Abstract
Chlamydia pneumoniae infection has been associated with cardiovascular diseases in seroepidemiological studies and by demonstration of the pathogen in atherosclerotic lesions. It has the capacity to infect several cell types, including monocyte-derived macrophages, which play an essential role in the development of atherosclerosis. However, the persistence of C. pneumoniae in mononuclear cells is poorly understood. To study the morphology and biological characteristics of the infection, human peripheral blood monocytes were infected with C. pneumoniae. Freshly isolated monocytes resisted the development of infectious progeny, and confocal and transmission electron microscopy showed that the morphology of the inclusions and chlamydial particles was abnormal. Addition of tryptophan or antibodies against gamma interferon did not diminish the inhibition of C. pneumoniae, suggesting that other factors are involved in the chlamydiostatic activity of the monocytes. Chlamydial mRNA was expressed at least 3 days after infection, however, and a capability for infected monocytes to induce a positive lymphocyte proliferative response was detected for up to 7 days, indicating that C. pneumoniae remains metabolically active in the monocytes in vitro. These results are in accordance with the hypothesis that C. pneumoniae may participate in the maintenance of local immunological response and inflammation via infected monocytes and thus enhance atherosclerosis.
Chlamydia pneumoniae is an obligate intracellular bacterium involved in a wide spectrum of respiratory tract infections (9, 13). A common feature of chlamydial infections is their tendency to persist, and they have been associated with many chronic conditions, including chronic bronchitis, adult-onset asthma, and coronary heart disease (10, 19, 22). In vitro, C. pneumoniae is able to infect a number of cell types, including monocyte-derived macrophages (6, 8, 11), which are pivotal to the development of atherosclerosis (17).
As professional phagocytic cells, monocytes/macrophages are responsible for the first host defense mechanisms during microbial infections and for regulation of other cells participating in immunological defense processes. Although the chronic characteristics of Chlamydia infections may be related to the capacity of the bacterium to be internalized by nonprofessional as well as professional phagocytes, the ability of the organism to escape the bactericidal systems of phagocytic cells (16) enables them to remain in these cells for long periods. It has been suggested that the residence of C. pneumoniae in circulating monocytes offers a means of distribution from the primary colonization region into other organs in order to initiate and participate in the maintenance of local immunological response and inflammation. However, the persistence of C. pneumoniae in mononuclear cells is poorly understood. In present study, we investigated the biological characteristics of C. pneumoniae infection in human monocytes in vitro.
MATERIALS AND METHODS
Organism.
C. pneumoniae Kajaani 7 (5) was propagated in HL cells, a cell line conventionally used to grow the bacteria, and chlamydial elementary bodies (EBs) were purified from the cells by Urografin (Schering AG, Berlin, Germany) density gradient centrifugation. Purified EBs were suspended in sucrose-phosphate-glutamic acid (0.2 M sucrose, 3.8 mM KH2PO4, 6.7 mM Na2HPO4, 5 mM l-glutamic acid [pH 7.4]) buffer and stored in small aliquots at −70°C until used.
Purification of PBMCs.
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized buffy coats provided by the Finnish Red Cross Blood Transfusion Service (Oulu, Finland) by using Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation. The PBMCs were washed three times with Hanks balanced salt solution (Sigma, St. Louis, Mo.), suspended in RPMI 1640 medium containing 10% human AB blood type serum (Finnish Red Cross Blood Transfusion Service, Helsinki, Finland), and distributed on plastic petri tissue culture dishes (107 cells/dish) for monocytes to adhere. After overnight incubation at 37°C in 5% CO2, nonadherent cells were removed by washing the plates twice with phosphate-buffered saline (PBS) and adherent cells were detached from the plastic surface by incubation the plates for 15 min at 4°C and using plastic cell rakes. Thereafter, the cells were allowed to adhere to the plastic surface again (2-h incubation), resulting in a harvested cell preparation containing more than 90% monocytes as determined by CD14 expression (with fluorescein isothiocyanate (FITC)-conjugated anti-CD14 monoclonal antibody; Becton Dickinson, Mountain View, Calif.).
T cells were purified from the isolated PBMCs by rosetting with 2-aminoethylisothiouronium hydrobromide (AET)-treated sheep erythrocytes. Briefly, 2 × 106 cells/ml were suspended with an equal volume of 3% AET-treated erythrocytes and incubated for 30 min on ice. The rosetted T cells were separated from nonrosetted cells (B cells and monocytes) by Ficoll-Paque density gradient centrifugation (1,600 rpm for 4 min). Following lysis of the sheep erythrocytes (with 0.9% NH3Cl for 15 min at 4°C), the obtained T cells were washed and suspended in RPMI 1640 medium containing 60% human AB serum and 7.5% dimethyl sulfoxide and stored in aliquots of 3 × 106 cells at −150°C until used for the lymphocyte proliferation experiments. The T-cell preparations were made up of >95% of CD3-positive cells (anti-CD3 FITC-conjugated monoclonal antibody; Becton Dickinson) and contained both CD4 and CD8 lymphocytes.
Infection of monocytes.
Monocytes were seeded on 24-well plates at a density of 4 × 105 to 5 × 105 cells per well, allowed to adhere, and infected with purified EBs of C. pneumoniae (6 × 106 inclusion-forming units [IFU]/well) by centrifugation (550 × g for 60 min). The infected cells were grown at 37°C under 5% CO2 in RPMI 1640 medium supplemented with 10% AB serum, 2 mM glutamine, and 20 μg of streptomycin per ml. For fluorescence microscopy studies, the cells were grown on coverslips. All experiments were performed in triplicate, and the cells were harvested 3 days after infection unless otherwise stated.
To study if gamma interferon (IFN-γ) is essential in restricting the growth of C. pneumoniae in monocytes, as previously found for other cell types (21), monocytes were treated with 200 μg of tryptophan (Sigma) per ml or with 0.5 μg of anti-IFN-γ antibody (Genzyme, Cambridge, Mass.) per ml, which both antagonize the influence of IFN-γ. The agents were added to the cultures simultaneously with the infection. The number of inclusions and the infectivity of the progeny were studied after 3 days of incubation as described below. For analysis of chlamydial viability in monocytes by PCR, 50 ng of IFN-γ (Genzyme) per ml was added to the cultures simultaneously with the infection to investigate if IFN-γ enhances the inhibition of chlamydial mRNA production. The concentrations of the added supplements were based on our preliminary studies with macrophages (data not shown).
Analysis of chlamydial infectivity.
After 3 days of incubation, the infected monocytes growing on coverslips were washed with phosphate-buffered saline, fixed with methanol, and stained directly with Chlamydia genus-specific FITC-conjugated monoclonal antibody (Pathfinder Chlamydia confirmation system; Kallestad Diagnostics). The number of chlamydial inclusions in the whole sample was counted with a Leitz Aristoplan fluorescence microscope.
The infectivity of the C. pneumoniae progeny was analyzed by performing repassages on freshly prepared confluent HL-cell layers growing on coverslips. Monocytes were harvested 3 days postinfection, and the cell suspension was mechanically disrupted and centrifuged on HL cells (550 × g for 60 min). The infected HL-cell monolayers were grown at 37°C under 5% CO2 in RPMI 1640 medium supplemented with 7.5% fetal calf serum, 2 mM glutamine, 20 μg of streptomycin per ml, and 0.5 μg of cycloheximide per ml. After 3 days of incubation, the cells were fixed and stained as above and the presence of chlamydial inclusions was studied or the cells were repassaged again on fresh HL cells.
Confocal microscopy of the infected monocytes.
Monocytes were infected, fixed, and stained with FITC-conjugated anti-Chlamydia antibody as described above and studied by confocal microscopy (Aristoplan CLSM fluorescence microscope; Leica Lasertechnic) equipped with a 75 mM air-cooled argon-krypton laser (Omnichrome; Chino). FITC was excited at a wavelength of 488 nm, and rhodamine was excited at 556 nm. The samples were scanned in 512- by 512-pixel formats in the x-y and x-z directions.
Transmission electron microscopy.
For transmission electron microscopy, fresh confluent monolayers of HL cells were infected with 105 to 106 IFU of C. pneumoniae by centrifugation, and the monocytes were infected as above. Cells were detached from the culture dish with plastic rakes 72 h postinfection, pelleted, fixed in 2.5% glutaraldehyde solution, postfixed with 1% OsO4 in 0.1 M phosphate buffer, dehydrated in acetone, and embedded in Epon LX 112. Thin sections were cut with a Reichert Ultracut E-ultramicrotome (Reichert-Jung, Vienna, Austria), stained with uranyl acetate and lead citrate, and examined under a Philips 410 transmission electron microscope.
Analysis of C. pneumoniae-specific mRNA.
Chlamydial mRNA transcripts of 16S rRNA, 60-kDa heat shock protein (HSP60), and 60-kDa cysteine-rich outer membrane protein (Crp60) were measured by reverse transcription-PCR (RT-PCR) in infected monocytes (4 × 105 to 5 × 105 cells; 6 × 106 IFU) 3, 7, and 10 days after infection. RNA was extracted from the cells with the RNeasy Midi kit (Qiagen, Hilden, Germany) as specified by the manufacturer, divided into two 100-μl aliquots, and stored at −70°C.
RNA was transformed to cDNA by RT with a commercial kit (Perkin-Elmer Cetus, Norwalk, Conn.) as specified by the manufacturer. Chlamydial DNA was amplified by PCR. The reaction mixture contained 1 mM deoxynucleoside triphosphates, 2 mM MgCl2, 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.0 U of Taq polymerase (Perkin-Elmer), 50 pmol of each primer, and 5 μl of cDNA in a total volume of 25 μl. After 5 min of initial denaturation at 94°C, the samples were subjected to 50 cycles of denaturation (94°C for 30 s), annealing (55°C for 30 s), and extension (72°C for 30 s) with a Perkin-Elmer Cetus GeneAmp 9600 thermocycler, followed by a final extension for 6 min at 72°C after the cycling had been completed.
The primers for the C. pneumoniae genes were synthesized at the University of Helsinki Institute for Biotechnology (Helsinki, Finland). The primers for 16S rRNA were CpnA (5′TGA CAA CTG TAG AAA TAC AGC3′) and CpnB (5′CGC CTC TCT CCT ATA AAT3′), as originally published by Gaydos et al. (7). The GE-1 (5′ACG TCA CGT AGT TAT AGA TAA GAG3′) and GE-2 (5′AAG TAG CTG GAG AGG TAT CCA CGG3′) primers were designed to recognize a sequence from the gene coding for HSP60; and the Crp60A (5′GAA GAG GGA CGA TGT CAA CCT3′) and Crp60B (5′ACT TGT AGG AGT TGT TTC TGG AT3′) primers were designed to recognize a sequence from the Crp60. RT-PCR amplification of the chromosomal β-actin gene was used to confirm the presence of undegraded and amplifiable RNA. The amplification products were separated and visualized by electrophoresis on 2% agarose gels containing ethidium bromide.
Analysis of the effect of infected monocytes on lymphocyte activation.
Presentation of C. pneumoniae antigen by the infected monocytes was studied by the lymphocyte blast transformation test. Freshly isolated monocytes (105 cells/well) were seeded on flat-bottom 96-well tissue culture plates (Nunc, Roskilde, Denmark) and infected immediately by adding 8 × 102 or 8 × 103 IFU of C. pneumoniae to each well. Tuberculin-purified protein derivative (Statens Serum Institute, Copenhagen, Denmark; final concentration, 10 μg/ml) was used as a control antigen. Purified T cells (105 cells/well) were added to the monocytes 0, 5, 7, or 10 days after infection, and the cultures were incubated in a humidified atmosphere of 5% CO2 at 37°C for a further 6 days. [methyl-3H]thymidine (0.2 μCi/well; Amersham Life Science, Little Chalfont, United Kingdom) was then added to the wells for 18 h. The cells from each well were harvested on nitrocellulose filters (Wallac, Turku, Finland) with an automatic cell harvester (Skatron AS, Lier, Norway), and the lymphocyte proliferation responses were measured in counts per minute (cpm) of radioactivity incorporated into the proliferating cells by using a liquid scintillation counter (Wallac).
RESULTS
Infectivity of C. pneumoniae in the monocyte cultures.
C. pneumoniae Kajaani 7 seemed capable of infecting monocytes, as shown by confocal microscopy 3 days after infection (Fig. 1). The exact number of inclusions was difficult to count, because over 90% of the monocytes contained multiple inclusions and chlamydial particles. Furthermore, the sizes and the staining intensities of the inclusions were remarkably diverse. However, when the infectivity of the progeny of the bacteria was studied by repassaging cell suspensions on fresh HL cells, no inclusions at all were found even after two repassages in spite of the large numbers of inclusions in primarily infected monocytes. To test whether the abortive effect of the monocytes on the growth of C. pneumoniae is influenced by IFN-γ, the IFN-γ antagonists anti-IFN-γ antibody or tryptophan were added to the infected monocyte cultures. However, neither the growth nor the infectivity of C. pneumoniae was influenced. Despite the treatments, the inclusions were still clearly abnormal in morphology, the progeny was still noninfectious, and after repassages on fresh HL cells, no inclusions were found.
FIG. 1.
A confocal micrograph of C. pneumoniae-infected human monocytes stained with FITC-conjugated anti-Chlamydia antibody. The monocyte contains several inclusions that are variable in size and staining intensity.
Electron microscopy of the infected monocytes.
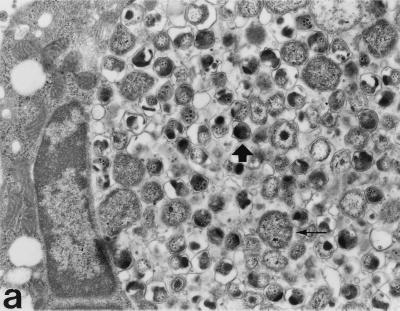
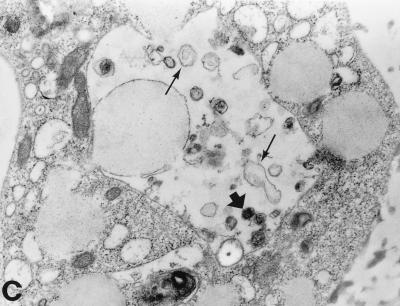
The morphology of the inclusions and the chlamydial particles was also studied by transmission electron microscopy. In monocytes, the inclusions were smaller and contained fewer chlamydial particles than in HL cells. As shown in Fig. 2B and C, the monocyte inclusions contained a few mature-looking EBs, abnormal reticulate bodies (RBs), and “envelope ghosts” similar to those detected in C. trachomatis-infected, antibiotic-treated epithelial cells (23). Normal RBs, such as those seen in HL cells (Fig. 2A), were not found.
FIG. 2.
Transmission electron micrographs of C. pneumoniae-infected HL cells and monocytes 72 h postinfection. (a) Large inclusions filled with C. pneumoniae particles are found in the HL cells. Mature EBs are small, with an electrodense core (thick arrow), while RBs are large, with a coarse inner structure (thin arrow). (b) Monocytes can carry very small multiple inclusions (arrows), some containing only one C. pneumoniae particle. A thin membrane always covers these inclusions. (c) The inclusions in monocytes contain far fewer C. pneumoniae particles than do those in HL cells. Only a few mature-looking EBs can be seen (thick arrow), and the RBs are clearly transformed (thin arrows). Magnification, ×10,400.
Metabolic activity of C. pneumoniae in the monocytes.
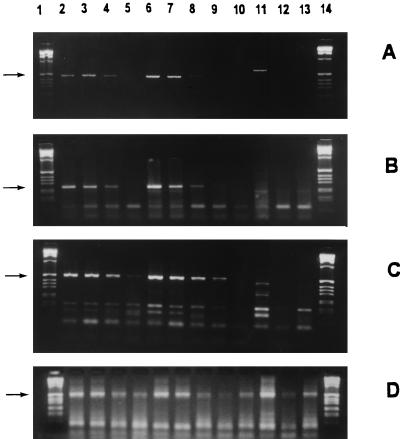
To study the metabolic activity of C. pneumoniae in monocytes, mRNA transcripts of 16S rRNA, Crp60, and HSP60 were analyzed (Fig. 3A to C). C. pneumoniae-specific 16S rRNA and Crp60 were expressed for at least up to 3 days after the infection. With the HSP60 primers, the RT-PCR was positive for 7 days postinfection. Administration of IFN-γ to the cell cultures had no significant effect on RNA expression of the bacteria. The PCR amplification of C. pneumoniae mRNA transcripts was negative in noninfected control cells. RT-PCR for β-actin mRNA was positive at all time points, indicating that the monocytes in the cell culture medium were viable (Fig. 3D).
FIG. 3.
Viability of C. pneumoniae in monocytes, studied by RT-PCR. (A to C) The mRNA expression of gene transcripts for 16S rRNA (463 bp); (A) and for Crp60 (252 bp); (B) was positive up to 3 days after infection, and that for HSP60 (507 bp); (C) was positive up to 7 days after infection. (D) The chromosomal β-actin gene of monocytes was expressed at all the time points. IFN-γ treatment of the infected monocytes seemed not to be able to inhibit gene expression. The arrows indicate the amplification products. Lanes: 1 and 14, molecular weight markers; 2 to 5, infected monocytes; 6 to 9, infected, IFN-γ treated cells; 10 to 13, uninfected cells. For each set of lanes, the samples were obtained right after infection and on days 1, 3, and 7 after infection, respectively.
Capacity of C. pneumoniae-infected monocytes to induce a lymphocyte proliferative response.
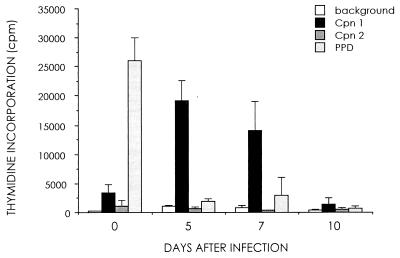
The characteristics of C. pneumoniae infection in monocytes were further analyzed by a lymphocyte proliferation assay. As shown in Fig. 4, the lymphocyte proliferative responses were positive at both antigen concentrations (8 × 102 and 8 × 103 IFU/well) when the T cells were added to the monocyte cultures on the day of infection (day 0). The monocytes infected with a higher challenge dose of C. pneumoniae induced a positive lymphocyte proliferation response even when the T cells were added to the cultures 5 or 7 days after infection. The purified protein derivative response, used as a positive control, declined soon after the antigenic challenge.
FIG. 4.
Ability of C. pneumoniae-infected monocytes to induce lymphocyte activation. Purified T cells were added to the infected monocyte cultures at different time points and incubated for 6 days, and lymphocyte proliferation responses were measured. Clear specific T-cell activation is seen up to 7 days. Values represent means and standard deviations of triplicate measurements. Cpn 1, C. pneumoniae (8 × 103 IFU/well); Cpn 2, C. pneumoniae (8 × 102 IFU/well). PPD, purified protein derivative.
DISCUSSION
We demonstrated in this study that freshly isolated human monocytes, although clearly invaded by C. pneumoniae, seem to resist the development of infectious progeny of the bacteria. Transmission electron microscopy showed that the inclusions contained chlamydial particles at different developmental stages, and chlamydial mRNA synthesis was detected up to 7 days post infection by RT-PCR. The data suggests that C. pneumoniae remains metabolically active in human monocytes in vitro, which is comparable to the growth characteristics of C. trachomatis serovar K (12, 20). We also showed that the infected monocytes could induce a positive lymphocyte proliferation response for at least 7 days, which further supports the viability of the bacteria in monocytes.
The chlamydicidal activity of human monocytes has previously been shown to involve most C. trachomatis serovars (23) and C. pneumoniae (11), but not C. psittaci, which is capable of limited growth in monocytes (14, 18). The mechanisms of the growth limitation are mostly obscure, however. IFN-γ is a central cytokine regulating the bactericidal activity of phagocytizing cells, and it is important in host defense against chlamydiae (2, 3, 4, 18, 23). However, excess amounts of anti-IFN-γ antibody or tryptophan could not reverse the inhibition of the production of infectious C. pneumoniae progeny in monocytes, as previously also found with C. trachomatis serovar K (12). This indicates that in addition to IFN-γ, other factors are involved in the inhibition mechanisms in freshly isolated monocytes.
In contrast to the monocytes, human macrophages support the growth of infective C. pneumoniae (6, 8, 11). The fate of chlamydiae in monocytes and in monocyte-derived macrophages seems to be dependent on the bacterial strain and is different between the oculogenital and LGV biovars of C. trachomatis. The LGV biovar is more resistant to the bactericidal effect of macrophages than is the trachoma biovar, suggesting that the well-adapted growth in macrophages may reflect the disease syndromes associated with different biovars (23). The differences in growth patterns may be linked to the degree of the respiratory burst of the cells, which decreases markedly when monocytes mature into macrophages (1). This in turn suggests that the lysosomal degradation of phagocytized microbial organisms is weakened in macrophages relative to monocytes.
Most chlamydial strains that successfully replicate within phagocytes have means of impairing host defenses, and this often involves inhibition of phagolysosomal fusion (16). No data have been published on how C. pneumoniae survives in the host cells, but our results suggest that its fate depends on the host cells themselves. Since C. pneumoniae-infected monocytes are able to induce the proliferation of CD4-positive lymphocytes, binding of degraded chlamydial antigens by HLA class II molecules must have occurred in the lysosomal vesicles, indicating that phagolysosomal fusion had not been totally avoided. On the other hand, in spite of the relatively low inoculum used in this study, the infected monocytes did carry inclusions containing chlamydial particles at various developmental stages. The simultaneously detected metabolic activity of C. pneumoniae in the monocytes suggests that replication occurs to some degree even though the development of infective EBs is hindered.
C. pneumoniae has recently been shown to spread from the respiratory tract to other organs via infected monocytes/macrophages (15) that are known to be central in the development of atherosclerosis (17). Together with these results, the well-adapted capability of C. pneumoniae to remain metabolically active in mononuclear phagocytes can be considered to support the hypothesis that C. pneumoniae is involved in maintaining long-standing local inflammation in vessel walls and thus is enhancing the development of atherosclerotic lesions.
ACKNOWLEDGMENTS
This work was supported by grants from Helsingin Sanomat Centennial Foundation, Finland, and the Sigrid Juselius Foundation.
We thank Juha Tuukkanen and Jouni Lakkakorpi (Department of Anatomy, University of Oulu) for guidance in confocal microscopy and Marja Syrjäkallio-Ylitalo (Department of Pathology, University of Oulu) for preparation of the electron micrographs.
REFERENCES
- 1.Auger M J, Ross J A. The biology of macrophage. In: Lewis C E, McGee J O, editors. The macrophage. New York, N.Y: Oxford University Press; 1992. p. 15. [Google Scholar]
- 2.Byrne G I, Carlin J M, Merkert T P, Arter D L. Long-term effects of gamma interferon on Chlamydia-infected host cells: microbicidal activity follows microbistasis. Infect Immun. 1989;57:1318–1320. doi: 10.1128/iai.57.4.1318-1320.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlin J M, Weller J B. Potentiation of interferon-mediated inhibition of Chlamydia infection by interleukin-1 in human macrophage cultures. Infect Immun. 1995;63:1870–1875. doi: 10.1128/iai.63.5.1870-1875.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlin J M, Borden E C, Byrne G I. Interferon-induced indoleamine 2,3-dioxygenase activity inhibits Chlamydia psittaci replication in human macrophages. J Interferon Res. 1989;9:329–337. doi: 10.1089/jir.1989.9.329. [DOI] [PubMed] [Google Scholar]
- 5.Ekman M R, Grayston J T, Visakorpi R, Kleemola M, Kuo C C, Saikku P. An epidemic of infections due to Chlamydia pneumoniae in military conscripts. Clin Infect Dis. 1993;17:420–425. doi: 10.1093/clinids/17.3.420. [DOI] [PubMed] [Google Scholar]
- 6.Gaydos C A, Summersgill J T, Sahney N N, Ramirez J A, Quinn T C. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect Immun. 1996;64:1614–1620. doi: 10.1128/iai.64.5.1614-1620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaydos C A, Quinn T C, Eiden J J. Identification of Chlamydia pneumoniae by DNA amplification of the 16S rRNA gene. J Clin Microbiol. 1992;30:796–800. doi: 10.1128/jcm.30.4.796-800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godzik K L, O’Brien E R, Wang S-K, Kuo C-C. In vitro susceptibility of human vascular wall cells to infection with Chlamydia pneumoniae. J Clin Microbiol. 1995;33:2411–2414. doi: 10.1128/jcm.33.9.2411-2414.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grayston J T, Campbell L A, Kuo C C, Mordhorst C H, Saikku P, Thom D H, Wang S-P. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J Infect Dis. 1990;161:618–625. doi: 10.1093/infdis/161.4.618. [DOI] [PubMed] [Google Scholar]
- 10.Hahn D L, Dodge R W, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-asthma. JAMA. 1991;266:225–230. [PubMed] [Google Scholar]
- 11.Kaukoranta-Tolvanen S-S, Teppo A-M, Laitinen K, Saikku P, Linnavuori K, Leinonen M. Growth of Chlamydia pneumoniae in cultured human peripheral blood mononuclear cells and induction of a cytokine response. Microb Pathog. 1996;21:215–221. doi: 10.1006/mpat.1996.0056. [DOI] [PubMed] [Google Scholar]
- 12.Koehler L, Nettelnbreker E, Hudson A P, Ott N, Gerard H C, Branigan P J, Schumacher H R, Drommer W, Zeidler H. Ultrastructural and molecular analyses of the persistence of Chlamydia trachomatis (serovar K) in human monocytes. Microb Pathog. 1997;22:133–142. doi: 10.1006/mpat.1996.0103. [DOI] [PubMed] [Google Scholar]
- 13.Kuo C C, Jackson L A, Campbell L A, Grayston J T. Chlamydia pneumoniae (TWAR) Clin Microbiol Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manor E, Sarov I. Fate of Chlamydia trachomatis in human monocytes and monocyte-derived macrophages. Infect Immun. 1986;54:90–95. doi: 10.1128/iai.54.1.90-95.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moazed T C, Kuo C C, Grayston J T, Campbell L A. Evidence of systemic dissemination of Chlamydia pneumoniae via macrophages in the mouse. J Infect Dis. 1998;177:1322–1325. doi: 10.1086/515280. [DOI] [PubMed] [Google Scholar]
- 16.Rank R G, Bavoil P M. Prospects for a vaccine against Chlamydia genital disease. II. Immunity and vaccine development. Bull Inst Pasteur. 1996;94:55–82. [Google Scholar]
- 17.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 18.Rothermel C D, Rubin B Y, Jaffe E A, Murray H W. Oxygen-independent inhibition of intracellular Chlamydia psittaci growth by human monocytes and interferon-γ-activated macrophages. J Immunol. 1986;137:689–692. [PubMed] [Google Scholar]
- 19.Saikku P, Leinonen M, Mattila K, Ekman M-R, Nieminen M S, Mäkelä P H, Huttunen J K, Valtonen V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;ii:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 20.Schmitz E, Nettelnbreker E, Zeidler H, Hammer M, Manor E, Wollenhaupt J. Intracellular persistence of chlamydial major outer-membrane protein, lipopolysaccharide and ribosomal RNA after non-productive infection of human monocytes with Chlamydia trachomatis serovar K. J Med Microbiol. 1993;38:278–285. doi: 10.1099/00222615-38-4-278. [DOI] [PubMed] [Google Scholar]
- 21.von Hertzen L, Leinonen M, Surcel H-M, Karjalainen J, Saikku P. Measurement of sputum antibodies in the diagnosis of acute and chronic respiratory infections associated with Chlamydia pneumoniae. Clin Diagn Lab Immunol. 1995;2:454–457. doi: 10.1128/cdli.2.4.454-457.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wyrick P B, Davis C H, Raulston J E, Knight S T, Choong J. Effect of clinically relevant culture conditions on antimicrobial susceptibility of Chlamydia trachomatis. Clin Infect Dis. 1994;19:931–936. doi: 10.1093/clinids/19.5.931. [DOI] [PubMed] [Google Scholar]
- 23.Yong E C, Chi E Y, Kuo C C. Differential antimicrobial activity of human mononuclear phagocytes against the human biovars of Chlamydia trachomatis. J Immunol. 1987;139:1297–1302. [PubMed] [Google Scholar]