Abstract
Background
Globally, the human immunodeficiency virus has been recognized as a major public health concern. The direct toxicity of antiretroviral medicines or their active metabolites causes liver cell destruction by different mechanisms, inducing immune-mediated inflammation, oxidative stress, and other mechanisms. On the other hand, the virus itself also produces hepatotoxicity. Therefore, this systematic review and meta-analysis aimed to assess the pooled prevalence of hepatotoxicity among HIV-infected patients in Ethiopia.
Methods
PubMed, Science Direct, Cochrane Library, Web of Science, and ResearchGate databases were used to find relevant articles. As well, various professional associations were searched to retrieve grey literature. The Newcastle–Ottawa Quality Assessment Scale was used to assess the quality of recruited studies. The data were extracted using Microsoft Excel, and the meta-analysis was carried out using STATA 14 software. I2 and Cochran’s Q test were employed to assess the presence of heterogeneity between studies. A random effect model was used. The funnel plot and Egger’s statistics were used to assess publication bias. Moreover, subgroup analysis and sensitivity analysis were also done.
Results
The pooled prevalence of hepatotoxicity among HIV patients in Ethiopia was 25.45% (95% CI = 20.06–30.84%). There was high heterogeneity, with an I2 value of 93.7%. Subgroup analysis by HAART status showed a higher pooled prevalence of hepatotoxicity among HIV patients taking HAART (23.63%) than among HAART naive patients (7.29%). In subgroup analysis, the pooled prevalence of hepatotoxicity among HIV/Tb co-infected and HIV mono-infected patients was 26.3% and 17.94%, respectively.
Conclusion
The current systematic review and meta-analysis showed a high prevalence of hepatotoxicity among HIV-infected patients. Therefore, regular monitoring of hepatotoxicity among HIV-infected patients is required in order to avoid liver damage and other complications.
Systematic review registration PROSPERO (2022:CRD42022334704)
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-022-07838-w.
Keywords: Hepatotoxicity, Liver enzyme elevation, Antiretroviral therapy, HIV/AIDS, Ethiopia
Introduction
Since the beginning of the twenty-first century, the human immunodeficiency virus (HIV) has been recognized as a major public health concern around the world [1, 2]. According to figures from 2019, HIV/AIDS is a pandemic disease with 38.0 million people living with the virus worldwide. Sub-Saharan Africa accounts for around 25.7 million of these people. In addition, 730,000 new HIV infections were reported in Eastern and Southern Africa in 2019 [3]. To meet the UNAIDS 90-90-90 targets, most African countries will have integrated community-based interventions into HIV healthcare service delivery by the beginning of 2020. Significant progress has been made in Sub-Saharan Africa in meeting the UNAIDS 90-90-90 objectives by the end of 2019. However, several African countries, like Ethiopia, have not yet met these targets [4]. By 2019, 87% of people living with HIV (PLHIV) in Eastern and Southern Africa recognized their status, 72% of PLWH were on treatment, and 65% of PLHIV in Eastern and Southern Africa were virally suppressed [5].
In HIV-infected patients, non-AIDS causes of morbidity and mortality are becoming more common. Liver illnesses have risen to become one of the top causes of death not related to AIDS. When evaluating and caring for these patients, a full understanding of the mechanisms underlying the development of liver disease is critical. HIV-related morbidity and mortality have decreased, and the burden of opportunistic infections (OIs) has declined significantly since the advent of highly active antiretroviral treatment (HAART) [6]. Because of the rapid scale-up of antiretroviral therapy, HIV-related morbidity and mortality have decreased considerably, even in underdeveloped countries [7].
Despite the fact that Ethiopia has had a free ART service since 2005, the prevalence of HIV and other HIV-related diseases such as hepatotoxicity is still too high [8]. HAART enhanced the quality of life of HIV patients by considerably reducing the frequency of opportunistic infections, morbidity, and mortality, despite a considerable change in the quality of life [9, 10]. However, drug-induced liver disease, hyperlipidemia, hyperglycemia, and lactic acidosis are still typical HAART adverse effects [11]. Because of asymptomatic or symptomatic side effects, patients commonly discontinue medication [12–14]. Poor adherence to HAART increases the chance of drug-resistant virus strains developing and can result in hepatotoxicity [15, 16].
Measuring liver enzymes is the most common way of determining hepatotoxicity. Hepatotoxicity grades are broadly classified as mild when an ALT value is between 1.25 and 2.5 upper reference limit, moderate when it is between 2.5 and 5.0 upper reference limit, and severe when it is between 5.0 and 10 upper reference limit [17, 18]. HIV and antiretroviral medications both cause abnormalities in liver enzymes. Biochemical monitoring revealed aberrant liver functions and symptoms in patients, indicating that anti-HIV medications should be stopped, and anti-HIV treatments were temporarily stopped due to hepatotoxicity [19].
Antiretroviral (ARV) medications harm liver cells either directly or through their active metabolites. On the other hand, immune-mediated injury, oxidative stress, mitochondrial injury, lipotoxicity, cytotoxicity, toxic metabolite build-up, gut microbial translocation, and systemic inflammation, on the other hand, are among HIV viral pathogenesis and liver injury mechanisms [12]. Because of the strong link between HIV and tuberculosis, all antiretroviral medicines, as well as several anti-TB drugs, have prevalent and problematic side events that cause adherence issues, hospitalization, and the risk of hepatotoxicity [20–22].
Toxicity may be linked to the drug's dose in cases of predicted liver injury. Patients on nevirapine-containing regimens, for example, experienced liver damage and skin rashes [23]. Studies indicate that several ARV medications have been linked to hepatotoxicity and nephrotoxicity [24, 25]. Despite the fact that numerous studies have been undertaken in various parts of Ethiopia, there is a lack of consistency in the extent and overall incidence of HAART and HIV virus-related hepatotoxicity. There has never been a study that establishes the overall prevalence of hepatotoxicity in HIV-positive patients. As a result, this was the first of its kind to be a systematic review and meta-analysis with the purpose of presenting comprehensive data on the prevalence of hepatotoxicity.
Methods
Protocol registration
The current systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement (Additional file 1: Table S1) [26]. The study protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number (2022:CRD42022334704).
Search strategy
A standard search strategy is used in PubMed, and later it is modified according to each specific database to get the best relevant results. Search strategies are constructed to include free-text terms (e.g., in the title and abstract) and any appropriate subject indexing (e.g., MeSH) expected to retrieve eligible studies, with the help of an expert in the review topic field. The three reviewers systematically searched different databases like Google Scholar, PubMed, Science Direct, Cochrane Library, EMBASE, Web of Science, and ResearchGate from March to May 22, 2022, using the combination of following keywords: “hepatotoxicity’’ OR “liver enzyme elevation” OR “biochemical alteration” OR “antiretroviral therapy” AND “HIV/AIDS” AND Ethiopia. Furthermore, grey literature was systematically searched in professional associations such as the Ethiopian Public Health Association (EPHA) and the Ethiopian Medical Laboratory Association (EMLA), university databases, and national annual conferences. The references from the retrieved articles, relevant reviews, and previous meta-analyses were searched for additional studies not identified by the database search. Searched articles were entered into Endnote Software to avoid duplicates and the list was consolidated into one list. The remaining articles were screened for their titles and abstracts independently by reviewers. Any disagreement during screening was resolved by consensus (Additional file 3).
Inclusion and exclusion criteria
The inclusion and exclusion criteria were determined after formulating the research question by using PI/ECO (Population, Intervention, Exposure, Comparison, and Outcome). Only original studies that reported the prevalence of hepatotoxicity among HIV/AIDS patients in Ethiopia were included in this systematic review and meta-analysis. In addition, studies reporting the prevalence of hepatotoxicity among HIV/AIDS patients co-infected with Mycobacterium tuberculosis (TB) were also included. These are articles with information that answers our research question. Likewise, no restrictions were applied regarding region, patient age, gender, and date of publication. We have narrowed our search to literature written in English. For duplicate studies, the first version or the one with all the necessary data was used. Exclusion criteria are mostly unrelated, duplicated, unavailable full texts, abstract-only papers, case reports, case series, letters to the editor, conference proceedings, or review pieces.
Data extraction and quality assessment
Three reviewers (Ousman M., Ermiyas A., and Habtye B.) independently selected the studies using the eligibility criteria based on the title, abstract, and full text. These reviewers then retrieved data from the entire text of potentially eligible studies using standardized data extraction forms compared the results, and resolved inconsistencies through consensus-based discussions. For identification purposes, each study was assigned a unique number, and the following descriptive data was extracted: name of the principal author; year of publication; regions where the study was conducted; methods (design and settings), total sample size; overall prevalence of hepatotoxicity; prevalence of hepatotoxicity before HAART; prevalence of hepatotoxicity after HAART; HIV/Tb co-infection status; and grading of hepatotoxicity (mild to severe). When studies lacked enough methodological information or the information was confusing, the authors were approached via an official email address or phone number for clarification. Three independent reviewers assessed the quality of the included studies using the Newcastle–Ottawa Quality Assessment Scale for recruited studies (NOS) [27]. Cohort studies’ quality was assessed using a 9-point scoring system, while cross-sectional and case–control studies' quality was assessed using an 8-point scoring system (Additional file 2: Table S2-S4).
Statistical analysis
The final meta-analysis was carried out using STATA version 14 using the metan commands. Because of the high heterogeneity, the pooled effect size and 95% confidence interval (95% CI) were calculated using a random-effects meta-analysis model. The I2 statistic and Cochran’s Q test with its related p-value were used to analyse the heterogeneity among studies. For a pooled analysis, an I2 statistic value of less than 25% was equivalent to no heterogeneity, 25–50% was equivalent to low heterogeneity, more than 50% was considered moderate, and 75% was considered high heterogeneity [28, 29]. The aggregate prevalence and 95% CI were presented using forest plots. Subgroup analysis was performed according to the publication year, study design, HIV/Tb co-infection status, ART status, and hepatotoxicity grading to investigate the source of the substantial heterogeneity reported. We ran a sensitivity analysis to see how a single study affected the pooled effect size. Any asymmetry in a funnel plot, as well as statistical significance in Egger’s regression test (p-value < 0.05), indicated publishing bias. As a result, the random effect analysis was used to do a nonparametric trim and fill analysis [30].
Results
Description of included studies
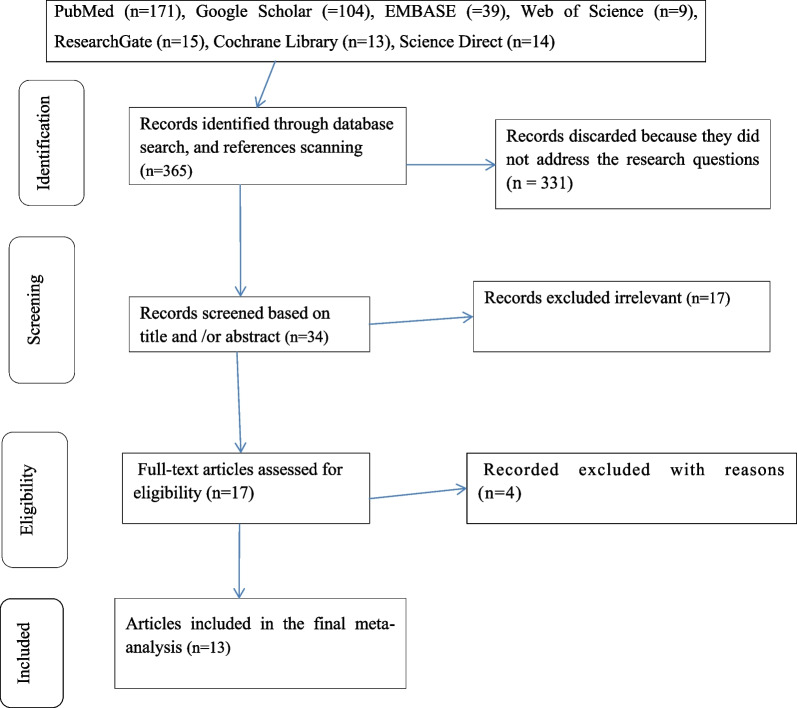
The current systematic review and meta-analysis found 365 abstracts and citations using electronic and reference scanning searches. Three hundred and fifty-two (352 total) studies were removed after duplicates and irrelevant studies were also removed based on titles and abstracts. The articles that were included were of good quality. Finally, a total of 13 studies were included in this systematic review and meta-analysis. The steps in the article's search and retrieval procedure are depicted in (Fig. 1).
Fig. 1.
Flow chart of studies’ search and retrieval process
Characteristics of the included studies
In this study, 13 original articles consisting of 3676 study participants were included [31–43]. The research was carried out in Ethiopia’s three (3) regions and one city administration. The regional breakdown of the studies was as follows: Five investigations were conducted in the Amhara area; one in Tigray; one in the South region; and the remaining six in Addis Ababa, Ethiopia’s capital. The earliest study [43] was published in 2008, and the most current study [36] was published in 2021. The sample size of the included studies varied from 84 to 1060 participants. Eight studies reported the prevalence of hepatotoxicity among patients with both HIV and TB infections, while the other five studies reported the prevalence of hepatotoxicity among HIV patients. The study participants’ mean or median age ranged from 32 to 39.7 years (Table 1).
Table 1.
The characteristics of the studies those were included in Ethiopia, 2022
| Authors (Year) | Region | Study design | Mean/median age (year) | Sample size | HIV/Tb co-infection status | Total prevalence of hepatotoxicity (%) | Overall hepatotoxicity on treatment naïve (%) | Overall hepatotoxicity on ART (%) | Degree of hepatotoxicity | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild (%) | Moderate (%) | Severe (%) | |||||||||
| Yazie (2021) [31] | Addis Ababa | Prospective cohort | 39.7 ± 10 | 63 | HIV | 41.3 | NA | 41.3 | 38.2 | 3.2 | NA |
| Baynes et al. (2017) [32] | Amhara | Retrospective cohort | NA | 275 | HIV | 23.3 | 6.5 | 23.2 | 2.2 | 10.9 | 3.6 |
| Shiferaw et al. (2016) [33] | Amhara | Cross-sectional | 36.29 ± 10.27 | 328 | HIV | 12.2 | 22 | 20.1 | 6.7 | 3.7 | 3 |
| Mulu et al. (2013) [34] | Amhara | Cross-sectional | 35 | 269 | HIV | 32 | NA | 32 | 22.3 | 7.8 | 1.84 |
| Tesfa et al. (2019) [35] | Amhara | Cross-sectional | 37.37 | 152 | HIV | 17.1 | 9.2 | 25 | 23.7 | 1.3 | NA |
| Gebremicael et al. (2021) [36] | Addis Ababa | Prospective cohort | 32 | 316 | HIV/Tb | 29.1 | 22.8 | 38 | 21.7 | 9.6 | 1.2 |
| Woldu et al. (2014) [37] | Tigray | Case–control | 33.6 ± 17 | 100 | HIV/Tb | 60 | NA | 55 | 45 | 8 | 7 |
| Yimer et al. (2014) [38] | Addis Ababa | Prospective cohort | NA | 1060 | HIV/Tb | 15 | NA | 15 | 8.02 | 4.9 | 2.08 |
| Yimer et al. (2011) [39] | Addis Ababa | Prospective cohort | NA | 353 | HIV/Tb | 30 | NA | 30 | NA | 11.6 | 18.4 |
| Yimer et al. (2012) [40] | Addis Ababa | Prospective cohort | 34 | 285 | HIV/Tb | 24.1 | 8.4 | 15.7 | NA | 11.6 | 18.4 |
| Hassen Ali et al. (2013) [41] | Oromia | Case–control | 32.1 ± 8.5 | 288 | HIV/Tb | 11.5 | NA | NA | 6.3 | NA | 5.2 |
| Zeleke et al. (2020) [42] | Amhara | Cross-sectional | NA | 84 | HIV/Tb | 20.2 | NA | NA | NA | NA | NA |
| Yimer et al. (2008) [43] | Addis Ababa | Prospective cohort | NA | 103 | HIV/Tb | 25.2 | NA | NA | NA | NA | NA |
NA Not available
Prevalence of hepatotoxicity among HIV infected patients
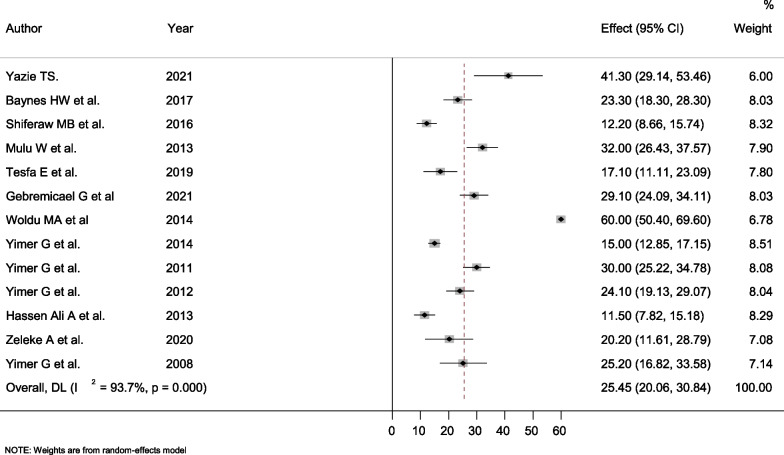
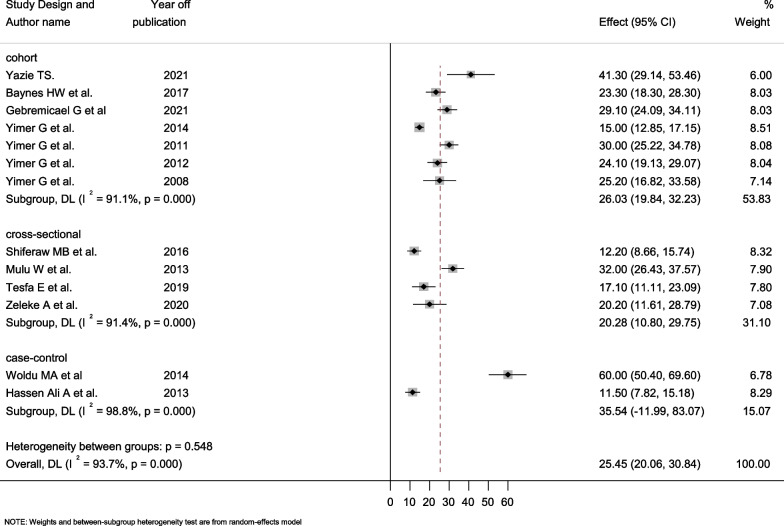
In this study, the prevalence of hepatotoxicity ranged from 11.5% reported in Jimma [41] to 60% reported in Mekele [37]. The current systematic review and meta-analysis indicated that the overall pooled prevalence of hepatotoxicity among HIV-infected patients in Ethiopia was 25.45% (95% CI = 20.06–30.84%). High heterogeneity was observed with a value of Q test (Tau-squared) of 87.73 (degree of freedom, d.f = 12, p-value < 0.001), and I2 was determined as 93.75% for the degree of inconsistency (Fig. 2).
Fig. 2.
Forest plot showing the pooled prevalence of hepatotoxicity among HIV infected patients
Subgroup analysis
ART status
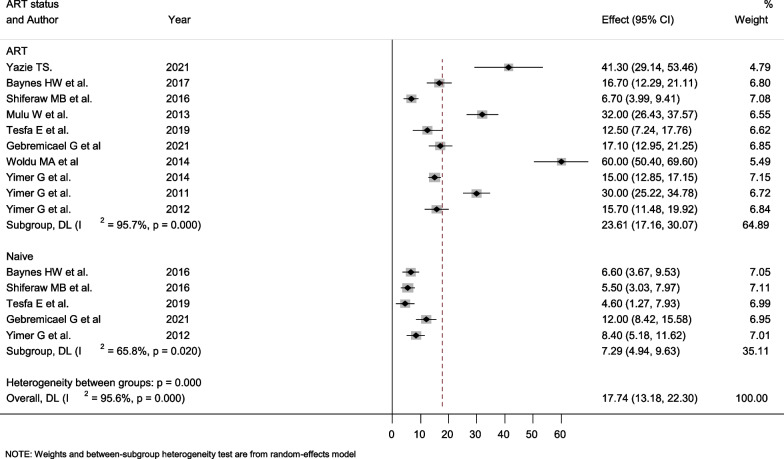
To investigate the source of high heterogeneity, subgroup analysis was done based on ART status, hepatotoxicity grade, study design, publication year, and HIV/TB co-infection. The prevalence of hepatotoxicity among treatment naive and treatment-taking groups has not been stated in all the investigations. The prevalence of hepatotoxicity among treatment-naive patients ranged from 4.6% (95% CI; 1.27–79.93%) to 8.4% (95% CI; 5.18–11.62%) with a pooled prevalence of 7.29% (95% CI; 4.94–9.63%). On the other hand, the pooled prevalence of hepatotoxicity among treatment-taking patients was exceptionally high. The computed prevalence ranges from 6.7% (95% CI; 3.99–9.41%) to 60% (95% CI; 50.4–69.6%) with a pooled prevalence of 23.61% (95% CI; 17.16–30.07%). Treatment-taking and treatment-naive groups’ also demonstrated substantial heterogeneity, with a value of I2 of 95.7% and 95.6%, respectively (Fig. 3).
Fig. 3.
Forest plot showing the pooled prevalence of hepatotoxicity among ART taking and naïve HIV/AIDS patients
Subgroup analysis by degree of hepatotoxicity
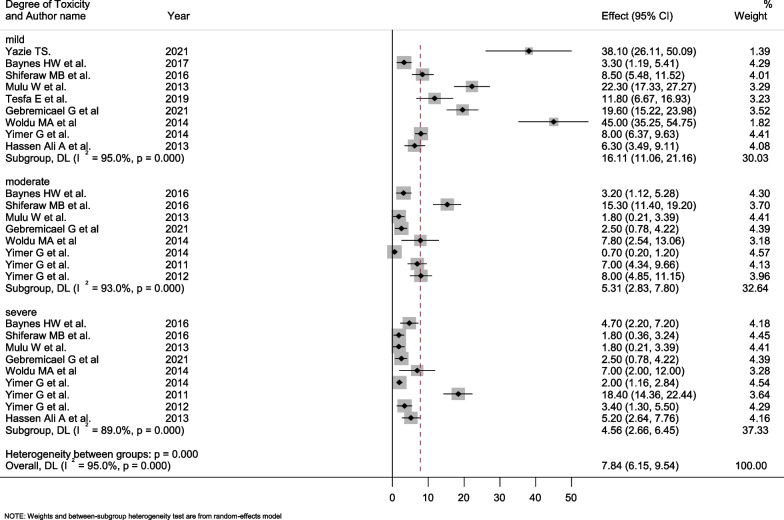
In this meta-analysis, the selected studies had the highest prevalence of mild hepatotoxicity, ranging from 3.3% (95% CI: 1.19–5.41) to 45.0% (95% CI: 35.25–54.75), with a pooled prevalence of 16.11% (95% CI: 11.06–21.16). The pooled prevalence of severe hepatotoxicity among the reported studies was 4.56% (95% CI: 2.66–6.45), which varied from 1.8% (95% CI: 0.21–3.39) to 18.4% (95% CI: 14.36–22.44). All levels of hepatotoxicity showed significant heterogeneity (Fig. 4).
Fig. 4.
Forest plot showing the pooled prevalence of hepatotoxicity grading among treatment taking and naïve HIV/AIDS patients
Subgroup analysis by study design and year of publication
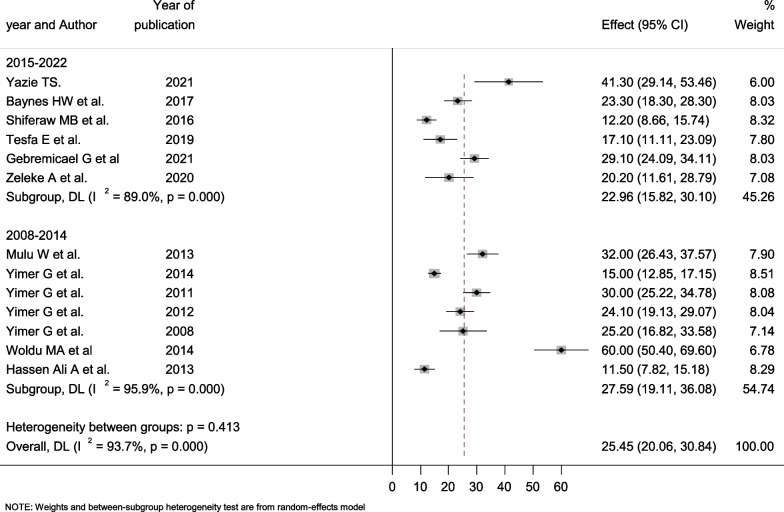
Seven studies employed cohort study designs, four were cross-sectional, and the remaining two used case–control study designs. The case–control studies' estimated prevalence of hepatotoxicity ranged from 11.5% (95% CI: 7.82–15.18) to 60.0% (95% CI: 50.40–69.60) with a pooled prevalence of 35.54% (95% CI: 11.99–83.07). The determined pooled prevalence was lower among cross-sectional studies at 20.28% (95% CI: 10.80–29.75), which varied from 12.2% (95% CI: 8.66–15.74) to 32% (95% CI: 26.45–37.57). In all designs, there was markedly high heterogeneity, with an I2 value of 91.1% and 98.8% Fig. 5. Moreover, we grouped all the studies into two groups based on their publication year, i.e., 2008–2014 and 2015–2022. The pooled prevalence of hepatotoxicity was 27.59% (95% CI: 19.11–36.08) and 22.29% (95% CI: 15.82–30.10) in studies conducted between 2000 and 2014. According to the results from this meta-analysis, the pooled prevalence of hepatotoxicity decreased somewhat from 2008 to 2014 to 2015–2022. There was significant variation within and across groups (Fig. 6).
Fig. 5.
Forest plot showing the pooled prevalence of hepatotoxicity among HIV/AIDS patients by study design
Fig. 6.
Forest plot showing the pooled prevalence of hepatotoxicity among HIV/AIDS patients by year of publication
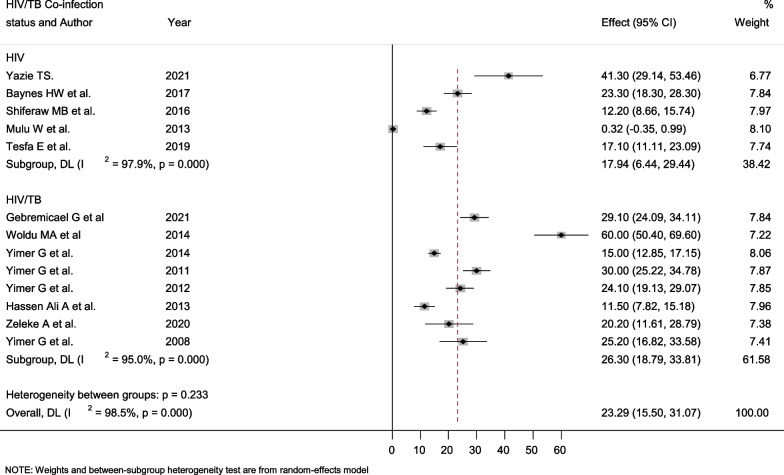
Subgroup analysis by HIV/Tb Co-infection status
The majority of the studies included in this meta-analysis reported hepatotoxicity among HIV/TB co-infected patients. The current meta-analysis found that HIV/TB co-infected patients had a higher pooled prevalence of hepatotoxicity than HIV mono-infected patients [26.3% (95% CI: 18.79–33.81)] versus [17.94% (95% CI: 6.44–29.44)]. In both groups, high levels of heterogeneity I2 were observed (Fig. 7).
Fig. 7.
Forest plot showing the pooled prevalence of hepatotoxicity among HIV/AIDS patients by HIV/Tb Co-infection status
Publication bias
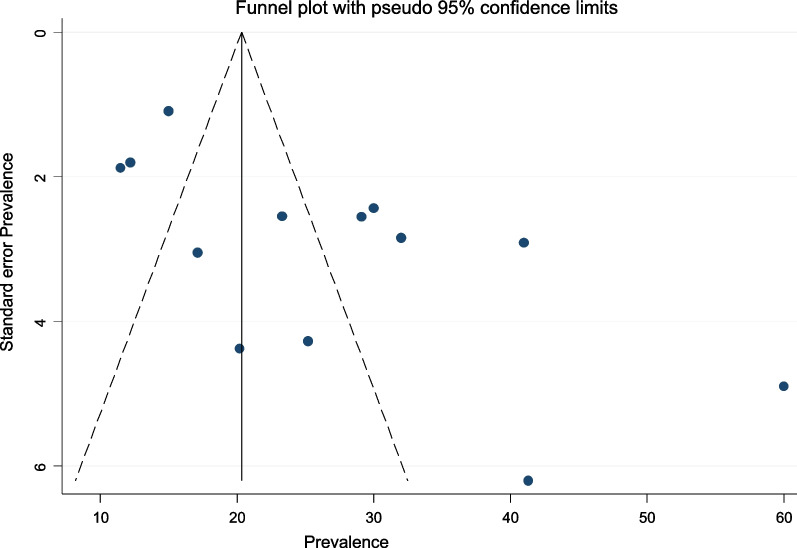
The prevalence of hepatotoxicity among HIV patients in this meta-analysis did not show a totally symmetrical depiction of the prevalence reported by the various studies. Egger's test statistics also indicated the presence of publication bias with a p-value of 0.006. This was also supported by the asymmetry of the funnel plot (Fig. 8).
Fig. 8.
Bias assessment plot of reported prevalence rates of hepatotoxicity among HIV-infected individuals across studies published in Ethiopia between 2008 and May, 2022
Trim and fill analysis of pooled prevalence of hepatotoxicity among HIV-infected patients
Based on trim and fill analysis, after adding six studies, the pooled prevalence of hepatotoxicity among HIV-infected in Ethiopia was 16.42% (95% CI 10.63–22.20) at a p-value < 0.001 (Table 2).
Table 2.
Trim and fill analysis of overall pooled prevalence of hepatotoxicity among HIV-infected patients in Ethiopia, 2022
| Meta-analysisa | |||||
|---|---|---|---|---|---|
| Method | Pooled est | 95% CI | Z-value | p-value | No. of studies |
| Fixed | 19.58 | 18.33–20.83 | 30.683 | < 0.001 | 13 |
| Random | 25.45 | 20.06–30.84 | 9.250 | < 0.001 | |
| Iteration | Estimate | Tn | # to trim | Diff |
|---|---|---|---|---|
| 1 | 19.58 | 69 | 4 | 91 |
| 2 | 16.94 | 80 | 6 | 22 |
| 3 | 15.71 | 83 | 6 | 6 |
| 4 | 15.71 | 83 | 6 | 0 |
| Filled meta-analysisb | |||||
|---|---|---|---|---|---|
| Method | Pooled est | 95% CI | Z-value | p-value | No. of studies |
| Fixed | 15.71 | 14.58–16.84 | 27.32 | < 0.001 | 19 |
| Random | 16.416 | 10.63–22.20 | 5.558 | < 0.001 | |
aTest for heterogeneity: Q = 191.844 on 12 degrees of freedom (p = 0.000)
Trimming estimator: Linear
Meta-analysis type: Fixed-effects mode
bTest for heterogeneity: Q = 426.787 on 18 degrees of freedom (p ≤ 0.001)
Moment-based estimate of between studies variance = 153.14
Sensitivity analysis
A sensitivity analysis was used to see how a single study affected the pooled effect size. When one study was removed at a time, the resulting pooled effect size was within the 95% confidence interval of the combined pooled effect size, indicating that no single study influenced the impact size (Table 3).
Table 3.
Sensitivity analysis
| S. No. | Study omitted | Estimate | 95% CI |
|---|---|---|---|
| 1 | Yazie (2021) | 24.41 | 18.98–29.85 |
| 2 | Baynes et al. (2017) | 25.69 | 19.86–31.52 |
| 3 | Shiferaw et al. (2016) | 26.69 | 20.89–32.49 |
| 4 | Mulu et al. (2013) | 24.86 | 19.34–30.37 |
| 5 | Tesfa et al. (2019) | 26.20 | 20.42–31.97 |
| 6 | Gebremicael et al. (2021) | 25.14 | 19.50–30.78 |
| 7 | Woldu et al. (2014) | 22.75 | 18.18–27.31 |
| 8 | Yimer et al. (2014) | 26.54 | 20.36–32.72 |
| 9 | Yimer et al. (2011) | 25.04 | 19.45–30.63 |
| 10 | Yimer et al. (2012) | 25.62 | 19.80–31.43 |
| 11 | Hassen Ali et al. (2013) | 26.74 | 21.00–32.47 |
| 12 | Zeleke et al. (2020) | 25.87 | 20.19–31.56 |
| 13 | Yimer et al. (2008) | 25.49 | 19.82–31.15 |
| 14 | Combined | 25.45 | 20.06–30.84 |
Discussion
Antiretroviral therapy (ART) has considerably reduced morbidity and death among HIV-positive people around the world [44]. By 2016, an estimated 19.5 million people had gotten ART around the world [45]. ART treatment, despite its enormous health benefits, has some side effects, particularly hepatic impairment, which contributes to morbidity and mortality in HIV patients [46, 47]. In HIV-infected patients, multiple mechanisms of liver damage have been identified, including HIV infection [48], hepatitis virus co-infections, ART-related neurotoxic effects [49], AIDS-related neoplasm [50], and age-related co-morbid conditions [51].
There is no published study that assesses the nationwide pooled prevalence of hepatotoxicity among HIV-infected individuals that we are aware of. As a result, this meta-analysis was the first to present comprehensive information on the magnitude of hepatotoxicity in HIV-positive people. In almost all of the investigations, the occurrence of hepatotoxicity was not associated with age or sex. According to our findings, the countrywide prevalence of hepatotoxicity among HIV-infected patients was 25.45% (95% CI = 20.06–30.84%). However, it differs from other cohort studies in Uganda (4.2%) [52], Botswana (1.1%) [53], the Netherlands (7.9%) [54], Taiwan (4.9%) [55], a randomised trial in Boehringer Ingelheim (10%) [56], and South Africa (17%) [23], and a review done by Kontorinis N et al. found (18%) [57]. In contrast, a study in Cameroon (42.1%) [58], Nigeria (36.4%) [19], and Iran (32% [59]) found a high prevalence of hepatotoxicity. The difference could be due to the differences in the prevalence of risk factors for liver disease like hepatitis B and C and other opportunistic infections; indiscriminate use of drugs; hepatotoxicity used; follow-up duration; and the definition criteria of hepatotoxicity. The increased prevalence in the current study may be brought on by additional hepatotoxicity risk factors.
This high risk of hepatotoxicity highlights the necessity for a national policy that includes hepatotoxicity screening tests as part of comprehensive care for HIV patients. Furthermore, the extent of hepatotoxicity was found to vary significantly in these investigations, ranging from 11.5% in Oromia, Jimma [41] to 60%in Tigray, Mekele [37]. As a result, there was a lot of heterogeneity (I2 = 93.7%). This disparity could be explained by changes in sample size, study design, the definition of high liver enzyme levels, data processing method, and study participants’ socio-demographic and clinical features. The gap may also be due to the small number of studies that were conducted; only one study was conducted in each of Oromia and Tigray, and most of them were in Addis Ababa and the Amhara region.
The random effect pooled prevalence of hepatotoxicity was higher in study patients who were on treatment at 23.61% (95% CI; 17.16–30.07%) compared to their treatment-naive counterparts at 7.29%, according to the subgroup analysis by ART status (95% CI; 4.94–9.63%). The rate of hepatotoxicity (23.61%) in the current study was lower than that observed in patients with HIV who had received ART in Nigeria (74.2%) [60], Iran (33%) [59], and similar to that in Cameroonian patients (22.6%) [61]. The current result was higher than a study done by Raffaele et al. who reported that (2.9%) of the treatment receiving group experienced hepatotoxicity [62]. Moreover, the prevalence of hepatotoxicity among treatment-naive individuals in the current study was lower than that seen in Tanzanian patients (13%) [63] and North American patients (15%) [64].
According to these findings, the use of HAART has significantly increased hepatotoxicity in HIV-infected patients. It is undeniable that the use of HAART has drastically improved the quality of life of HIV-infected people by extending their lives. The main problems of HAART in long-term usage include the potential for hepatocyte derangements due to the drug’s direct toxicity, functional disturbance, mitochondrial injury, and/or active metabolites, all of which can be life-threatening. The virus causes hepatotoxicity by causing systemic inflammation or oxidative stress pathogenesis, and the condition is further exacerbated by HAART hepatocyte damage [65, 66]. There was also a considerably higher variability in both the medication taking and treatment naive groups, with I2 of 95.7% and 95.6%, respectively. A prior study found that 14–20% of HIV-infected individuals receiving antiretroviral medication (ART) experienced hepatotoxicity [67, 68].
Hepatotoxicity, which can range from mild to severe, is a major health problem that leads to high rates of morbidity, mortality, and hospitalization [69]. The current meta-analysis found that the pooled prevalence of mild hepatotoxicity was 16.11%, which was lower than the studies in Iran (23.1%) [59], and Nigeria (37.2%) [19]. Of the studies used for this review, the highest rate of mild hepatotoxicity was 45% in a study conducted by Woldu et al. in the Tigray region [37], followed by 38.1% in Addis Ababa by Yazie [31]. In the Amhara region, Baynes HW et al. found the lowest incidence of mild hepatotoxicity [32]. On the other hand, the overall rate of severe hepatotoxicity was 4.56% (95% CI: 2.66–6.45). This result was higher than the studies in Iran (1.5%) [59], the USA (2%) [70], and Nigeria (3.2%) [19]. Furthermore, previous studies found that the prevalence of hepatotoxicity ranged between 1 and 18% [71–73]. Of the studies recruited for this review, Yimer et al. found the highest rate of severe hepatotoxicity at 18.4% [39], whereas Shiferaw et al. found the lowest at 1.8% [33]. The findings underlined the difficulty in assessing and controlling hepatotoxicity associated with antiretroviral therapy and viral hepatotoxicity [74].
In the two case–control studies, subgroup analysis revealed a high pooled overall prevalence of hepatotoxicity of 35.54% (95% CI: 11.99–83.07). In contrast, cross-sectional studies reported the lowest overall frequency of hepatotoxicity at 20.28% (95% CI: 10.80–29.75). This disparity could be related to differences in study methodology, sample size, and study participants' sociocultural backgrounds. Likewise, poor documentation and record-keeping can result in a low prevalence of hepatotoxicity in a retrospective analysis. A subgroup analysis depending on the year of publication was also undertaken. According to this meta-analysis, the overall pooled prevalence of hepatotoxicity across studies completed between 2008 and 2014 G.C. was 27.59% (95% CI: 19.11–36.08). This is higher than the pooled prevalence of hepatotoxicity among the recently conducted studies, which is 22.29% (95% CI: 15.82–30.10). This decrease in hepatotoxicity over the last year could be attributed to the development of our health system, the growth of health facilities, the reduction of opportunistic infections, and the careful monitoring of HIV patients for organ toxicity and injuries.
In this meta-analysis, we attempted to conduct a subgroup analysis based on HIV/Tb co-infection status. According to a random effect meta-analysis, the total pooled prevalence of hepatotoxicity among HIV/Tb co-infected adults (26.3%) was higher than that of non-co-infected people (17.94%). Because the HIV virus weakens the immune system, HIV-positive people are more likely to develop active tuberculosis (Tb), which can exacerbate liver damage. Several prior studies [41, 75–79] have reported the hepatotoxicity of anti-Tb medications, which supports our finding. For example, a study in Cameroon discovered that 52.9% of Tb/HIV co-infected people develop hepatotoxicity [58]. On the other hand, a study in China found that 4.2% of HIV/Tb co-infected patients had hepatotoxicity [80]. This underscores the significance of closer and more frequent monitoring of liver function and other organs in individuals using anti-Tb drugs.
The presence of publication bias in this meta-analysis was revealed by the symmetry of the funnel plot and Egger’s test results. The sensitivity analysis, on the other hand, revealed that no single study had an impact on the total pooled effect size. Despite the fact that research selection criteria were well established and study selection was carried out by three independent reviewers, we were unable to completely eliminate publication bias, which may have influenced our findings. The following restrictions apply to this study: Even after subgroup analysis for multiple variables, there is still a lot of heterogeneity. Due to inconsistent risk factor assessments by the recruited studies, it was unable to examine parameters such as CD4 T cell count, sex, age, underlying illness status, type of HAART regimen, and HAART regimen duration that were linked to the pooled prevalence of hepatotoxicity in HIV-infected patients.
Conclusion
According to the current systematic review and meta-analysis, hepatotoxicity is prevalent among HIV-infected people. People who had HIV/Tb co-infection and were on antiretroviral therapy (ART) had a higher overall pooled prevalence of hepatotoxicity. Many anti-HIV and anti-Tb medications have the potential to harm the liver. This comprehensive result emphasizes the significance of regular monitoring in order to avoid organ damage, particularly hepatotoxicity and HIV/AIDS-related complications. The findings of this study will also assist policymakers and other stakeholders. Furthermore, the data could be used in future research and decision-making based on evidence.
Supplementary Information
Additional file 1: PRISMA 2020 checklist.
Additional file 2: Quality assessment form for included studies.
Additional file 3: The syntax for each database.
Acknowledgements
Not applicable.
Author contributions
OM involved in the conception of the research idea; OM, EA: undertook data extraction, analysis, interpretation, and manuscript write-up. OM, EA, HB, and EE: undertook the acquisition of data, interpreted the results, and drafted the manuscript. OM, EA, AG, HB, MF, MT, MN, and TF: participated in the study design, acquisition of data, and revised the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
All the datasets used and/or analyzed during the current study are available in the manuscript.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent to publication
Not applicable.
Competing interests
The authors declare that they have no potential competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ousman Mohammed, Email: ousmanabum@gmail.com.
Ermiyas Alemayehu, Email: ermiyas0009@gmail.com.
Habtye Bisetegn, Email: habtiye21@gmail.com.
Mihret Tilahun, Email: tilahunmihret21@gmail.com.
Alemu Gedefie, Email: alemugedefie@gmail.com.
Endris Ebrahim, Email: endris.index@gmail.com.
Mesfin Fiseha, Email: mesfinfiseha40@gmail.com.
Mogesie Necho, Email: nechomoges2014@gmail.com.
Temesgen Fiseha, Email: temafiseha@gmail.com.
References
- 1.Okolie MN, Eghafona NO, Omoregie R. Anti-human immunodeficiency virus agents. J Med Lab Sci. 2003;12(1):1–14. [Google Scholar]
- 2.De Cock KM, Mbori-Ngacha D, Marum E. Shadow on the continent: public health and HIV/AIDS in Africa in the 21st century. The Lancet. 2002;360(9326):67–72. doi: 10.1016/S0140-6736(02)09337-6. [DOI] [PubMed] [Google Scholar]
- 3.Adebisi YA, Rabe A, Ekpenyong A, Okereke M, Sarah OA. Towards 90–90-90 target: COVID-19 and HIV response in Africa. J Infect Dis Epidemiol. 2021;7:191. [Google Scholar]
- 4.Dean HD, Fenton KA. Integrating a social determinants of health approach into public health practice: a five-year perspective of actions implemented by CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Public Health Rep. 2013;128(6_suppl3):5–11. doi: 10.1177/00333549131286S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pepperrell T, Hill A, Moorhouse M, Clayden P, McCann K, Sokhela S, et al. Phase 3 trials of new antiretrovirals are not representative of the global HIV epidemic. J Virus Erad. 2020;6(2):70–73. doi: 10.1016/S2055-6640(20)30019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elmore K, Nelson R, Gant Z, Jeffries C, Broeker L, Mirabito M, et al. Data harmonization process for creating the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Atlas. Public Health Rep. 2014;129(1_suppl1):63–69. doi: 10.1177/00333549141291S110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiertiburanakul S, Boettiger D, Lee MP, Omar SF, Tanuma J, Ng OT, et al. Trends of CD4 cell count levels at the initiation of antiretroviral therapy over time and factors associated with late initiation of antiretroviral therapy among Asian HIV-positive patients. J Int AIDS Soc. 2014;17(1):18804. doi: 10.7448/IAS.17.1.18804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulissa Z, Jerene D, Lindtjørn B. Patients present earlier and survival has improved, but pre-ART attrition is high in a six-year HIV cohort data from Ethiopia. PLoS ONE. 2010;5(10):e13268. doi: 10.1371/journal.pone.0013268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan K, Khan AH, Sulaiman SA, Soo CT, Ahsan R. Adverse effect of highly active anti-retroviral therapy (HAART) in HIV/AIDS patients. Indian J Pharm Pract. 2014;7(3):6–11. [Google Scholar]
- 10.Chu KM, Manzi M, Zuniga I, Biot M, Ford NP, Rasschaert F, et al. Nevirapine-and efavirenz-associated hepatotoxicity under programmatic conditions in Kenya and Mozambique. Int J STD AIDS. 2012;23(6):403–407. doi: 10.1258/ijsa.2009.009328. [DOI] [PubMed] [Google Scholar]
- 11.Federal HI, Prevention AI. Control Office Federal Ministry of Health. Guidelines for prevention of mother-to-child transmission of HIV in Ethiopia. 2007
- 12.Kaspar MB, Sterling RK. Mechanisms of liver disease in patients infected with HIV. BMJ Open Gastroenterol. 2017;4(1):e000166. doi: 10.1136/bmjgast-2017-000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Rust G, Cardarelli K, Felizzola J, Fransua M, Stringer HG., Jr Adherence to highly active antiretroviral therapy impact on clinical and economic outcomes for medicaid enrollees with human immunodeficiency virus and hepatitis C coinfection. AIDS Care. 2015;27(7):829–835. doi: 10.1080/09540121.2015.1021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long L, Fox M, Sanne I, Rosen S. The high cost of second-line antiretroviral therapy for HIV/AIDS in South Africa. AIDS. 2010;24(6):915–919. doi: 10.1097/QAD.0b013e3283360976. [DOI] [PubMed] [Google Scholar]
- 15.Mensah KB, Adu-Gyamfi PK, Boakye-Gyasi E. HAART therapy in Ghana: assessment of adverse drug reaction reports of patients at an HIV clinic and a teaching hospital. J Basic Clin Pharm. 2017; 8(3).
- 16.Megerso A, Garoma S, Tolosa Eticha TW, Daba S, Tarekegn M, Habtamu Z. Predictors of loss to follow-up in antiretroviral treatment for adult patients in the Oromia region, Ethiopia. HIV/AIDS (Auckland, NZ) 2016;8:83. doi: 10.2147/HIV.S98137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chadwick EG, Pinto J, Yogev R, Alvero CG, Hughes MD, Palumbo P, et al. Early initiation of lopinavir/ritonavir in infants less than 6 weeks of age: pharmacokinetics and 24 week safety and efficacy. Pediatr Infect Dis J. 2009;28(3):215. doi: 10.1097/INF.0b013e31818cc053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng YT, Yang CJ, Chang SY, Lin SW, Tsai MS, Liu WC, et al. Incidence and risk factors of skin rashes and hepatotoxicity in HIV-infected patients receiving nevirapine-containing combination antiretroviral therapy in Taiwan. Int J Infect Dis. 2014;29:12–17. doi: 10.1016/j.ijid.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Hamza M, Maifada YA, Musa B, Mijinyawa MS, Muhammad BM, Garba HA. Prevalence and risk factors for hepatotoxicity among patients with HIV/AIDS on highly active antiretroviral therapy in North-Western Nigeria. Sub-Saharan Afr J Med. 2014;1(4):175. [Google Scholar]
- 20.Walker UA. Antiretroviral therapy-induced liver alterations. Curr Opin HIV AIDS. 2007;2(4):293–298. doi: 10.1097/COH.0b013e328122dbaa. [DOI] [PubMed] [Google Scholar]
- 21.Núñez M. Clinical syndromes and consequences of antiretroviral-related hepatotoxicity. Hepatology. 2010;52(3):1143–1155. doi: 10.1002/hep.23716. [DOI] [PubMed] [Google Scholar]
- 22.Bonacini M, Inductivo-Yu I. Highly active antiretroviral therapy-induced liver injury. Curr Drug Saf. 2008;3(1):4–13. doi: 10.2174/157488608783333916. [DOI] [PubMed] [Google Scholar]
- 23.Sanne I, Mommeja-Marin H, Hinkle J, Bartlett JA, Lederman MM, Maartens G, et al. Severe hepatotoxicity associated with nevirapine use in HIV-infected subjects. J Infect Dis. 2005;191(6):825–829. doi: 10.1086/428093. [DOI] [PubMed] [Google Scholar]
- 24.Abubakar MG, Abduljalil MM, Nasiru YI. Changes in liver function enzymes of HIV/AIDS patients treated with antiretroviral drugs (ARVS) in specialist hospital, Sokoto, Nigeria. Niger J Basic Appl Sci. 2014;22(3–4):85–89. [Google Scholar]
- 25.Nagarwal N, Iyer D, Gabbi C, Saha P, Patel SG, Mo Q, et al. HIV-1 viral protein R (Vpr) induces fatty liver in mice via LXRα and PPARα dysregulation: implications for HIV-specific pathogenesis of NAFLD. Sci Rep. 2017;7(1):1–5. doi: 10.1038/s41598-017-13835-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group* Preferred reporting items fsor systematic reviews and meta-analyses: the PRISMA statement. Ann Internal Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 27.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000.
- 28.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 31.Yazie TS. Derangement of liver enzymes, hyperglycemia, anemia, and associated factors among HIV-infected patients treated with tenofovir disoproxil fumarate-based regimen in Ethiopia: a prospective cohort study. BioMed Res Int. 2021; 2021. [DOI] [PMC free article] [PubMed]
- 32.Baynes HW, Tegene B, Gebremichael M, Birhane G, Kedir W, Biadgo B. Assessment of the effect of antiretroviral therapy on renal and liver functions among HIV-infected patients: a retrospective study. HIV/AIDS (Auckland, NZ) 2017;9:1. doi: 10.2147/HIV.S120979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiferaw MB, Tulu KT, Zegeye AM, Wubante AA. Liver enzymes abnormalities among highly active antiretroviral therapy experienced and HAART naïve HIV-1 infected patients at Debre Tabor Hospital, North West Ethiopia: a comparative cross-sectional study. AIDS Res Treatment. 2016; 2016. [DOI] [PMC free article] [PubMed]
- 34.Mulu W, Gidey B, Chernet A, Alem G, Abera B. Hepatotoxicity and associated risk factors in HIV-infected patients receiving antiretroviral therapy at Felege Hiwot Referral Hospital, Bahirdar, Ethiopia. Ethiop J Health Sci. 2013;23(3):217–226. doi: 10.4314/ejhs.v23i3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tesfa E, Siefu D, Belayneh Y, Mekonnen Z. Liver enzyme elevation in patients taking HAART compared with treatment naïve controls at Debre Berhan Referral Hospital: a comparative cross-sectional study, Northeast Ethiopia. BMC Res Notes. 2019;12(1):1–7. doi: 10.1186/s13104-019-4748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gebremicael G, Tola HH, Gebreegziaxier A, Kassa D. Incidence of hepatotoxicity and factors associated during highly active antiretroviral therapy in people living with HIV in Ethiopia: a prospective cohort study. HIV/AIDS (Auckland, NZ) 2021;13:329. doi: 10.2147/HIV.S283076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woldu MA, Zedwe AG, Jimma LL. Assesment of drug induced hepatotoxicity in patients treated for TB/HIV coinfections in ayder referral hospital ART clinic, Mekelle, Ethiopia. 2014.
- 38.Yimer G, Gry M, Amogne W, Makonnen E, Habtewold A, Petros Z, et al. Evaluation of patterns of liver toxicity in patients on antiretroviral and anti-tuberculosis drugs: a prospective four arm observational study in Ethiopian patients. PLoS ONE. 2014;9(4):e94271. doi: 10.1371/journal.pone.0094271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yimer G, Ueda N, Habtewold A, Amogne W, Suda A, Riedel KD, et al. Pharmacogenetic & pharmacokinetic biomarker for efavirenz based ARV and rifampicin based anti-TB drug induced liver injury in TB-HIV infected patients. PLoS ONE. 2011;6(12):e27810. doi: 10.1371/journal.pone.0027810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yimer G, Amogne W, Habtewold A, Makonnen E, Ueda N, Suda A, et al. High plasma efavirenz level and CYP2B6* 6 are associated with efavirenz-based HAART-induced liver injury in the treatment of naive HIV patients from Ethiopia: a prospective cohort study. Pharmacogenomics J. 2012;12(6):499–506. doi: 10.1038/tpj.2011.34. [DOI] [PubMed] [Google Scholar]
- 41.Hassen Ali A, Belachew T, Yami A, Ayen WY. Anti-tuberculosis drug induced hepatotoxicity among TB/HIV co-infected patients at Jimma University Hospital, Ethiopia: nested case-control study. PLoS ONE. 2013;8(5):e64622. doi: 10.1371/journal.pone.0064622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeleke A, Misiker B, Yesuf TA. Drug-induced hepatotoxicity among TB/HIV co-infected patients in a referral hospital, Ethiopia. BMC Res Notes. 2020;13(1):1–5. doi: 10.1186/s13104-019-4872-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yimer G, Aderaye G, Amogne W, Makonnen E, Aklillu E, Lindquist L, et al. Anti-tuberculosis therapy-induced hepatotoxicity among Ethiopian HIV-positive and negative patients. PLoS ONE. 2008;3(3):e1809. doi: 10.1371/journal.pone.0001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wada N, Jacobson LP, Cohen M, French A, Phair J, Muñoz A. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984–2008. Am J Epidemiol. 2013;177(2):116–125. doi: 10.1093/aje/kws321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahy MI, Sabin KM, Feizzadeh A, Wanyeki I. Progress towards 2020 global HIV impact and treatment targets. J Int AIDS Soc. 2021;24:e25779. doi: 10.1002/jia2.25779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goehringer F, Bonnet F, Salmon D, Cacoub P, Paye A, Chêne G, ANRS EN20 Mortalité 2010 Study Group et al. Causes of death in HIV-infected individuals with immunovirologic success in a national prospective survey. AIDS Res Hum Retrovir. 2017;33(2):187–193. doi: 10.1089/AID.2016.0222. [DOI] [PubMed] [Google Scholar]
- 47.Neukam K, Mira JA, Collado A, Rivero-Juárez A, Monje-Agudo P, Ruiz-Morales J, et al. Liver toxicity of current antiretroviral regimens in HIV-infected patients with chronic viral hepatitis in a real-life setting: the HEPAVIR SEG-HEP Cohort. PLoS ONE. 2016;11(2):e0148104. doi: 10.1371/journal.pone.0148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Towner WJ, Xu L, Leyden WA, Horberg MA, Chao CR, Tang B, et al. The effect of HIV infection, immunodeficiency and antiretroviral therapy on the risk of hepatic dysfunction. J Acquir Immune Defic Syndr (1999). 2012;60(3):321. doi: 10.1097/QAI.0b013e31824e9ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moodie EE, Pant Pai N, Klein MB. Is antiretroviral therapy causing long-term liver damage? A comparative analysis of HIV-mono-infected and HIV/hepatitis C co-infected cohorts. PLoS ONE. 2009;4:e4517. doi: 10.1371/journal.pone.0004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lefkowitch JH. Pathology of AIDS-related liver disease. Dig Dis. 1994;12(6):321–330. doi: 10.1159/000171468. [DOI] [PubMed] [Google Scholar]
- 51.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalyesubula R, Kagimu M, Opio KC, Kiguba R, Semitala CF, Schlech WF, et al. Hepatotoxicity from first line antiretroviral therapy: an experience from a resource limited setting. Afr Health Sci. 2011; 11(1). [PMC free article] [PubMed]
- 53.Bussmann H, Wester CW, Ndwapi N, Grundmann N, Gaolathe T, Puvimanasinghe J, et al. Five year outcomes of initial patients treated in Botswana’s National Antiretroviral treatment program. AIDS (London, England) 2008;22(17):2303. doi: 10.1097/QAD.0b013e3283129db0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wit FW, Weverling GJ, Weel J, Jurriaans S, Lange JM. Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. J Infect Dis. 2002;186(1):23–31. doi: 10.1086/341084. [DOI] [PubMed] [Google Scholar]
- 55.Wu PY, Cheng CY, Liu CE, Lee YC, Yang CJ, Tsai MS, et al. Multicenter study of skin rashes and hepatotoxicity in antiretroviral-naive HIV-positive patients receiving non-nucleoside reverse-transcriptase inhibitor plus nucleoside reverse-transcriptase inhibitors in Taiwan. PLoS ONE. 2017;12(2):e0171596. doi: 10.1371/journal.pone.0171596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stern JO, Robinson PA, Love J, Lanes S, Imperiale MS, Mayers DL. A comprehensive hepatic safety analysis of nevirapine in different populations of HIV infected patients. JAIDS J Acquir Immune Defic Syndr. 2003;34:S21–33. doi: 10.1097/00126334-200309011-00005. [DOI] [PubMed] [Google Scholar]
- 57.Kontorinis N, Dieterich D. Hepatotoxicity of antiretroviral therapy. AIDS Rev. 2003;5(1):36–43. [PubMed] [Google Scholar]
- 58.Enoh JE, Cho FN, Manfo FP, Ako SE, Akum EA. Abnormal levels of liver enzymes and hepatotoxicity in HIV-positive, TB, and HIV/TB-coinfected patients on treatment in Fako Division, Southwest Region of Cameroon. BioMed Res Int. 2020; 2020. [DOI] [PMC free article] [PubMed]
- 59.Hashempour T, Moayedi J, Mousavi Z, Esmaeli M, Asadzadeh A, Hasanshahi Z, et al. Incidence of hepatotoxicity in Iranian patients with HIV on antiretroviral therapies and its correlation with virologic response to HIV treatment. Lab Med. 2021;52(4):369–374. doi: 10.1093/labmed/lmaa106. [DOI] [PubMed] [Google Scholar]
- 60.Odiba AS, Ukegbu CY, Omeje KO. Transaminase [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)] activity of HIV and HBV co-infected female patients on drugs and female patients not on drugs. J Pharm Biol Sci. 2014;9:24–29. [Google Scholar]
- 61.Lucien K, Clement A, Fon N, Weledji P, Ndikvu C. The effects of antiretroviral treatment on liver function enzymes among HIV-infected out patients attending the central hospital of Yaounde, Cameroon. Afr J Clin Exp Microbiol. 2010; 11(3).
- 62.Bruno R, Sacchi P, Maiocchi L, Zocchetti C, Filice G. Hepatotoxicity and nelfinavir: a meta-analysis. Clin Gastroenterol Hepatol. 2005;3(5):482–488. doi: 10.1016/s1542-3565(05)00162-x. [DOI] [PubMed] [Google Scholar]
- 63.Nagu TJ, Kanyangarara M, Hawkins C, Hertmark E, Chalamila G, Spiegelman D, et al. Elevated alanine aminotransferase in antiretroviral-naïve HIV-infected African patients: magnitude and risk factors. HIV Med. 2012;13(9):541–548. doi: 10.1111/j.1468-1293.2012.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sterling RK, Chiu S, Snider K, Nixon D. The prevalence and risk factors for abnormal liver enzymes in HIV-positive patients without hepatitis B or C coinfections. Dig Dis Sci. 2008;53(5):1375–1382. doi: 10.1007/s10620-007-9999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ong MM, Latchoumycandane C, Boelsterli UA. Troglitazone-induced hepatic necrosis in an animal model of silent genetic mitochondrial abnormalities. Toxicol Sci. 2007;97(1):205–213. doi: 10.1093/toxsci/kfl180. [DOI] [PubMed] [Google Scholar]
- 66.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283(20):13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qin F, Jiang J, Qin C, Huang Y, Liang B, Xu Y, et al. Liver damage in patients living with HIV on antiretroviral treatment with normal baseline liver function and without HBV/HCV infection: an 11-year retrospective cohort study in Guangxi, China. BMJ Open. 2019;9(4):e023140. doi: 10.1136/bmjopen-2018-023140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodriguez-Rosado RG, Garcia-Samaniego J, Soriano V. Hepatotoxicity after introduction of highly active antiretroviral therapy. AIDS. 1998;12(10):1256. doi: 10.1097/00002030-199810000-00025. [DOI] [PubMed] [Google Scholar]
- 69.Heil EL, Townsend ML, Shipp K, Clarke A, Johnson MD. Incidence of severe hepatotoxicity related to antiretroviral therapy in HIV/HCV coinfected patients. AIDS research and treatment. 2010; 2010. [DOI] [PMC free article] [PubMed]
- 70.Selik RM, Byers RH, Jr, Dworkin MS. Trends in diseases reported on US death certificates that mentioned HIV infection, 1987–1999. J Acquir Immune Defic Syndr (1999) 2002;29(4):378–387. doi: 10.1097/00126334-200204010-00009. [DOI] [PubMed] [Google Scholar]
- 71.Van Leth F, Phanuphak P, Ruxrungtham K, Baraldi E, Miller S, Gazzard B, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. The Lancet. 2004;363(9417):1253–1263. doi: 10.1016/S0140-6736(04)15997-7. [DOI] [PubMed] [Google Scholar]
- 72.Núñez M, Lana R, Mendoza JL, Martín-Carbonero L, Soriano V. Risk factors for severe hepatic injury after introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;27:426–431. doi: 10.1097/00126334-200108150-00002. [DOI] [PubMed] [Google Scholar]
- 73.Van Leeuwen R, Katlama C, Murphy RL, Squires K, Gatell J, Horban A, et al. A randomized trial to study first-line combination therapy with or without a protease inhibitor in HIV-1-infected patients. AIDS. 2003;17(7):987–999. doi: 10.1097/00002030-200305020-00007. [DOI] [PubMed] [Google Scholar]
- 74.Reisler R, Liou S, Servoss J, Robbins G, Theodore D, Murphy R, et al. Incidence of hepatotoxicity and mortality in 21 adult antiretroviral treatment trials. In: Program and abstracts of The 1st IAS Conference on HIV Pathogenesis and Treatment 2001; (43):8–11.
- 75.Shakya R, Rao BS, Shrestha B. Incidence of hepatotoxicity due to antitubercular medicines and assessment of risk factors. 2004; 38(6): 1074–1079. [DOI] [PubMed]
- 76.Senaratne WV, Pinidiyapathirage MJ, Perera GA, Wickremasinghe AR. Anti-tuberculosis drug inducd hepatitis–a Sri Lankan experience. Age (years) 2006;11(20):44. doi: 10.4038/cmj.v51i1.1369. [DOI] [PubMed] [Google Scholar]
- 77.Makhlouf HA, Helmy A, Fawzy E, El-Attar M, Rashed HA. A prospective study of antituberculous drug-induced hepatotoxicity in an area endemic for liver diseases. Hep Intl. 2008;2(3):353–360. doi: 10.1007/s12072-008-9085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pukenyte E, Lescure FX, Rey D, Rabaud C, Hoen B, Chavanet P, et al. Incidence of and risk factors for severe liver toxicity in HIV-infected patients on anti-tuberculosis treatment. Int J Tuberc Lung Dis. 2007;11(1):78–84. [PubMed] [Google Scholar]
- 79.Lima MD, Melo HR. Hepatotoxicity induced by antituberculosis drugs among patients coinfected with HIV and tuberculosis. Cad Saude Publica. 2012;28:698–708. doi: 10.1590/s0102-311x2012000400009. [DOI] [PubMed] [Google Scholar]
- 80.Mo P, Zhu Q, Teter C, Yang R, Deng L, Yan Y, et al. Prevalence, drug-induced hepatotoxicity, and mortality among patients multi-infected with HIV, tuberculosis, and hepatitis virus. Int J Infect Dis. 2014;28:95–100. doi: 10.1016/j.ijid.2014.06.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: PRISMA 2020 checklist.
Additional file 2: Quality assessment form for included studies.
Additional file 3: The syntax for each database.
Data Availability Statement
All the datasets used and/or analyzed during the current study are available in the manuscript.