Abstract
Background and aims
A novel non–insulin-based metabolic score for insulin resistance (METS‐IR) index has been proposed as a simple and reliable alternative insulin resistance (IR) marker, but its the predictive value in asymptomatic adults with coronary artery calcification (CAC) remains unclear.
Methods and results
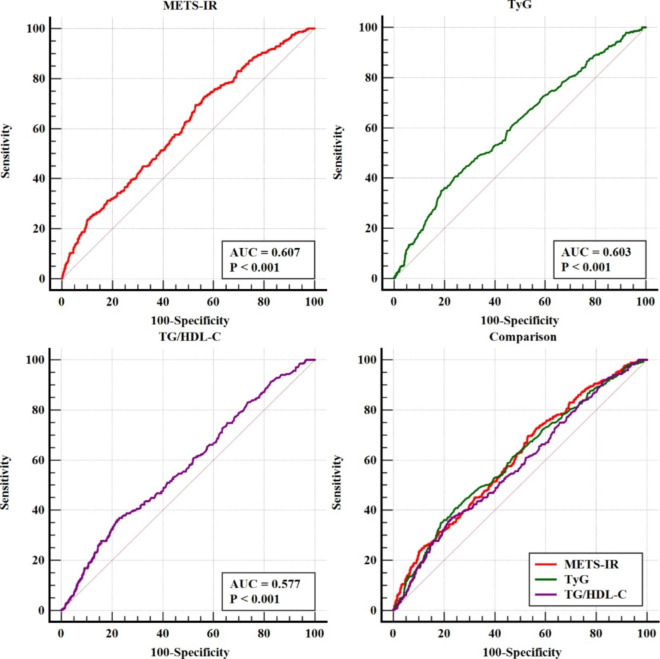
We enrolled 1576 participants without cardiovascular disease (CVD), who underwent multidetector computed tomography. Logistic regression, restricted cubic spline models and receiver operating characteristic (ROC) curves were used to examine the association between METS-IR, the ratio of triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) and triglyceride glucose index (TyG index) and CAC. In multivariate logistic regression analysis, the increase in METS-IR was independently associated with a higher prevalence of CAC (all P < 0.05 in Models 1–3). Furthermore, restricted cubic splines indicated that the significance of METS-IR in predicting CAC was higher than that of other IR indexes. In ROC curve analysis, without considering the P value, the area under the curve of CAC predicted by METS-IR was higher than that of other IR indexes (METS-IR, 0.607; TyG index, 0.603; TG/HDL-C, 0.577).
Conclusion
Compared with other IR indexes, METS-IR may have better discrimination ability in predicting the incidence of CAC in asymptomatic adults without CVD.
Keywords: Insulin resistance, Metabolic score for insulin resistance, Triglyceride glucose index, Coronary artery calcification
Introduction
At present, cardiovascular disease (CVD) has been established as the main cause of morbidity and mortality all over the world, which has greatly increased the physical and economic burden on people [1]. Over the years, although secondary prevention strategies for CVD including drug therapy, revascularization and rehabilitation have been broadly developed and applied, the risk of recurrence and mortality of cardiovascular complications is still comparatively high in patients with CVD, especially those with high risk factors [2]. Therefore, the primary prevention of CVD is particularly important, such as identifying and correcting risk factors in high-risk groups of CVD. Coronary atherosclerosis has been recognized as the leading cause of CVD, so timely detection and prevention of coronary atherosclerosis has become the main strategy for the primary prevention of CVD. Coronary artery calcification (CAC) is a highly reliable and robust biomarker for coronary atherosclerosis and is closely related to the traditional risk factors for CVD, which plays an important role in the primary prevention of CVD [3–5]. Several studies have unanimously indicated that CAC assessed by multidetector computed tomography (CT), an imaging technique used to noninvasively quantify coronary calcium, can be used as a reliable and repeatable predictor and prognostic factor for CVD, independent of other traditional risk factors [4, 6, 7]. Current guidelines also show that screening for CAC is very important to improve cardiovascular risk assessment and guide the use of preventive treatment in the middle-and low-risk populations without CVD and asymptomatic individuals [8, 9]. Therefore, it is very important to identify CAC and its risk factors if we want to develop new therapeutic targets and customize risk reduction strategies that match individual risk levels.
Insulin resistance (IR), a component of metabolic syndrome, has been shown to be associated with a high risk of a variety of diseases, including diabetes, hypertension, obesity and coronary artery disease (CAD) [10]. Pathophysiological studies have shown that IR promotes hyperglycemia, dyslipidemia and a proinflammatory state, which may be the main reason for the progression of CAC [11]. In addition, IR is not only the cause of up to 40% of myocardial infarction, but also the most important single risk factor for CAD in young adults [12]. At present, there are a variety of methods to assess IR. First, euglycaemic-hyperinsulinaemic clamp (EHC) as the gold standard for the evaluation of IR was first proposed in the 1970s, and then it was widely used, but it was limited in large-scale clinical studies and epidemiological investigations because of its laborious, expensive, complex and time-consuming characteristics [13]. Subsequently, the homeostasis model assessment for IR (HOMA-IR) derived from fasting insulin and glucose levels was first reported in the 1980s and has been shown to have a strong correlation with IR assessed by EHC [14]. HOMA-IR is the most widely used method to assess IR when fasting insulin data are available. However, because fasting insulin is not an indicator of routine measurement, its application in some studies is also limited. In view of this, some researchers have developed several alternative indicators of noninsulin–based IR, such as the ratio of triglycerides to high-density lipoprotein cholesterol (TG/HDL-C) and the triglyceride glucose index (TyG index), which are easier to obtain and cheaper in clinical practice [15, 16]. Since the TyG index was developed to evaluate IR, a large number of studies have shown that TyG, a surrogate marker closely related to HOMA-IR, could independently predict the occurrence and prognosis of hypertension, diabetes, stroke and other CVD, even better than HOMA-IR [17–19]. However, IR is related not only to glucose and lipid metabolism, but also to nutritional status and body fat distribution. Therefore, when evaluating metabolic related diseases, these two markers of IR, which do not include nutritional components and body fat distribution, may also have certain defects.
Therefore, Bello-Chavolla OY et al. developed a new non–insulin-based metabolic score for IR (METS‐IR) in 2018, and showed that METS-IR was a very valuable score for assessing cardiac metabolic risk in healthy and high-risk subjects, as well as a promising screening tool for IR [20]. In addition, they also indicated that METS-IR was better than the TyG index and TG/HDL-C in the diagnosis of type 2 diabetes in Mexican participants, which might be because the formula of METS-IR included not only glucose and lipid metabolism indicators, but also nutritional indicators, which also increased the degree of fit between METS-IR and EHC [20]. Since the development of METS-IR, some studies have shown that METS-IR is associated with adipokine disorder, inflammatory activity, arterial stiffness, hypertension, diabetes and ischemic heart disease [20–26]. IR is very important in the occurrence and development of CVD, but there are a variety of assessment tools for IR, and invasive or expensive assessment tools in clinical settings are limited, so it is very meaningful to find a noninvasive, low-cost and effective assessment tool for IR in clinical settings. However, the relationship between METS-IR and CAC has not yet been reported. Based on this, to fill this knowledge gap and identify more effective IR assessment methods in clinical settings, the present study was designed with the aims of: (1) identifying the potential association between METS-IR and CAC; and (2) determining whether METS-IR has better discrimination ability in predicting the incidence of CAC in asymptomatic adults without CVD, compared with other non–insulin‐based IR indexes.
Subjects, materials and methods
Study population
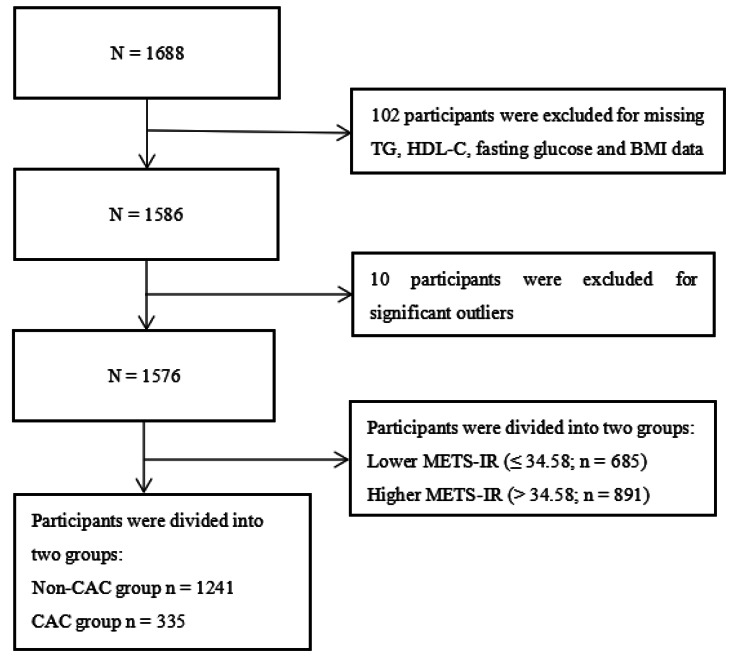
The present study was a secondary analysis based on a cross-sectional study performed by Choi SY et al. at the Seoul National University Hospital Healthcare System Gangnam Center from January 2014 to March 2016, they aimed to use a genome-wide association study (GWAS) among 1688 participants to explore single nucleotide polymorphisms (SNPs) that may be associated with severe CAC in an asymptomatic Korean population, more details can be found elsewhere [27]. Participants with a history of CVD and symptoms of CAD and participants with missing TG, HDL-C, fasting glucose, body mass index (BMI) and CAC measurements were excluded. Ultimately, 1576 participants were finally enrolled in the current study (Fig. 1). The original study protocol was approved by the ethics committee of the Seoul National University Hospital. Informed consent was waived owing to the retrospective nature of the study, and the study was carried out in accordance with the principles of the Declaration of Helsinki as revised in 2008.
Fig. 1.
Flow chart of the study participants. TG triglyceride, HDL-C high-density lipoprotein cholesterol, BMI Body mass index, METS-IR metabolic score for insulin resistance, CAC coronary artery calcification
Data collection and definitions
Data from the present study were obtained from a free public database (https://figshare.com), which allowed researchers to download and use original data. After the authors of the original research shared the data, the raw data were protected by the data sharing policy, so we were free to use the data for secondary analysis without harming the rights and interests of the authors. However, when using these data, we needed to cite data sources [28]. In the original data file, the data variables needed for the present study were as follows: age, sex, smoking, diabetes, hypertension, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting glucose, total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C), HDL-C, high sensitivity C-reactive protein (Hs-CRP), creatinine, hemoglobin Alc (HbA1c) and CAC score (CACS). The details of the collection and measurement of the above data have been described elsewhere [27].
In this study, smoking status was divided into two groups: none or past and current. BMI was calculated as weight (kg)/height (m)2. Diabetes was defined as fasting blood glucose ≥ 126 mg/dl, HbA1c ≥ 6.5%, or taking hypoglycemic agents, or history of diabetes diagnosis. Hypertension was defined as SBP/DBP ≥ 140/90 mmHg or taking antihypertensive agents or history of hypertension diagnosis. The noninsulin-based markers of IR were calculated based on previously reported formulas, as follows: the TyG index was calculated as ln(fasting glucose [mg/dL] × fasting TG [mg/dL]/2) [15], and the METS-IR was determined as ln(2 × fasting glucose [mg/dL] + fasting TG [mg/dL]) × BMI [kg/m2] / ln(fasting HDL-C [mg/dL]) [20]. In this study, we divided participants into two groups by the optimal cutoff point of METS-IR determined by receiver operating characteristic (ROC) curve analysis: lower METS-IR (≤ 34.58; n = 685) and higher METS-IR (> 34.58; n = 891).
CAC measurements
All the participants underwent CAC measurements, with coronary CT performed by a 256-slice multidetector CT scanner (Brilliance iCT 256; Philips Medical Systems, Cleveland, Ohio) or a 16-slice scanner (Somatom Sensation 16; Siemens Medical Solutions, Forchheim, Germany), and with calcium scans performed by electrocardiogram-gated dose modulation; more details have been described in detail elsewhere [27, 29]. The CACS was calculated by using a software program (Rapidia 2.8; INFINITT, Seoul, Republic of Korea) and the previously reported Agatston score method [27, 30]. As in previous studies, the presence of CAC was defined as a CACS > 0 [31]. In this study, we divided participants into two groups: non-CAC (CACS = 0; n = 1241) and CAC (CACS > 0; n = 335).
Statistical analysis
Continuous variables were expressed as the mean ± SD or median (interquartile range), and comparisons between groups were performed using independent-sample T-tests or Mann-Whitney U tests. Categorical variables were presented as frequencies (%), and comparisons between groups were performed using chi-square tests or Fisher’s exact test. Logistic regression models with restricted cubic splines were performed to explore the potential nonlinear relationships between TG/HDL-C, TyG index and METS-IR and CAC. The relationship between the TG/HDL-C, TyG index and METS-IR and CAC was evaluated using logistic regression analysis in different models with adjustments for covariables with clinical importance and statistical significance. Model 1: adjusted for age and sex, Model 2: adjusted for variables included in Model 1 and smoking, diabetes, hypertension, and Model 3: adjusted for variables included in Model 2 and SBP, DBP, TC, LDL-C, creatinine, Hs-CRP, HbA1c. C-statistics derived from ROC curve analysis were used to test the discrimination ability of noninsulin-based markers of IR for CAC. DeLong’s test was performed to compare the area under the curve (AUC) of these markers. All statistical tests were performed with SPSS 19.0 (SPSS Inc., Chicago, Illinois, USA), MedCalc version 19.1 (MedCalc Software, Belgium) and R Programming Language (version 3.6.3). A two-tailed P value < 0.05 was regarded as statistically significant.
Results
Baseline characteristics of the study population
The baseline characteristics of the participants were showed in Table 1. The study included 1576 participants (mean age: 52.86 ± 7.05 years; 76.10% men). The participants were divided into two groups based on the optimal cutoff point of METS-IR. Compared with participants in the lower METS-IR group, those with higher METS-IR showed higher SBP, DBP, BMI, TG, LDL-C, Hs-CRP, creatinine, fasting glucose, HbA1c, TG/HDL-C and TyG index, lower HDL‑C, and a higher percentage of male, smoking, diabetes, hypertension and CAC.
Table 1.
Baseline characteristics of participants stratified by the optimal cutoff point of METS-IR.
| Total population | METS-IR ≤ 34.58 | METS-IR > 34.58 | P value | |
|---|---|---|---|---|
| N | 1576 | 685 | 891 | |
| Age (years) | 52.86 ± 7.05 | 53.09 ± 6.98 | 52.69 ± 7.10 | 0.256 |
| Male, n (%) | 1200 (76.10) | 399 (58.20) | 801 (89.90) | < 0.001 |
| Smoking, n (%) | 231 (14.70) | 68 (9.90) | 163 (18.30) | < 0.001 |
| Diabetes, n (%) | 181 (11.50) | 33 (4.80) | 148 (16.60) | < 0.001 |
| Hypertension, n (%) | 457 (29.00) | 143 (20.90) | 314 (35.20) | < 0.001 |
| SBP (mmHg) | 117.35 ± 13.51 | 113.91 ± 13.62 | 120.00 ± 12.83 | < 0.001 |
| DBP (mmHg) | 77.97 ± 10.30 | 75.24 ± 10.60 | 80.06 ± 9.56 | < 0.001 |
| Body mass index (kg/m2) | 24.23 ± 2.70 | 22.13 ± 1.73 | 25.85 ± 2.13 | < 0.001 |
| Total cholesterol (mg/dL) | 195.28 ± 33.85 | 195.93 ± 32.48 | 194.78 ± 34.88 | 0.502 |
| TG (mg/dL) | 125.45 ± 71.48 | 90.21 ± 41.85 | 152.55 ± 77.49 | < 0.001 |
| LDL‑C (mg/dL) | 123.35 ± 30.53 | 120.21 ± 29.82 | 125.74 ± 30.86 | 0.001 |
| HDL‑C (mg/dL) | 52.05 ± 11.94 | 59.75 ± 11.83 | 46.14 ± 8.01 | < 0.001 |
| Hs-CRP (mg/dL) | 0.05 (0.03, 0.12) | 0.04 (0.02, 0.10) | 0.07 (0.03, 0.14) | < 0.001 |
| Creatinine ( mg/dL) | 0.93 ± 0.18 | 0.88 ± 0.19 | 0.97 ± 0.16 | < 0.001 |
| Fasting glucose (mg/dL) | 101.50 ± 18.97 | 95.64 ± 13.20 | 106.00 ± 21.35 | < 0.001 |
| Hemoglobin Alc (%) | 5.79 ± 0.61 | 5.66 ± 0.43 | 5.90 ± 0.70 | < 0.001 |
| TG/HDL-C | 2.18 (1.40, 3.32) | 1.39 (0.97, 1.98) | 2.97 (2.08, 4.21) | < 0.001 |
| TyG index | 8.61 ± 0.58 | 8.27 ± 0.48 | 8.88 ± 0.50 | < 0.001 |
| METS-IR | 35.85 ± 5.91 | 30.63 ± 2.88 | 39.86 ± 4.29 | < 0.001 |
| CAC (%) | 335 (21.30) | 102 (14.90) | 233 (26.20) | < 0.001 |
Data were expressed as mean ± SD, median (interquartile range), or n (%)
METS-IR metabolic score for insulin resistance, SBP systolic blood pressure, DBP diastolic blood pressure, TG triglyceride, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, Hs-CRP high sensitivity C-reactive protein, TyG index triglyceride-glucose index, CACS coronary artery calcification score, CAC coronary artery calcification
Associations of the METS-IR, TyG index and TG/HDL-C with CAC
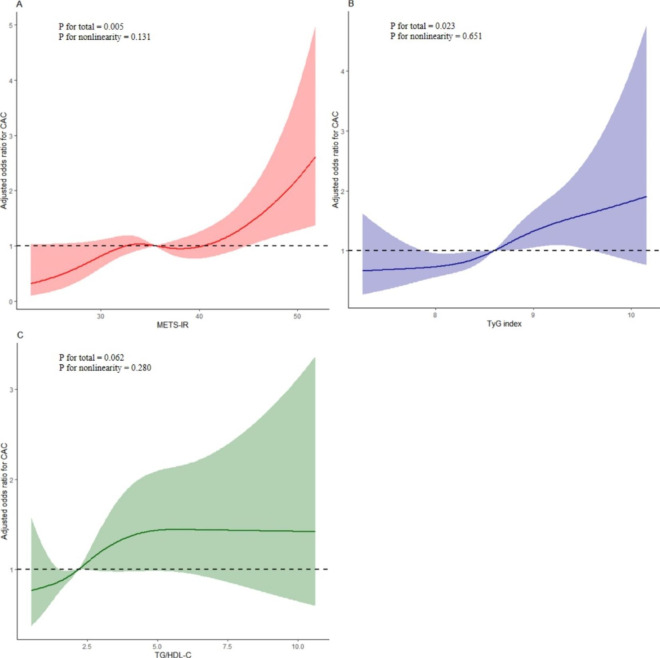
As shown in Table 3, in logistic regression analysis, three models (Models 1–3), including covariables with statistical significance (P < 0.1) and clinical significance in Table 2, were constructed to assess the predictive significance of METS-IR, TyG index and TG/HDL-C for CAC. With the increase in confounding factors, higher METS-IR, TyG index and TG/HDL-C remained independent risk predictors of CAC, despite their being regarded as nominal or continuous variables (all P < 0.05 in Models 1–3). Furthermore, logistic regression models with restricted cubic splines indicated that after full adjustments for the confounders (Model 3), the METS-IR, TyG index and TG/HDL-C were linearly correlated with CAC (all P for nonlinearity > 0.05), and demonstrated that the significance of METS-IR in predicting CAC was higher than that of the TyG index and TG/HDL-C (METS-IR, P for total = 0.005; TyG index, P for total = 0.023; TG/HDL-C, P for total = 0.062; respectively) (Fig. 2).
Table 3.
Multivariate logistic regression analysis of factors associated with the incidence of CAC
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||
| METS-IR | 1.064 (1.040, 1.089) | < 0.001 | 1.041 (1.017, 1.066) | 0.001 | 1.046 (1.019, 1.073) | 0.001 | |
| METS-IRa | 1.717 (1.304, 2.262) | < 0.001 | 1.406 (1.057, 1.871) | 0.019 | 1.499 (1.091, 2.060) | 0.013 | |
| TyG | 1.813 (1.442, 2.278) | < 0.001 | 1.415 (1.110, 1.804) | 0.005 | 1.439 (1.103, 1.878) | 0.007 | |
| TyGb | 2.079 (1.594, 2.710) | < 0.001 | 1.591 (1.198, 2.113) | 0.001 | 1.730 (1.264, 2.368) | 0.001 | |
| TG/HDL-C | 1.105 (1.038, 1.176) | 0.002 | 1.071 (1.004, 1.143) | 0.037 | 1.076 (1.003, 1.155) | 0.040 | |
| TG/HDL-Cc | 1.892 (1.443, 2.480) | < 0.001 | 1.685 (1.275, 2.228) | < 0.001 | 1.770 (1.301, 2.410) | < 0.001 | |
a The OR was examined regarding lower METS-IR as reference, b The OR was examined regarding lower TyG as reference, c The OR was examined regarding lower TG/HDL-C as reference.
Model 1: adjusted for age, sex
Model 2: adjusted for variables included in Model 1 and smoking, diabetes, hypertension
Model 3: adjusted for variables included in Model 2 and systolic blood pressure, diastolic blood pressure, total cholesterol, low-density lipoprotein cholesterol, creatinine, high sensitivity C-reactive protein, hemoglobin Alc. CAC coronary artery calcification, METS-IR metabolic score for insulin resistance, TyG triglyceride-glucose index, TG triglyceride, HDL-C high-density lipoprotein cholesterol, OR odd ratio, CI confidence interval
Table 2.
Univariate logistic regression analysis of the incidence of CAC.
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.056 | 1.038–1.075 | < 0.001 |
| Male | 2.344 | 1.671–3.288 | < 0.001 |
| Smoking | 1.726 | 1.343–2.218 | < 0.001 |
| Diabetes | 3.639 | 2.634–5.026 | < 0.001 |
| Hypertension | 2.467 | 1.919–3.171 | < 0.001 |
| Systolic blood pressure | 1.019 | 1.010–1.028 | < 0.001 |
| Diastolic blood pressure | 1.026 | 1.014–1.038 | < 0.001 |
| Body mass index | 1.136 | 1.086–1.188 | < 0.001 |
| Total cholesterol | 0.996 | 0.992–1.000 | 0.030 |
| Triglyceride | 1.003 | 1.001–1.004 | < 0.001 |
| Low-density lipoprotein cholesterol | 0.996 | 0.992–1.001 | 0.096 |
| High-density lipoprotein cholesterol | 0.983 | 0.972–0.993 | 0.001 |
| High sensitivity C-reactive protein | 1.267 | 0.855–1.878 | 0.239 |
| Creatinine | 1.915 | 0.973–3.768 | 0.060 |
| Fasting glucose | 1.023 | 1.016–1.029 | < 0.001 |
| Hemoglobin Alc | 1.982 | 1.635–2.403 | < 0.001 |
| Triglyceride/high-density lipoprotein cholesterol | 1.116 | 1.052–1.184 | < 0.001 |
| Triglyceride-glucose index | 1.899 | 1.533–2.354 | < 0.001 |
| Higher METS-IR | 2.024 | 1.564–2.620 | < 0.001 |
CAC coronary artery calcification, METS-IR metabolic score for insulin resistance, OR odd ratio, CI confidence interval
Fig. 2.
Restricted cubic spline plots of the association between METS-IR (A), TyG index (B) and TG/HDL-C (C) with CAC. The associations were adjusted for age, sex, smoking, diabetes, hypertension, systolic blood pressure, diastolic blood pressure, total cholesterol, low-density lipoprotein cholesterol, creatinine, high sensitivity C-reactive protein, hemoglobin Alc. CAC coronary artery calcification, METS-IR metabolic score for insulin resistance, TyG index triglyceride-glucose index, TG triglyceride, HDL-C high-density lipoprotein cholesterol
Discrimination ability of different models for CAC
In ROC curve analysis, the comparative analysis of the AUC of noninsulin-based markers of IR for predicting CAC showed that the discriminant ability of METS-IR and the TyG index were significantly higher than that of TG/HDL-C (all P for comparison < 0.05). In addition, without considering the P value, the AUC of CAC predicted by METS-IR was higher than that of the TyG index, and the addition of METS-IR to a baseline risk model had an incremental effect on the predictive value for CAC (Table 4; Fig. 3).
Table 4.
C-statistics of discrimination ability of different models for CAC.
| variables | AUC | 95% CI | P value | Z value | P for comparison |
|---|---|---|---|---|---|
| Univariate model | |||||
| TG/HDL-C | 0.577 | 0.552–0.601 | < 0.001 | 4.352 | Reference |
| TyG | 0.603 | 0.578–0.627 | < 0.001 | 5.871 | 0.001 |
| METS-IR | 0.607 | 0.583–0.631 | < 0.001 | 6.243 | 0.021 |
| Multivariate model | |||||
| Baseline risk modela | 0.692 | 0.669–0.715 | < 0.001 | 11.531 | Reference |
| Baseline risk modela with TG/HDL-C | 0.694 | 0.671–0.717 | < 0.001 | 11.659 | 0.486 |
| Baseline risk modela with TyG | 0.697 | 0.674–0.720 | < 0.001 | 11.841 | 0.257 |
| Baseline risk modela with METS-IR | 0.700 | 0.677–0.723 | < 0.001 | 12.100 | 0.176 |
a The baseline risk model included age, sex, smoking, diabetes, hypertension, systolic blood pressure, diastolic blood pressure, total cholesterol, low-density lipoprotein cholesterol, creatinine, high sensitivity C-reactive protein, hemoglobin Alc
CAC coronary artery calcification, TG triglyceride, HDL-C high-density lipoprotein cholesterol, TyG triglyceride-glucose index, METS-IR metabolic score for insulin resistance, AUC area under the curve, CI confidence interval
Fig. 3.
C-statistics evaluating incremental effects of different models. METS-IR metabolic score for insulin resistance, TyG triglyceride-glucose index, TG triglyceride, HDL-C high-density lipoprotein cholesterol, AUC area under the curve
Discussion
To the best of our knowledge, this cross-sectional study was the first report on the relationship between METS-IR and CAC. In this study, we retrospectively explored the potential association between METS-IR and CAC, and determined that METS-IR had better discrimination ability in predicting the incidence of CAC in asymptomatic adults without CVD than other non–insulin-based IR indexes. The main findings showed a significant positive and linear association between the METS-IR and CAC after adjusting for confounding factors. Moreover, the results also provided evidence that without considering the comparative P value, the predictive significance and discrimination ability of METS-IR for CAC were higher than those of the TyG index and TG/HDL-C, which elucidated that in addition to the TyG index and TG/HDL-C, METS-IR also played an important role in CAC.
IR is defined as the impaired biological response of major tissues such as adipose tissue, muscle and liver to insulin stimulation, which can damage glucose metabolism and lead to compensatory increases in β-cell insulin production and hyperinsulinemia [32]. Although IR has been proven to involve some genetic factors, it is mainly a secondary disease state related to excess body fat, which can lead to many pathological conditions such as dysfunction of blood glucose, blood pressure and blood lipids, visceral obesity, increased inflammatory markers, endothelial dysfunction and prothrombin state [32]. An abundance of studies have shown that IR, which is thought to occur more than 10 years earlier than type 2 diabetes (T2DM), is mainly associated with T2DM [33]; additionally, it is also associated with some metabolism-related diseases, including obesity, metabolic syndrome, arterial stiffness, CAC and CVD [10, 17, 18, 31, 32, 34–36].
Recently, a novel non–insulin-based substitute of IR combining glucose and lipid metabolism and body fat components was developed, called METS‐IR, which has been proven to be superior to the TyG index and TG/HDL-C in the diagnosis of diabetes [20], which was basically consistent with our main findings; that is, METS-IR might be superior to TyG and TG/HDL-C in the diagnosis of CAC. Consistent with a previous study [31], our study also found that a higher TyG index was related to a higher risk of CAC. Intriguingly, our study also obtained an unexpected result, namely, METS-IR may have better diagnostic performance in CAC. In addition, a large-scale epidemiological study involving 142,005 adults showed that in the fully adjusted model, only METS-IR was positively correlated with blood pressure levels (P < 0.001), while the TyG index and TG/HDL-C were not [21]. Similar to this study, Fan J et al. used logistic regression analysis to explore the relationship between TG/HDL-C, TyG index and METS-IR with prehypertension, and found that among the three indexes, after full adjustment, only METS-IR was positively associated with SBP and DBP, and only METS-IR was significantly correlated with prehypertension, regardless of the classification of waist circumference [22]. Moreover, Bello-Chavolla OY et al. also found that compared with TG/HDL-C, TyG index and HOMA-IR, the positive correlation coefficient between METS-IR and pulse wave velocity was higher, and METS‐IR had better discrimination ability for hypertension [23]. In addition, several studies have shown that METS-IR is associated with new-onset diabetes [25], ischemic heart disease [26] and metabolic syndrome [37]. While different from our research, Mirr M et al. have shown that the diagnostic performance of the TyG index and TG/HDL-C was better than METS-IR in metabolic syndrome [37]. The reason for this difference may be due to different research populations and specific diseases. Therefore, the diagnostic performance of these noninsulin-based markers of IR for metabolic related diseases still needs to be further explored in large-scale clinical studies and epidemiological investigations.
In summary, our study found that the METS-IR was better than the TyG index and TG/HDL-C at predicting the incidence of CAC in asymptomatic adults without CAD, which further verified that METS-IR may be used as a simple, low-cost and noninvasive marker to assess the prevalence of CAC. Although the mechanisms of the higher association of the METS-IR than the TyG index and TG/HDL-C with CAC have not been fully clarified, it may be attributed to the fact that METS-IR is superior to the TyG index and TG/HDL-C in evaluating IR. Alternatively, compared to the TG/HDL-C and TyG index, which represented IR in the liver and muscle, the METS-IR, due to the involvement of BMI, might serve as a better indicator of IR in adipose tissue, muscle and liver and a more valuable marker of CAC [38]. In addition, the biological mechanism of the association between METS-IR and CAC is still unclear and may be mediated by the following biological mechanisms. First, IR can promote dyslipidemia, visceral obesity, elevated inflammatory markers, endothelial dysfunction and prothrombin status [39], and then lead to the occurrence of CAC through these pathological mechanisms. Additionally, Ding L et al. also found that METS-IR was positively associated with inflammatory activity and adipokine disorder, all of which may lead to the occurrence and progression of CAC [24]. Second. As mentioned earlier, IR can disrupt glucose metabolism, leading to compensatory increases in β-cell insulin production and hyperinsulinemia, which in turn leads to diabetes and other metabolic diseases closely related to CVD and CAC [40, 41]. For example, Won et al. confirmed that IR, represented by the TyG index, is an independent predictor of CAC progression, especially in adults with less severe baseline CAC [42]. Third, due to the addition of BMI, METS-IR may be a better indicator of IR in muscle, liver and adipose tissue, and may play a more important role in the occurrence of CAC [43, 44].
Strengths
Overall, this study had several strengths. First, our study described the relationship between METS-IR and CAC for the first time, filling the gap in this research field. Second, our study confirmed the linear correlation between METS-IR and the risk of CAC. Third, we not only found that METS-IR was better than TyG and TG/HDL-C in distinguishing CAC, but also found that it could increase the ability of baseline models including traditional cardiovascular risk factors to predict CAC.
Limitations
Although this study obtained surprising results, it still had some limitations. First, this study was a cross-sectional study, which could not identify the causal relationship between METS-IR and CAC. Second, this study failed to explore insulin-based IR markers, such as HOMA-IR and QUICKI, and lacked a comprehensive comparison. Third, this study did not include data on anti-dyslipidemic and anti-hypertensive medications, which might miss potential confounding factors. Finally, the data of this study only came from the general population in a single center, so the findings might not be widely applicable. Despite these limitations, this study demonstrated for the first time the robustness of the correlation between METS-IR and CAC in asymptomatic patients without CVD.
Conclusion
Taken together, the evidence provided by us showed for the first time that the METS-IR was independently related to CAC, and compared with the TyG index and TG/HDL-C, the METS-IR had a stronger ability to distinguish CAC, which elucidated the important role of METS-IR in determining CAC in the general population. The findings supported the importance of controlling METS-IR and its components in reducing CAC risk, and it is of great significance in the actual clinical environment and epidemiological investigation.
Acknowledgements
We thank the investigators, the staff, and the participants of the orginal study for their valuable contributions.
Author contributions
Zhenwei Wang and Xu Huang conceived and designed the study. Zhenwei Wang and Xu Huang were responsible for the management and retrieval of data, contributed to initial data analysis and interpretation. Zhenwei Wang drafted the initial manuscript. Naifeng Liu and Jingjie Li revised the manuscript. Xiaofang Hui revised the manuscript, responded to the reviewers’ comments and embellished the accuracy and fluency of English expression of the manuscript. Naifeng Liu was the guarantor of this work and had full access to all the data in the study and take responsibility for its integrity and the accuracy of the data analysis. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Data can be downloaded from from a public database (https://figshare.com)
Copyright and License Policy
The original data used in this article is authorized by the CC-BY 4.0 license, and the original data has not been changed. After appropriate reference, this article can be exempted from liability. The license link is as follows: https://creativecommons.org/licenses/by/4.0/
Declarations
Declaration of competing interest
The authors have no conflicts of interest to disclose.
Ethics approval and consent to participate
The original study protocol was approved by the ethics committee of the Seoul National University Hospital. The need for informed consent was waived because of the retrospective nature of the study, and the study was carried out in accordance with the principles of the Declaration of Helsinki as revised in 2008.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhenwei Wang and Xiaofang Hui contributed equally to this work and shared frst authorship.
References
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9):e139–596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J. 2015;36(19):1163–70. doi: 10.1093/eurheartj/ehu505. [DOI] [PubMed] [Google Scholar]
- 3.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, et al. Arterial Calcification and Not Lumen Stenosis Is Highly Correlated With Atherosclerotic Plaque Burden in Humans: A Histologic Study of 723 Coronary Artery Segments Using Nondecalcifying Methodology. J Am Coll Cardiol. 1998;31(1):126–33. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 4.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary Calcium Score and Cardiovascular Risk. J Am Coll Cardiol. 2018;72(4):434–47. doi: 10.1016/j.jacc.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin H, Weir-McCall JR, Leipsic JA, Son J, Sellers SL, Shao M, et al. The Relationship Between Coronary Calcification and the Natural History of Coronary Artery Disease. JACC Cardiovasc Imaging. 2021;14(1):233–42. doi: 10.1016/j.jcmg.2020.08.036. [DOI] [PubMed] [Google Scholar]
- 6.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 7.Carr JJ, Jacobs DRJ, Terry JG, Shay CM, Sidney S, Liu K, et al. Association of Coronary Artery Calcium in Adults Aged 32 to 46 Years With Incident Coronary Heart Disease and Death. JAMA Cardiol. 2017;2(4):391–9. doi: 10.1001/jamacardio.2016.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur J Prev Cardiol. 2016;23(11):NP1–96. doi: 10.1177/2047487316653709. [DOI] [PubMed] [Google Scholar]
- 9.Goff DCJ, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RBS, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293–302. doi: 10.1038/nrendo.2014.29. [DOI] [PubMed] [Google Scholar]
- 11.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14(5):575–85. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu A, Abbasi F, Reaven GM. Adiposity indices in the prediction of metabolic abnormalities associated with cardiovascular disease in non-diabetic adults. Nutr Metab Cardiovasc Dis. 2011;21(8):553–60. doi: 10.1016/j.numecd.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214-23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 16.Chiang J, Lai N, Chang J, Koo M. Predicting insulin resistance using the triglyceride-to-high-density lipoprotein cholesterol ratio in Taiwanese adults. Cardiovasc Diabetol. 2011;10:93. doi: 10.1186/1475-2840-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. 2021;20(1):76. doi: 10.1186/s12933-021-01268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pranata R, Huang I, Irvan, Lim MA, Vania R. The association between triglyceride-glucose index and the incidence of type 2 diabetes mellitus-a systematic review and dose-response meta-analysis of cohort studies. Endocrine. 2021;74(2):254–62. doi: 10.1007/s12020-021-02780-4. [DOI] [PubMed] [Google Scholar]
- 19.Guo W, Zhao L, Mo F, Peng C, Li L, Xu Y, et al. The prognostic value of the triglyceride glucose index in patients with chronic heart failure and type 2 diabetes: A retrospective cohort study. Diabetes Res Clin Pract. 2021;177:108786. doi: 10.1016/j.diabres.2021.108786. [DOI] [PubMed] [Google Scholar]
- 20.Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, Viveros-Ruiz T, Cruz-Bautista I, Romo-Romo A, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178(5):533–44. doi: 10.1530/EJE-17-0883. [DOI] [PubMed] [Google Scholar]
- 21.Liu XZ, Fan J, Pan SJ. METS-IR, a novel simple insulin resistance indexes, is associated with hypertension in normal-weight Chinese adults. J Clin Hypertens (Greenwich) 2019;21(8):1075–81. doi: 10.1111/jch.13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan J, Gao ST, Wang LJ, Qian ZL, Zhou ZQ, Liu XZ. Association of Three Simple Insulin Resistance Indexes with Prehypertension in Normoglycemic Subjects. Metab Syndr Relat Disord. 2019;17(7):374–9. doi: 10.1089/met.2019.0029. [DOI] [PubMed] [Google Scholar]
- 23.Bello Chavolla OY, Antonio Villa NE, Vargas Vázquez A, Martagón AJ, Mehta R, Arellano Campos O, et al. Prediction of incident hypertension and arterial stiffness using the non–insulin-based metabolic score for insulin resistance (METS-IR) index. J Clin Hypertens (Greenwich) 2019;21(8):1063–70. doi: 10.1111/jch.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding L, Gao Y, Li Y, Huang Y, Wang X, Qi X. Metabolic Score for Insulin Resistance Is Correlated to Adipokine Disorder and Inflammatory Activity in Female Knee Osteoarthritis Patients in a Chinese Population. Diabetes Metab Syndr Obes. 2020;13:2109–18. doi: 10.2147/DMSO.S249025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang M, Liu D, Qin P, Liu Y, Sun X, Li H, et al. Association of metabolic score for insulin resistance and its 6-year change with incident type 2 diabetes mellitus. J Diabetes. 2021;13(9):725–34. doi: 10.1111/1753-0407.13161. [DOI] [PubMed] [Google Scholar]
- 26.Yoon J, Jung D, Lee Y, Park B. The Metabolic Score for Insulin Resistance (METS-IR) as a Predictor of Incident Ischemic Heart Disease: A Longitudinal Study among Korean without Diabetes. J Pers Med. 2021;11(8):742. doi: 10.3390/jpm11080742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi S, Shin E, Choe EK, Park B, Lee H, Park HE, et al. Genome-wide association study of coronary artery calcification in asymptomatic Korean populations. PLoS ONE. 2019;14(3):e0214370. doi: 10.1371/journal.pone.0214370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CHOI SU-YEON. Clinical characteristics_CACS_GWAS_n = 1688. figshare. Dataset. 2019. doi:10.6084/m9.figshare.7853588.v1.
- 29.Liu Y, Wu M, Xu J, Sha D, Xu B, Kang L. Association between Triglyceride and glycose (TyG) index and subclinical myocardial injury. Nutr Metab Cardiovasc Dis. 2020;30(11):2072–6. doi: 10.1016/j.numecd.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Lee SY, Hyun YY, Lee KB, Kim H. Low serum magnesium is associated with coronary artery calcification in a Korean population at low risk for cardiovascular disease. Nutr Metab Cardiovasc Dis. 2015;25(11):1056–61. doi: 10.1016/j.numecd.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte MJ, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 31.Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol. 2017;16(1):108. doi: 10.1186/s12933-017-0589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman AM, Pennings N. Insulin Resistance. [Updated 2021 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507839/.
- 33.Park HM, Lee HS, Lee Y, Lee J. The triglyceride-glucose index is a more powerful surrogate marker for predicting the prevalence and incidence of type 2 diabetes mellitus than the homeostatic model assessment of insulin resistance. Diabetes Res Clin Pract. 2021;180:109042. doi: 10.1016/j.diabres.2021.109042. [DOI] [PubMed] [Google Scholar]
- 34.Wang S, Shi J, Peng Y, Fang Q, Mu Q, Gu W, et al. Stronger association of triglyceride glucose index than the HOMA-IR with arterial stiffness in patients with type 2 diabetes: a real-world single-centre study. Cardiovasc Diabetol. 2021;20(1):82. doi: 10.1186/s12933-021-01274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Q, Zhang T, Cheng Y, Ma Y, Xu Y, Yang J, et al. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Cardiovasc Diabetol. 2020;19(1):108. doi: 10.1186/s12933-020-01086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou S, Xu Y. Association of the triglyceride-glucose index and major adverse cardiac and cerebrovascular events in female patients undergoing percutaneous coronary intervention with drug-eluting stents: A retrospective study. Diabetes Res Clin Pract. 2021;181:109073. doi: 10.1016/j.diabres.2021.109073. [DOI] [PubMed] [Google Scholar]
- 37.Mirr M, Skrypnik D, Bogdański P, Owecki M. Newly proposed insulin resistance indexes called TyG-NC and TyG-NHtR show efficacy in diagnosing the metabolic syndrome. J Endocrinol Invest. 2021;44(12):2831–43. doi: 10.1007/s40618-021-01608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han T, Cheng Y, Tian S, Wang L, Liang X, Duan W, et al. Changes in triglycerides and high-density lipoprotein cholesterol may precede peripheral insulin resistance, with 2-h insulin partially mediating this unidirectional relationship: a prospective cohort study. Cardiovasc Diabetol. 2016;15(1):154. doi: 10.1186/s12933-016-0469-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53(7):1270–87. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye Z, Xie E, Gao Y, Li P, Tu Y, Guo Z, et al. The triglyceride glucose index is associated with future cardiovascular disease nonlinearly in middle-aged and elderly Chinese adults. BMC Endocr Disord. 2022;22(1):242. doi: 10.1186/s12902-022-01157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferreira-Hermosillo A, Ibarra-Salce R, Rodríguez-Malacara J, Molina-Ayala MA. Comparison of indirect markers of insulin resistance in adult patients with Double Diabetes. BMC Endocr Disord. 2020;20(1):87. doi: 10.1186/s12902-020-00570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Won KB, Park EJ, Han D, Lee JH, Choi SY, Chun EJ, et al. Triglyceride glucose index is an independent predictor for the progression of coronary artery calcification in the absence of heavy coronary artery calcification at baseline. Cardiovasc Diabetol. 2020;19(1):34. doi: 10.1186/s12933-020-01008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee H, Park HE, Yoon JW, Choi SY. Clinical Significance of Body Fat Distribution in Coronary Artery Calcification Progression in Korean Population [published correction appears in Diabetes Metab J. 2021 Nov;45(6):974]. Diabetes Metab J. 2021;45(2):219 – 30. doi:10.4093/dmj.2019.0161. [DOI] [PMC free article] [PubMed]
- 44.Russo D, Morrone LF, Errichiello C, De Gregorio MG, Imbriaco M, Battaglia Y, et al. Impact of BMI on cardiovascular events, renal function, and coronary artery calcification. Blood Purif. 2014;38(1):1–6. doi: 10.1159/000362862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be downloaded from from a public database (https://figshare.com)
Copyright and License Policy
The original data used in this article is authorized by the CC-BY 4.0 license, and the original data has not been changed. After appropriate reference, this article can be exempted from liability. The license link is as follows: https://creativecommons.org/licenses/by/4.0/