Abstract
Background
Risk profiles for premature cardiovascular disease (CVD) are unclear. This study aimed to examine baseline risk profiles for incident CVD by age at onset in Chinese population.
Methods
A total of 97,841 participants without CVD were enrolled from the Kailuan cohort study. Four age groups were examined (< 55, 55 to < 65, 65 to < 75, and ≥ 75 years) for CVD onset. Risk profiles included clinical, lipid, metabolic, and inflammatory risk factors and biomarkers.
Results
Of the clinical factors, diabetes was associated with the highest relative risk for incident CVD in participants younger than 55 years (sub-distributional hazard ratio [sHR], 4.08; 95% confidence interval [CI], 3.47–4.80). Risk factors that were also noted for CVD onset in participants younger than 55 years included hypertension, metabolism syndrome, overweight or obese, dyslipidemia, and smoking. Among the biomarkers, insulin resistance measured by triglyceride-glucose index had the highest sHR (1.42; 95% CI, 1.35–1.49) for CVD in participants younger than 55 years. In comparison, weaker but significant associations with CVD in participants younger than 55 years were noted for most lipids, metabolic biomarkers, and inflammatory biomarkers. Most risk factors and biomarkers had associations that attenuated with increasing age at onset. Some biomarkers had similar CVD age association, while a few had no association with CVD onset at any age.
Conclusions
These findings showed that diabetes and insulin resistance, in addition to hypertension, metabolism syndrome, overweight or obese, dyslipidemia, and smoking, appeared to be the strongest risk factors for premature onset of CVD, and most risk factors had attenuated relative rates at older ages.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-022-02592-x.
Keywords: Cardiovascular disease, Risk factors, Young adults, Age-related risk
Background
Cardiovascular disease (CVD) is the leading cause of premature death worldwide [1]. Premature CVD generally refers to having a history of CVD events before the age of 55 and 65 for men and women, respectively [2, 3]. Despite advances in CVD prevention and management, outcomes among young adults have been suboptimal. The clinical significance of identifying individuals at risk of premature CVD in reducing the burden of premature morbidity and mortality is now increasingly being recognized, due to the incidence of CVD among young adults has been stagnating or increasing [4–7]. However, evidence on characterization of biomarker profiles that identify premature CVD has been inadequate.
The reasons for suboptimal outcomes are multifactorial, including temporal trends in age-based differences in risk factors, clinical presentation, acute management, or use of preventive therapies [8–11]. Literatures regarding the determinants on premature CVD were limited. Most biomarker studies of premature CVD have been cross-sectionally designed in the European population and reported differences in the levels of serum lipids for premature vs conventional CVD, as well as differences by sex for premature CVD [11–14]. Emerging evidence suggested that CVD risk may also be associated with novel biomarkers related to lipoprotein subfractions, inflammation, and metabolic pathways, and not merely with levels of standard risk factors [15–17]. However, characterization of biomarker profiles that identify premature CVD has been inadequate. Recently, the Women’s Health Study investigated more than 50 risk factors and biomarkers related to coronary heart disease among women [18]. To our knowledge, there was no large prospective study on the association of an extensive panel of novel and traditional biomarkers according to age at the time of incident CVD among Chinese population.
To address these knowledge gaps, we investigated the relevant risk of clinical risk factors and lipid, metabolic, and inflammation biomarkers with incident CVD in a large community-based prospective cohort.
Methods
Study population
The study population were from the Kailuan study, which is an ongoing prospective cohort study conducted in Tangshan, China. The details on Kailuan study have been previously described [19–21]. Briefly, since June 2006, a total of 101,510 participants (81,110 men and 20,400 women, aged 18–98 years) were enrolled in the first survey from 11 hospitals and underwent questionnaire assessments, clinical examinations, and laboratory tests. All participants were followed biennially to update their status on the aforementioned parameters. In the present study, we excluded participants with a history of myocardial infraction or stroke (n = 3669) at baseline; ultimately, a total of 97,841 participants were included in the current analysis. The study was performed according to the guidelines of the Helsinki Declaration and was approved by the Ethics Committee of Kailuan General Hospital (approval number: 2006–05) and Beijing Tiantan Hospital (approval number: 2010–014-01). All the participants agreed to take part in the study and provided written informed consent.
Incident CVD ascertainment
Incident CVD was a composite of first stroke or myocardial infarction. Assessment of CVD has been described previously [21–23]. The database of CVD diagnoses was obtained from the Municipal Social Insurance Institution and Hospital Discharge Register and was updated annually. An expert panel collected and reviewed the annual discharge records from 11 hospitals in the Kailuan community to identify patients who were suspected of CVD. Incident stroke was diagnosed based on neurological signs, clinical symptoms, and neuroimaging tests, including computed tomography or magnetic resonance, according to the World Health Organization criteria [24]. Myocardial infarction was diagnosed according to the criteria of the World Health Organization based on clinical symptoms, changes in the serum concentrations of cardiac enzymes and biomarkers, and electrocardiographic results [22, 25].
Risk factors assessment
Baseline risk factors were collected via a standardized questionnaire by trained staff, including age, sex, education level, family income, physical activity, smoking, alcohol intake, and medical history (hypertension, diabetes, and dyslipidemia). Educational level was classified as illiterate or primary school, middle school, and high school or above. Income was categorized into > 800 and ≤ 800 yuan/month. Smoking and alcohol intake habits were stratified into never, former, or current. Physical activity included both physical activity in leisure time and at work and then was categorized as active physical activity (≥ 80 min per week at leisure or manual work) physical inactivity (< 80 min per week, or none at leisure time, or mental work) [26]. Body mass index (BMI) was calculated as weight in kilograms divided by the height in meters squared and were categorized as overweight (BMI 25.0 to < 28.0) and obsess (BMI ≥ 28.0) [27]. Blood pressure was measured in the seated position using a mercury sphygmomanometer, and the mean results of three measurements of systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded. Hypertension was defined as SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg, any use of the antihypertensive drug, or a self-reported history of hypertension [28]. Diabetes was defined as fasting blood glucose (FBG) ≥ 7.0 mmol/L, any use of glucose-lowering drugs, or a self-reported history of diabetes. Dyslipidemia was defined as any self-reported history or use of lipid-lowering drugs, or total cholesterol (TC) ≥ 5.17 mmol/L or triglyceride (TG) ≥ 1.69 mmol/L or low-density lipoprotein cholesterol (LDL-C) ≥ 3.62 mmol/L or high-density lipoprotein cholesterol (HDL-C) ≤ 1.04 mmol/L. Metabolic syndrome was defined according to the ATP-III criteria [29].
Biomarker measurements
Fasting blood samples were collected in the morning after an 8- to 12-h overnight fast and transfused into vacuum tubes containing EDTA. Plasma was separated from blood immediately and stored at 4 °C. All the blood samples were analyzed using an auto-analyzer (Hitachi 747, Hitachi, Tokyo, Japan) on the day of the blood draw. The biochemical indicators included FBG, serum lipids (TC, TG, LDL-C, HDL-C, TC/HDL-C, TG/HDL-C, non-HDL-C, remnant cholesterol [calculated as TC-LDL-C-HDL-C]), serum creatinine, high-sensitivity C-reactive protein (hs-CRP), white blood cell count, neutrophil count, and platelet count. The triglyceride-glucose index (TyG) was calculated as ln (fasting TG [mg/dl] × FBG [mg/dl]/2) [30]. Estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration creatinine equation [31].
Statistical analysis
We divided the age at CVD onset into 4 age groups (< 55, 55 to < 65, 65 to < 75, and ≥ 75 years) and participants contributed to advancing age groups over time until the occurrence of incident CVD or censoring (death or the end of the follow-up), and calculated CVD incidence rates. The baseline characteristics were presented as mean ± standard deviation (SD) or frequency with percentage as appropriate. Differences in the characteristics across 4 age categories were tested using analysis of variance or the Kruskal–Wallis test for continuous variables according to distribution, and using chi-square for categorical variables.
Considering the proportion of non-CVD death was over 5%, we used stratified competing risk models with the counting process method, stratified by the 4 age groups, in which non-CVD death was regarded as a competing risk event. We estimated adjusted sub-distributional hazard ratio (sHR) with 95% confidence interval (CI) for per SD increment of each biomarker and for clinical categories of risk factors with clinical cutoff points. The basic adjusted model included sex, educational level, and family income, then each risk factor was added individually to the basic adjusted model stratified by age groups. In additional analyses, we examine the associations between risk factors and incident CVD in the models that included covariates mentioned above plus the following additional covariates (physical activity, smoking, drinking, BMI, hypertension, diabetes, dyslipidemia, SBP, and DBP). The proportional hazard assumptions were evaluated by visualization of Schoenfeld residuals, and no potential violation was observed. The population-attributable risk for clinical risk factors was calculated with a method previously described [32]. Likelihood ratio tests were used to evaluate the interaction between each individual risk factor and age groups in the basic adjusted model.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). All statistical tests were 2-sided, and P < 0.05 was considered statistically significant.
Results
Baseline characteristics
Of 97,841 participants included in the analysis, 90,197 (92.24%) participants did not develop CVD and incident CVD occurred in 7587 patients (7.76%) during the study period. Most baseline characteristics differed between CVD cases and non-cases (Table 1, Additional file 1: Table S1) and across age groups (Additional file 1: Table S2). The prevalence of most clinical risk factors and levels of serum lipids and metabolic and inflammatory biomarkers were higher in cases than in non-cases.
Table 1.
Baseline characteristics of the participants with and without CVD
| Characteristics | No. (%) | Noncases (n = 90,197) |
|||
|---|---|---|---|---|---|
| Incidence CVD | |||||
| At age < 55 y (n = 1241) |
At 55 to < 65 y (n = 2693) |
At 65 to < 75 y (n = 2273) |
At age ≥ 75 y (n = 1380) |
||
| Clinical risk factors | |||||
| Age, y | 44.29 ± 5.56 | 53.37 ± 4.20 | 62.50 ± 4.95 | 72.66 ± 5.51 | 50.96 ± 12.59 |
| Men, n (%) | 1097 (88.40) | 2399 (89.08) | 2028 (89.22) | 1246 (90.29) | 71,048 (78.77) |
| High school or above, n (%) | 55 (4.49) | 66 (2.54) | 56 (2.62) | 36 (3.06) | 6389 (7.371) |
| Income > 800 yuan/month, n (%) | 143 (11.69) | 296 (11.44) | 254 (11.92) | 191 (16.26) | 12,427 (14.34) |
| Current smoker, n (%) | 570 (46.68) | 1112 (42.92) | 767 (35.62) | 302 (24.34) | 29,577 (33.85) |
| Current drinker, n (%) | 546 (44.64) | 1000 (38.60) | 730 (33.97) | 354 (28.55) | 32,760 (37.48) |
| Physical inactivity, n (%) | 133 (10.88) | 229 (8.88) | 111 (5.23) | 53 (4.51) | 7631 (8.82) |
| Hypertension, n (%) | 720 (58.02) | 1734 (64.39) | 1556 (68.46) | 946 (68.55) | 36,969 (40.99) |
| Diabetes, n (%) | 175 (14.10) | 486 (18.05) | 434 (19.09) | 209 (15.14) | 7438 (8.25) |
| Dyslipidemia, n (%) | 557 (44.88) | 1183 (43.93) | 980 (43.11) | 513 (37.17) | 31,145 (34.53) |
| Metabolism syndrome, n (%) | 249 (20.06) | 642 (23.84) | 583 (25.65) | 316 (22.90) | 11,967 (13.27) |
| Body mass index, kg/m.2 | 26.18 ± 3.61 | 25.91 ± 3.46 | 25.54 ± 3.37 | 25.12 ± 3.42 | 24.96 ± 3.49 |
| Overweight or obese, n (%) | 748 (60.27) | 1562 (58.00) | 1224 (53.85) | 675 (48.91) | 42,098 (46.67) |
| Systolic blood pressure, mm Hg | 138.26 ± 23.68 | 141.36 ± 22.75 | 144.56 ± 22.96 | 144.45 ± 21.27 | 129.64 ± 20.41 |
| Diastolic blood pressure, mm Hg | 90.05 ± 14.57 | 89.71 ± 13.18 | 88.00 ± 11.95 | 84.04 ± 10.73 | 82.96 ± 11.58 |
| Lipids profile | |||||
| Total cholesterol, mmol/L | 5.21 ± 1.15 | 5.12 ± 1.18 | 5.11 ± 1.22 | 5.02 ± 1.20 | 4.93 ± 1.14 |
| Triglycerides, mmol/L | 2.05 ± 1.62 | 1.97 ± 1.58 | 1.81 ± 1.45 | 1.63 ± 1.21 | 1.66 ± 1.36 |
| LDL cholesterol, mmol/L | 2.44 ± 0.93 | 2.40 ± 0.94 | 2.39 ± 1.14 | 2.33 ± 1.08 | 2.34 ± 0.90 |
| HDL cholesterol, mmol/L | 1.54 ± 0.40 | 1.55 ± 0.41 | 1.57 ± 0.44 | 1.59 ± 0.49 | 1.55 ± 0.40 |
| Total/HDL cholesterol | 3.58 ± 1.37 | 3.48 ± 1.12 | 3.48 ± 1.55 | 3.39 ± 1.47 | 3.39 ± 3.26 |
| Triglyceride/HDL cholesterol | 1.44 ± 1.37 | 1.36 ± 1.18 | 1.28 ± 1.48 | 1.13 ± 1.17 | 1.17 ± 2.26 |
| Non-HDL-C, mmol/L | 3.67 ± 1.10 | 3.56 ± 1.17 | 3.53 ± 1.21 | 3.42 ± 1.21 | 3.38 ± 1.11 |
| Remnant cholesterol, mmol/L | 1.23 ± 1.15 | 1.16 ± 1.25 | 1.14 ± 1.32 | 1.10 ± 1.36 | 1.04 ± 1.15 |
| Metabolic and Inflammatory | |||||
| Fasting blood glucose, mmol/L | 5.86 ± 2.17 | 6.04 ± 2.29 | 5.92 ± 2.18 | 5.69 ± 1.99 | 5.43 ± 1.61 |
| Triglyceride-glucose index | 8.90 ± 0.75 | 8.90 ± 0.73 | 8.81 ± 0.71 | 8.69 ± 0.68 | 8.64 ± 0.69 |
| eGFR, ml/min/1.73/m.2 | 86.25 ± 21.83 | 80.9 ± 23.98 | 76.6 ± 27.72 | 71.43 ± 40.79 | 82.56 ± 25.47 |
| Creatinine, μmol/L | 94.50 ± 41.98 | 94.74 ± 40.29 | 93.55 ± 33.05 | 95.49 ± 35.58 | 91.56 ± 29.85 |
| Serum uric acid, μmol/L | 301.01 ± 88.77 | 295.31 ± 84.63 | 306.58 ± 89.47 | 314.79 ± 94.12 | 287.85 ± 83.18 |
| Hs-CRP, mg/L | 2.49 ± 5.32 | 2.66 ± 4.60 | 3.29 ± 7.05 | 3.94 ± 8.60 | 2.33 ± 6.48 |
| White blood cell count, *109/L | 7.20 ± 1.98 | 7.04 ± 2.77 | 7.10 ± 13.09 | 6.50 ± 2.13 | 6.83 ± 9.86 |
| Neutrophil count, *109/L | 4.34 ± 1.50 | 4.18 ± 1.71 | 4.13 ± 2.36 | 3.91 ± 1.29 | 3.97 ± 2.91 |
| Platelet, *109/L | 219.23 ± 82.37 | 205.74 ± 56.82 | 198.56 ± 79.37 | 189.93 ± 78.41 | 211.22 ± 746.16 |
| Red blood cell count, *109/L | 4.96 ± 0.50 | 4.89 ± 0.48 | 5.37 ± 16.04 | 4.67 ± 0.48 | 5.05 ± 26.46 |
Noncases did not develop cardiovascular disease during follow-up
CVD Cardiovascular disease, eGFR Estimated glomerular filtration rate, HDL High-density lipoprotein, hs-CRP High-sensitivity C-reactive protein, LDL Low-density lipoprotein
During median follow-up of 12.99 years, CVD incidence per 1000 person-years ranged from 4.69 (95% CI, 4.44–4.96) for CVD onset less than 55 years to 7.50 (95% CI, 7.11–7.90) for CVD onset at 75 years or older (Additional file 1: Table S3).
Clinical risk factors
Diabetes had the highest relative risk for incident CVD onset less than 55 years; the adjusted sHR was 4.08 (95% CI, 3.47–4.80). For incident CVD onset 55 years or older, hypertension had the highest relative risk; the adjusted sHR was 2.61 (95% CI, 2.41–2.82) and attenuated with age, with a risk of 1.59 (95% CI, 1.43–1.78) at onset in those 75 years or older. Increased risks were also noted for CVD onset in participants younger than 55 years for metabolism syndrome (sHR, 3.38; 95% CI, 2.94–3.88), overweight or obese (sHR, 1.71; 95% CI, 1.52–1.92), dyslipidemia (sHR, 1.62; 95% CI, 1.45–1.82), and smoking (sHR, 1.49; 95% CI, 1.33–1.67), which also attenuated with age (Fig. 1 and Table 2).
Fig. 1.
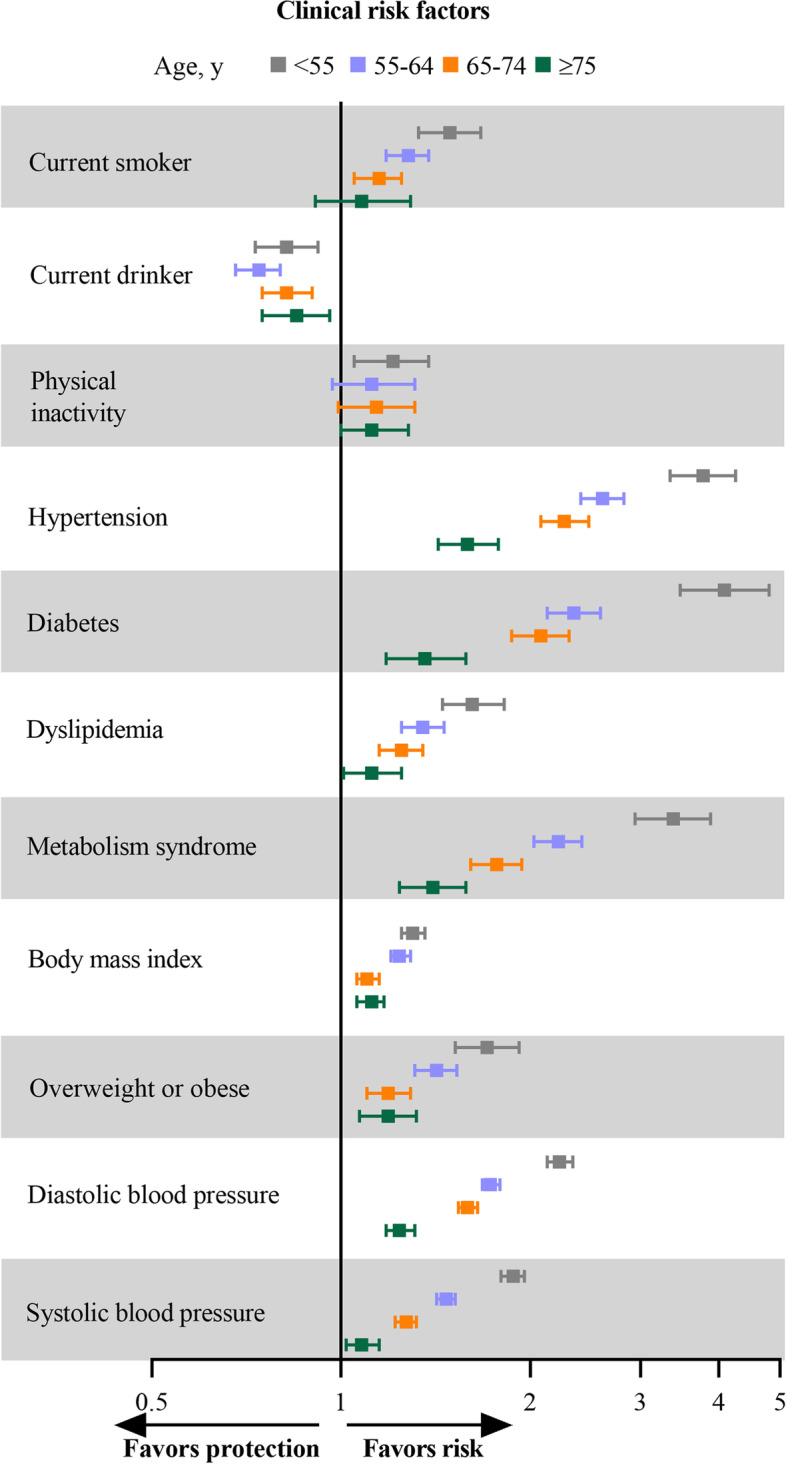
Sub-distributional hazard ratios with 95% confidence intervals for the association between clinical risk factors and risk of cardiovascular disease in different CVD-onset age groups. Model was adjusted for gender, educational level, and family income, and interactions between the risk factors of interest and age groups
Table 2.
Associations of risk factors with incident CVD by age at onset
| Incident CVD, adjusted sHR (95%CI) | P for interaction | ||||
|---|---|---|---|---|---|
| At age < 55 y | At age 55 to 65 y | At age 65 to 75 y | At age ≥ 75 y | ||
| Clinical risk factors | |||||
| Current smoker | 1.49(1.33–1.67) | 1.28(1.18–1.38) | 1.15(1.05–1.25) | 1.08(0.91–1.29) | 0.0006 |
| Current drinker | 0.82(0.73–0.92) | 0.74(0.68–0.80) | 0.82(0.75–0.90) | 0.85(0.75–0.96) | 0.8373 |
| Physical inactivity | 1.21(1.05–1.38) | 1.12(0.97–1.31) | 1.14(0.99–1.31) | 1.12(1.00–1.28) | 0.0844 |
| Hypertension | 3.77(3.34–4.25) | 2.61(2.41–2.82) | 2.27(2.08–2.48) | 1.59(1.43–1.78) | < 0.0001 |
| Diabetes | 4.08(3.47–4.80) | 2.35(2.13–2.59) | 2.08(1.87–2.31) | 1.36(1.18–1.58) | < 0.0001 |
| Dyslipidemia | 1.62(1.45–1.82) | 1.35(1.25–1.46) | 1.25(1.15–1.35) | 1.12(1.01–1.25) | < 0.0001 |
| Metabolism syndrome | 3.38(2.94–3.88) | 2.22(2.03–2.42) | 1.77(1.61–1.94) | 1.40(1.24–1.58) | < 0.0001 |
| BMI, per SD increment | 1.30(1.25–1.36) | 1.24(1.20–1.29) | 1.10(1.06–1.15) | 1.12(1.06–1.17) | < 0.0001 |
| Overweight or obese | 1.71(1.52–1.92) | 1.42(1.31–1.53) | 1.19(1.10–1.29) | 1.19(1.07–1.32) | < 0.0001 |
| Systolic BP, per SD increment | 2.22(2.12–2.34) | 1.73(1.67–1.78) | 1.58(1.52–1.63) | 1.24(1.18–1.30) | < 0.0001 |
| Diastolic BP, per SD increment | 1.88(1.80–1.96) | 1.47(1.42–1.51) | 1.27(1.22–1.32) | 1.09(1.03–1.15) | < 0.0001 |
| Lipids, per SD increment | |||||
| Total cholesterol | 1.33(1.27–1.38) | 1.10(1.06–1.14) | 1.07(1.03–1.12) | 1.07(1.02–1.13) | < 0.0001 |
| Triglycerides | 1.15(1.11–1.19) | 1.10(1.07–1.13) | 1.08(1.05–1.12) | 1.09(1.03–1.15) | 0.0193 |
| LDL cholesterol | 1.05(0.98–1.14) | 1.01(0.97–1.06) | 1.04(1.00–1.08) | 1.03(0.99–1.08) | 0.8382 |
| HDL cholesterol | 1.14(1.07–1.21) | 1.04(1.00–1.09) | 1.04(1.00–1.08) | 0.98(0.93–1.03) | 0.0023 |
| Total/HDL cholesterol | 1.03(1.01–1.05) | 1.04(1.00–1.07) | 1.00(0.99–1.01) | 1.01(1.00–1.02) | 0.0549 |
| Triglycerides/HDL cholesterol | 1.01(1.00–1.01) | 1.14(1.09–1.19) | 1.07(1.03–1.11) | 1.04(1.01–1.07) | < 0.0001 |
| Non–HDL cholesterol | 1.32(1.26–1.38) | 1.09(1.05–1.13) | 1.06(1.01–1.10) | 1.08(1.03–1.14) | < 0.0001 |
| Remnant cholesterol | 1.31(1.25–1.38) | 1.08(1.04–1.12) | 1.02(0.98–1.06) | 1.05(0.99–1.10) | < 0.0001 |
| Metabolic, per SD increment | |||||
| Fasting blood glucose | 1.31(1.24–1.38) | 1.22(1.19–1.25) | 1.16(1.13–1.19) | 1.09(1.05–1.14) | < 0.0001 |
| Triglyceride-glucose index | 1.42(1.35–1.49) | 1.26(1.22–1.31) | 1.19(1.15–1.24) | 1.14(1.09–1.21) | < 0.0001 |
| eGFR | 0.78(0.73–0.83) | 0.79(0.75–0.84) | 0.74(0.67–0.81) | 0.96(0.85–1.09) | 0.0030 |
| Creatinine | 1.00(0.93–1.06) | 1.04(1.01–1.07) | 1.07(1.04–1.11) | 1.04(1.00–1.08) | 0.1459 |
| Serum uric acid | 1.14(1.08–1.21) | 1.10(1.05–1.14) | 1.18(1.13–1.23) | 1.07(1.02–1.12) | 0.0076 |
| Inflammatory, per SD increment | |||||
| Hs–CRP | 1.12(1.08–1.17) | 1.03(1.01–1.05) | 1.06(1.03–1.09) | 1.03(1.00–1.05) | 0.0001 |
| White blood cell count | 1.07(1.01–1.12) | 1.00(0.99–1.01) | 1.02(0.99–1.04) | 0.97(0.94–1.00) | 0.1470 |
| Neutrophil count | 1.07(1.05–1.10) | 1.03(1.01–1.04) | 1.06(1.04–1.09) | 1.00(0.97–1.03) | 0.0248 |
| Platelet | 1.00(0.99–1.00) | 0.44(0.28–0.69) | 0.68(0.37–1.24) | 0.96(0.55–1.65) | 0.0022 |
| Red blood cell count | 0.00(0.00–0.86) | 1.01(0.98–1.04) | 1.01(0.98–1.04) | 0.97(0.94–1.00) | 0.0708 |
sHRs (95% CI) were obtained from stratified competing risk models with non-CVD death as a competing risk, adjusted for gender, educational level, and family income, and interactions between the risk factors of interest and age groups
CI Confidence interval, CVD Cardiovascular disease, eGFR Estimated glomerular filtration rate, HDL High-density lipoprotein, hs-CRP High-sensitivity C-reactive protein, LDL Low-density lipoprotein, SD Standard deviation, sHR Sub-distributional hazard ratio
Lipids profiles
Significant associations with CVD onset in participants younger than 55 years were noted (per SD increase) for TC (sHR, 1.33; 95% CI, 1.27–1.38), TG (sHR, 1.15; 95% CI, 1.11–1.19), HDL-C (sHR, 1.14; 95% CI, 1.07–1.21), non-HDL-C (sHR, 1.32; 95% CI, 1.26–1.38), and remnant cholesterol (sHR, 1.31; 95% CI, 1.25–1.38) and attenuated with age. These biomarkers showed associations with incident CVD in almost all age groups. However, no significant difference was noted for LDL-C and TC/HDL-C with CVD by increasing age (Fig. 2 and Table 2).
Fig. 2.
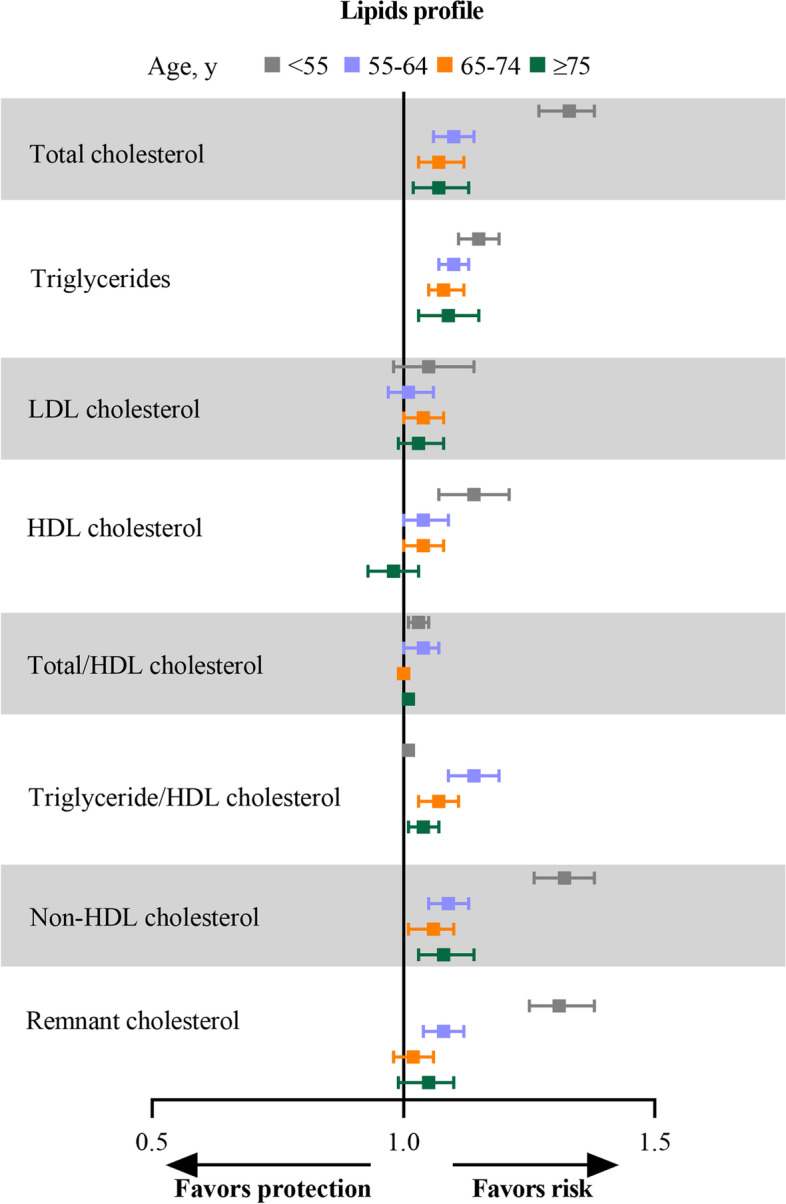
Sub-distributional hazard ratios with 95% confidence intervals for the association between lipids and risk of cardiovascular disease in different CVD-onset age groups. Model was adjusted for gender, educational level, and family income, and interactions between the risk factors of interest and age groups. Abbreviations: LDL, low-density lipoprotein; HDL, high-density lipoprotein
Metabolic and inflammatory biomarkers
The TyG index had the highest relative risk of incident CVD onset at any age, for CVD onset younger than 55 years; the adjusted sHR for per SD increase was 1.42 (95% CI, 1.35–1.49) and attenuated with age, with a risk of 1.14 (95% CI, 1.09–1.21) at onset in those 75 years or older. Other biomarkers, including FBG (sHR, 1.31; 95% CI, 1.24–1.38), SUA (sHR, 1.154; 95% CI, 1.08–1.21), hs-CRP (sHR, 1.12; 95% CI, 1.08–1.17), and neutrophil count (sHR, 1.07; 95% CI, 1.04–1.12), showed positive associations with incident CVD; eGFR (sHR, 0.78; 95% CI, 0.73–0.83) showed negative association with incident CVD which all attenuated with age (Fig. 3 and Table 2).
Fig. 3.
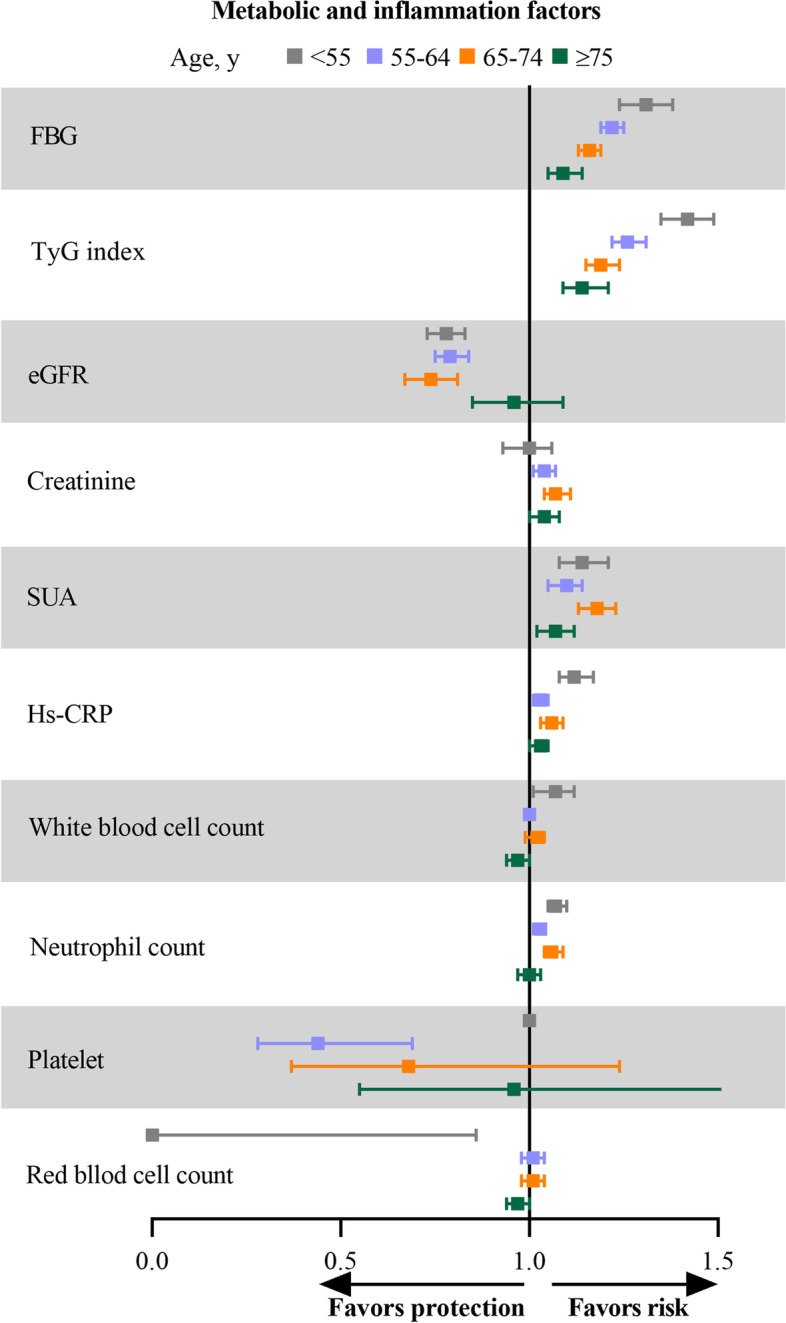
Sub-distributional hazard ratios with 95% confidence intervals for the association of metabolic and inflammatory biomarkers with risk of cardiovascular disease in different CVD-onset age groups. Model was adjusted for gender, educational level, and family income, and interactions between the risk factors of interest and age groups. Abbreviations: eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; hs-CRP, high sensitivity C-reactive protein; SUA, serum uric acid; TyG index, triglyceride glucose index
Additional analyses
We examined the associations with incident CVD using models further adjusted for additional covariates (physical activity, smoking, drinking, BMI, hypertension, diabetes, dyslipidemia, SBP, and DBP) and evaluated the associations using separate models for each clinical risk factor at a time. The associations of biomarkers with incident CVD are generally preserved in the models (Additional file 1: Table S4).
Population-attributable risk
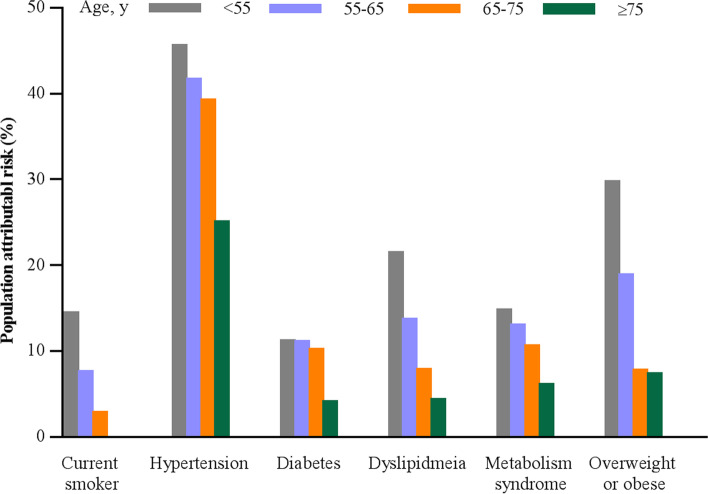
The population-attributable risk for the clinical risk factors attenuated with age (Fig. 4 and Additional file 1: Table S5). Of the categorical clinical risk factors analyzed, hypertension had the highest population-attributable risk in all age groups, which ranged from 45.4% (95% CI, 41.7–48.9%) for incident CVD onset younger than 55 years to 24.9% (95% CI, 19.0–30.7%) for onset in those 75 years or older, while smoking had the lowest population-attributable risk in all age groups.
Fig. 4.
Population attribution risk for risk factors and incident cardiovascular disease across age categories
Discussion
In this large prospective study, we identified risk profiles associated with the risk of CVD occurring at younger ages. Of the clinical factors, diabetes was associated with the highest relative risk for incident CVD in participants younger than 55 years, in addition to hypertension, metabolism syndrome, overweight or obese, dyslipidemia, and smoking, which were also strong risk factors for premature CVD. Among the biomarkers examined in participants with CVD younger than 55 years, the TyG index reflecting insulin resistance had the highest magnitude of relative risk, which was greater than the association of FBG, lipids, or inflammatory biomarkers. Most clinical risk factors and biomarkers of cardiovascular risk showed age-attenuated associations with incident CVD. These findings underscore the importance of diabetes and insulin resistance as major determinants of premature CVD, as well as other modified major risk factors that can be addressed with lifestyle or preventive interventions.
Early life exposure of cardiovascular risk factors has been emerging as potentially important risk factor for premature CVD. Diabetes mellitus has been reported to be associated with excess cardiovascular morbidity and mortality in all age groups, while the most pronounced effect was observed in young people [19, 33]. A 23-year follow-up of the Da Qing Diabetes Study showed that young-onset diabetes was a risk factor for premature death and cardiovascular disease [33]. In line with prior findings, our study found that a history of diabetes was associated with a fourfold increase in the risk of premature CVD. Accumulating evidence supports that diabetes in younger people has a more rapid deterioration of β-cell function than is seen in later-onset diabetes [34]. This loss of β-cell function might result in higher SBP and LDL-C concentrations due to increased oxidative stress and activation of renin-angiotensin system, which would accelerate atherosclerosis and increase the risk of premature CVD [35]. Observations of blood pressure in young adults confirmed the effect of hypertension on CVD events [36]. Our data showed that a history of hypertension was associated with a 3.8-fold higher risk of premature CVD. Existing evidence suggested that early-onset hypertension has been found to be more affected by hereditary susceptibility [37]. Similarly, obese and overweight were associated with incident CVD and could be targeted to reduce the risk of CVD, in particular younger adults. Behavioral modification could also target smoking, as it was associated with a higher risk of incident CVD, especially in young adults. Smoking remains a major public health problem across all ages but in particular for younger individuals, and smoking cessation initiatives should remain part of efforts to reduce cardiovascular risk across all ages [38]. It should be noted that, in our study, current drinking tended to have protective effects on the risk of premature CVD, which was supported by several previous studies [39–41], demonstrating that alcohol consumption, especially moderate drinking was associated with decreased risk of CVD. Possible mechanisms may be that alcohol has been shown to raise HDL-C and apolipoprotein A-I, which are inversely associated with the risk of CVD. Additionally, alcohol consumption may also influence atherosclerotic plaque composition and provide stabilizing benefits.
Many previous studies have evaluated the associations between cholesterol levels and CVD risk in young adults. Studies on dyslipidemia and premature CVD measured biomarkers at the time of the acute CVD event and reported on the prevalence of dyslipidemia or risk of CVD based on unadjusted models, reaching disparate conclusions. These studies showed mixed results with a higher, lower, or similar prevalence of dyslipidemia in younger vs older adults [42–44]. Three large cohorts of younger men from the Chicago Heart Association Detection Project in Industry, Chicago People Gas Company, and Multiple Risk Factor Intervention Trial studies demonstrate a continuous, graded relationship of serum cholesterol level to long-term risk of CVD, substantial absolute risk, and absolute excess risk of CVD death for younger men with elevated serum cholesterol levels [45]. Data from the Coronary Artery Risk Development In Young Adults study indicated that non-optimal HDL-C at commonly observed levels during young adulthood was independently associated with coronary atherosclerosis two decades later [46]. Our present results are consistent with these previous observations and provide additional evidence for the atherogenicity of serum lipids (TC, TG, HDL-C, non-HDL-C, and remnant cholesterol) at different ages, and the effects were more pronounced in young adults.
In the present study, insulin resistance measured by the TyG index had the strongest association with premature CVD out of approximately 10 biomarkers examined. Insulin resistance is a chronic disorder that leads to deleterious changes in the blood vessel wall and premature CVD; the TyG index developed from TG and FBG was a simple and reliable surrogate for insulin resistance and was highly correlated with the hyperinsulinemic-euglycemic clamp and homeostasis model assessment of insulin resistance [20]. In our study, the TyG index had a greater association with CVD occurring in participants at younger ages and up to 75 years than all the other biomarkers. The TyG index potentially links insulin resistance and its concomitant thermogenic dyslipidemia with future risk of both diabetes and premature CVD. Chronic kidney disease is an emerging public health problem and can be regarded as a premature CVD entity [47]; our study showed that decreased eGFR and increased SUA were significantly associated with the risk of CVD, especially among young adults. This finding was supported by a previous study that hyperuricemia at an early age was associated with a higher risk of CVD than later-onset hyperuricemia [48].
The positive associations of inflammatory biomarkers with CVD, which was more pronounced for premature CVD, were supported by the growing evidence on the role of inflammation in initial and recurrent cardiovascular events. The Health, Aging and Body Composition study showed hs-CRP is less predictive of CVD in older compared with younger adults [49]. Lower platelet counts were reported to be associated with increased risk of thrombotic events, our study found the association between platelet counts and CVD differed by age of CVD onset, and increased platelet counts may be a protective role in CVD among adults with 55 to 65 years of CVD onset. In addition, neutrophil counts, as a ubiquitous biomarker of acute infection and inflammation, were strongly associated with the incidence of CVD. The findings were extended via our study by showing the positive association between neutrophil counts was more evident in younger adults. Several cardiovascular disease risk factors were associated with high inflammation levels, including smoking, blood pressure, diabetes, BMI, and abdominal adiposity [50], suggesting that inflammation that accompanies excess adiposity states, such as diabetes and insulin resistance, could be even more relevant for CVD occurring at a younger age.
In this study, the incidence rates of CVD increased with age, consistent with age being a substantial risk factor. Analysis of the relative rates of risk factors for incident CVD should not, however, imply that risk factors are more important at younger vs older ages. Therefore, the importance of primary CVD prevention among older adults is not diminished by the observed attenuation of relative rates of risk factors with incident CVD in older individuals. Rather, these results suggest a stronger relative association of risk factors with younger vs older ages and emphasize the need for improved primary prevention among younger adults. The age-related attenuation of relative risk has implications for cardiovascular risk modeling depending on the age group in which CVD occurs. Young adults are unaware of their heightened cardiometabolic and mortality risk, which would still translate into a great disease burden. Improving modifiable clinical risk factors could substantially reduce CVD risk.
Strengths of this study included the large number of participants, long follow-up period, and the collection of information on various lifestyle factors and biomarkers. The study also has several limitations. First, lifestyle and medical history were self-reported, which may be subject to recall bias. Second, there are challenges to compare the risks associated with clinical categorical variables vs continuous biomarkers. Similar issues affect the population-attributable risks, which depend on the magnitude of risk association with the prevalence of the risk factors in the population. Third, our study was conducted among Chinese; thus, the results may not be generalized to other populations.
Conclusions
In conclusion, our study has identified risk factors and biomarkers associated with the risk of CVD occurring in adults at younger vs older ages. The most substantial risk of premature CVD was associated with diabetes and insulin resistance biomarkers, as well as hypertension, metabolic syndrome, overweight or obese, dyslipidemia, and smoking. Most lipid profiles and metabolic and inflammatory biomarkers were also associated with premature CVD risk, albeit their relative magnitude was less than the TyG index. Although the relative risk of CVD was attenuated with age, cardiometabolic risk factors prevention and management remained important at all ages. These findings highlight the importance of early identification, screening, stratification, and treatment to decrease the burden of premature CVD and mortality.
Supplementary Information
Additional file 1: Table S1. Baseline characteristics of participants with and without incident CVD. Table S2. Baseline characteristics of participants by different age. Table S3. Incidence rate of CVD in different age group. Table S4. Multivariable-adjusted associations of risk factors with incident CVD by age at onset. Table S5. PAR and 95% CI for cardiovascular disease by risk factors.
Acknowledgements
We thank all study participants, their relatives, the members of the survey teams at the 11 regional hospitals of the Kailuan Medical Group, and the project development and management teams at the Beijing Tiantan Hospital and the Kailuan Group.
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- eGFR
Estimated glomerular filtration rate
- FBG
Fasting blood glucose
- HDL-C
High-density lipoprotein cholesterol
- hs-CRP
High-sensitivity C-reactive protein
- LDL-C
Low-density lipoprotein cholesterol
- SBP
Systolic blood pressure
- SD
Standard deviation
- sHR
Sub-distributional hazard ratio
- TC
Total cholesterol
- TG
Triglyceride
- TyG index
Triglyceride-glucose index
Authors’ contributions
XT and SC wrote the manuscript. AW, XT, and YZ1 (Yingting Zuo) researched the data. SC, YZ2 (Yijun Zhang), XZ, and QX researched the data and contributed to the discussion. YL and SW contributed to the discussion and reviewed/edited the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2018YFC1312400 and 2018YFC1312402) and Beijing Municipal Administration of Hospitals Incubating Program (PX2020021).
Availability of data and materials
Data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.
Declarations
Ethics approval and consent to participate
The study was performed according to the guidelines of the Helsinki Declaration and was approved by the Ethics Committee of Kailuan General Hospital (approval number: 2006–05) and Beijing Tiantan Hospital (approval number: 2010–014-01). All participants agreed to take part in the study and provided informed written consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xue Tian and Shuohua Chen contributed equally to this work.
Contributor Information
Yanxia Luo, Email: lyx100@ccmu.edu.cn.
Shouling Wu, Email: drwusl@163.com.
Anxin Wang, Email: wanganxin@bjtth.org.
References
- 1.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Nam BH, D'Agostino RB, Sr, Levy D, Murabito JM, Wang TJ, Wilson PW, O'Donnell CJ. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA. 2004;291(18):2204–2211. doi: 10.1001/jama.291.18.2204. [DOI] [PubMed] [Google Scholar]
- 3.Stone NJ, Robinson JG, Lichtenstein AH, BaireyMerz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2018;15(4):230–240. doi: 10.1038/nrcardio.2017.154. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50(22):2128–2132. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 6.George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995–2008. Ann Neurol. 2011;70(5):713–721. doi: 10.1002/ana.22539. [DOI] [PubMed] [Google Scholar]
- 7.Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, Flaherty ML, Khatri P, Ferioli S, De Los Rios La Rosa F et al. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79(17):1781–1787. doi: 10.1212/WNL.0b013e318270401d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucholz EM, Strait KM, Dreyer RP, Lindau ST, D'Onofrio G, Geda M, Spatz ES, Beltrame JF, Lichtman JH, Lorenze NP, et al. Editor’s choice-sex differences in young patients with acute myocardial infarction: A VIRGO study analysis. Eur Heart J Acute Cardiovasc Care. 2017;6(7):610–622. doi: 10.1177/2048872616661847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelletier R, Humphries KH, Shimony A, Bacon SL, Lavoie KL, Rabi D, Karp I, Tsadok MA, Pilote L. Sex-related differences in access to care among patients with premature acute coronary syndrome. CMAJ. 2014;186(7):497–504. doi: 10.1503/cmaj.131450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao M, Vaartjes I, Graham I, Grobbee D, Spiering W, Klipstein-Grobusch K, Woodward M, Peters SA. Sex differences in risk factor management of coronary heart disease across three regions. Heart. 2017;103(20):1587–1594. doi: 10.1136/heartjnl-2017-311429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pancholy SB, Shantha GP, Patel T, Cheskin LJ. Sex differences in short-term and long-term all-cause mortality among patients with ST-segment elevation myocardial infarction treated by primary percutaneous intervention: a meta-analysis. JAMA Intern Med. 2014;174(11):1822–1830. doi: 10.1001/jamainternmed.2014.4762. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 13.Singh A, Collins BL, Gupta A, Fatima A, Qamar A, Biery D, Baez J, Cawley M, Klein J, Hainer J, et al. Cardiovascular risk and statin eligibility of young adults after an MI: Partners YOUNG-MI Registry. J Am Coll Cardiol. 2018;71(3):292–302. doi: 10.1016/j.jacc.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avezum A, Makdisse M, Spencer F, Gore JM, Fox KA, Montalescot G, Eagle KA, White K, Mehta RH, Knobel E, et al. Impact of age on management and outcome of acute coronary syndrome: observations from the Global Registry of Acute Coronary Events (GRACE) Am Heart J. 2005;149(1):67–73. doi: 10.1016/j.ahj.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Stegemann C, Pechlaner R, Willeit P, Langley SR, Mangino M, Mayr U, Menni C, Moayyeri A, Santer P, Rungger G, et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation. 2014;129(18):1821–1831. doi: 10.1161/CIRCULATIONAHA.113.002500. [DOI] [PubMed] [Google Scholar]
- 16.Würtz P, Havulinna AS, Soininen P, Tynkkynen T, Prieto-Merino D, Tillin T, Ghorbani A, Artati A, Wang Q, Tiainen M, et al. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation. 2015;131(9):774–785. doi: 10.1161/CIRCULATIONAHA.114.013116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mora S, Buring JE, Ridker PM, Cui Y. Association of high-density lipoprotein cholesterol with incident cardiovascular events in women, by low-density lipoprotein cholesterol and apolipoprotein B100 levels: a cohort study. Ann Intern Med. 2011;155(11):742–750. doi: 10.7326/0003-4819-155-11-201112060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dugani SB, Moorthy MV, Li C, Demler OV, Alsheikh-Ali AA, Ridker PM, Glynn RJ, Mora S. Association of lipid, inflammatory, and metabolic biomarkers with age at onset for incident coronary heart disease in women. JAMA Cardiol. 2021;6(4):437–447. doi: 10.1001/jamacardio.2020.7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao M, Song L, Sun L, Wang M, Wang C, Yao S, Li Y, Yun C, Zhang S, Sun Y, et al. Associations of type 2 diabetes onset age with cardiovascular disease and mortality: the Kailuan study. Diabetes Care. 2021;44(6):1426–1432. doi: 10.2337/dc20-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang A, Tian X, Zuo Y, Chen S, Meng X, Wu S, Wang Y. Change in triglyceride-glucose index predicts the risk of cardiovascular disease in the general population: a prospective cohort study. Cardiovasc Diabetol. 2021;20(1):113. doi: 10.1186/s12933-021-01305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C, Yuan Y, Zheng M, Pan A, Wang M, Zhao M, Li Y, Yao S, Chen S, Wu S, et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. J Am Coll Cardiol. 2020;75(23):2921–2930. doi: 10.1016/j.jacc.2020.04.038. [DOI] [PubMed] [Google Scholar]
- 22.Jin C, Chen S, Vaidya A, Wu Y, Wu Z, Hu FB, Kris-Etherton P, Wu S, Gao X. Longitudinal change in fasting blood glucose and myocardial infarction risk in a population without diabetes. Diabetes Care. 2017;40(11):1565–1572. doi: 10.2337/dc17-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu S, Song Y, Chen S, Zheng M, Ma Y, Cui L, Jonas J. Blood pressure classification of 2017 associated with cardiovascular disease and mortality in young Chinese adults. Hypertension. 2020;76(1):251–258. doi: 10.1161/HYPERTENSIONAHA.119.14239. [DOI] [PubMed] [Google Scholar]
- 24.Stroke Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20(10):1407–1431. doi: 10.1161/01.STR.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 25.Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas A, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90(1):583–612. doi: 10.1161/01.CIR.90.1.583. [DOI] [PubMed] [Google Scholar]
- 26.Wendel-Vos GC, Schuit AJ, Saris WH, Kromhout D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol. 2003;56(12):1163–1169. doi: 10.1016/S0895-4356(03)00220-8. [DOI] [PubMed] [Google Scholar]
- 27.Chen C, Lu FC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17(Suppl):1–36. [PubMed] [Google Scholar]
- 28.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 29.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 30.Ma X, Dong L, Shao Q, Cheng Y, Lv S, Sun Y, Shen H, Wang Z, Zhou Y, Liu X. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):31. doi: 10.1186/s12933-020-01006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol. 2017;3(9):1228–1236. doi: 10.1001/jamaoncol.2016.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hui Y, Wang J, An Y, Gong Q, Li H, Zhang B, Shuai Y, Chen Y, Hu Y, Li G. Premature death and risk of cardiovascular disease in young-onset diabetes: a 23-year follow-up of the Da Qing Diabetes Study. Endocrine. 2019;65(1):46–52. doi: 10.1007/s12020-019-01928-7. [DOI] [PubMed] [Google Scholar]
- 34.Zhou S, Meng X, Wang S, Ren R, Hou W, Huang K, Shi H. A 3-year follow-up study of β-cell function in patients with early-onset type 2 diabetes. Exp Ther Med. 2016;12(2):1097–1102. doi: 10.3892/etm.2016.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liguori A, Abete P, Hayden JM, Cacciatore F, Rengo F, Ambrosio G, Bonaduce D, Condorelli M, Reaven PD, Napoli C. Effect of glycaemic control and age on low-density lipoprotein susceptibility to oxidation in diabetes mellitus type 1. Eur Heart J. 2001;22(22):2075–2084. doi: 10.1053/euhj.2001.2655. [DOI] [PubMed] [Google Scholar]
- 36.Miura K, Daviglus ML, Dyer AR, Liu K, Garside DB, Stamler J, Greenland P. Relationship of blood pressure to 25-year mortality due to coronary heart disease, cardiovascular diseases, and all causes in young adult men: the Chicago heart association detection project in industry. Arch Intern Med. 2001;161(12):1501–1508. doi: 10.1001/archinte.161.12.1501. [DOI] [PubMed] [Google Scholar]
- 37.Niiranen TJ, McCabe EL, Larson MG, Henglin M, Lakdawala NK, Vasan RS, Cheng S. Heritability and risks associated with early onset hypertension: multigenerational, prospective analysis in the framingham heart study. BMJ. 2017;357:j1949. doi: 10.1136/bmj.j1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tolstrup JS, Hvidtfeldt UA, Flachs EM, Spiegelman D, Heitmann BL, Bälter K, Goldbourt U, Hallmans G, Knekt P, Liu S, et al. Smoking and risk of coronary heart disease in younger, middle-aged, and older adults. Am J Public Health. 2014;104(1):96–102. doi: 10.2105/AJPH.2012.301091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song RJ, Nguyen XT, Quaden R, Ho YL, Justice AC, Gagnon DR, Cho K, O'Donnell CJ, Concato J, Gaziano JM, et al. Alcohol consumption and risk of coronary artery disease (from the Million Veteran Program) Am J Cardiol. 2018;121(10):1162–1168. doi: 10.1016/j.amjcard.2018.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukamal KJ, Jensen MK, Grønbaek M, Stampfer MJ, Manson JE, Pischon T, Rimm EB. Drinking frequency, mediating biomarkers, and risk of myocardial infarction in women and men. Circulation. 2005;112(10):1406–1413. doi: 10.1161/CIRCULATIONAHA.105.537704. [DOI] [PubMed] [Google Scholar]
- 41.Zhang XY, Shu L, Si CJ, Yu XL, Liao D, Gao W, Zhang L, Zheng PF. Dietary patterns, alcohol consumption and risk of coronary heart disease in adults: a meta-analysis. Nutrients. 2015;7(8):6582–6605. doi: 10.3390/nu7085300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377(9765):557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goliasch G, Wiesbauer F, Blessberger H, Demyanets S, Wojta J, Huber K, Maurer G, Schillinger M, Speidl WS. Premature myocardial infarction is strongly associated with increased levels of remnant cholesterol. J Clin Lipidol. 2015;9(6):801–806.e801. doi: 10.1016/j.jacl.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Wei Y, Qi B, Xu J, Zhou G, Chen S, Ouyang P, Liu S. Age- and sex-related difference in lipid profiles of patients hospitalized with acute myocardial infarction in East China. J Clin Lipidol. 2014;8(6):562–567. doi: 10.1016/j.jacl.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA. 2000;284(3):311–318. doi: 10.1001/jama.284.3.311. [DOI] [PubMed] [Google Scholar]
- 46.Pletcher MJ, Bibbins-Domingo K, Liu K, Sidney S, Lin F, Vittinghoff E, Hulley SB. Nonoptimal lipids commonly present in young adults and coronary calcium later in life: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Ann Intern Med. 2010;153(3):137–146. doi: 10.7326/0003-4819-153-3-201008030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stenvinkel P. Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease. J Intern Med. 2010;268(5):456–467. doi: 10.1111/j.1365-2796.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- 48.Li L, Zhao M, Wang C, Zhang S, Yun C, Chen S, Cui L, Wu S, Xue H. Early onset of hyperuricemia is associated with increased cardiovascular disease and mortality risk. Clin Res Cardiol. 2021;110(7):1096–1105. doi: 10.1007/s00392-021-01849-4. [DOI] [PubMed] [Google Scholar]
- 49.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Tracy RP, Rubin SM, Harris TB, Pahor M. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study) Am J Cardiol. 2003;92(5):522–528. doi: 10.1016/S0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- 50.Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res. 2005;66(2):265–275. doi: 10.1016/j.cardiores.2004.12.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Baseline characteristics of participants with and without incident CVD. Table S2. Baseline characteristics of participants by different age. Table S3. Incidence rate of CVD in different age group. Table S4. Multivariable-adjusted associations of risk factors with incident CVD by age at onset. Table S5. PAR and 95% CI for cardiovascular disease by risk factors.
Data Availability Statement
Data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.