Abstract
Periodontitis is a chronic inflammatory disease which gradually destroys the supporting tissues of the teeth, leading to tooth loss in adults. The lesions are characterized by a persistence of inflammatory cells in gingival and periodontal connective tissues. To understand what mechanisms are involved in the establishment of chronic lesions, we hypothesized that infiltrating lymphocytes might be resistant to apoptosis. However, both Bcl-2 and Bcl-xL were weakly detected in lymphocytes from the lesions, compared with those from peripheral blood, suggesting that these cells are susceptible to apoptosis. Nevertheless, very few apoptotic cells were observed in tissue sections from the lesions. Lymphocytes from the lesions expressed mRNA encoding Fas, whereas Fas-ligand mRNA was very weakly expressed in lymphocytes from the lesions and in periodontal tissues. Since the results indicated that lymphocytes in the lesions might be susceptible to Fas-mediated apoptosis but lack the death signal, we next investigated if these lymphocytes actually undergo apoptosis by the addition of anti-Fas antibodies in vitro. Fas-positive lymphocytes from the lesions underwent apoptosis by these antibodies, but Fas-negative lymphocytes and Fas-positive peripheral lymphocytes did not undergo apoptosis by these antibodies. These results indicate that lymphocytes in the lesions are susceptible to activation-induced cell death and are induced to die by apoptosis after the addition of exogenous Fas ligand.
Most chronic inflammatory diseases destroy the target tissues or organs gradually, as seen in the joints of rheumatoid arthritis patients, leading eventually to permanent loss of function. Chronic inflammatory periodontitis, the major cause of tooth loss in adults, is one such disease. It is initially caused by an infection by a mixture of oral anaerobic bacteria of the gingival sulcus (15), leading to a gradual destruction of supporting tissues of the tooth, which are composed of gingival and periodontal connective tissues, cementum, and alveolar bone. The established connective tissue lesion is then infiltrated by a massive accumulation of mononuclear leukocytes, primarily composed of T and B lymphocytes and plasma cells (7, 17). Unlike other acute infectious diseases, periodontal disease is characterized by a persistence of these inflammatory cells (7, 17), and the clinical course of the disease often has a chronic outcome (15). In addition, unlike other chronic infectious diseases such as tuberculosis or leprosy, periodontal disease does not seem to be caused by disease-specific bacteria with high virulence (5) which multiply in host cells and cause specific pathological lesions persisting for long periods (1, 9). Thus, it seems that infiltrating inflammatory cells are somehow acting to prolong the clinical course of the disease. So far, several hypotheses have been made to account for the molecular mechanisms underlying the establishment of chronic periodontal lesions (18, 19). Wassenaar et al. (18) have established T-cell clones from inflamed gingiva and examined the antigen specificity of the clones. They found that the majority of the T cells in the lesions recognized a tissue-oriented autoantigen such as denatured collagen type I and suggested that such autoimmune reactions might contribute to the chronicity of the disease (18). On the other hand, Yamamoto et al. proposed that the absence of interleukin-4 in inflamed gingiva inhibits apoptotic cell death in the accumulated macrophages and hence may contribute to the chronicity of the disease (19). Since these previous reports had suggested that accumulated inflammatory cells might be resistant to apoptosis, which then leads the cells to a phenotype of longevity, we decided to investigate the expression profiles of apoptosis-related molecules in chronically inflamed human periodontal lesions and infiltrating inflammatory cells and to investigate whether these molecules could function as effector or target molecules for apoptosis.
There is increasing evidence that the Fas/Fas-ligand system plays a key role in the control of activation-induced suicide of T cells and peripheral clonal deletion of autoreactive T and B lymphocytes and activated B lymphocytes (for a review, see the work of Nagata [11]), while the Bcl-2 family proteins play a role in the deletion of activated lymphocytes by neglect (passive cell death) (for a review, see the work of Parijs and Abbas [14]).
The Bcl-2 protein is known to interact with Bax, which forms a heterodimer with Bcl-2 and counteracts the protective role of apoptosis by Bcl-2 (6). Overexpression of Bcl-2 reduces the formation of Bax homodimers and inhibits apoptosis, while overexpression of Bax reduces Bcl-2 homodimers and accelerates cell death, suggesting that the ratio of Bcl-2 to Bax is important in regulating passive cell death and survival. Another member of the Bcl-2 family, Bcl-xL, is also reported to inhibit apoptosis in various cell types (14).
In this context, we first investigated the levels of Bcl-2 and Bcl-xL proteins in infiltrating lymphocytes and compared them with those in peripheral lymphocytes. We suggest that the level of Bcl-2 family proteins is relatively low in infiltrating cells, although relatively no detectable apoptotic cells were observed in the lesions. We then investigated whether Fas or Fas ligand was expressed in inflamed gingival tissues and lymphocytes accumulated at these sites, and we suggest that lymphocytes accumulated in chronic periodontal lesions may be susceptible to Fas-mediated apoptosis. In addition, we report that Fas-positive lymphocytes isolated from these lesions are easily led to apoptosis by stimulation with anti-Fas antibody, which mimics the function of Fas ligand, while those from peripheral lymphocytes are resistant to apoptosis upon the same stimulation. And we suggest that lack of Fas-mediated apoptosis in activated lymphocytes could, at least in part, contribute to the chronicity of the disease and that exogenous Fas ligand could be a candidate to provide protection against the disease having chronic outcomes.
MATERIALS AND METHODS
Subjects.
Thirty patients diagnosed as having chronic marginal periodontitis (adult periodontitis) who visited Okayama University Dental School Hospital were enrolled in the study. All patients had a history of chronic periodontitis for more than 5 years and were diagnosed according to the criteria described by Murayama et al. (10). Tissue samples were obtained from inflamed gingiva and granulation tissues resected during periodontal surgery. Prior to tissue resection, informed consent of the donor was obtained. In some cases, peripheral lymphocytes were also isolated from the same donors.
Antibodies.
Anti-Fas antibody, CH-11 (immunoglobulin M [IgM]; Medical & Biological Laboratories, Nagoya, Japan), was used for apoptosis induction assay, and mouse IgM (Cappel, Durham, N.C.) was used as the isotype control. ZB4 (IgG1; Medical & Biological Laboratories), which inhibits the action of CH-11, was used as the neutralizing antibody. UB2 (IgG1; Medical & Biological Laboratories), which recognizes Fas but does not transduce the apoptosis signal, was used for the detection and selection of Fas-positive cells, and mouse IgG1 (Cappel) was used as the isotype control. For the detection of Bcl-2 and Bcl-xL proteins by Western blotting, mouse monoclonal antibodies (clone 124; DAKO, Glostrup, Denmark) and rabbit polyclonal antibodies (Transduction Laboratories, Lexington, Ky.) were used, respectively. To examine lymphocyte subsets by flow cytometric analyses, mouse anti-human CD3 conjugated with fluorescein isothiocyanate (FITC), anti-human CD19 conjugated with R-phycoerythrin (RPE), anti-human CD4 conjugated with FITC, and anti-human CD8 conjugated with RPE (IgG1; DAKO) were used. To stimulate peripheral lymphocytes for the induction of Fas-ligand mRNA, anti-human CD3 (NU-T3; IgG2a; Nichirei Corp., Tokyo, Japan) was used.
Isolation of periodontal lymphocytes.
Mononuclear leukocytes from inflamed gingiva and periodontal granulation tissues were isolated by the method described by Modlin et al. (8) with a slight modification, since the original method was developed to isolate lymphocytes from skin biopsy specimens of leprosy patients. Briefly, the tissue specimen in AIM V medium (Gibco BRL, Grand Island, N.Y.) containing 125 ng of amphotericin B per ml, proteinase inhibitors (1 μg of α2-macroglobulin per ml, 0.1 μg of α1-antitrypsin per ml, 200 μg of EDTA-2Na per ml, 0.5 μg of aprotinin per ml, and 0.5 μg of E-64 per ml), and 10 U of heparin sodium per ml was cut into 1-mm3 pieces with surgical scalpels and extruded through a 73.7-μm-pore-size mesh filter with glass rods. Mononuclear cells were then isolated by Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) centrifugation. The majority of the isolated cells (more than 99%) showed forward and side scatter identical to those of peripheral blood lymphocytes when judged by flow cytometry; thus, we considered these cells as lymphocyte-rich fractions (periodontal lymphocytes).
In some experiments, Fas-positive and -negative cells were separated from whole-cell suspensions by using a mini-MACS magnetic cell sorting kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Briefly, the cells were incubated with 20 μg of UB2 per ml for 30 min. UB2-positive cells were then isolated with microbeads conjugated with rat anti-mouse IgG (Miltenyi Biotec GmbH) according to the manufacturer’s instructions, while UB2-negative cells were considered Fas negative.
Reverse transcription-PCR.
The detection of mRNA encoding Fas, Fas ligand, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed by reverse transcription-PCR. Total RNA was isolated with TRIZOL reagent (Gibco BRL) from frozen sections of the tissue samples and from periodontal lymphocytes, followed by the synthesis of cDNA by reverse transcription with oligo(dT)12–18 primer and SuperScript II RNase H− reverse transcriptase (Life Technologies, Grand Island, N.Y.). The set of primers used for each amplification was as follows: Fas (5′-CACTATTGCTGGAGTCATG-3′ and 5′-CTGAGTCACTAGTAATGTCC-3′) (14), Fas ligand (5′-GCACACAGCATCATCTTTGG-3′ and 5′-ATGTAAGAAGACCCTCACTG-3′), and GAPDH (5′-TGGTATCGTGGAAGGACTCATGAC-3′ and 5′-ATGCCAGTGAGCTTCCCGTTCAGC-3′) (12). The annealing temperature was calculated with Oligo Macintosh computer software (version 4.05; National Biosciences, Plymouth, Minn.). About 1/10 of the cDNA sample was used for each amplification, which was done for 40 cycles with a thermal cycler (ASTEC, Fukuoka, Japan). One-fifth of the amplified products was subjected to 2% agarose gel electrophoresis, and the DNA was visualized by staining the gel with ethidium bromide. The amount of mRNA encoding Fas and Fas ligand was estimated by the intensity of the staining of amplified cDNA relative to that encoding GAPDH with NIH Image software.
Western immunoblotting.
The detection of Bcl-2 and Bcl-xL proteins in periodontal lymphocytes and in peripheral lymphocytes was performed by Western immunoblotting. Proteins were extracted by the method of Ohta et al. (13). Briefly, cells from periodontal and peripheral lymphocytes were lysed with cell lysis buffer containing 10 mM Tris-HCl (pH 7.6), 150 mM NaCl, and 1% Triton X-100, with a commercially obtained mixture of proteinase inhibitors (1 tablet of Complete per 25 ml; Boehringer Mannheim GmbH, Mannheim, Germany), which was followed by the measurement of protein concentration (protein assay; Bio-Rad Laboratories, Richmond, Calif.). Thirty micrograms of each protein sample was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by blotting onto an Immobilon polyvinylidene difluoride membrane (Millipore Corp., Bedford, Mass.). Primary antibodies as described above for the detection of Bcl-2 and Bcl-xL were used at a concentration of 1 μg/ml. Immunoreactive proteins were detected by enhanced chemiluminescence (ECL; Amersham Life Science, Little Chalfont, Buckinghamshire, United Kingdom) according to the manufacturer’s instructions. The relative amounts of intracellular Bcl-2 and Bcl-xL proteins in the periodontal lymphocytes versus those in the peripheral lymphocytes were estimated with NIH Image software.
In vitro induction of apoptosis.
For induction of apoptosis in cells from periodontal lesions and peripheral blood, the cells were first incubated for an hour at 37°C with or without 1 μg of ZB4 per ml. To the wells containing cells preincubated with ZB4 was added 250 ng of CH-11 per ml, all of which was incubated for 36 h. Similarly, to the wells containing cells preincubated without ZB4 was added either CH-11 or the same amount of mouse IgM, all of which was incubated for 36 h. The method used to detect apoptotic cells is described below.
Flow cytometric analyses of Fas-positive cells and lymphocyte subsets and detection of apoptotic cells.
Surface expression of Fas antigen on periodontal and peripheral lymphocytes was measured by flow cytometric analysis. The cells were incubated either with 20 μg of UB2 per ml or with the same amount of mouse IgG1 (Cappel) for 30 min, followed by incubation with FITC-conjugated anti-mouse IgG1 (Cappel) for 30 min. The cells (105) were then subjected to flow cytometric analysis (EPICS XL; Coulter, Hialeah, Fla.). The ratio of Fas-positive cells was calculated as follows: (number of UB2-positive cells − number of mouse IgG1-positive cells)/total cell number.
In some experiments, Fas-positive and -negative lymphocytes were examined for their lymphocyte subsets. Either Fas-positive or -negative periodontal lymphocytes were incubated with FITC-conjugated anti-human CD3 and RPE-conjugated anti-human CD19 (DAKO) for 30 min on ice according to the manufacturer’s instructions. Simultaneously, parts of the same samples were incubated with FITC-conjugated anti-human CD4 and RPE-conjugated anti-human CD8 (DAKO) for 30 min on ice. These samples (105 cells per sample) were subjected to dual-color flow cytometric analyses (EPICS XL) to examine the ratio of T and B lymphocytes and the ratio of CD4 to CD8.
For the detection of apoptotic cells in periodontal and peripheral lymphocytes, an ApoAlert annexin V apoptosis kit (Clontech, Palo Alto, Calif.) was used. The fluorescence intensity of the FITC-conjugated annexin V was read on a flow cytometer (EPICS XL; Coulter) according to the manufacturer’s instructions.
Histological detection of apoptotic cells in periodontal lesions.
The labeling of DNA breaks in situ was performed to detect apoptotic cells in periodontal lesions according to the in situ end-labeling method with an in situ apoptosis detection kit (Takara Biomedicals, Kyoto, Japan) as described by Gavrieli et al. (3). Briefly, slide-fixed tissue sections were incubated with 15 mg of proteinase K per ml for 30 min at 37°C, followed by incubation with TdT buffer (30 mM Tris-HCl buffer [pH 7.2], 140 mM sodium cacodylate, 1 mM cobalt chloride) containing terminal deoxynucleotidyl transferase (TdT) (0.3 U/ml; Life Technologies) and digoxigenin-labeled 11-dUTP (Boehringer Mannheim) in a humid atmosphere for 1 h at 37°C. The reaction was terminated by transferring the slides to buffer containing 300 mM sodium chloride and 30 mM sodium citrate for 15 min at room temperature. The sections were washed and incubated with peroxidase-conjugated sheep antidigoxigenin (1:500; Boehringer Mannheim) for 1 h at room temperature. After the slides were rinsed, the peroxidase complex was developed with a diaminobenzidine detection kit (Funakoshi Co., Tokyo, Japan). The brown precipitate, an indication of the presence of DNA fragmentation, was examined by bright-field microscopy. In negative controls, the TdT was omitted from the buffer containing digoxigenin-labeled 11-dUTP. The same tissue treated with 20 U of DNase I (Sigma, St. Louis, Mo.) per ml was used as a positive control. In addition, rat mammary gland provided by Takara Biomedicals was used to confirm that apoptotic cells were actually detected.
RESULTS
Expression of Bcl-2 and Bcl-xL proteins in periodontal and peripheral lymphocytes.
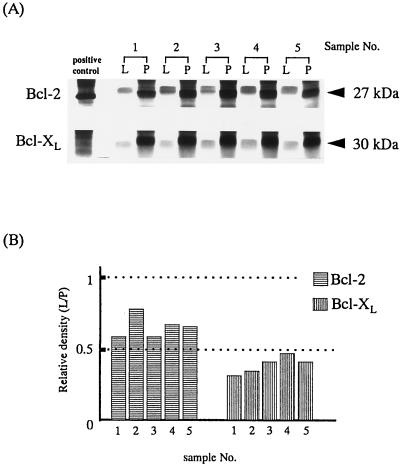
Intracellular expression levels of Bcl-2 and Bcl-xL were compared between periodontal and peripheral lymphocytes obtained from five donors (patients 1 to 5) by Western immunoblotting. As indicated in Fig. 1A, both Bcl-2 and Bcl-xL were weakly detected in periodontal lymphocytes compared with peripheral lymphocytes. The relative intensity of Bcl-2 and Bcl-xL proteins in periodontal lymphocytes compared with that in peripheral lymphocytes is shown in Fig. 1B. The amount of Bcl-2 protein in periodontal lymphocytes was estimated to be about 50 to 75% of that in peripheral lymphocytes, while the amount of Bcl-xL protein in periodontal lymphocytes was estimated to be below 50% of that in peripheral lymphocytes.
FIG. 1.
Bcl-2 and Bcl-xL protein levels are compared between periodontal and peripheral lymphocytes. (A) Thirty micrograms of each cell lysate was subjected to Western immunoblotting analysis. Cell lysate from HL-60 cells was used as the positive control for Bcl-2, while that from Jurkat cells was used as the positive control for Bcl-xL. Both Bcl-2 and Bcl-xL proteins were detected more weakly in periodontal lymphocytes (lanes L) than in peripheral lymphocytes (lanes P). (B) Data are expressed as the relative density of Bcl-2 or Bcl-xL in periodontal lymphocytes (L) versus that in peripheral lymphocytes (P).
Histological observation of apoptotic cells in chronically inflamed periodontal lesions.
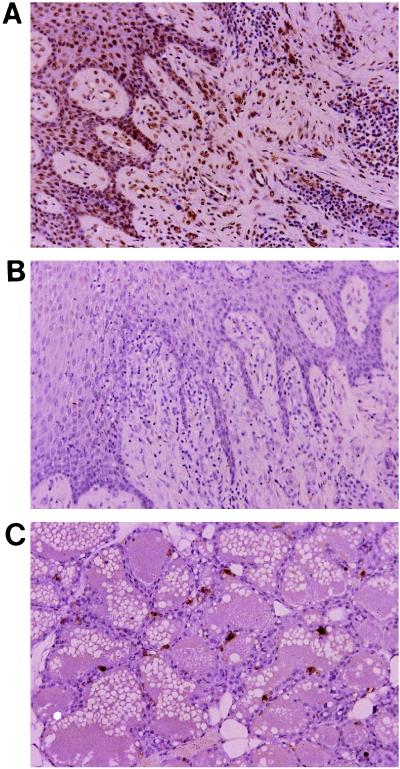
To determine to what extent infiltrating mononuclear cells are apoptotic in periodontal lesions, tissue sections were examined by the in situ end-labeling technique. As indicated in Fig. 2B, basically no apoptotic cells were observed in chronically inflamed periodontal lesions, while many DNA breaks were observed in the same tissue treated with DNase (Fig. 2A) and in rat mammary gland serving as the positive control (Fig. 2C). Additionally, freshly isolated periodontal lymphocytes were not found to be apoptotic, as judged by annexin V staining. Furthermore, these lymphocytes did not show any affinity for annexin V for at least 6 h after isolation.
FIG. 2.
No apoptotic cells were observed in the periodontal lesions (B). Apoptotic cells were observed in the control (same tissue treated with DNase [A] and rat mammary gland tissues [C]).
Expression of Fas and Fas-ligand mRNA in periodontal tissues and in periodontal lymphocytes.
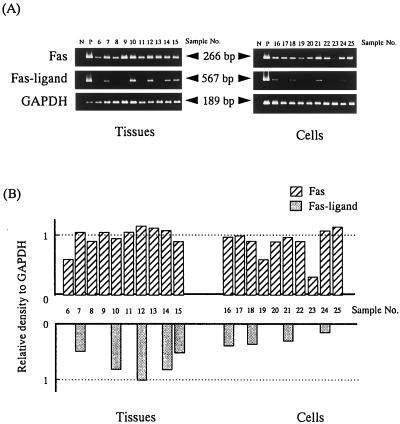
mRNA encoding Fas and Fas ligand was detected in both frozen periodontal tissue sections (patients 6 to 15) and periodontal lymphocytes (patients 16 to 25). As indicated in Fig. 3A, Fas mRNA was detected in all (10 of 10) periodontal tissues (Fig. 3A) as well as in periodontal lymphocytes (9 of 10) (Fig. 3A). In contrast, mRNA encoding Fas ligand was very weakly detected in periodontal tissues (5 of 10) and in periodontal lymphocytes (4 of 10) (Fig. 3A). For semiquantitation of the PCR products, the relative intensity of amplified Fas and Fas-ligand cDNA versus that of the internal control (GAPDH) was calculated. The results are shown in Fig. 3B. In both periodontal tissues and periodontal lymphocytes, the amount of Fas mRNA was estimated to be higher than that of Fas ligand. As for peripheral lymphocytes, mRNA encoding Fas ligand was not detected by reverse transcription-PCR in both periodontally diseased and healthy subjects. However, these lymphocytes turned to be positive for Fas-ligand mRNA when these cells were stimulated with NU-T3 (positive control for Fas-ligand mRNA shown in Fig. 3A).
FIG. 3.
Fas and Fas-ligand mRNA levels in periodontal tissues and in periodontal lymphocytes are compared. Fas mRNA was detected in almost all tissue and cell samples, while Fas-ligand mRNA was only very weakly detected (A). HeLa cells were used as a positive control for Fas mRNA (lane P in top rows), while CD3-stimulated (62.5 ng of NU-T3 per ml for 24 h) peripheral T cells were used as the control for Fas ligand (lane P in middle rows). The relative intensity of the amplified cDNA encoding Fas and Fas ligand compared with that of an internal control (GAPDH) was calculated (B). In all cases, Fas mRNA was more intensively detected than was Fas-ligand mRNA.
Distribution of Fas-positive cells in mononuclear lymphocytes isolated from periodontal lesions.
The ratio of Fas-positive cells in periodontal and peripheral lymphocytes from five donors (patients 26 to 30) was determined by the indirect immunofluorescence method. The results are shown in Table 1. The ratio of Fas-positive cells in periodontal lymphocytes varied from patient to patient (31 to 67%; mean, 46%), while that of peripheral lymphocytes was almost consistent throughout the samples (60 to 70%; mean, 65%). In some samples, Fas-positive and -negative periodontal lymphocytes were further examined for their lymphocyte subsets. In Fas-positive periodontal lymphocytes, 50% were CD3 positive and 5% were CD19 positive. Among CD3-positive T lymphocytes, 75% of them were found to be CD4 positive, while 25% were CD8 positive. On the other hand, 30% were found to be CD3 positive and 4% were found to be CD19 positive among Fas-negative periodontal lymphocytes. Among CD3-positive T lymphocytes, 50% were CD4 positive, while the other 50% were CD8 positive.
TABLE 1.
Ratios of Fas-positive mononuclear cells in periodontal lesions and in peripheral blooda
| Patient no. | Ratio of Fas-positive cells (%)
|
|
|---|---|---|
| Periodontal | Peripheral | |
| 26 | 38 | 65 |
| 27 | 53 | 68 |
| 28 | 67 | 61 |
| 29 | 31 | 70 |
| 30 | 40 | 60 |
| Total (mean ± SD) | 46 ± 14 | 65 ± 4 |
The ratios of Fas-positive mononuclear cells to total mononuclear cells in periodontal lesions (periodontal) and in peripheral blood (peripheral) from the same donors are shown. Data were obtained by the indirect immunofluorescence method with a flow cytometer.
In vitro induction of Fas-mediated apoptosis in periodontal lymphocytes.
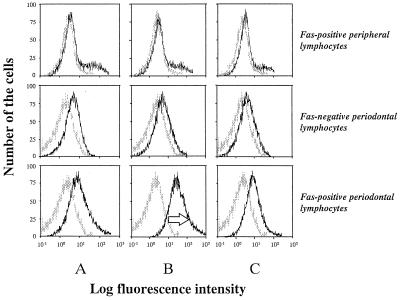
Fas-mediated apoptosis was induced either in Fas-positive peripheral lymphocytes or in Fas-positive and -negative periodontal lymphocytes by the addition of CH-11 to the culture medium. The results are shown in Fig. 4. As indicated, Fas-positive periodontal lymphocytes underwent apoptotic cell death 36 h following stimulation with CH-11, as judged by annexin V staining (lower histogram of column B). In contrast, Fas-positive peripheral lymphocytes did not undergo apoptosis in 36 h (upper histogram of column B). In addition, apoptotic cell death in Fas-positive periodontal lymphocytes was markedly inhibited by preincubation with ZB4 (lower histogram of column A) or by stimulation with control IgM (lower histogram of column C). However, in periodontal lymphocytes, the inhibitory effect of apoptosis by ZB4 was not complete. Furthermore, incubation with control IgM resulted in a slight increase in annexin V-positive cells, suggesting that Fas-independent cell death occurred in these periodontal lymphocyte populations (columns A and C).
FIG. 4.
Fas-positive periodontal lymphocytes underwent apoptotic cell death in 36 h following stimulation with anti-Fas antibody (250 ng of CH-11 per ml), as judged by annexin V labeling technique (as indicated by arrow in lower histogram of column B), while Fas-positive peripheral lymphocytes did not die from apoptosis (upper histogram of column B). A small number of the cells among periodontal lymphocytes preincubated with neutralizing antibody (1 μg of ZB4 per ml) (column A) and incubated with control IgM (250 ng/ml) (column C) underwent apoptosis.
DISCUSSION
The current study can be summarized as follows: lymphocytes in periodontal lesions may be susceptible to apoptosis (death by neglect); however, very few inflammatory cells accumulated in the periodontal lesions were found to be apoptotic; although many accumulated cells expressed functional Fas on their cell surface, very few cells expressed Fas ligand; and thus these periodontal lymphocytes are led to death by stimulation with anti-Fas antibody (CH-11) in vitro.
Both Bcl-2 and Bcl-xL proteins were found to be expressed more weakly in lymphocytes from periodontal lesions than in those from peripheral blood obtained from the same donors. It is generally believed that the ratio of Bcl-2 to Bax is very important in regulating cell death (6). In fact, we have investigated expression of mRNA encoding both Bcl-2 and Bax from the same samples, and the results indicated that the expression of Bax was highly superior to that of Bcl-2 in lymphocytes isolated from periodontal lesions (our unpublished data). In addition, another inhibitor of apoptosis, Bcl-xL, was also more weakly expressed in cells obtained from periodontal lesions than in cells from peripheral lymphocytes. These results suggest that, if Bcl-2 family members are involved, the intracellular environment of lymphocytes from periodontal lesions is susceptible to apoptosis (death by neglect), although relatively few apoptotic cells were observed in tissue sections isolated from the lesions. In addition, no apoptotic cells were observed among freshly isolated periodontal lymphocytes, suggesting that the majority of the infiltrated cells are viable.
Since periodontal lesions are always exposed to continuous stimulation from bacterial antigens, we then hypothesized that repeated antigenic stimulation might enhance the possibility of activation-induced cell death in these cells, but this type of cell death might not function well in the lesions. Therefore, we decided to investigate the interaction between Fas and Fas ligand in periodontal lesions, since the Fas-Fas-ligand pathway has been suggested to be involved in the deletion of both activated and autoreactive T and B lymphocytes (11). The results obtained indicated that Fas is more abundantly expressed in the lymphoid cells and tissues of periodontal lesions than is Fas ligand. Therefore, we speculated that lymphocytes accumulated in periodontal lesions might actually be susceptible to Fas-mediated apoptosis but are lacking a sufficient quantity of death signal, leading to the persistence of these cells, which further accelerates the chronicity of the disease. To test this speculation, we investigated whether Fas-mediated apoptosis is actually induced in these cells isolated from periodontal lesions when the cells are forced to die by the addition of anti-Fas antibodies in vitro. The results confirmed that Fas-positive lymphocytes from periodontal lesions, not from peripheral lymphocytes, were induced to die by apoptosis after stimulation with anti-Fas antibodies, which are known to mimic the function of Fas ligand. This cell death seemed to be strictly Fas mediated, since coincubation of the cells with anti-Fas neutralizing antibodies markedly reduced the ratio of dead cells in Fas-positive lymphocytes. However, this inhibitory effect was not complete, and part of the periodontal lymphocytes underwent cell death with control IgM, suggesting that Fas-independent cell death such as passive cell death occurred to some extent in the in vitro situation. Then the question arises as to what kinds of cells these Fas-positive and -negative lymphocytes accumulated in periodontal lesions are. About 50% of Fas-positive cells isolated from the lesions were found to be CD3+ T cells, and the ratio of CD4 to CD8 in this T-cell population was approximately 3/1, while about 30% of Fas-negative cells were found to be T cells, and the ratio of CD4 to CD8 was near 1/1. This observation is particularly interesting since CD8+ T lymphocytes are believed to be a major cell type expressing functional Fas ligand. Chronic periodontal lesions have been reported to be characterized by the accumulation of CD4+ RO memory cells, while few CD4+ RA cells were found in the lesions (20). We speculate that these Fas-positive cells may be either CD4+ RO memory cells or antigen-activated T cells which have been repeatedly stimulated by antigens. A further study confirming this speculation is currently in progress and will be reported elsewhere.
It has been suggested that soluble Fas ligand or anti-Fas antibodies mimicking the function of Fas ligand could potentially be therapeutic agents in the reduction of inflammatory reactions and clinical symptoms in the joints of chronic rheumatoid arthritis patients (2, 4). The results of our current study indicate that we could utilize a similar approach to down-regulate unfavorable immune reactions in periodontal lesions and to diminish the clinical symptoms of the disease. In fact, it has recently been suggested that inflammatory host cell-derived products, rather than bacterial products, are more important for inflammatory tissue destruction in chronic periodontal disease (5). Thus, it has become our next subject to confirm the effects of anti-Fas antibodies or Fas ligand as an anti-inflammatory reagent in appropriate animal models. In addition, our understanding of the molecular basis for the chronicity of the disease may enhance our knowledge of the pathogenesis of other chronic inflammatory diseases seen in various organs or tissues.
ACKNOWLEDGMENTS
We thank L. Keleher for her help in preparing the manuscript.
This work is supported in part by a Grant-in-Aid for Scientific Research (A) (no. 09307042, 10307047) from the Ministry of Education, Science, Sports and Culture of Japan and in part by “Funds for Comprehensive Research on Aging and Health” from the Ministry of Health and Welfare of Japan.
REFERENCES
- 1.Boddingius J, Dijkman H. In situ locations of Mycobacterium leprae-specific antigens. Immunoelectronoptical studies. Acta Leprol. 1989;7:107–112. [PubMed] [Google Scholar]
- 2.Fujisawa K, Asahara H, Okamoto K, Aono H, Hasunuma T, Kobata T, Iwakura Y, Yonehara S, Sumida T, Nishioka K. Therapeutic effect of the anti-Fas antibody on arthritis in HTLV-I tax transgenic mice. J Clin Invest. 1996;98:271–278. doi: 10.1172/JCI118789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasunuma T, Hoa T T M, Aono H, Asahara H, Yonahara S, Yamamoto K, Sumida T, Gay S, Nishioka K. Induction of Fas-dependent apoptosis in synovial infiltrating cells in rheumatoid arthritis. Int Immunol. 1996;8:1595–1602. doi: 10.1093/intimm/8.10.1595. [DOI] [PubMed] [Google Scholar]
- 5.Jotwani R, Cutler C W. Adult periodontitis—specific bacterial infection or chronic inflammation? J Med Microbiol. 1998;47:187–188. doi: 10.1099/00222615-47-3-187. [DOI] [PubMed] [Google Scholar]
- 6.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 7.McGhee M L, Ogawa T, Pitts A M, Moldoveanu Z, Mestecky J, McGhee J R, Kiyono H. Cellular analysis of functional mononuclear cells from chronically inflamed gingival tissue. Reg Immunol. 1989;2:103–110. [PubMed] [Google Scholar]
- 8.Modlin R L, Mehra V, Wong L, Fujimiya Y, Chang W, Horwitz D A, Bloom B R, Rea T H, Pattengale P K. Suppressor T lymphocytes from lepromatous leprosy skin lesions. J Immunol. 1986;137:2831–2834. [PubMed] [Google Scholar]
- 9.Mshana R N, Humber D P, Harboe M, Belehu A. Demonstration of mycobacterial antigens in nerve biopsies from leprosy patients using peroxidase-antiperoxidase immunoenzyme technique. Clin Immunol Immunopathol. 1983;29:359–368. doi: 10.1016/0090-1229(83)90039-9. [DOI] [PubMed] [Google Scholar]
- 10.Murayama Y, Nagai A, Okamura K, Kurihara H, Nomura Y, Kokeguchi S, Kato K. Serum immunoglobulin G antibody to periodontal bacteria. Adv Dent Res. 1988;2:339–345. doi: 10.1177/08959374880020022401. [DOI] [PubMed] [Google Scholar]
- 11.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 12.Nobori T, Miura K, Wu D J, Lois A, Takabayashi K, Carson D A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994;368:753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- 13.Ohta K, Iwai K, Kasahara Y, Taniguchi N, Krajewski S, Reed J C, Miyawaki T. Immunoblot analysis of cellular expression of Bcl-2 family proteins, Bcl-2, Bax, Bcl-X and Mcl-1, in human peripheral blood and lymphoid tissues. Int Immunol. 1995;7:1817–1825. doi: 10.1093/intimm/7.11.1817. [DOI] [PubMed] [Google Scholar]
- 14.Parijs L V, Abbas K. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280:243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 15.Socransky S S, Haffajee A D. The nature of periodontal diseases. Ann Periodontol. 1997;2:3–10. doi: 10.1902/annals.1997.2.1.3. [DOI] [PubMed] [Google Scholar]
- 16.Tachibana O, Nakazawa H, Lampe J, Watanabe K, Kleihues P, Ohgaki H. Expression of Fas/APO-1 during the progression of astrocytomas. Cancer Res. 1995;55:5528–5530. [PubMed] [Google Scholar]
- 17.Taubman M A, Stoufi E D, Ebersole J L, Smith D J. Phenotypic studies of cells from periodontal disease tissues. J Periodont Res. 1984;19:587–590. doi: 10.1111/j.1600-0765.1984.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 18.Wassenaar A, Reinhardus C, Thepen T, Abraham-Inpijn L, Kievits F. Cloning, characterization, and antigen specificity of T-lymphocyte subsets extracted from gingival tissue of chronic adult periodontitis patients. Infect Immun. 1995;63:2147–2153. doi: 10.1128/iai.63.6.2147-2153.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto M, Kawabata K, Fujihashi K, McGhee J R, Van Dyke T E, Bamberg T V, Hiroi T, Kiyono H. Absence of exogenous interleukin-4-induced apoptosis of gingival macrophages may contribute to chronic inflammation in periodontal diseases. Am J Pathol. 1996;148:331–339. [PMC free article] [PubMed] [Google Scholar]
- 20.Yamazaki K, Nakajima T, Aoyagi T, Hara K. Immunohistological analysis of memory T lymphocytes and activated B lymphocytes in tissues with periodontal disease. J Periodont Res. 1993;28:324–334. doi: 10.1111/j.1600-0765.1993.tb01076.x. [DOI] [PubMed] [Google Scholar]