Abstract
Background
Chromatin contacts are essential for gene-expression regulation; however, obtaining a high-resolution genome-wide chromatin contact map is still prohibitively expensive owing to large genome sizes and the quadratic scale of pairwise data. Chromosome conformation capture (3C)-based methods such as Hi-C have been extensively used to obtain chromatin contacts. However, since the sparsity of these maps increases with an increase in genomic distance between contacts, long-range or trans-chromatin contacts are especially challenging to sample.
Results
Here, we create a high-density reference genome-wide chromatin contact map using a meta-analytic approach. We integrate 3600 human, 6700 mouse, and 500 fly Hi-C experiments to create species-specific meta-Hi-C chromatin contact maps with 304 billion, 193 billion, and 19 billion contacts in respective species. We validate that meta-Hi-C contact maps are uniquely powered to capture functional chromatin contacts in both cis and trans. We find that while individual dataset Hi-C networks are largely unable to predict any long-range coexpression (median 0.54 AUC), meta-Hi-C networks perform comparably in both cis and trans (0.65 AUC vs 0.64 AUC). Similarly, for long-range expression quantitative trait loci (eQTL), meta-Hi-C contacts outperform all individual Hi-C experiments, providing an improvement over the conventionally used linear genomic distance-based association. Assessing between species, we find patterns of chromatin contact conservation in both cis and trans and strong associations with coexpression even in species for which Hi-C data is lacking.
Conclusions
We have generated an integrated chromatin interaction network which complements a large number of methodological and analytic approaches focused on improved specificity or interpretation. This high-depth “super-experiment” is surprisingly powerful in capturing long-range functional relationships of chromatin interactions, which are now able to predict coexpression, eQTLs, and cross-species relationships. The meta-Hi-C networks are available at https://labshare.cshl.edu/shares/gillislab/resource/HiC/.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13059-022-02790-z.
Keywords: Hi-C, Meta-analysis, Coexpression, eQTL
Background
The physical associations generated by chromatin contacts are a critical factor to regulate and determine gene-expression patterns [1–4]. Functional chromatin contacts can form across a wide range of genomic distances within a chromosome (cis) or across chromosomes (trans). Although trans contacts are non-random [5] and there is evidence of trans-regulatory interactions [6, 7], studying the functional role of these interactions is difficult due to the high sparsity of available chromatin contact maps in trans.
Obtaining high-density chromatin contact maps at all genomic distances and in trans is not yet feasible with most existing maps being essentially probabilistic in nature, capturing some fraction of likely present contacts in a distance-dependent manner. Genome-wide contact maps can be obtained using chromosome conformation capture (3C)-based technologies such as Hi-C [8]. From Hi-C experiments, an nxn chromatin contact matrix is generated where the genome is divided into n equally sized bins and contact frequency between each bin-pair is obtained by summing the pair-ended reads spanning between a pair of bins (Fig. 1A). The bin size is also referred to as “resolution” and is dependent on the sequencing depth of the experiment — at low sequencing depth, the matrix is generated using large bin sizes (low resolution) to reduce the sparsity of the matrix. The commonly used sizes of these bins can range from 1 KB to 1 MB. However, due to large genome sizes and the quadratic scale of pairwise data, obtaining these chromatin contact matrices at high resolution would require prohibitively expensive sequencing at even 1X depth in the pairwise space. Capturing long-range and trans chromatin contacts is made more difficult since the frequency of contacts decreases with an increase in genomic distance between contacting loci in cis [8]. And in trans, the contacts are at least 2 orders of magnitude less frequent [5] while also having a larger search space than cis.
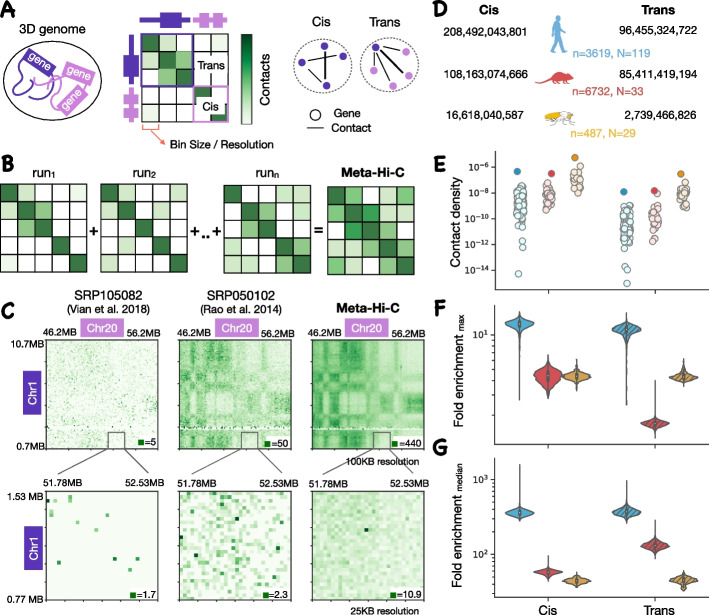
Fig. 1.
Creating the meta-Hi-C network. A Genes are co-localized in chromatin 3D structure through frequent chromatin contacts. Each chromosome is divided up into “bins” of a specific size referred to as resolution and the chromatin contact matrix represents the number of pair-ended reads spanning between a pair of bins. The contact matrix can be represented with networks where nodes are genes and edges are interaction frequencies. The cis and trans networks consist of intra-chromosome and inter-chromosome edges respectively. B Contact maps from individual Hi-C experiments are aggregated to create a meta-Hi-C contact map. C Visual comparison of our meta-Hi-C and two individual Hi-C contact matrices: Vian et al. [9] (a typical experiment) and Rao et al. [10] (densest experiment in trans) at 100-KB (top) and 25-KB resolutions (bottom). The maximum contact frequency is given at the bottom of each map. The high-intensity region observed in our meta-Hi-C contact matrix at 25-KB resolution belongs to gene UBEJ2 on Chr1 and ZFP64 on Chr20. Both these genes are also strongly coexpressed in our coexpression network with a coexpression strength of 0.98 on a genome-wide rank-standardized scale of 0 to 1. D Total number of contacts in cis and trans meta-Hi-C network of human, mouse, and fly. n and N are the numbers of runs and projects aggregated in each species respectively. E Contact density (total contacts/number of 1-bp bins) across individual projects in cis and trans for each species. Individual points are individual experiments and the darker shades of points are the values for the meta-Hi-C network. Distribution of fold enrichment of contacts among genes in the meta-Hi-C network relative to the maximum (F) or median (G) number of contacts for that gene among individual network in cis and trans. The total number of genes in F and G are 23,465, 20,672, and 9636 in human, mouse and fly respectively
To overcome the sequencing-depth barrier, targeted 3C-based techniques such as ChiA-PET [11] and Capture-Hi-C [12] are widely used to obtain high-resolution contact maps for specific proteins or selected loci respectively. Alternatively, several in silico methods have taken the advantage of existing limited-resolution contact maps to either generate higher resolution maps using machine learning approaches [13–16] and/or detect statistically significant interactions by background fitting [10, 17]. However, with a few exceptions [18, 19], most of the available methods are only tested to enhance cis interactions because longer range interactions are essentially unavailable within any given data set.
In this work, we propose a meta-analysis approach where we leverage several hundreds of available chromatin contact matrix generated from Hi-C-based experiments to create a dense genome-wide chromatin contact matrix for three species: human, mouse, and fly. We show that these meta-Hi-C chromatin contact matrices are valuable for capturing long-range and trans-chromosomal interactions. We evaluated the effectiveness of contact maps using three criteria; chromatin contact matrix was used to predict (1) gene-expression profiles, (2) target genes for eQTLs, and (3) conservation across pairs of species (human-mouse, human-fly, and mouse-fly). Our reference networks complement a very diverse array of efforts in genomics, from those focused on more targeted experiments in Hi-C which now have an overall “null” with which to compare individual results, to genome interpretation methods, whether interpreting variants, expression patterning, or regulatory sequence.
Results
Meta-Hi-C network predicts coexpression at greater resolution and scale than individual networks
In brief, for building the meta-Hi-C matrix, we uniformly processed 3619, 6732, and 487 Hi-C runs for human, mouse, and fly respectively. The runs were obtained after querying sequence read archive (SRA) with field limitations of given species and Hi-C as experiment strategy. A genome-wide chromatin contact matrix at several resolutions (1KB, 5KB, 10KB, 25KB, 40KB, 100KB, 250KB, and 500KB) was created for each run after mapping the reads to the same reference genome for each species. Within SRA, all the runs belonging to a study are grouped together as a project. A project can consist of multiple runs, which can include biological or technical replicates across multiple tissues or cell types. Chromatin contact matrices within a project were aggregated to create a project-level Hi-C matrix. There were 119, 33, and 29 projects for human, mouse, and fly respectively (Fig. 1D). The meta-Hi-C matrix for each species was created after further aggregating all project-level Hi-C matrix within a species (Fig. 1B). For subsequent analysis, the genome-wide Hi-C matrix was mapped to genes to create Hi-C networks where nodes are genes and edges are the interaction frequency between genes (Fig. 1A). To determine contact frequency between each gene pair we use the maximum contact frequency between each bin in which genes reside. The genome-wide Hi-C networks were divided into cis and trans depending on if the edge connects two genes in the same chromosome or different chromosomes respectively. Figure 1D–G highlights the comprehensively greater depth of the meta-Hi-C network. To validate the predictive power of the meta-Hi-C network, we benchmarked it against Hi-C networks inferred from individual projects for each species.
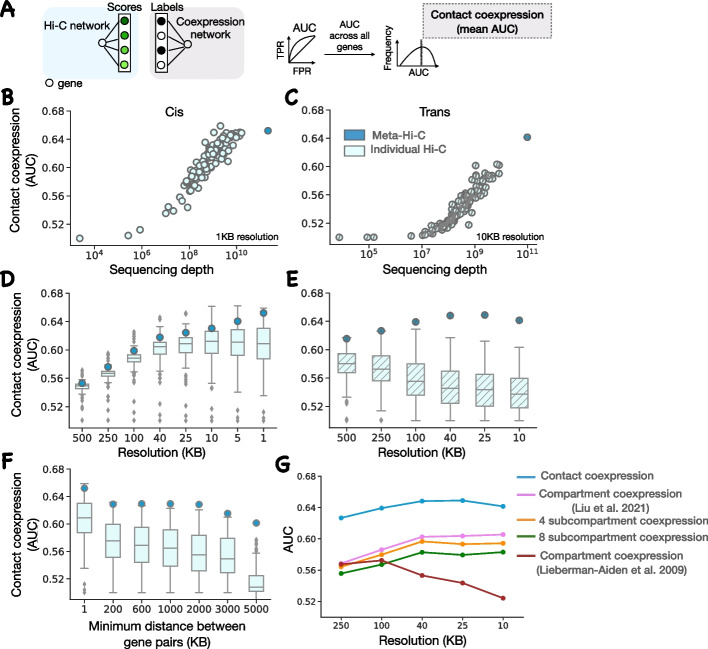
As our first performance test, we assessed the tendency for spatially co-localized genes to be coexpressed [20, 21], using previously derived shared patterns of expression in independent data [22]. The underlying hypothesis is that spatial proximity may be a useful way to organize regulatory relationships, as in the case of linear sequence, thus yielding shared spatial relationships for genes that are coexpressed. Thus, while perfect performance at predicting coexpression is not expected, the genome-wide scale of the assessment makes it useful for assessing cis and trans effects. For each gene, we measure the ability of interaction frequency to predict the gene’s top 1% coexpression partners. We call this measure “contact coexpression” (Fig. 2A) and is expressed as an AUC (area under the ROC curve) with possible values ranging between 0 and 1. A score of 1 indicates that interaction frequency perfectly predicts coexpression; 0.5 indicates no relationship.
Fig. 2.
Meta-Hi-C network benchmarking. A Contact coexpression metric schematic. Circles represent genes and lines represent edges of that gene in respective networks. For each target gene, we use its ranked edges in the Hi-C network to predict the top 1% of its edges in the coexpression network. We perform this task for every gene and then report contact coexpression as the average AUC across all genes. B Contact coexpression for the individual and meta-Hi-C network in cis as a function of sequencing depth at 1-KB resolution C Same as (B) but in trans and at 10-KB resolution. D The boxplot shows the distribution of contact coexpression for each project at various resolutions in cis. Circles represent the performance of the cis meta-Hi-C network. E Same as (D) but using trans networks. F The boxplot shows the distribution of contact coexpression in cis at 1-KB resolution for each project at various distance thresholds. G Comparison of contact coexpression score of the meta-Hi-C network and compartment coexpression score at various resolutions. We called compartments in each individual network and then aggregated those calls, capturing the probability of sharing a compartment across data. The compartment coexpression metric captures the ability of aggregated compartment preference to predict coexpression. Subcompartment coexpression is defined analogously to compartment coexpression. Compartments were called using either the Liu et al. [23] method or the Liberman-Aiden et al. [8] method
We evaluated the contact coexpression as a function of the sequencing depth of the Hi-C network in cis (Fig. 2B) and trans (Fig. 2C) for all individual Hi-C networks and meta-Hi-C network in human. We find that performance is linearly dependent on the log of sequencing depth and meta-analysis provides additional coverage. We find that in cis the best powered individual experiments are close to the saturation depth that maximizes performance (Fig. 2B), although performing substantially worse in trans. In trans, the meta-Hi-C network acts like a “super-experiment”, where the additional coverage fully converts into substantial additional performance (Fig. 2C). We found similar results for mouse (Additional file 1: Fig. S1C and S1D) and fly (Additional file 1: Fig. S2C and S2D). Although contact coexpression scores in cis and trans are similar (0.63 AUC cis vs 0.64 AUC trans at 10KB resolution), the search space in trans is at least three times larger when compared to cis, making a direct comparison of the aggregate “strength” of cis and trans relationships non-trivial.
The resolution of the Hi-C matrix is an important parameter for obtaining gene networks, and hence, we evaluated the contact coexpression of individual Hi-C and meta-Hi-C networks at several resolutions. We find that for the individual networks performance increases with an increase in resolution, plateaus, and then slightly falls off in cis (Fig. 2D). In essence, improved resolution is useful in cis because the coverage is adequate for it to provide a useful signal until the very finest resolution where most experiments begin to decline, although the meta-Hi-C network continues to slightly increase, as might be expected. In contrast, in trans (Fig. 2E), the performance monotonically falls with an increase in resolution for individual experiments. However, the trans pattern for meta-Hi-C networks strongly resembles that of individual experiments in cis, increasing and then plateauing with improvements in resolution. This suggests unlike individual networks, meta-Hi-C networks are dense enough to be analyzed at high resolutions even in trans. We found similar results for mouse (Additional file 1: Fig. S1A and S1B) and fly (Additional file 1: Fig. S2A and S2B).
The Hi-C matrix in cis has high density of contacts at bins near the diagonal and the contact density decreases exponentially as the distance between the bins increases so that even Hi-C networks with higher contact density on average will be highly sparse at distant bins. This makes it difficult to capture functional contacts between distant gene pairs from a Hi-C matrix. Hence, we evaluated the contact coexpression of individual Hi-C networks and meta-Hi-C networks at various linear distance thresholds in cis. We find that for long-range contacts (minimum distance between gene pairs> 600 KB) the additional sequencing depth of meta-Hi-C networks when compared to individual Hi-C networks fully converts into additional performance (Fig. 2F). However, for both individual networks and meta-Hi-C network, the performance decreases in the absence of short-range contacts. This could be due to a higher number of short-range regulatory interactions or due to the similarity of the chromatin environment for nearby genes.
Chromatin can be folded into structural patterns at different length scales [24]. At a large genomic scale, the genome is spatially segregated into two compartments [8] and further up to 8 subcompartments [23]. At a shorter genomic scale (< 1 MB), chromosomes fold into topologically associated domains (TADs). Since the interaction between genes is partly constrained to occur within the same structural units (compartment/subcompartment/TADs), we sought to determine if the contact coexpression performance of the meta-Hi-C network can be explained with these genomic structures. In each individual network, we identified the compartment of each gene and then binarized the compartment preference for each gene pair: so each experiment becomes a binary network of gene pairs found in the same compartment (1) or a different compartment (0). The binarized individual networks are then aggregated, capturing the probability of sharing a compartment across all data. For each gene, we measure the ability of aggregated compartment frequency to predict the gene’s top 1% coexpression partners. We call this measure “compartment coexpression” and it is expressed as an AUC with possible values ranging between 0 and 1. A score of 1 indicates that the same compartment frequency perfectly predicts coexpression; 0.5 indicates no relationship. We also defined “subcompartment coexpression” and “TAD coexpression” analogously to compartment coexpression.
We compared TAD coexpression (defined only in cis), compartment coexpression, and subcompartment coexpression with meta-Hi-C contact coexpression at several resolutions. We used two different methods for calling compartments: an older PCA-based method Liberman-Aiden et al. [8] and a comparatively recent method Calder [23]. In cis, we find that compartment and subcompartment coexpression is comparable to or better than contact coexpression while TAD coexpression is lower than compartment at up to 10-KB resolution (Additional file 1: Fig. S3). TADs are often considered functional genomic units and genes within the same TADs tend to be coexpressed [25]. However, unlike compartment and contact coexpression, TAD coexpression does not capture long-range interactions (average TAD size is smaller than 1 MB). This likely explains the non-random yet low performance of TAD coexpression (AUC 0.55). We also evaluated the conservation of TADs and boundaries across all individual Hi-C matrices (Additional file 1: Fig. S4). The number of TADs conserved across experiments decreases relatively rapidly and we did not find any TAD which was conserved across all the experiments. In trans, we find that compartment coexpression and subcompartment coexpression performances are lower than contact coexpression performance, suggesting other trans interactions contribute (Fig. 2G).
Meta-Hi-C network effectively captures more eQTL interactions
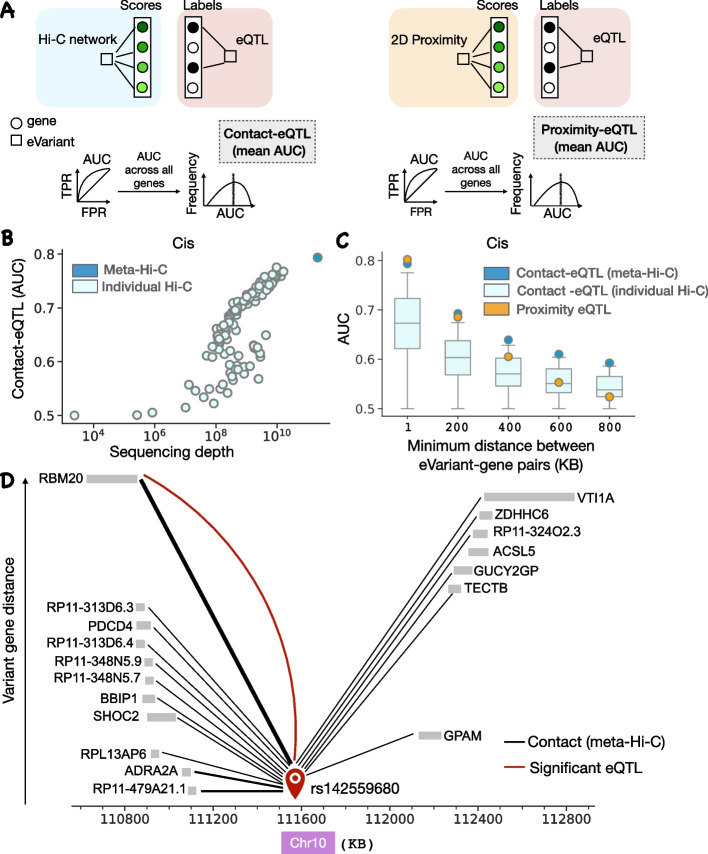
For our second performance assessment, we tested the hypothesis that genetic variants (eVariant) regulate gene expression of the target gene (eGene) via physical contact [26, 27]. The set of eQTLs was obtained from GTEx (Section Methods). For each eVariant, the interaction frequency with all genes falling in unique contact map bins at 1-KB resolution was used to predict the eGene (Fig. 3A). This is termed “contact-eQTL” and is expressed as an AUC with possible values ranging between 0 and 1, with 1 and 0 meaning that the eVariant and target eGenes have the highest and lowest interaction frequency respectively when compared to all eVariant and non-eGene interactions. Similar to the previous benchmarking test, we find that performance is linearly dependent on the log of sequencing depth and meta-analysis provides additional coverage; the meta-Hi-C network has higher performance when compared to any of the individual Hi-C networks (Fig. 3B). This emphasizes the significance of dense Hi-C networks in identifying eQTLs.
Fig. 3.
Meta-Hi-C network captures long-range eQTLs. A Contact-eQTL and proximity-eQTL metric schematic. For contact-eQTL and proximity-eQTL, for each variant, the edges are ranked by contact frequency or inverse of the genomic distance from the gene respectively. The labels are obtained from eQTL associations (Section Methods). We perform this task for every variant and then report the average AUC across all variants. B Contact-eQTL for individual Hi-C network and meta-Hi-C network at 1-KB resolution as a function of sequencing depth. C The boxplot shows the distribution of contact-eQTL for each project at various minimum distance thresholds. Circles represent the contact-eQTL of the meta-Hi-C network and proximity-eQTL. D An example comparing contact-eQTL with proximity-eQTL. All genes associated with eVariant rs142559680 along with their sequence position on the X axis. The vertical separation between gene and variant increases as the distance between the variant and gene TSS increases. The thickness of the variant gene edge increases with an increase in the contact frequency in the meta-Hi-C network. Although gene RP11-479A21.1 is the closest to the variant, gene RBM20 has the strongest contact with the variant and is also the only significantly associated gene with the variant
We further evaluated the ability of meta-Hi-C networks to predict target genes for variants by comparing their performance with a linear genomic distance-based predictor, the current standard approach. The distance between the variant and gene transcription start site (TSS) remains almost the only metric widely used to annotate target genes for variants [28]. For each eVariant, the inverse of linear distance (1/TSS) with all genes is ranked and then used to predict the eGene (Fig. 3A). This is termed “proximity-eQTL” and is expressed as an AUC with possible values ranging between 0 and 1, with 1 and 0 meaning that eGenes are the closest and farthest from the eVariant respectively. We compared contact-eQTL of individual Hi-C networks and meta-Hi-C networks at various linear distance thresholds (Fig. 3C). We reassuringly find that the meta-Hi-C network outperforms individual Hi-C networks at all distance thresholds. Interestingly, we find that contact-eQTL (meta-Hi-C) outperforms proximity-eQTL for long range eQTLs (minimum distance between the eVariant-gene pairs > 200KB). Furthermore, the performance for both contact-eQTL and proximity-eQTL decreases in the absence of short-range contacts. This is in agreement with our previous observation where we find that contact coexpression decreases in the absence of short-range contacts.
Trans-chromosomal chromatin contacts show evolutionary conservation
Having established that meta-Hi-C networks are well powered to capture meaningful contacts, we now use them to study the conservation of genomic contacts between species. Since chromatin contacts regulate gene expression, it is reasonable to expect some conservation of these contacts across species even in the context of large scale genomic alteration and, in the reverse, divergence in contacts across species can help explain regulatory evolution [29, 30]. We evaluated the conservation of contacts across species in three different ways; we compare the contact coexpression scores for ortholog genes in each species pair, and we use the Hi-C network of one species to predict either Hi-C network (“contact conservation”) or coexpression network in another species.
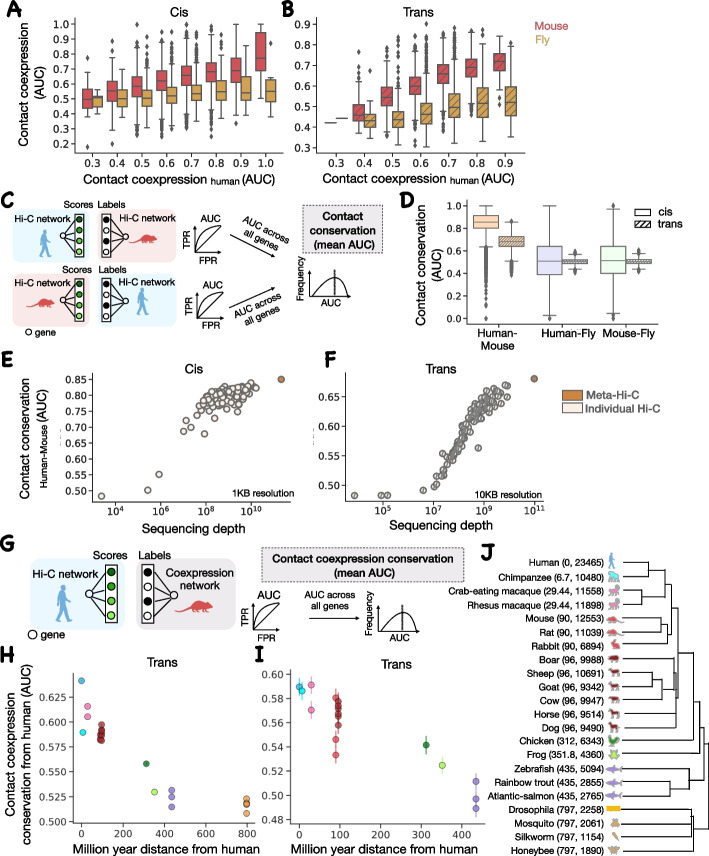
Before directly comparing the contact map across species, we first compared the contact coexpression scores for 1:1 orthologous genes across species. We find a strong linear relationship between human and mouse scores and a somewhat weaker relationship between human and fly scores in both cis (Fig. 4A) and trans (Fig. 4B). This suggests that if a gene is spatially co-regulated in one species, it is likely to be spatially co-regulated across other species.
Fig. 4.
Chromatin contacts are conserved across species in both cis and trans. Contact coexpression in cis (A) and trans (B) for 1:1 orthologs in human-mouse and human-fly. C Contact conservation schematic. For each gene, ranked edges in human Hi-C network are used to predict the top 10% of mouse Hi-C network edges. This task is repeated for each gene and in both directions and the average AUC is reported as human-mouse contact conservation. D The distribution of contact conservation score across genes in each direction using the meta-Hi-C network for various pairs of species. human-mouse contact conservation for individual Hi-C network and meta-Hi-C network as a function of sequencing depth in cis (E) and trans (F). G Contact coexpression conservation schematic. For each gene, ranked edges in the human Hi-C network are used to predict the top 1% of mouse coexpression network edges. This task is repeated for each gene and we report the average AUC as mouse contact coexpression conservation with human. H Contact coexpression conservation with human for several species. I Same as (H) but only using the same set of 429 ortholog genes across species. The error bars represent a 68% confidence interval. In cis, the Hi-C network is analyzed at 1-KB resolution, and in trans, the Hi-C network at 10-KB resolution is used for human and mouse and 1-KB resolution for fly. J The dendrogram shows phylogenetic relationships between species used for contact coexpression conservation analysis. Million year distance from human and number of 1:1 orthologs with human is listed in the parenthesis
We next characterized the degree to which gene contacts are conserved by directly comparing the meta-Hi-C network across species. Each gene’s shared neighborhood is defined by ranking all edges in the chromatin contact network and then using it to predict the gene’s top 10% of edges in another species. We call this “contact conservation” and again treat it as a prediction task with 1 meaning perfect contact conservation, 0.5 consistent with random reordering of neighborhoods, and 0 meaning that contacting partners have reversed (Fig. 4C). For the trans conservation score, only the trans gene pairs in both species are used, similarly for cis analysis. As expected, we find that the contact conservation is higher for human-mouse (AUC> 0.8) when compared with human-fly (AUC 0.5) or mouse-fly (AUC 0.51) (Fig. 4D) in both cis and trans.
We also re-validated the power of the meta-Hi-C network: we compared the contact conservation scores for individual and meta-Hi-C networks at the highest available resolution. We reassuringly find that the meta-Hi-C network outperforms individual projects in both cis and trans (Fig. 4E, F). This again suggests that the meta-Hi-C network is efficient in capturing chromatin contacts when compared to individual networks. Although the conservation of cis chromatin structure across species is not surprising and is evident in the presence of syntenic regions between species, the conservation of trans-chromatin contacts is noteworthy. It suggests that the trans-chromatin structure is likely selected for preservation to maintain function.
We further investigated the evolution of trans chromatin contacts in human by comparing the degree to which the human contacts can predict coexpression across several species. This method allowed us to extend our analysis to species for which the meta-Hi-C network is not available. Each gene’s neighborhood is defined by ranking all edges in the chromatin contact network of one species and then used to predict the gene’s top 1% of coexpressed gene pairs in another species. We call this “contact coexpression conservation” and calculate the AUC as above (Fig. 4G). When contact coexpression conservation is plotted along with the phylogenetic distance across species, we find that the performance decreases with an increase in phylogenetic distance using trans meta-Hi-C networks (Fig. 4H). This suggests that the contacts diverge as the species pair becomes distant across evolution. As expected, we also find that the strongest coexpressed genes have the most contact coexpression conservation (Additional file 1: Fig. S5). The number of 1:1 orthologs also decreases with an increase in the phylogenetic distance (Fig. 4J), and it seemed possible that our observation was dominated by the number of ortholog pairs between species. To eliminate this possibility, we redid our analysis but using only the same set of ortholog genes (429 genes) in each species and our result persisted (Fig. 4I). Species more than 100 million years of distance (mya) from humans have stronger divergence in contacts when compared to species within the Mammalia Class. The species included in Fig. 4I were limited to the Chordata phylum to ensure a reasonable number of genes in the analysis.
Data availability and online tool
In order to facilitate the broad adoption of meta-Hi-C by the community, we have made data available via an online tool (https://gillisweb.cshl.edu/HiC/). Contact data can be obtained in two ways: (a) network download: direct download of the desired resolution and species meta-Hi-C contact matrix in cis or trans available at https://labshare.cshl.edu/shares/gillislab/resource/HiC in HiCMatrix format (https://github.com/deeptools/HiCMatrix) and (b) gene vector download: contact frequency with every genomic loci at the chosen resolution and for any desired gene found in the respective species. The downloaded file is in a bed file format which can be uploaded to the UCSC genome browser for further analysis as desired.
Discussion
In this work, we created a high-depth, genome-wide chromatin contact map using a meta-analytic approach, validated it, and further used it to reveal chromatin structure to function relationships. We find that for the three species analyzed in this study (human, mouse, and fly), chromatin contacts strongly predicted the coexpression of genes. We also show that chromatin contacts are better than linear proximity for predicting eQTLs when high-resolution chromatin contact data is available. Our results persist even when only long-range chromatin contacts are analyzed. Additionally, we find that trans-chromosomal contacts show evidence of conservation between human and mouse. In contrast, we do not find any conservation between these mammalian networks and fly (human-fly AUC 0.50, mouse-fly AUC 0.51). This is striking in its nearly exact correspondence with the null, but the very large amount of data combined with the dramatically different genomes appears to leave no average signal when analyzed across homologs. It remains plausible that a model trained specifically to account for the broad cross-species differences would find subtler biological overlaps our direct assessments miss.
Meta-Hi-C networks are an effective means for capturing otherwise hard to characterize long-range interactions providing potentially uniquely important practical applications. One important application for a wide area of genomics is their ability to prioritize distant target genes for variants. We expect these networks to be powerful training data for future machine learning attempts to predict chromosomal contacts, an important area of ongoing research [13–16]. Additionally, meta-Hi-C networks can be used with other cell-type-specific ‘omics datasets such as ChIP-Seq to reveal cell-type-specific enhancer-promoter contacts. Previously, Nasser et al. [31, 32] used averaged Hi-C data across 10 cell types in their ABC model to accurately make cell-type-specific enhancer-gene predictions. Thus, the continuing evolution of methods with improved specificity is likely to complement our better-powered but less condition-specific meta-analytic approach.
Within the Hi-C analysis, and even outside of it, aggregation of data is well appreciated to be a useful strategy. Reproducible biological replicates within the same study are often combined to increase the density of Hi-C data thereby capturing more interactions [10, 33]. Our approach can be thought of as the most extreme version of this idea, combining experiments as broadly as possible to capture statistical relationships that are common. This is most useful if the depth is a major limitation, as in trans contacts, as it comes with the cost of a loss of condition-specificity. Thus, the route forward for the field as a whole will doubtless involve both improved specificity, integration, and interpretive methods.
In summary, our study sheds new light on the functional role of long-range and trans-chromosomal contacts and provides a critical resource for use by a wide range of genomics research.
Conclusions
In this study, we leveraged the hundreds of available Hi-C maps by aggregating them to build high-depth meta-Hi-C maps for human, mouse, and fly. These maps act like a “super-experiment” where additional depth leads to surprising power in capturing long-range functional relationships. These maps are able to predict coexpression, eQTLs, and cross-species relationships. The availability of the meta-Hi-C maps complements the ongoing efforts in identifying functional chromatin contacts and provides significant evidence for the functionality of trans chromatin contacts. The meta-Hi-C networks are available for download via an online tool at https://gillisweb.cshl.edu/HiC/.
Methods
Hi-C data sources
The Hi-C data for each species were obtained from SRA search (https://www.ncbi.nlm.nih.gov/sra/) with the field limitations of “Organism”: [“Homo sapiens,” “Mus musculus,” “Drosophila melanogaster”], “Strategy”: “hi c.” We found 3913, 8431, and 502 runs for human, mouse, and fly respectively. We also added 268, 17, and 25 runs manually that were labeled OTHER in SRA, but were deemed to be valid Hi-C data based on publication details. After manual additions, filtering out runs without available restriction enzyme information, and excluding runs that failed to process, we had 3621, 6733, and 487 samples for human, mouse, and fly.
SRA projects processed for human are SRP050102 [10], SRP012412 [34], SRP152979 [35], SRP152879 [36], SRP118999 [37], SRP154953 [38], SRP199098 [39], SRP234115 [40], SRP094854, SRP149906 [36], SRP165933 [41], SRP212226 [14], SRP117084 [42], SRP218691, SRP233368 [43], SRP106040 [44], SRP125488, SRP250432, SRP141473 [45], ERP107279, SRP224133 [46], SRP184300, SRP168606 [29], SRP216194 [47], SRP178527 [48], SRP173234 [49], SRP239849 [50], SRP106379, SRP162098 [51], SRP120957, DRP005280, SRP150259 [52], SRP170743, SRP131003 [53], SRP158113, SRP186190, SRP212073 [54], SRP133031 [55], SRP135798, SRP131871 [56], SRP158276 [57], SRP114754, SRP267107 [58], SRP227918 [59], SRP271101 [60], SRP186277, SRP115913 [61], SRP157799 [62], SRP110964, SRP194362 [63], SRP151075 [64], SRP157894 [65], SRP160101, SRP157048 [66], SRP221518 [67], SRP225696 [68], ERP104251, SRP105082 [9], SRP223060 [69], SRP234897 [70], SRP250333 [71], SRP113633 [72], SRP186012 [73], SRP199225 [74], SRP107176 [75], SRP105181 [76], SRP066852, SRP095110, SRP162056, SRP201909 [77], SRP153415 [78], SRP127183 [79], ERP118600, SRP274139 [80], SRP115572 [81], SRP099610 [82], SRP108500 [83], SRP195614 [84], SRP235557 [85], SRP264796 [86], SRP197114, SRP132233 [87], SRP244334, SRP113478, SRP107148, SRP083971, SRP192392 [88], SRP145420 [89], SRP152361 [90], SRP141229, SRP261300, SRP154986 [91], SRP261299, SRP150629 [92], SRP111140, SRP130935 [93], SRP165232, SRP098826, SRP102403 [94], SRP154399, SRP090318 [95], DRP005173 [96], SRP107149, SRP245657, SRP149124, SRP060755, SRP163366 [97], SRP071243, SRP272124 [98], SRP103077, SRP163908, SRP170855 [99], SRP217227 [73], SRP132876 [100], SRP100408 [101], SRP105086, SRP076397, SRP182670, and SRP109036.
SRA projects processed for mouse are SRP217487 [102], SRP101928 [103], SRP075985 [104], SRP105082 [9], SRP165933 [41], SRP261290 [105], SRP118601 [106], SRP107774 [107, 108], SRP226118, SRP252213 [109], SRP229756, SRP250878, SRP131117 [110], SRP247488 [111], SRP223513 [112], SRP119332 [113], SRP268173 [67], SRP270993 [114], SRP179647 [115], SRP255620 [116], SRP100871 [117], SRP192917 [14], SRP156597 [118], SRP227097 [67], ERP114475, SRP249897 [119], SRP096571 [120], SRP144391 [121], SRP110616 [122], SRP292639 [119], SRP194410 [123], SRP200567 [124], and SRP218950 [118].
SRA projects processed for fly are ERP122732 [125], SRP165773 [126], SRP119928 [127], ERP112882, SRP050096 [128], SRP223221 [129], SRP097891 [130], ERP016479, SRP104256 [131], SRP186730 [132], SRP230396 [133], SRP107636 [134], SRP073988 [135], SRP111713 [136], SRP107637 [134], SRP195621 [84], SRP193880, SRP168946 [137], SRP107556 [134], SRP158369 [138], SRP110166, SRP165772 [139], ERP112723, SRP219433, SRP156199 [140], SRP199618 [141], SRP132075 [142], SRP140881, and SRP181908 [143].
In total, we aggregated 119 human projects, 33 mouse projects, and 29 fly projects. Additional file 2: Tables S1, S2 and S3 further summarize the number of runs, sequencing depth, and cell-type information for each project in respective species.
Hi-C data processing pipeline
All runs were reprocessed from short read sequence data to reduce differential computational noise across experiments. Restriction enzymes were identified for each sample from the literature. SRA files were downloaded using prefetch, then converted to paired FASTQ files using fasterq-dump. FASTQ files were processed using the HiCUP tool [144], with the alteration that short reads were aligned using the STAR aligner, instead of the default Bowtie2 [145]. HiCUP truncates the reads based on restriction site, aligns them, and filters artifactual and duplicated data. Reads were aligned to the hg38, mm10, and dm6 in human, mouse, and fly respectively. Output SAM files were converted to indexed and compressed Pairs files using the bam2pairs tool. Finally, pairwise chromosome-chromosome contact matrices were generated at single base-pair resolution.
Building chromatin contact matrix
To obtain a chromatin contact matrix, each chromosome is divided up into “bins” of a specific size. The number of base pairs in each bin represents the “resolution” of the matrix. The contact frequency for each bin pair is obtained by summing the reads falling in that bin. The chromatin contact matrix was generated at 8 resolutions (1KB, 5KB, 10KB, 25KB, 40KB, 100KB, 250KB, and 500KB) in cis for all species and trans for only fly. For human and mouse, trans chromatin contact matrices at 1-KB and 5-KB resolutions were not processed due to high memory requirements (more than 2TB). These files were written in HiCMatrix (https://github.com/deeptools/HiCMatrix) h5 format. For each species, we excluded sex chromosomes and considered only autosomes (human: chr1 to chr22, mouse:chr1 to chr19 and fly: chr2L, chr2R, chr3L, chr3R, chr4). The contact frequency of each genomic pair coordinate was summed across runs to generate a project-level chromatin contact matrix. The sequencing depth of a project in cis and trans is obtained by summing all the contacts in cis and trans respectively. The contact frequency was KR-normalized separately for the cis and trans networks to adjust for nonuniformities in coverage introduced due to experimental bias [146] using the hicCorrectMatrix tool of HiCexplorerV3.6 [147]. All project-level chromatin contact matrices within each species were further summated to create species-level meta-Hi-C maps. Gene transcription start site (TSS) and transcription end site (TES) were used to determine the bins in which the gene resides. A list of genes, TSS, and TES were obtained as GTF files from ENSEMBL (September 2019). To determine the contact frequency between each gene pair, we use the maximum contact frequency between each bin in which genes reside. This method was previously used by Babaei et al. [21].
Coexpression data
The coexpression network used in this study is a “high confidence gene” aggregated coexpression network available at https://labshare.cshl.edu/shares/gillislab/resource/CoCoCoNet/ which was generated using the method previously described in CoCoCoNet [22]. In brief, several bulk RNA-seq datasets were obtained from NCBI’s SRA database (unique SRA Study IDs). Networks for each dataset are built by calculating the Spearman correlation between all pairs of genes, then ranking the correlation coefficients for all gene-gene pairs, with NAs assigned the median rank. Each network is then rank standardized and normalized by dividing by the maximum rank. Aggregate networks are then generated by averaging rank standardized networks from individual datasets. There are 23,465, 20,672, and 9636 genes (sex chromosomes are excluded) in the coexpression network for human, mouse, and fly.
Compartment, subcompartment, and TAD assignment
Gene compartments were either identified using “hicPCA” (tool of HiCexplorerV3.6 [147] which is based on the Lieberman-Aiden et al. method [8] using each chromosome KR-normalized cis-contact matrix or Calder2.0 [23] available at github (https://github.com/CSOgroup/CALDER2.0). Gene density was used for A or B compartment assignment when the “hicPCA” tool was used. TADs and TAD boundaries were identified using TopDom [148]. A TAD is overlapping across two Hi-C experiments at a given resolution if the TAD’s start and end positions are the same in both experiments.
eQTL data source and processing
A list of tissue-specific “significant” variant gene pair associations and “all” variant gene pair associations (including non-significant associations) across 54 tissues along with the distance between the variant and gene TSS (at bp resolution) were obtained from the GTEx Portal v8 at https://gtexportal.org. Since the meta-Hi-C network is not tissue-specific, we combined the data across tissues to generate a set of unique “significant” and “all” variant gene pair associations. To obtain a list of “non-significant” gene pair associations, “significant” variant gene pair associations were removed from “all” variant gene pair associations data. All variants in the coding regions and up to 1KB of any gene TSS and TES were removed. For performance score, 1-KB cis chromatin contact matrix is used, and for each eVariant, only genes in unique bins are tested. The total number of variants tested is 1,574,194 at 1-KB resolution. The total number of variant gene pair tested is 54,220,988 among which 4,319,205 pairs are significant.
Cross-species analysis
A list of 1:1 orthologs for a pair of species was obtained from OrthoDB [149]. Species divergence time was sourced from Timetree [150].
Supplementary Information
Additional file 1 Supplementary Figures S1-S5 with each legend. Fig S1. Meta-Hi-C network benchmarking in mouse. Fig S2. Meta-Hi-C network benchmarking in human. Fig S3. TAD coexpression vs contact coexpression. Fig S4. TAD and boundary conservation across experiments. Fig. S5. Contact coexpression conservation at various coexpression thresholds.
Additional file 2 Table S1. Details of each individual Hi-C project used for building human meta-Hi-C network. Table S2. Details of each individual Hi-C project used for building mouse meta-Hi-C network. Table S3. Details of each individual Hi-C project used for building fly meta-Hi-C network.
Acknowledgements
We acknowledge the IT support for scientific computing at CSHL for providing access to the Elzar cluster that has contributed to the results reported here. We thank members of the Gillis lab, particularly Benjamin D. Harris for assistance with code writing, Stephan Fischer and Risa Karakida Kawaguchi for comments on the manuscript, and, John Hover and John Lee for assisting with data download and data management.
Peer review information
Wenjing She was the primary editor of this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Review history
The review history is available as Additional file 3.
Authors’ contributions
J.G. conceived the study. R.L. and J.G. designed experiments, analyzed data, and wrote the manuscript. R.L. performed the experiments. N.F and R.L. designed the web tool. N.F parsed raw data and performed data curation. The authors read and approved the final manuscript.
Funding
J.G and R.L. are supported by NIH grants R01MH113005 and R01LM012736. The high-performance cluster Elzar utilized for this work is supported by NIH grant S10OD028632-01.
Availability of data and materials
The meta-Hi-C matrix for human, mouse, and fly are available for download from the online tool at https://gillisweb.cshl.edu/HiC/ or direct download at https://labshare.cshl.edu/shares/gillislab/resource/HiC/ or Figshare [151].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ruchi Lohia, Email: lohia@cshl.edu.
Nathan Fox, Email: nchristopherfox@gmail.com.
Jesse Gillis, Email: jesse.gillis@utoronto.ca.
References
- 1.Diament A, Tuller T. Modeling three-dimensional genomic organization in evolution and pathogenesis. Semin Cell Dev Biol. 2019;90:78–93. doi: 10.1016/j.semcdb.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Delaneau O, Zazhytska M, Borel C, Giannuzzi G, Rey G, Howald C, et al. Chromatin three-dimensional interactions mediate genetic effects on gene expression. Science. 2019;364(6439). 10.1126/science.aat8266. [DOI] [PubMed]
- 3.Xu H, Zhang S, Yi X, Plewczynski D, Li MJ. Exploring 3D chromatin contacts in gene regulation: The evolution of approaches for the identification of functional enhancer-promoter interaction. Comput Struct Biotechnol J. 2020;18:558–70. 10.1016/j.csbj.2020.02.013. [DOI] [PMC free article] [PubMed]
- 4.Hafner A, Boettiger A. The spatial organization of transcriptional control. Nature Reviews Genetics 2022. 2022;1–16. 10.1038/s41576-022-00526-0. [DOI] [PubMed]
- 5.Sarnataro S, Chiariello AM, Esposito A, Prisco A, Nicodemi M. Structure of the human chromosome interaction network. PLoS ONE. 2017;12(11):e0188201. doi: 10.1371/journal.pone.0188201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dekker J, Misteli T. Long-range chromatin interactions. Cold Spring Harb Perspect Biol. 2015;7(10):a019356. [DOI] [PMC free article] [PubMed]
- 7.Maass PG, Barutcu AR, Rinn JL. Interchromosomal interactions: a genomic love story of kissing chromosomes. J Cell Biol. 2019;218(1):27–38. [DOI] [PMC free article] [PubMed]
- 8.Lieberman-Aiden E, Van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326(5950):289–93. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vian L, Pȩkowska AP, Rao SSP, Kieffer-Kwon KR, Jung S, Baranello L, et al. The energetics and physiological impact of cohesin extrusion. Cell. 2018;173(5):1165. doi: 10.1016/j.cell.2018.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao SSP, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159(7):1665–80. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang R, Yu M, Li G, Chee S, Liu T, Schmitt AD, et al. Mapping of long-range chromatin interactions by proximity ligation-assisted ChIP-seq. Cell Res. 2016;26(12):1345–8. doi: 10.1038/cr.2016.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dryden NH, Broome LR, Dudbridge F, Johnson N, Orr N, Schoenfelder S, et al. Unbiased analysis of potential targets of breast cancer susceptibility loci by Capture Hi-C. Genome Res. 2014;24(11):1854–68. doi: 10.1101/gr.175034.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fudenberg G, Kelley DR, Pollard KS. Predicting 3D genome folding from DNA sequence with Akita. Nat Methods. 2020;17(11):1111–7. doi: 10.1038/s41592-020-0958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Chen H, Li R, Taft DA, Yao G, Bai F, et al. Spatial clustering and common regulatory elements correlate with coordinated gene expression. PLoS Computational Biology. 2019;15(3):e1006786. doi: 10.1371/journal.pcbi.1006786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, An L, Xu J, Zhang B, Zheng WJ, Hu M, et al. Enhancing Hi-C data resolution with deep convolutional neural network HiCPlus. Nat Commun. 2018;9(1):1–9. doi: 10.1038/s41467-018-03113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwessinger R, Gosden M, Downes D, Brown RC, Oudelaar AM, Telenius J, et al. DeepC: predicting 3D genome folding using megabase-scale transfer learning. Nat Methods. 2020;17(11):1118–24. doi: 10.1038/s41592-020-0960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ay F, Bailey TL, Noble WS. Statistical confidence estimation for Hi-C data reveals regulatory chromatin contacts. Genome Res. 2014;24(6):999–1011. doi: 10.1101/gr.160374.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bulathsinghalage C, Liu L. Network-based method for regions with statistically frequent interchromosomal interactions at single-cell resolution. BMC Bioinformatics. 2020;21(Suppl 14):369. doi: 10.1186/s12859-020-03689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong K, Ma J. Revealing Hi-C subcompartments by imputing inter-chromosomal chromatin interactions. Nat Commun. 2019;10(1):5069. doi: 10.1038/s41467-019-12954-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varrone M, Nanni L, Ciriello G, Ceri S. Exploring chromatin conformation and gene co-expression through graph embedding. Bioinformatics. 2020;36(Supplement_2):i700-8. [DOI] [PubMed]
- 21.Babaei S, Mahfouz A, Hulsman M, Lelieveldt BPF, de Ridder J, Reinders M. Hi-C chromatin interaction networks predict co-expression in the mouse cortex. PLoS Comput Biol. 2015;11(5):e1004221. [DOI] [PMC free article] [PubMed]
- 22.Lee J, Shah M, Ballouz S, Crow M, Gillis J. CoCoCoNet: conserved and comparative co-expression across a diverse set of species. Nucleic Acids Res. 2020;48(W1):W566–71. doi: 10.1093/nar/gkaa348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Nanni L, Sungalee S, Zufferey M, Tavernari D, Mina M, et al. Systematic inference and comparison of multi-scale chromatin sub-compartments connects spatial organization to cell phenotypes. Nat Commun. 2021;12(1):1–11. doi: 10.1038/s41467-021-22666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCord RP, Kaplan N, Giorgetti L. Chromosome conformation capture and beyond: toward an integrative view of chromosome structure and function. Mol Cell. 2020;77(4):688–708. [DOI] [PMC free article] [PubMed]
- 25.Nora EP, Lajoie BR, Schulz EG, Giorgetti L, Okamoto I, Servant N, et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485(7398):381–5. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Yang J, Zhang Y, Wang J. Discover novel disease-associated genes based on regulatory networks of long-range chromatin interactions. Methods. 2021;189:22–33. doi: 10.1016/j.ymeth.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong N, Jung I. Long-range chromatin interactions in pathogenic gene expression control. Transcription. 2020;11(5):211–6. doi: 10.1080/21541264.2020.1843958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stacey D, Fauman EB, Ziemek D, Sun BB, Harshfield EL, Wood AM, et al. ProGeM: a framework for the prioritization of candidate causal genes at molecular quantitative trait loci. Nucleic Acids Res. 2019;47(1):e3. doi: 10.1093/nar/gky837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eres IE, Luo K, Hsiao CJ, Blake LE, Gilad Y. Reorganization of 3D genome structure may contribute to gene regulatory evolution in primates. PLoS Genet. 2019;15(7):e1008278. doi: 10.1371/journal.pgen.1008278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krefting J, Andrade-Navarro MA, Ibn-Salem J. Evolutionary stability of topologically associating domains is associated with conserved gene regulation. BMC Biol. 2018;16(1):1–12. doi: 10.1186/s12915-018-0556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasser J, Bergman DT, Fulco CP, Guckelberger P, Doughty BR, Patwardhan TA, et al. Genome-wide enhancer maps link risk variants to disease genes. Nature. 2021;593(7858):238–43. doi: 10.1038/s41586-021-03446-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulco CP, Nasser J, Jones TR, Munson G, Bergman DT, Subramanian V, et al. Activity-by-contact model of enhancer-promoter regulation from thousands of CRISPR perturbations. Nat Genet. 2019;51(12):1664–9. doi: 10.1038/s41588-019-0538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Won H, de la Torre-Ubieta L, Stein JL, Parikshak NN, Huang J, Opland CK, et al. Chromosome conformation elucidates regulatory relationships in developing human brain. Nature. 2016;538(7626):523–7. doi: 10.1038/nature19847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heidari N, Phanstiel DH, He C, Grubert F, Jahanbani F, Kasowski M, et al. Genome-wide map of regulatory interactions in the human genome. Genome Res. 2014;24(12):1905. doi: 10.1101/gr.176586.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arvanitis M, Tampakakis E, Zhang Y, Wang W, Auton A. 23andMe Research Team, et al. Genome-wide association and multi-omic analyses reveal ACTN2 as a gene linked to heart failure. Nat Commun. 2020;11(1):1122. doi: 10.1038/s41467-020-14843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu L, Liu X, Huang WK, Giusti-Rodríguez P, Cui J, Zhang S, et al. Robust Hi-C maps of enhancer-promoter interactions reveal the function of non-coding genome in neural development and diseases. Mol Cell. 2020;79(3):521-534.e15. [DOI] [PMC free article] [PubMed]
- 37.Rao SSP, Huang SC, St Hilaire BG, Engreitz JM, Perez EM, Kieffer-Kwon KR, et al. Cohesin loss eliminates all loop domains. Cell. 2017;171(2):305-320.e24. [DOI] [PMC free article] [PubMed]
- 38.Lawlor N, Márquez EJ, Orchard P, Narisu N, Shamim MS, Thibodeau A, et al. Multiomic profiling identifies cis-regulatory networks underlying human pancreatic β cell identity and function. Cell Rep. 2019;26(3):788-801.e6. [DOI] [PMC free article] [PubMed]
- 39.Ochi Y, Kon A, Sakata T, Nakagawa MM, Nakazawa N, Kakuta M, et al. Combined cohesin-RUNX1 deficiency synergistically perturbs chromatin looping and causes myelodysplastic syndromes. Cancer Discov. 2020;10(6):836–53. [DOI] [PMC free article] [PubMed]
- 40.Stik G, Vidal E, Barrero M, Cuartero S, Vila-Casadesús M, Mendieta-Esteban J, et al. CTCF is dispensable for immune cell transdifferentiation but facilitates an acute inflammatory response. Nat Genet. 2020;52(7):655–61. doi: 10.1038/s41588-020-0643-0. [DOI] [PubMed] [Google Scholar]
- 41.El Khattabi L, Zhao H, Kalchschmidt J, Young N, Jung S, Van Blerkom P, et al. A pliable mediator acts as a functional rather than an architectural bridge between promoters and enhancers. Cell. 2019;178(5):1145-1158.e20. [DOI] [PMC free article] [PubMed]
- 42.Heinz S, Texari L, Hayes MGB, Urbanowski M, Chang MW, Givarkes N, et al. Transcription elongation can affect genome 3D structure. Cell. 2018;174(6):1522-1536.e22. [DOI] [PMC free article] [PubMed]
- 43.Kang H, Shokhirev MN, Xu Z, Chandran S, Dixon JR, Hetzer MW. Dynamic regulation of histone modifications and long-range chromosomal interactions during postmitotic transcriptional reactivation. Genes Dev. 2020;34(13–14):913–30. doi: 10.1101/gad.335794.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zirkel A, Nikolic M, Sofiadis K, Mallm JP, Brant L, Becker C, et al. Topological demarcation by HMGB2 is disrupted early upon senescence entry across cell types and induces CTCF clustering. bioRxiv. 2017;127522. 10.1101/127522.
- 45.Song M, Yang X, Ren X, Maliskova L, Li B, Jones IR, et al. Mapping cis-regulatory chromatin contacts in neural cells links neuropsychiatric disorder risk variants to target genes. Nat Genet. 2019;51(8):1252–62. doi: 10.1038/s41588-019-0472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wutz G, Ladurner R, St Hilaire BG, Stocsits RR, Nagasaka K, Pignard B, et al. ESCO1 and CTCF enable formation of long chromatin loops by protecting cohesinSTAG1 from WAPL. Elife. 2020;9:e52091. 10.7554/eLife.52091. [DOI] [PMC free article] [PubMed]
- 47.Kloetgen A, Thandapani P, Ntziachristos P, Ghebrechristos Y, Nomikou S, Lazaris C, et al. Three-dimensional chromatin landscapes in T cell acute lymphoblastic leukemia. Nat Genet. 2020;52(4):388–400. doi: 10.1038/s41588-020-0602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu L, Li QZ, Jin W, Lv H, Lin H. Revealing gene function and transcription relationship by reconstructing gene-level chromatin interaction. Comput Struct Biotechnol J. 2019;17:195–205. [DOI] [PMC free article] [PubMed]
- 49.Nir G, Farabella I, Pérez Estrada C, Ebeling CG, Beliveau BJ, Sasaki HM, et al. Walking along chromosomes with super-resolution imaging, contact maps, and integrative modeling. PLoS Genet. 2018;14(12):e1007872. doi: 10.1371/journal.pgen.1007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang J, McGovern A, Martin P, Duffus K, Ge X, Zarrineh P, et al. Analysis of chromatin organization and gene expression in T cells identifies functional genes for rheumatoid arthritis. Nat Commun. 2020;11(1):4402. doi: 10.1038/s41467-020-18180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrari R, de Llobet Cucalon LI, Di Vona C, Le Dilly F, Vidal E, Lioutas A, et al. TFIIIC binding to Alu elements controls gene expression via chromatin looping and histone acetylation. Mol Cell. 2020;77(3):475–487.e11. [DOI] [PMC free article] [PubMed]
- 52.Jacobson EC, Perry JK, Long DS, Olins AL, Olins DE, Wright BE, et al. Migration through a small pore disrupts inactive chromatin organization in neutrophil-like cells. BMC Biol. 2018;16(1). [DOI] [PMC free article] [PubMed]
- 53.Dily FL, Le Dily F, Vidal E, Cuartero Y, Quilez J, Nacht S, et al. Hormone control regions mediate opposing steroid receptor-dependent genome organizations. Genome Res. 2019;29(1):29–39. 10.1101/gr.243824.118. [DOI] [PMC free article] [PubMed]
- 54.Zhang X, Liu X, Du Z, Wei L, Fang H, Dong Q, et al. The loss of heterochromatin is associated with multiscale three-dimensional genome reorganization and aberrant transcription during cellular senescence. Genome Res. 2021;31(7):1121–35. 10.1101/gr.275235.121. [DOI] [PMC free article] [PubMed]
- 55.Rodriguez A. High HDL-cholesterol paradox: SCARB1-LAG3-HDL axis. Curr Atheroscler Rep. 2021;23(1):5. [DOI] [PMC free article] [PubMed]
- 56.Paulsen J, Liyakat Ali TM, Nekrasov M, Delbarre E, Baudement MO, Kurscheid S, et al. Long-range interactions between topologically associating domains shape the four-dimensional genome during differentiation. Nat Genet. 2019;51(5):835–43. doi: 10.1038/s41588-019-0392-0. [DOI] [PubMed] [Google Scholar]
- 57.Achinger-Kawecka J, Valdes-Mora F, Luu PL, Giles KA, Caldon CE, Qu W, et al. Epigenetic reprogramming at estrogen-receptor binding sites alters 3D chromatin landscape in endocrine-resistant breast cancer. Nat Commun. 2020;11(1):320. doi: 10.1038/s41467-019-14098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitter M, Gasser C, Takacs Z, Langer CCH, Tang W, Jessberger G, et al. Conformation of sister chromatids in the replicated human genome. Nature. 2020;586(7827):139–44. [DOI] [PMC free article] [PubMed]
- 59.Senigl F, Maman Y, Dinesh RK, Alinikula J, Seth RB, Pecnova L, et al. Topologically associated domains delineate susceptibility to somatic hypermutation. Cell Rep. 2019;29(12):3902-3915.e8. [DOI] [PMC free article] [PubMed]
- 60.Guo D, Xie Q, Jiang S, Xie T, Li Y, Huang X, et al. Synergistic alterations in the multilevel chromatin structure anchor dysregulated genes in small cell lung cancer. Comput Struct Biotechnol J. 2021;19:5946–59. doi: 10.1016/j.csbj.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wutz G, Várnai C, Nagasaka K, Cisneros DA, Stocsits RR, Tang W, et al. Topologically associating domains and chromatin loops depend on cohesin and are regulated by CTCF, WAPL, and PDS5 proteins. EMBO J. 2017;36(24):3573–99. doi: 10.15252/embj.201798004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iwasaki O, Tanizawa H, Kim KD, Kossenkov A, Nacarelli T, Tashiro S, et al. Involvement of condensin in cellular senescence through gene regulation and compartmental reorganization. Nat Commun. 2019;10(1):5688. doi: 10.1038/s41467-019-13604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dileep V, Wilson KA, Marchal C, Lyu X, Zhao PA, Li B, et al. Rapid irreversible transcriptional reprogramming in human stem cells accompanied by discordance between replication timing and chromatin compartment. Stem Cell Rep. 2019;13(1):193–206. [DOI] [PMC free article] [PubMed]
- 64.Tian L, Xu B, Shao Y, Ma X, Li Y, Newman S, et al. Abstract 1485: Allelic specificity of immunoglobulin heavy chain (IGH) translocation in B-cell acute lymphoblastic leukemia (B-ALL) unveiled by long-read sequencing. Cancer Res. 2018;78(13_Supplement):1485.
- 65.Guo Y, Perez AA, Hazelett DJ, Coetzee GA, Rhie SK, Farnham PJ. CRISPR-mediated deletion of prostate cancer risk-associated CTCF loop anchors identifies repressive chromatin loops. Genome Biol. 2018;19(1):160. doi: 10.1186/s13059-018-1531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ooi WF, Nargund AM, Lim KJ, Zhang S, Xing M, Mandoli A, et al. Integrated paired-end enhancer profiling and whole-genome sequencing reveals recurrent CCNE1 and IGF2 enhancer hijacking in primary gastric adenocarcinoma. Gut. 2020;69(6):1039–52. doi: 10.1136/gutjnl-2018-317612. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Cao J. GSimPy: a Python package for measuring group similarity. SoftwareX. 2020;12:100526.
- 68.Hyle J, Zhang Y, Wright S, Xu B, Shao Y, Easton J, et al. Acute depletion of CTCF directly affects MYC regulation through loss of enhancer-promoter looping. Nucleic Acids Res. 2019;47(13):6699–713. doi: 10.1093/nar/gkz462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ray-Jones H, Duffus K, McGovern A, Martin P, Shi C, Hankinson J, et al. Mapping DNA interaction landscapes in psoriasis susceptibility loci highlights KLF4 as a target gene in 9q31. BMC Biol. 2020;18(1):47. doi: 10.1186/s12915-020-00779-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choudhary MN, Friedman RZ, Wang JT, Jang HS, Zhuo X, Wang T. Co-opted transposons help perpetuate conserved higher-order chromosomal structures. Genome Biol. 2020;21(1):16. doi: 10.1186/s13059-019-1916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thiecke MJ, Wutz G, Muhar M, Tang W, Bevan S, Malysheva V, et al. Cohesin-dependent and independent mechanisms support chromosomal contacts between promoters and enhancers. Cell Rep. 2020;32(3):107929. 10.1016/j.celrep.2020.107929. [DOI] [PMC free article] [PubMed]
- 72.Kojic A, Cuadrado A, De Koninck M, Giménez-Llorente D, Rodríguez-Corsino M, Gómez-López G, et al. Distinct roles of cohesin-SA1 and cohesin-SA2 in 3D chromosome organization. Nat Struct Mol Biol. 2018;25(6):496–504. doi: 10.1038/s41594-018-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y, Haarhuis JHI, Sedeño Cacciatore Á, Oldenkamp R, van Ruiten MS, Willems L, et al. The structural basis for cohesin-CTCF-anchored loops. Nature. 2020;578(7795):472–6. doi: 10.1038/s41586-019-1910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lhoumaud P, Badri S, Rodriguez-Hernaez J, Sakellaropoulos T, Sethia G, Kloetgen A, et al. NSD2 overexpression drives clustered chromatin and transcriptional changes in a subset of insulated domains. Nat Commun. 2019;10(1):4843. doi: 10.1038/s41467-019-12811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo Z, Rhie SK, Lay FD, Farnham PJ. A prostate cancer risk element functions as a repressive loop that regulates HOXA13. Cell Rep. 2017;21(6):1411–7. [DOI] [PMC free article] [PubMed]
- 76.Belaghzal H, Dekker J, Gibcus JH. Hi-C 2.0: an optimized Hi-C procedure for high-resolution genome-wide mapping of chromosome conformation. Methods. 2017;123:56–65. [DOI] [PMC free article] [PubMed]
- 77.Casa V, Gines MM, Gusmao EG, Slotman JA, Zirkel A, Josipovic N, et al. Redundant and specific roles of cohesin STAG subunits in chromatin looping and transcription control. Genome Res. 2020;30(4):515–27. 10.1101/gr.253211.119. [DOI] [PMC free article] [PubMed]
- 78.Martinez-Soria N, McKenzie L, Draper J, Ptasinska A, Issa H, Potluri S, et al. The oncogenic transcription factor RUNX1/ETO corrupts cell cycle regulation to drive leukemic transformation. Cancer Cell. 2019;35(4):705. [DOI] [PMC free article] [PubMed]
- 79.Assi SA, Imperato MR, Coleman DJL, Pickin A, Potluri S, Ptasinska A, et al. Subtype-specific regulatory network rewiring in acute myeloid leukemia. Nat Genet. 2019;51(1):151–62. doi: 10.1038/s41588-018-0270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Richart L, Lapi E, Pancaldi V, Cuenca M, Pau ECdS, Madrid-Mencía M, et al. STAG2 loss-of-function affects short-range genomic contacts and modulates urothelial differentiation in bladder cancer cells. Nucleic Acids Res. 2021;49(19):11005–21. 10.1093/nar/gkab864. [DOI] [PMC free article] [PubMed]
- 81.Gibcus JH, Samejima K, Goloborodko A, Samejima I, Naumova N, Kanemaki M, et al. Mitotic chromosomes fold by condensin-dependent helical winding of chromatin loop arrays. bioRxiv. 2018;174649. 10.1101/174649.
- 82.Niskanen H, Tuszynska I, Zaborowski R, Heinäniemi M, Ylä-Herttuala S, Wilczynski B, et al. Endothelial cell differentiation is encompassed by changes in long range interactions between inactive chromatin regions. Nucleic Acids Res. 2018;46(4):1724–40. doi: 10.1093/nar/gkx1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nair SJ, Yang L, Meluzzi D, Oh S, Yang F, Friedman MJ, et al. Phase separation of ligand-activated enhancers licenses cooperative chromosomal enhancer assembly. Nat Struct Mol Biol. 2019;26(3):193–203. doi: 10.1038/s41594-019-0190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ray J, Munn PR, Vihervaara A, Lewis JJ, Ozer A, Danko CG, et al. Chromatin conformation remains stable upon extensive transcriptional changes driven by heat shock. Proc Natl Acad Sci U S A. 2019;116(39):19431–9. doi: 10.1073/pnas.1901244116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao Q, Dacre M, Nguyen T, Pjanic M, Liu B, Iyer D, et al. Molecular mechanisms of coronary disease revealed using quantitative trait loci for TCF21 binding, chromatin accessibility, and chromosomal looping. Genome Biol. 2020;21:135. [DOI] [PMC free article] [PubMed]
- 86.Shi C, Ray-Jones H, Ding J, Duffus K, Fu Y, Gaddi VP, et al. Chromatin looping links target genes with genetic risk loci for dermatological traits. J Invest Dermatol. 2021;141(8):1975–84. [DOI] [PMC free article] [PubMed]
- 87.Ghurye J, Rhie A, Walenz BP, Schmitt A, Selvaraj S, Pop M, et al. Integrating Hi-C links with assembly graphs for chromosome-scale assembly. PLoS Comput Biol. 2019;15(8):e1007273. doi: 10.1371/journal.pcbi.1007273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morf J, Wingett SW, Farabella I, Cairns J, Furlan-Magaril M, Jiménez-García LF, et al. RNA proximity sequencing reveals the spatial organization of the transcriptome in the nucleus. Nat Biotechnol. 2019;37(7):793–802. doi: 10.1038/s41587-019-0166-3. [DOI] [PubMed] [Google Scholar]
- 89.Barutcu AR, Blencowe BJ, Rinn JL. Differential contribution of steady-state RNA and active transcription in chromatin organization. EMBO Rep. 2019;20(10):e48068. doi: 10.15252/embr.201948068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akdemir KC, et al. Chromatin folding domains disruptions by somatic genomic rearrangements in human cancers. Nat Genet. 2019;10. [DOI] [PMC free article] [PubMed]
- 91.Kantidze OL, Luzhin AV, Nizovtseva EV, Safina A, Valieva ME, Golov AK, et al. The anti-cancer drugs curaxins target spatial genome organization. Nat Commun. 2019;10(1):1441. doi: 10.1038/s41467-019-09500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Canzio D, Nwakeze CL, Horta A, Rajkumar SM, Coffey EL, Duffy EE, et al. Antisense lncRNA transcription mediates DNA demethylation to drive stochastic protocadherin α promoter choice. Cell. 2019;177(3):639–653.e15. [DOI] [PMC free article] [PubMed]
- 93.Elbatsh AMO, Kim E, Eeftens JM, Raaijmakers JA, van der Weide RH, García-Nieto A, et al. Distinct roles for condensin’s two ATPase sites in chromosome condensation. Mol Cell. 2019;76(5):724–737.e5. [DOI] [PMC free article] [PubMed]
- 94.Dixon JR, Xu J, Dileep V, Zhan Y, Song F, Le VT, et al. Integrative detection and analysis of structural variation in cancer genomes. Nat Genet. 2018;50(10):1388–98. 10.1038/s41588-018-0195-8. [DOI] [PMC free article] [PubMed]
- 95.Lai B, Tang Q, Jin W, Hu G, Wangsa D, Cui K, et al. Trac-looping measures genome structure and chromatin accessibility. Nat Methods. 2018;15(9):741–7. doi: 10.1038/s41592-018-0107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kadota M, Nishimura O, Miura H, Tanaka K, Hiratani I, Kuraku S. Multifaceted Hi-C benchmarking: what makes a difference in chromosome-scale genome scaffolding? Gigascience. 2020;9(1). [DOI] [PMC free article] [PubMed]
- 97.Jacobson EC, Grand RS, Perry JK, Vickers MH, Olins AL, Olins DE, et al. Hi-C detects novel structural variants in HL-60 and HL-60/S4 cell lines. Genomics. 2020;112(1):151–62. doi: 10.1016/j.ygeno.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 98.Chignon A, Rosa M, Boulanger MC, Argaud D, Devillers R, Bon-Baret V, et al. Enhancer-associated aortic valve stenosis risk locus 1p21.2 alters NFATC2 binding site and promotes fibrogenesis. 2021. [DOI] [PMC free article] [PubMed]
- 99.Raviram R, Rocha PP, Luo VM, Swanzey E, Miraldi ER, Chuong EB, et al. Analysis of 3D genomic interactions identifies candidate host genes that transposable elements potentially regulate. Genome Biol. 2018;19(1):216. doi: 10.1186/s13059-018-1598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pan DZ, Garske KM, Alvarez M, Bhagat YV, Boocock J, Nikkola E, et al. Integration of human adipocyte chromosomal interactions with adipose gene expression prioritizes obesity-related genes from GWAS. Nat Commun. 2018;9(1):1512. doi: 10.1038/s41467-018-03554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lopez-Pajares V, Rubin A, Barajas B, Furlan-Magaril M, Mumbach M, Greenleaf W, et al. 464 Dynamic and stable enhancer-promoter contacts regulate epidermal terminal differentiation. J Invest Dermatol. 2017;137(5):S80. [Google Scholar]
- 102.Norrie JL, Lupo MS, Xu B, Al Diri I, Valentine M, Putnam D, et al. Nucleome dynamics during retinal development. Neuron. 2019;104(3):512-528.e11. [DOI] [PMC free article] [PubMed]
- 103.Stadhouders R, Vidal E, Serra F, Di Stefano B, Le Dily F, Quilez J, et al. Transcription factors orchestrate dynamic interplay between genome topology and gene regulation during cell reprogramming. Nat Genet. 2018;50(2):238–49. doi: 10.1038/s41588-017-0030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kieffer-Kwon KR, Nimura K, Rao SSP, Xu J, Jung S, Pekowska A, et al. Myc regulates chromatin decompaction and nuclear architecture during B cell activation. Mol Cell. 2017;67(4):566-578.e10. [DOI] [PMC free article] [PubMed]
- 105.Collins PL, Purman C, Porter SI, Nganga V, Saini A, Hayer KE, et al. DNA double-strand breaks induce H2Ax phosphorylation domains in a contact-dependent manner. Nat Commun. 2020;11(1):3158. doi: 10.1038/s41467-020-16926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim YH, Marhon SA, Zhang Y, Steger DJ, Won KJ, Lazar MA. Rev-erbα dynamically modulates chromatin looping to control circadian gene transcription. Science. 2018;359(6381):1274–7. doi: 10.1126/science.aao6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chan WF, Coughlan HD, Zhou JHS, Keenan CR, Bediaga NG, Hodgkin PD, et al. Pre-mitotic genome re-organisation bookends the B cell differentiation process. Nat Commun. 2021;12(1):1344. doi: 10.1038/s41467-021-21536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Johanson TM, Lun ATL, Coughlan HD, Tan T, Smyth GK, Nutt SL, et al. Transcription-factor-mediated supervision of global genome architecture maintains B cell identity. Nat Immunol. 2018;19(11):1257–64. doi: 10.1038/s41590-018-0234-8. [DOI] [PubMed] [Google Scholar]
- 109.Chen CCL, Deshmukh S, Jessa S, Hadjadj D, Lisi V, Andrade AF, et al. Histone H3.3G34-mutant interneuron progenitors co-opt PDGFRA for gliomagenesis. Cell. 2020;183(6):1617-1633.e22. [DOI] [PMC free article] [PubMed]
- 110.Wang Y, Wang H, Zhang Y, Du Z, Si W, Fan S, et al. Reprogramming of meiotic chromatin architecture during spermatogenesis. Mol Cell. 2019;73(3):547-561.e6. [DOI] [PubMed]
- 111.Kriz AJ, Colognori D, Sunwoo H, Nabet B, Lee JT. Balancing cohesin eviction and retention prevents aberrant chromosomal interactions, polycomb-mediated repression, and X-inactivation. Mol Cell. 2021;81(1):1970-1987.e9. [DOI] [PMC free article] [PubMed]
- 112.Jiang Q, Ang JYJ, Lee AY, Cao Q, Li KY, Yip KY, et al. G9a plays distinct roles in maintaining DNA methylation, retrotransposon silencing, and chromatin looping. Cell Rep. 2020;33(4):108315. [DOI] [PubMed]
- 113.Barutcu AR, Rasim Barutcu A, Maass PG, Lewandowski JP, Weiner CL, Rinn JL. A TAD boundary is preserved upon deletion of the CTCF-rich Firre locus. Nat Commun. 2018;9(1). [DOI] [PMC free article] [PubMed]
- 114.Zhu Y, Denholtz M, Lu H, Murre C. Calcium signaling instructs NIPBL recruitment at active enhancers and promoters via distinct mechanisms to reconstruct genome compartmentalization. Genes Dev. 2021;35(1–2):65–81. doi: 10.1101/gad.343475.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kaaij LJT, Mohn F, van der Weide RH, de Wit E, Bühler M. The ChAHP complex counteracts chromatin looping at CTCF sites that emerged from SINE expansions in mouse. Cell. 2019;178(6):1437-1451.e14. [DOI] [PubMed]
- 116.Gnan S, Flyamer IM, Klein KN, Castelli E, Rapp A, Maiser A, et al. Nuclear organisation and replication timing are coupled through RIF1-PP1 interaction. Nat Commun. 2021;12(1):2910. 10.1038/s41467-021-22899-2. [DOI] [PMC free article] [PubMed]
- 117.Siersbæk R, Madsen JGS, Javierre BM, Nielsen R, Bagge EK, Cairns J, et al. Dynamic rewiring of promoter-anchored chromatin loops during adipocyte differentiation. Mol Cell. 2017;66(3):420-435.e5. [DOI] [PubMed]
- 118.Du Z, Zheng H, Kawamura YK, Zhang K, Gassler J, Powell S, et al. Polycomb group proteins regulate chromatin architecture in mouse oocytes and early embryos. Mol Cell. 2020;77(4):825-839.e7. [DOI] [PubMed]
- 119.Tan L, Ma W, Wu H, Zheng Y, Xing D, Chen R, et al. Changes in genome architecture and transcriptional dynamics progress independently of sensory experience during post-natal brain development. Cell. 2021;184(3):741–758.e17. doi: 10.1016/j.cell.2020.12.032. [DOI] [PubMed] [Google Scholar]
- 120.Schwarzer W, Abdennur N, Goloborodko A, Pekowska A, Fudenberg G, Loe-Mie Y, et al. Two independent modes of chromatin organization revealed by cohesin removal. Nature. 2017;551(7678):51–6. doi: 10.1038/nature24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miura H, Takahashi S, Poonperm R, Tanigawa A, Takebayashi SI, Hiratani I. Single-cell DNA replication profiling identifies spatiotemporal developmental dynamics of chromosome organization. Nat Genet. 2019;51(9):1356–68. doi: 10.1038/s41588-019-0474-z. [DOI] [PubMed] [Google Scholar]
- 122.Brandão HB, Gassler J, Imakaev M, Flyamer IM, Ladstätter S, Bickmore WA, et al. A mechanism of cohesin-dependent loop extrusion organizes mammalian chromatin structure in the developing embryo. Biophys J. 2018;114(3):255a.
- 123.Chatzidaki EE, Powell S, Dequeker BJH, Gassler J, Silva MCC, Tachibana K. Ovulation suppression protects against chromosomal abnormalities in mouse eggs at advanced maternal age. Curr Biol. 2021;31(18):4038–4051.e7. doi: 10.1016/j.cub.2021.06.076. [DOI] [PubMed] [Google Scholar]
- 124.Silva MCC, Powell S, Ladstätter S, Gassler J, Stocsits R, Tedeschi A, et al. Wapl releases Scc1-cohesin and regulates chromosome structure and segregation in mouse oocytes. J Cell Biol. 2020;219(4). [DOI] [PMC free article] [PubMed]
- 125.Simmons E, Vaid R, Bing XY, Levine M, Mannervik M, Vaquerizas JM. Independence of chromatin conformation and gene regulation during Drosophila dorsoventral patterning. Nat Genet. 2021;53(4):487–99. doi: 10.1038/s41588-021-00799-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.AlHaj Abed J, Erceg J, Goloborodko A, Nguyen SC, McCole RB, Saylor W, et al. Highly structured homolog pairing reflects functional organization of the Drosophila genome. Nat Commun. 2019;10(1):4485. doi: 10.1038/s41467-019-12208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cattoni DI, Cardozo Gizzi AM, Georgieva M, Di Stefano M, Valeri A, Chamousset D, et al.. Single-cell absolute contact probability detection reveals chromosomes are organized by multiple low-frequency yet specific interactions. Nat Commun. 2017;8(1). [DOI] [PMC free article] [PubMed]
- 128.Li L, Lyu X, Hou C, Takenaka N, Nguyen HQ, et al. Widespread rearrangement of 3D chromatin organization underlies polycomb-mediated stress-induced silencing. Mol Cell. 2015;58(2):216–31. [DOI] [PMC free article] [PubMed]
- 129.Gisselbrecht SS, Palagi A, Kurland JV, Rogers JM, Ozadam H, Zhan Y, et al. Transcriptional silencers in Drosophila serve a dual role as transcriptional enhancers in alternate cellular contexts. Mol Cell. 2020;77(2):324-337.e8. [DOI] [PMC free article] [PubMed]
- 130.Schauer T, Ghavi-Helm Y, Sexton T, Albig C, Regnard C, Cavalli G, et al. The Drosophila dosage compensation complex activates target genes by chromosome looping within the active compartment. bioRxiv. 2017;101634. 10.1101/101634.
- 131.Ramírez F, Bhardwaj V, Arrigoni L, Lam KC, Grüning BA, Villaveces J, et al. High-resolution TADs reveal DNA sequences underlying genome organization in flies. Nat Commun. 2018;9(1):189. doi: 10.1038/s41467-017-02525-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Loubiere V, Papadopoulos GL, Szabo Q, Martinez AM, Cavalli G. Widespread activation of developmental gene expression characterized by PRC1-dependent chromatin looping. Sci Adv. 2020;6(2):eaax4001. doi: 10.1126/sciadv.aax4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zenk F, Zhan Y, Kos P, Löser E, Atinbayeva N, Schächtle M, et al. HP1 drives de novo 3D genome reorganization in early Drosophila embryos. Nature. 2021;593(7858):289–93. doi: 10.1038/s41586-021-03460-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Szabo Q, Jost D, Chang JM, Cattoni DI, Papadopoulos GL, Bonev B, et al. TADs are 3D structural units of higher-order chromosome organization in Drosophila. Sci Adv. 2018;4(2):eaar8082. 10.1126/sciadv.aar8082. [DOI] [PMC free article] [PubMed]
- 135.Cubeñas-Potts C, Rowley MJ, Lyu X, Li G, Lei EP, Corces VG. Different enhancer classes in Drosophila bind distinct architectural proteins and mediate unique chromatin interactions and 3D architecture. Nucleic Acids Res. 2017;45(4):1714–30. doi: 10.1093/nar/gkw1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Q, Sun Q, Czajkowsky DM, Shao Z. Sub-kb Hi-C in D. melanogaster reveals conserved characteristics of TADs between insect and mammalian cells. Nat Commun. 2018;9(1):1–8. doi: 10.1038/s41467-017-02526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chathoth KT, Zabet NR. Chromatin architecture reorganization during neuronal cell differentiation in Drosophila genome. Genome Res. 2019;29(4):613–25. doi: 10.1101/gr.246710.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rowley MJ, Lyu X, Rana V, Ando-Kuri M, Karns R, Bosco G, et al. Condensin II counteracts cohesin and RNA polymerase II in the establishment of 3D chromatin organization. Cell Rep. 2019;26(11):2890-2903.e3. [DOI] [PMC free article] [PubMed]
- 139.Erceg J, AlHaj Abed J, Goloborodko A, Lajoie BR, Fudenberg G, Abdennur N, et al. The genome-wide multi-layered architecture of chromosome pairing in early Drosophila embryos. Nat Commun. 2019;10(1):4486. doi: 10.1038/s41467-019-12211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gutierrez-Perez I, Rowley MJ, Lyu X, Valadez-Graham V, Vallejo DM, Ballesta-Illan E, et al. Ecdysone-induced 3D chromatin reorganization involves active enhancers bound by pipsqueak and polycomb. Cell Rep. 2019;28(10):2715-2727.e5. [DOI] [PMC free article] [PubMed]
- 141.Ulianov SV, Zakharova VV, Galitsyna AA, Kos PI, Polovnikov KE, Flyamer IM, set al. Order and stochasticity in the folding of individual Drosophila genomes. Nat Commun. 2021;12(1):41. [DOI] [PMC free article] [PubMed]
- 142.Ulianov, Doronin, Khrameeva, Kos, Starikov, Galitsyna, et al. Nuclear lamina maintains global spatial organization of chromatin in Drosophila cultured cells. In: 2018 IEEE International Conference on Bioinformatics and Biomedicine (BIBM). vol. 0; 2018. p. 2493.
- 143.Kribelbauer JF, Loker RE, Feng S, Rastogi C, Abe N, Rube HT, et al. Context-dependent gene regulation by homeodomain transcription factor complexes revealed by shape-readout deficient proteins. Mol Cell. 2020;78(1):152-167.e11. [DOI] [PMC free article] [PubMed]
- 144.Wingett S, Ewels P, Furlan-Magaril M, Nagano T, Schoenfelder S, Fraser P, et al. HiCUP: pipeline for mapping and processing Hi-C data. F1000Res. 2015;4:1310. doi: 10.12688/f1000research.7334.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature Methods. 2012;9(4):357–9. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Knight PA, Ruiz D. A fast algorithm for matrix balancing. IMA J Numer Anal. 2012;33(3):1029–47. [Google Scholar]
- 147.Wolff J, Rabbani L, Gilsbach R, Richard G, Manke T, Backofen R, et al. Galaxy HiCExplorer 3: a web server for reproducible Hi-C, capture Hi-C and single-cell Hi-C data analysis, quality control and visualization. Nucleic Acids Res. 2020;48(W1):W177–84. doi: 10.1093/nar/gkaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shin H, Shi Y, Dai C, Tjong H, Gong K, Alber F, et al. TopDom: an efficient and deterministic method for identifying topological domains in genomes. Nucleic Acids Res. 2016;44(7):e70. doi: 10.1093/nar/gkv1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kriventseva EV, Kuznetsov D, Tegenfeldt F, Manni M, Dias R, Simão FA, et al. OrthoDB v10: sampling the diversity of animal, plant, fungal, protist, bacterial and viral genomes for evolutionary and functional annotations of orthologs. Nucleic Acids Res. 2019;47(D1):D807–11. doi: 10.1093/nar/gky1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kumar S, Stecher G, Suleski M, Hedges SB. TimeTree: a resource for timelines, timetrees, and divergence times. Mol Biol Evol. 2017;34(7):1812–9. [DOI] [PubMed]
- 151.Lohia R, Nathan F, Gillis J. High depth genome wide chromatin contact matrix. Datasets. Figshare. 2022. 10.25452/figshare.plus.21280752.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1 Supplementary Figures S1-S5 with each legend. Fig S1. Meta-Hi-C network benchmarking in mouse. Fig S2. Meta-Hi-C network benchmarking in human. Fig S3. TAD coexpression vs contact coexpression. Fig S4. TAD and boundary conservation across experiments. Fig. S5. Contact coexpression conservation at various coexpression thresholds.
Additional file 2 Table S1. Details of each individual Hi-C project used for building human meta-Hi-C network. Table S2. Details of each individual Hi-C project used for building mouse meta-Hi-C network. Table S3. Details of each individual Hi-C project used for building fly meta-Hi-C network.
Data Availability Statement
In order to facilitate the broad adoption of meta-Hi-C by the community, we have made data available via an online tool (https://gillisweb.cshl.edu/HiC/). Contact data can be obtained in two ways: (a) network download: direct download of the desired resolution and species meta-Hi-C contact matrix in cis or trans available at https://labshare.cshl.edu/shares/gillislab/resource/HiC in HiCMatrix format (https://github.com/deeptools/HiCMatrix) and (b) gene vector download: contact frequency with every genomic loci at the chosen resolution and for any desired gene found in the respective species. The downloaded file is in a bed file format which can be uploaded to the UCSC genome browser for further analysis as desired.
The meta-Hi-C matrix for human, mouse, and fly are available for download from the online tool at https://gillisweb.cshl.edu/HiC/ or direct download at https://labshare.cshl.edu/shares/gillislab/resource/HiC/ or Figshare [151].