19 (HR 2.5 [95% CI 0.8 –7.6]) with time to CROF were attenuated (Table 4). Older age remained significantly associated with time to CROF in both models. The direction of the association of both genotypes and CROF did not change when restricting the analysis to Caucasian participants (data not shown). Cyclophosphamide dose was not included in the multivariable models, as this was normalized by body size.
Table 4:
Adjusted hazard ratios (HR) for chemotherapy-related ovarian failure for CYP2C19 and GSTA1
| CYP2C19 model Adjusted HR (95% CI) | GSTA1 model Adjusted HR (95% CI) | |
|---|---|---|
|
CYP2C19
(AA vs. AG/GG) |
2.5 (0.8 – 7.6) | - |
|
GSTA1
(CC vs. AC/AA) |
- | 0.24 (0.06–1.0) |
| Age at chemotherapy | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) |
| Tamoxifen | 1.5 (0.6–3.6) | 1.6 (0.8–3.5) |
Discussion
In this study of 115 young breast cancer patients undergoing cyclophosphamide-based chemotherapy, inter-individual variations in CYP2C19 and GSTA1, two enzymes involved in cyclophosphamide metabolism, may be related to post-treatment ovarian function. Breast cancer survivors who were homozygous for the CYP2C19 minor allele had significantly shorter time to CROF compared to survivors who had at least one major allele, although the association was attenuated in adjusted analysis. Participants who were homozygous for the minor allele of GSTA1 had significantly longer time to ovarian failure compared to survivors who had at least one major allele; however, this relationship was also attenuated after adjusting for age and tamoxifen use. While these findings suggest that genetic variation in chemotherapy metabolism is associated with the gonadotoxic effects of cancer treatments, larger replicative studies are required to establish more firmly the directionality and magnitude of the relationship between these candidate SNPs and ovarian function after cyclophosphamide exposure.
For GSTA1, an association between homozygosity for the minor allele and longer time to ovarian failure was observed in unadjusted analysis. Though the hazard ratio remained similar in magnitude, the association just missed statistical significance after adjusting for age and tamoxifen exposure. GSTA1 encodes one of several glutathione S-transferase enzymes that detoxify alkylating agents including 4-hydroxy-cyclophosphamide (Hayes and Pulford, 1995, Sweeney et al., 2000b). This candidate SNP was selected because of prior data showing that breast cancer patients who were homozygous for the GSTA1 minor allele had improved survival compared to those with genotypes of at least one major allele (Sweeney et al., 2000b). We hypothesized that breast cancer survivors homozygous for the GSTA1 minor allele would have increased risk of ovarian failure, but found the directionality of the association in this cohort to be opposite of our hypothesis. The most likely explanation for our results is that the association between genotype and biological activation and elimination of cyclophosphamide is complex. There are multiple routes for cyclophosphamide activation and detoxification (Dirven et al., 1994b, Zhong et al., 2006, Pinto et al., 2009). Several in vitro and in vivo studies have shown mixed results regarding validation of enzyme activation and detoxification of cyclophosphamide (Dirven et al., 1994a, Timm et al., 2005). Therefore, replicative studies are required to establish more firmly the directionality and magnitude of the relationship between this candidate SNP and ovarian function after cyclophosphamide exposure.
The CYP2C19 SNP was of interest because prior data demonstrated an association with premature ovarian failure in the context of cyclophosphamide exposure and lupus. Takada et al. demonstrated that women who were homozygous for the CYP2C19 major allele had increased risk of premature ovarian failure, whereas those who were homozygous for the minor allele were more likely to experience end-stage renal disease, presumably from varying levels of exposure to active cyclophosphamide (Takada et al., 2004). While we also found an association between this CYP2C19 SNP and ovarian failure, this association was no longer significant following adjustment for confounding, in part due to the small number of participants homozygous (n=5) for the minor allele. It is possible that the functional significance of this SNP varies by the amount of cyclophosphamide exposure. For example, one pharmacogenomic study that examined the association between CYP2C19 genotype with serial cyclophosphamide plasma concentrations showed a genotype-phenotype relationship only in the subset of individuals receiving lower doses of cyclophosphamide (Timm et al., 2005). Breast cancer patients receive significantly higher doses of cyclophosphamide than those with lupus, which may partly explain why we did not see the same genotype-phenotype relationship.
We did not replicate our prior finding that homozygosity for the CYP3A4*1B major allele was related to longer time to ovarian failure in breast cancer survivors who were younger than 45 (HR 0.3, 95% CI 0.07–0.9) (Su et al., 2010). The homozygous AA allele frequency for this SNP is low in reference populations (<1 to 7%) (van Schaik, 2008), consistent with our finding of no participants with this genotype. Because of low genotype frequency, we were not adequately powered to reproduce our prior observation.
Several limitations of our study should be considered. The total number of women experiencing the outcome CROF was 32 (28%), limiting the power of the study to adjust for multiple confounders. Ovarian failure was measured by menstrual bleeding, the current gold standard, but we do not have longitudinal ovarian reserve measures such as AMH to corroborate menstrual pattern. The strengths of our study include the prospective collection of frequent menstrual diaries, relatively large cohort of women, young median age, and long follow up time.
Conclusions
In this study of 115 premenopausal women, we show that genetic variations in CYP2C19 and GSTA1 may be potential biomarkers for risk of chemotherapy-related ovarian failure. With advances in precision medicine, mechanistic studies are needed to improve understanding of how genetic polymorphism(s) function and interact, in order to personalize cancer treatment for efficacy and minimizing toxicities. We advocate for replicative studies and if these findings hold true, to consider these SNPs as risk factors for ovarian failure, with the understanding that the mechanisms by which cyclophosphamide is metabolized and by which ovarian failure occur are complex.
IMPACT STATEMENT.
What is already known on this subject?
Young breast cancer survivors face important potential implications of chemotherapy-related ovarian failure (CROF). Little is known about individual risk for CROF. Cyclophosphamide, a particularly gonadotoxic drug commonly used in breast cancer treatment, is metabolized by various cytochrome p450 enzymes. Studies have shown genetic variation in p450 enzymes is associated with differential clinical outcomes after cyclophosphamide treatment: breast cancer patients homozygous for GSTA1 minor allele had improved overall survival; lupus patients homozygous for CYP2C19 minor allele had increased risk for CROF; and CYP3A4*1B I was associated with decreased risk for CROF.
What do the results of this study add?
We show a surprising opposite direction for the risk of CROF in breast cancer patients with GSTA1 and CYP2C19 variants, while we did not show a significant risk for genetic variation in CYP3A4 (which had previously been shown to have a protective effect) or GSTP1.
What are the implications of these findings for clinical practice and/or further research?
This study shows the complexity of genetic variation in predicting outcomes to treatment. We advocate for future replicative studies to validate GSTA1 and CYP2C19 and definitively negate CYP3A4 and GSTP1 as biomarkers for risk of CROF after cyclophosphamide treatment. Understanding genetic variation in chemotherapy metabolism has the potential to individualize treatment regimens to maximize efficacy and minimize toxicity.
Acknowledgements:
We acknowledge and are grateful to the patients and their families who participated in this clinical trial
Funding:
This work was supported by the California Breast Cancer Research Program [20OB-0144, 2014-2018] and the National Institutes of Health R01 [HD080952-04, 2014-2019]
Footnotes
Declaration of interest statement: The authors have no conflicts of interest to disclose.
References
- Aithal GP, Day CP, Leathart JB & Daly AK, 2000. Relationship of polymorphism in CYP2C9 to genetic susceptibility to diclofenac-induced hepatitis. Pharmacogenetics, 10, 511–8. [DOI] [PubMed] [Google Scholar]
- Ambrosone CB, Sweeney C, Coles BF, Thompson PA, Mcclure GY, Korourian S, Fares MY, Stone A, Kadlubar FF & Hutchins LF, 2001. Polymorphisms in glutathione S-transferases (GSTM1 and GSTT1) and survival after treatment for breast cancer. Cancer Res, 61, 7130–5. [PubMed] [Google Scholar]
- Amirimani B, Ning B, Deitz AC, Weber BL, Kadlubar FF & Rebbeck TR, 2003. Increased transcriptional activity of the CYP3A4*1B promoter variant. Environ Mol Mutagen, 42, 299–305. [DOI] [PubMed] [Google Scholar]
- Amirimani B, Walker AH, Weber BL & Rebbeck TR, 1999. RESPONSE: re: modification of clinical presentation of prostate tumors by a novel genetic variant in CYP3A4. J Natl Cancer Inst, 91, 1588–90. [DOI] [PubMed] [Google Scholar]
- Anderson RA & Cameron DA, 2011. Pretreatment serum anti-mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab, 96, 1336–43. [DOI] [PubMed] [Google Scholar]
- Anon, American Cancer Society: Cancer Treatment and Survivorship Facts & Figures 2014–2015. Atlanta: American Cancer Society; 2014. [online]. [Accessed Access Date 8/2019] [Google Scholar]
- Bines J, Oleske DM & Cobleigh MA, 1996. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol, 14, 1718–29. [DOI] [PubMed] [Google Scholar]
- Byrne J, Fears TR, Gail MH, Pee D, Connelly RR, Austin DF, Holmes GF, Holmes FF, Latourette HB, Meigs JW & et al. , 1992. Early menopause in long-term survivors of cancer during adolescence. Am J Obstet Gynecol, 166, 788–93. [DOI] [PubMed] [Google Scholar]
- Choi JY, Nowell SA, Blanco JG & Ambrosone CB, 2006. The role of genetic variability in drug metabolism pathways in breast cancer prognosis. Pharmacogenomics, 7, 613–24. [DOI] [PubMed] [Google Scholar]
- De Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA & Goldstein JA, 1994. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem, 269, 15419–22. [PubMed] [Google Scholar]
- Demichele A, Gimotty P, Botbyl J, Aplenc R, Colligon T, Foulkes AS & Rebbeck TR, 2007. In response to “Drug metabolizing enzyme polymorphisms predict clinical outcome in a node-positive breast cancer cohort”. J Clin Oncol, 25, 5675–7. [DOI] [PubMed] [Google Scholar]
- Dirven HA, Van Ommen B.& Van Bladeren PJ, 1994a. Involvement of human glutathione S-transferase isoenzymes in the conjugation of cyclophosphamide metabolites with glutathione. Cancer Res, 54, 6215–20. [PubMed] [Google Scholar]
- Dirven HA, Venekamp JC, Van Ommen B.& Van Bladeren PJ, 1994b. The interaction of glutathione with 4-hydroxycyclophosphamide and phosphoramide mustard, studied by 31P nuclear magnetic resonance spectroscopy. Chem Biol Interact, 93, 185–96. [DOI] [PubMed] [Google Scholar]
- Dolleman M, Depmann M, Eijkemans MJ, Heimensem J, Broer SL, Van Der Stroom EM, Laven JS, Van Rooij IA, Scheffer GJ, Peeters PH, Van Der Schouw YT, Lambalk CB & Broekmans FJ, 2014. Anti-Mullerian hormone is a more accurate predictor of individual time to menopause than mother’s age at menopause. Hum Reprod, 29, 584–91. [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group, 2005. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet, 365, 1687–717. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Greendale GA, Petersen L, Zibecchi L, Kahn B.& Belin TR, 2000. Managing menopausal symptoms in breast cancer survivors: results of a randomized controlled trial. J Natl Cancer Inst, 92, 1054–64. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Ennis M, Pritchard KI, Trudeau M.& Hood N, 1999. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol, 17, 2365–70. [DOI] [PubMed] [Google Scholar]
- Gor PP, Su HI, Gray RJ, Gimotty PA, Horn M, Aplenc R, Vaughan WP, Tallman MS, Rebbeck TR & Demichele A, 2010. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: a retrospective cohort study. Breast Cancer Res, 12, R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM & De Villiers TJ, 2012. Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab, 97, 1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD & Pulford DJ, 1995. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol, 30, 445–600. [DOI] [PubMed] [Google Scholar]
- Jordan VC, 2003. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov, 2, 205–13. [DOI] [PubMed] [Google Scholar]
- Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS & Schuetz E, 2001. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet, 27, 383–91. [DOI] [PubMed] [Google Scholar]
- Lang T, Klein K, Fischer J, Nussler AK, Neuhaus P, Hofmann U, Eichelbaum M, Schwab M.& Zanger UM, 2001. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics, 11, 399–415. [DOI] [PubMed] [Google Scholar]
- Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, Rosenberg R, Przypyszny M, Rein A.& Winer EP, 2004. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol, 22, 4174–83. [DOI] [PubMed] [Google Scholar]
- Petrek JA, Naughton MJ, Case LD, Paskett ED, Naftalis EZ, Singletary SE & Sukumvanich P, 2006. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol, 24, 1045–51. [DOI] [PubMed] [Google Scholar]
- Pinto N, Ludeman SM & Dolan ME, 2009. Drug focus: Pharmacogenetic studies related to cyclophosphamide-based therapy. Pharmacogenomics, 10, 1897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scripture CD, Sparreboom A.& Figg WD, 2005. Modulation of cytochrome P450 activity: implications for cancer therapy. Lancet Oncol, 6, 780–9. [DOI] [PubMed] [Google Scholar]
- Sharda SV, Gulati S, Tripathi G, Jafar T, Kumar A, Sharma RK & Agrawal S, 2008. Do glutathione-S-transferase polymorphisms influence response to intravenous cyclophosphamide therapy in idiopathic nephrotic syndrome? Pediatr Nephrol, 23, 2001–6. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD & Jemal A, 2019. Cancer statistics, 2019. CA Cancer J Clin, 69, 7–34. [DOI] [PubMed] [Google Scholar]
- Su HI, Sammel MD, Velders L, Horn M, Stankiewicz C, Matro J, Gracia CR, Green J.& Demichele A, 2010. Association of cyclophosphamide drug-metabolizing enzyme polymorphisms and chemotherapy-related ovarian failure in breast cancer survivors. Fertil Steril, 94, 645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland MC & Osborne CK, 1991. Tamoxifen in premenopausal patients with metastatic breast cancer: a review. J Clin Oncol, 9, 1283–97. [DOI] [PubMed] [Google Scholar]
- Sweeney C, Ambrosone CB, Joseph L, Stone A, Hutchins LF, Kadlubar FF & Coles BF, 2003. Association between a glutathione S-transferase A1 promoter polymorphism and survival after breast cancer treatment. Int J Cancer, 103, 810–4. [DOI] [PubMed] [Google Scholar]
- Sweeney C, Farrow DC, Schwartz SM, Eaton DL, Checkoway H.& Vaughan TL, 2000a. Glutathione S-transferase M1, T1, and P1 polymorphisms as risk factors for renal cell carcinoma: a case-control study. Cancer Epidemiol Biomarkers Prev, 9, 449–54. [PubMed] [Google Scholar]
- Sweeney C, Mcclure GY, Fares MY, Stone A, Coles BF, Thompson PA, Korourian S, Hutchins LF, Kadlubar FF & Ambrosone CB, 2000b. Association between survival after treatment for breast cancer and glutathione S-transferase P1 Ile105Val polymorphism. Cancer Res, 60, 5621–4. [PubMed] [Google Scholar]
- Takada K, Arefayene M, Desta Z, Yarboro CH, Boumpas DT, Balow JE, Flockhart DA & Illei GG, 2004. Cytochrome P450 pharmacogenetics as a predictor of toxicity and clinical response to pulse cyclophosphamide in lupus nephritis. Arthritis Rheum, 50, 2202–10. [DOI] [PubMed] [Google Scholar]
- Timm R, Kaiser R, Lotsch J, Heider U, Sezer O, Weisz K, Montemurro M, Roots I.& Cascorbi I, 2005. Association of cyclophosphamide pharmacokinetics to polymorphic cytochrome P450 2C19. Pharmacogenomics J, 5, 365–73. [DOI] [PubMed] [Google Scholar]
- Van Schaik RH, 2008. CYP450 pharmacogenetics for personalizing cancer therapy. Drug Resist Updat, 11, 77–98. [DOI] [PubMed] [Google Scholar]
- Walshe JM, Denduluri N.& Swain SM, 2006. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol, 24, 5769–79. [DOI] [PubMed] [Google Scholar]
- Warne GL, Fairley KF, Hobbs JB & Martin FI, 1973. Cyclophosphamide-induced ovarian failure. N Engl J Med, 289, 1159–62. [DOI] [PubMed] [Google Scholar]
- Zhong S, Huang M, Yang X, Liang L, Wang Y, Romkes M, Duan W, Chan E.& Zhou SF, 2006. Relationship of glutathione S-transferase genotypes with side-effects of pulsed cyclophosphamide therapy in patients with systemic lupus erythematosus. Br J Clin Pharmacol, 62, 457–72. [DOI] [PMC free article] [PubMed] [Google Scholar]