Abstract
Problem:
Pregnancy represents a state of systemic immune activation that is primarily driven by alterations in circulating innate immune cells. Recent studies have suggested that cellular adaptive immune components, T cells and B cells, also undergo changes throughout gestation. However, the phenotypes and functions of such adaptive immune cells are poorly understood. Herein, we utilized high-dimensional flow cytometry and functional assays to characterize T-cell and B-cell responses in pregnant and non-pregnant women.
Methods:
Peripheral blood mononuclear cells from pregnant (n = 20) and non-pregnant (n = 25) women were used for phenotyping of T-cell and B-cell subsets. T-cell proliferation and B-cell activation were assessed by flow cytometry after in vitro stimulation, and lymphocyte cytotoxicity was evaluated by using a cell-based assay. Statistical comparisons were performed with linear mixed-effects models.
Results:
Pregnancy was associated with modestly enhanced basal activation of peripheral CD4+ T cells. Both CD4+ and CD8+ T cells from pregnant women showed increased activation-induced proliferation; yet, a reduced proportion of these cells expressed activation markers compared to non-pregnant women. There were no differences in peripheral lymphocyte cytotoxicity between study groups. A greater proportion of B cells from pregnant women displayed memory-like and activated phenotypes, and such cells exhibited higher activation following stimulation.
Conclusion:
Maternal circulating T cells and B cells display distinct responses during pregnancy. The former may reflect the unique capacity of T cells to respond to potential threats without undergoing aberrant activation, thereby preventing systemic inflammatory responses that can lead to adverse perinatal consequences.
Keywords: adaptive immunity, B cell, cytotoxicity, flow cytometry, maternal circulation, T cell

Maternal peripheral T cells and B cells display distinct responses during pregnancy. Pregnancy drives enhanced activation and proliferative capacity of T cells; yet, these cells exhibit diminished activation in response to stimulation. Moreover, pregnancy is accompanied by increased memory-like and activated B cells.
1 |. INTRODUCTION
Pregnancy represents a state of mild intravascular inflammation that can be broadly characterized by enhanced innate immune responses to defend against pathogenic threats.1–3 Specifically, prior studies have indicated that the maternal circulation contains increased numbers or frequencies of activated and functional myeloid cells (i.e., monocytes and granulocytes)4–11 as well as elevated concentrations of humoral innate immune components such as complement.12–16 More recently, the application of omics platforms to the maternal circulation provided further evidence of innate immune activation and demonstrated a correlation between alterations in innate immune-related processes and advancing gestational age.17–22 Yet, these comprehensive studies also hinted at systemic alterations in adaptive immune signatures, primarily T cells, during pregnancy and, in particular, prior to the onset of physiologic or pathologic labor.18–21,23,24 Such observations may have clinical implications for the monitoring and prediction of the premature onset of labor leading to preterm birth. Indeed, the aberrant activation of maternal T cells has also been associated with the pathogenesis of preeclampsia.25–29 Furthermore, changes in B-cell phenotypes have been reported in the periphery30 and at the maternal-fetal interface31 throughout gestation and in the pathology of preterm labor, respectively. Therefore, the cellular responses driven by the adaptive limb of immunity during pregnancy warrant further investigation.
The conventional belief is that circulating T cells are skewed toward a Th2-like phenotype throughout gestation.32–37 Accordingly, a number of clinical investigations noted that some autoimmune disorders (e.g., multiple sclerosis and rheumatoid arthritis) are temporarily alleviated during pregnancy.38–46 This suppression also seems to extend to the maternal-fetal interface where multiple protective mechanisms exist to prevent T-cell activation, such as exhaustion or senescence,47–49 local silencing of T-cell chemotactic signals and trafficking,50–52 and expansion of regulatory T cells.53–63 Importantly, single-cell RNA signatures derived from T cells infiltrating the maternal-fetal interface can be tracked in the maternal circulation and may serve as biomarkers for obstetrical disease.21,23,64 Hence, investigating the functional status of circulating T cells during pregnancy may provide a window on the events taking place at the maternal-fetal interface. Although a large body of research has focused on examining the phenotypes and functions of T cells and B cells throughout gestation,3,31,65–68 little is known of the potential pregnancy-specific functional differences in such adaptive immune cells. In the current study, we utilized high-dimensional flow cytometry together with functional assays to characterize T-cell and B-cell cellular responses in the periphery of pregnant and non-pregnant women.
2 |. METHODS
2.1 |. Human subjects and clinical specimens
Peripheral blood samples were collected from healthy pregnant and non-pregnant women enrolled under research protocols at the Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), U. S. Department of Health and Human Services (DHHS), Wayne State University (Detroit, MI, USA), and the Detroit Medical Center (Detroit, MI, USA). The collection and use of biological specimens for research purposes were approved by the Institutional Review Boards of Wayne State University and the Detroit Medical Center. All patients provided written informed consent prior to sample collection. The present study included pregnant women (n = 20), predominantly African-American, whose peripheral blood was collected in the third trimester prior to the administration of any medication, with a median gestational age of 39.1 weeks at sampling, prior to the onset of labor. The control study group comprised healthy non-pregnant women (n = 27) of reproductive age from the same community.
2.2 |. Stimulation of T-cell proliferation
Peripheral blood was obtained by venipuncture and collected in Vacutainer K3 EDTA tubes (BD Biosciences, San Jose, CA, USA). Peripheral blood mononuclear cells (PBMCs) were isolated by Lymphoprep density gradient (Axis Shield, Oslo, Norway), per manufacturer instructions. Isolated PBMCs were centrifuged at 300 × g for 5 min and resuspended in phosphate-buffered saline (PBS) at a density of 1×106 cells/mL. Next, PBMCs were stained with 1 μL/mL CellTrace Violet dye (Thermo Fisher Scientific, Life Technologies Corporation, Carlsbad, CA, USA) for 20 min at 37°C. The staining reaction was quenched by adding complete RPMI 1640 medium (Thermo Fisher Scientific, Life Technologies Limited, Paisley, UK) [enriched with 5% human serum (Sigma-Aldrich, St Louis, MO, USA) and 1% Penicillin-Streptomycin (Thermo Fisher Scientific)] and by allowing the suspension to incubate at room temperature (RT) for 2 min. The PBMCs were then centrifuged at 300 × g for 5 min, resuspended in complete RPMI 1640 medium, and counted, using ViaStain AOPI Staining Solution (Nexcelom Bioscience, Lawrence, MA, USA) and a Nexcelom Bioscience Cellometer Auto 2000. An aliquot containing 1×106 cells was set aside for basal (day 0) immunophenotyping. The remaining cell suspension volume was divided into control and stimulated samples. Control suspensions were treated with 55 μM 2-mercaptoethanol (Life Technologies Corporation, Grand Island, NY, USA); stimulated solutions were treated with 55 μM 2-mercaptoethanol, Dynabeads™ Human T-activator CD3/CD28 (Thermo Fisher Scientific) at a ratio of 1:1 cells:beads and with 2000 U/mL recombinant human IL-2 (BD Biosciences). Each sample was seeded in triplicate, for control and stimulated cells, at a density of 1×105 cells per well in a 96-well U bottom plate. The plate was incubated at 37°C with 5% CO2 for six days.
2.3 |. T-cell phenotyping for basal and proliferated samples
Following six days of incubation, PBMCs were collected, washed, and resuspended in PBS. For basal immunophenotyping, 1×106 cells were resuspended in PBS. Cell suspensions were incubated with 0.5 μL/mL Fixable Viability Stain 575 V (BD Biosciences) for 15 min in the dark at RT. Next, PBMCs were washed and incubated with extracellular fluorochrome-conjugated anti-human mAbs (Supplemental Table 1) for 30 min in the dark at 4°C. Cells were washed in stain buffer (BD Biosciences), then fixed and permeabilized by using the Foxp3/Transcription Factor Staining Buffer Set (Thermo Fisher Scientific), per manufacturer instructions. Intranuclear staining was performed with fluorochrome-conjugated anti-human mAbs (Supplemental Table 1), which were added to cell suspensions and then incubated for 30 min in the dark at 4°C. Finally, cells were washed in Foxp3 Permeabilization Buffer (Thermo Fisher Scientific) and resuspended in 0.5 mL of stain buffer for analysis by flow cytometry.
CountBright absolute counting beads (Thermo Fisher Scientific) were added prior to analysis. Flow cytometry acquisition was performed on a BD LSRFortessa flow cytometer, using FACSDiva software version 6.0. The analysis was performed and the figures were created by using FlowJo software version 10 (FlowJo, Ashland, OR, USA). T-cell subsets were identified based on the gating strategy presented in Supplemental Figure 1.
2.4 |. Peripheral lymphocyte cytotoxicity assay
Target K-562 cells (ATCC, Manassas, VA, USA) – myelogenous leukemia cells that lack MHC class I and II expression69–71 – were cultured in complete RPMI 1640 medium [enriched with 10% fetal bovine serum and 1% Penicillin-Streptomycin], collected, centrifuged at 300 × g for 5 min, and resuspended in PBS. Next, cells were incubated with 1 μL/mL carboxyfluorescein diacetate succinimidyl ester (CFSE; Thermo Fisher Scientific) at 37°C with 5% CO2 for 20 min. To stop the reaction, complete RPMI 1640 medium was added and the suspension was incubated at RT for 2 min. The cells were resuspended in complete RPMI 1640 medium and counted, using ViaStain AOPI Staining Solution and a Nexcelom Bioscience Cellometer Auto 2000.
Peripheral blood was obtained by venipuncture and collected in Vacutainer K3 EDTA tubes. PBMCs were isolated by Lymphoprep density gradient, per manufacturer instructions. Target (K-562) cells and PBMCs were mixed in sterile FACS tubes in the following ratios (PBMCs:target cells): 0:1 6:1, 12:1, 25:1, and 50:1. The resulting cell suspensions were centrifuged at 300 × g for 5 min, resuspended in complete RPMI 1640 culture medium, and transferred to a 96-well U-bottom plate. The plate was centrifuged at 100 × g for 2 min and then incubated at 37°C with 5% CO2 for 4 h. Following incubation, cell suspensions were transferred to FACS tubes, diluted with PBS, and centrifuged at 300 × g for 5 min. Cell pellets were resuspended in PBS and incubated with 1 μL/mL 7-aminoactinomycin D (7-AAD; Thermo Fisher Scientific) in the dark at 4°C for 15 min. Cell suspensions were centrifuged at 300 × g for 5 min and resuspended in 0.5 mL of stain buffer for analysis by flow cytometry.
CountBright absolute counting beads (Thermo Fisher Scientific) were added prior to analysis. Flow cytometry acquisition was performed on a BD LSRFortessa flow cytometer, using FACSDiva software version 6.0. Viable target cells were classified as CFSE+7AAD−, while killed target cells were classified as CFSE+7AAD+. Viable and dead lymphocytes were classified as CFSE−7AAD− and CFSE−7AAD+, respectively. The percentage of killed target cells was calculated as follows: # of CFSE+7AAD+ cells / (# of CFSE+7AAD+ cells + # of CFSE+7AAD− cells). The analysis was performed and the figures were created by using FlowJo software version 10.
2.5 |. B-cell phenotyping
PBMCs were isolated and counted as described above. An aliquot of 1×106 cells was used for phenotyping. The cells were incubated with 1.0 μL/mL Fixable Viability Stain 510(BD Biosciences) for 15 min in the dark at RT. Next, PBMCs were washed and incubated with extracellular fluorochrome-conjugated anti-human mAbs (Supplemental Table 2) for 30 min in the dark at 4°C. The cells were then washed once with stain buffer and resuspended in 0.5 mL of stain buffer for analysis by flow cytometry.
CountBright absolute counting beads were added prior to analysis. Flow cytometry acquisition was performed on a BD LSRFortessa flow cytometer, using FACSDiva software version 6.0. The analysis was performed and the figures were created by using FlowJo software version 10. B-cell subsets were identified based on the gating strategy presented in Supplemental Figure 2.
2.6 |. B-cell activation assay
PBMCs were isolated and counted as described above. For the control and stimulated arms of the B-cell activation assay, PBMCs were seeded in sterile FACS tubes with 2.5×105 cells. The control suspension received no treatment; the stimulated suspension was treated with 10 μg/mL F (ab’) 2-goat anti-human IgG, IgM (H+L) (Functional grade, Life Technologies Corporation, Carlsbad, CA, USA). The cells were incubated at 37°C for 30 min. Next, an equivalent volume of Phosflow Fix Buffer I (BD Biosciences) was added and the cells were incubated at 37°C for 10 min. Cells were washed twice with Permeabilization Solution I (BD Biosciences), per manufacturer instructions. After resuspension in Permeabilization Solution I, anti-human fluorophore-conjugated mAb Phospho-BKT (Supplemental Table 1) was added and incubated in the dark at 4°C for 30 min. After 15 min, the fluorophore-conjugated anti-human CD19 mAb (Supplemental Table 1) was added, and the incubation was resumed under the same conditions. Next, the cells were washed twice with Permeabilization Solution I. Finally, the cell pellets were resuspended in 0.5 mL stain buffer for analysis via flow cytometry.
CountBright absolute counting beads were added prior to analysis. Flow cytometry acquisition was performed on a BD LSRFortessa flow cytometer, using FACSDiva software version 6.0. The analysis was performed and the figures were created by using FlowJo software version 10. Fold change in B-cell activation was calculated as follows: [Stimulated (MFImAb – MFIIsotype)] / [Control (MFImAb – MFIIsotype)]. Any fold changes < 1 were considered to be “no change” and assigned a value of 1.0, which did not impact the significance of the results. B-cell activation was determined based on the gating strategy presented in Supplemental Figure 3.
2.7 |. Statistical analysis
Statistical analyses for baseline T-cell phenotyping, stimulated and control proliferated T-cell phenotyping, and B-cell phenotyping were performed by using the R statistical programming language. Linear mixed-effects models72 were fit for the comparison of stimulated and control T-cell flow cytometry data and between study groups to account for repeated measurements. The data obtained by flow cytometry were modeled as proportions. For T-cell baseline (day 0) phenotyping and B-cell phenotyping, the proportion of cells with a given phenotype was compared between pregnant and non-pregnant study groups, and a p-value < .05 was considered statistically significant. For T-cell proliferated (day 6) phenotyping, involving interactions between control and stimulated samples within both study groups, a false discovery rate-adjusted p-value73 (q-value) < .05 was considered statistically significant. For heatmap representations of immunophenotyping results, flow cytometry data were transformed into Z-scores by subtracting the mean and dividing by the standard deviation. Of note, the heatmaps were generated to display the proportion of cells with a given phenotype in pregnant vs. non-pregnant women, which included control and stimulated samples for the T-cell proliferation analyses. Phenotypes listed in the heatmap were thus not statistically compared to one another. Statistical analyses for PBMC cytotoxicity and B-cell activation were performed by using the Shapiro-Wilk test for normality followed by the Mann-Whitney U-test and GraphPad Prism software version 9.0.0 for Windows (GraphPad Software, San Diego, CA, USA, www.graphpad.com). A p-value < .05 was considered statistically significant.
3 |. RESULTS
3.1 |. Pregnancy is associated with a modest increase in activated CD4+ T cells
Pregnancy includes the selective modulation of the adaptive immune system at the maternal-fetal interface19,48,49,52,62,74–78 and in the periphery.18,20,21,23,79 Therefore, we first sought to uncover differences in the systemic baseline (day 0) T-cell subset composition as a function of pregnancy. PBMCs were isolated from non-pregnant and pregnant women for phenotyping via flow cytometry (Figure 1A), using the gating strategy presented in Supplemental Figure 1. The relative proportions of CD4+ and CD8+ T cells with the characterized phenotypes in each patient are presented in Figure 1B. While there were no pregnancy-specific differences in the proportions of total CD4+ or CD8+ T cells, pregnancy was associated with a significantly higher basal proportion of CD4+ T cells expressing the early activation marker, CD69 (Figure 1C),80,81 although the effect size was small. In addition, a significantly higher proportion of cells co-expressed CD69 and the co-inhibitory receptor PD-182–84 among CD4+ T cells isolated from pregnant compared to non-pregnant women (Figure 1D). The co-expression of CD69 and PD-1 is likely to be indicative of prolonged T-cell activation.85,86 These data suggest that pregnancy is associated with a modest enhancement in the baseline activation of peripheral CD4+ T cells.
FIGURE 1.
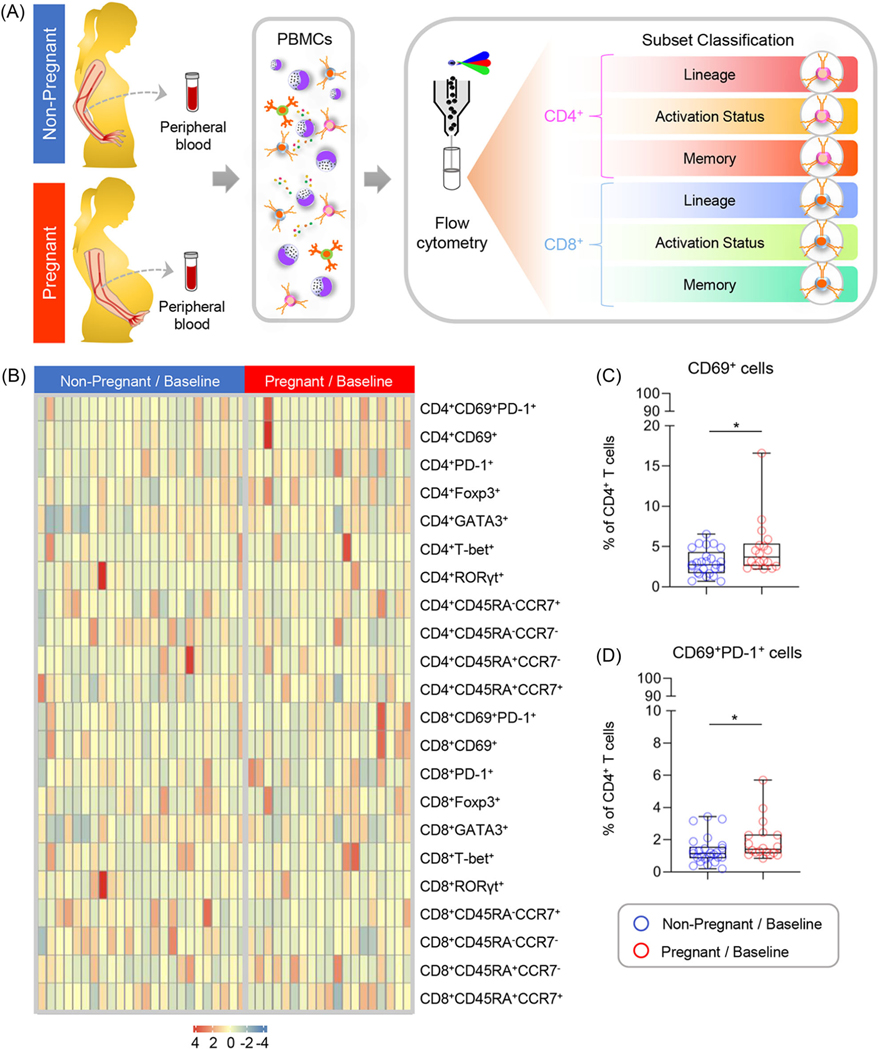
Comparison of basal T-cell subset composition between non-pregnant and pregnant women. (A) Peripheral blood samples were collected from non-pregnant (n = 25, indicated in blue) and pregnant (n = 18, indicated in red) women to isolate peripheral blood mononuclear cells (PBMCs) for T-cell phenotyping at baseline (day 0). (B) Heatmap representation showing the basal proportion of T cells with various immunophenotypes from non-pregnant (indicated in blue) and pregnant (indicated in red) women. The color key indicates the relative proportion of T cells with the various immunophenotypes considered, which were not compared among each other. (C) Proportion of CD4+ T cells expressing CD69 and (D) co-expressing CD69 and PD-1 at baseline from non-pregnant (blue circles) and pregnant (red circles) women. Data are presented as box-and-whisker plots where midlines indicate medians, boxes indicate interquartile ranges, and whiskers indicate minimum/maximum ranges. *p < .05
3.2 |. Circulating T cells display a pregnancy-specific increase in proliferative capacity with diminished susceptibility to activation
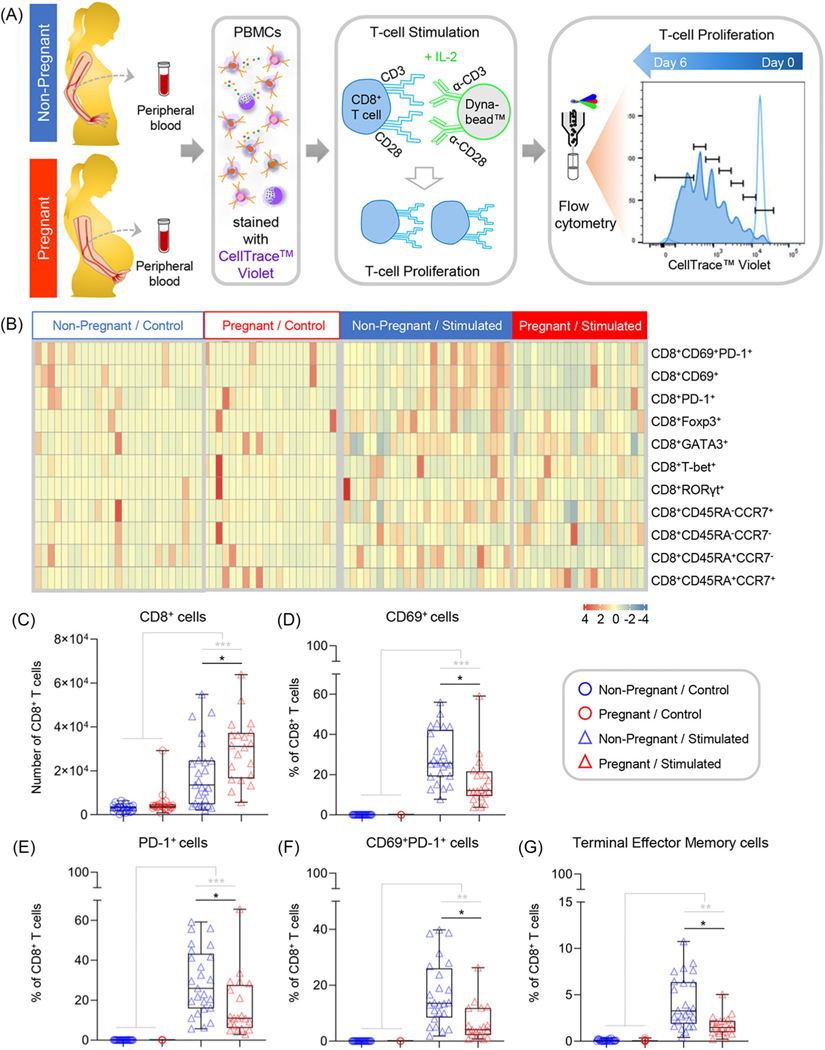
Given the baseline differences in peripheral T-cell activation between non-pregnant and pregnant women, we next considered whether pregnancy was associated with altered function, including proliferative capacity, of such cells. Accordingly, PBMCs isolated from pregnant and non-pregnant women were stimulated with anti-CD3/anti-CD28 and rhIL-2 to assess pregnancy-specific differences in CD4+ (Figure 2A) and CD8+ (Figure 3A) T-cell proliferation. As expected, significant changes in subset proportions (Figure 2B&3B) and absolute numbers (Figure 2C&3C) were observed in T cells derived from non-pregnant and pregnant women following stimulation (Extended Dataset 1); here, we focused on the phenotypic and functional differences between study groups. Both CD4+ (Figure 2C) and CD8+ T cells (Figure 3C) had a significantly higher proliferative capacity, as determined by absolute cell counts, in pregnant compared to non-pregnant women. Next, we analyzed the proliferated CD4+ (Figure 2B) and CD8+ (Figure 3B) T-cell subset composition of the two study groups relative to controls that were cultured under identical conditions without stimulation. Strikingly, we found that the proportion of T cells expressing the activation marker CD69 was significantly reduced in stimulated CD4+ (Figure 2D) and CD8+ (Figure 3D) T cells from pregnant compared to non-pregnant women. Furthermore, a significantly decreased proportion of CD4+ (Figure 2E) and CD8+ (Figure 3E) T cells expressed PD-1 in pregnant compared to non-pregnant women following stimulation. The proportion of cells co-expressing CD69 and PD-1 was also found to be significantly lower in CD4+ (Figure 2F) and CD8+ (Figure 3F) T cells from pregnant women. Finally, a significantly lower proportion of CD8+ terminally differentiated effector memory T cells (CD45RA+CCR7−), characterized by low proliferative capacity and rapid effector function,87,88 was observed in pregnant compared to non-pregnant women (Figure 3G). Taken together, these results demonstrate that CD4+ and CD8+ T cells isolated from pregnant women have an increased capacity for proliferation; however, when proliferated, pregnancy-derived T cells show a reduced proportion of cells expressing CD69 and PD-1, suggesting that pregnancy modulates T-cell responses.
FIGURE 2.
Comparison of CD4+ T-cell proliferation between non-pregnant and pregnant women. (A) Peripheral blood samples were collected from non-pregnant (n = 25, indicated in blue) and pregnant (n = 20, indicated in red) women to isolate peripheral blood mononuclear cells (PBMCs) for in vitro stimulation with anti-CD3/anti-CD28 and recombinant human IL-2. Cells were cultured for 6 days prior to phenotyping. Controls were cultured in parallel without stimulation. (B) Heatmap representation showing the proportion of CD4+ T cells with various immunophenotypes from non-pregnant (indicated in blue) and pregnant (indicated in red) women with (stimulated) or without (control) stimulation. The color key indicates the relative proportion of T cells with the various immunophenotypes considered, which were not compared among each other. (C) Absolute number of CD4+ T cells in control and proliferated samples from non-pregnant (blue symbols) and pregnant (red symbols) women. (D-F) Proportion of proliferated CD4+ T cells with the phenotype of (D) CD4+CD69+, (E) CD4+PD-1+, and (F) CD4+CD69+PD-1+. Data are presented as box-and-whisker plots where midlines indicate medians, boxes indicate interquartile ranges, and whiskers indicate minimum/maximum ranges. Each dot represents the mean of three biological replicates per sample. Grey lines and asterisks represent within-group differences between control and stimulated samples (i.e., pregnant control vs. pregnant stimulated), while black lines and asterisks represent significant differences between groups after stimulation (i.e., pregnant stimulated vs. non-pregnant stimulated). *p < .05; ***p < .001
FIGURE 3.
Comparison of CD8+ T-cell proliferation between non-pregnant and pregnant women. (A) Peripheral blood samples were collected from non-pregnant (n = 25, indicated in blue) and pregnant (n = 20, indicated in red) women to isolate peripheral blood mononuclear cells (PBMCs) for in vitro stimulation with anti-CD3/anti-CD28 and recombinant human IL-2. Cells were cultured for 6 days prior to phenotyping. Controls were cultured in parallel without stimulation. (B) Heatmap representation showing the proportion of CD8+ T cells with various immunophenotypes from non-pregnant (indicated in blue) and pregnant (indicated in red) women with (stimulated) or without (control) stimulation. The color key indicates the relative proportion of T cells with the various immunophenotypes considered, which were not compared among each other. (C) Absolute number of CD8+ T cells in control and proliferated samples from non-pregnant (blue symbols) and pregnant (red symbols) women. (D-G) Proportion of proliferated CD8+ T cells with the phenotype of (D) CD8+CD69+, (E) CD8+PD-1+, (F) CD4+CD69+PD-1+, and (G) CD8+CD45RA+CCR7− (terminal effector memory). Data are presented as box-and-whisker plots where midlines indicate medians, boxes indicate interquartile ranges, and whiskers indicate minimum/maximum ranges. Each dot represents the mean of three biological replicates per sample. Grey lines and asterisks represent within-group differences between control and stimulated samples (i.e., pregnant control vs. pregnant stimulated), while black lines and asterisks represent significant differences between groups after stimulation (i.e., pregnant stimulated vs. non-pregnant stimulated). *p < .05; **p < .01; ***p < .001
3.3 |. Pregnancy does not alter peripheral lymphocyte cytotoxicity
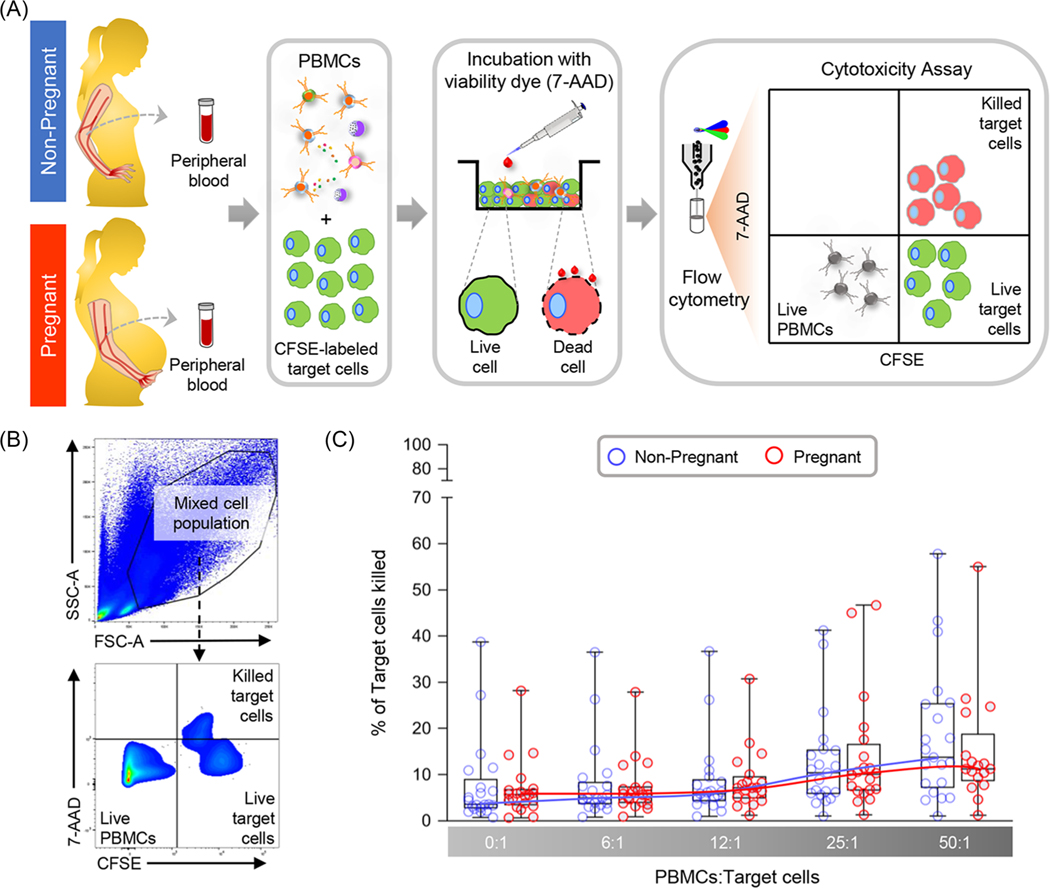
Having observed pregnancy-specific differences in lymphocyte activation status, we wondered whether the cytotoxicity of circulating lymphocytes would differ based on pregnancy status. Cytotoxic lymphocytes directly kill target cells through the release of granules, which represents an important mechanism of defense against viruses and intracellular bacteria.89,90 Hence, we isolated PBMCs from pregnant and non-pregnant women and incubated them with CFSE-labeled target cells (Figure 4A). Flow cytometry was used to quantify the number of killed target cells (Figure 4B). No significant differences were found between the pregnant and non-pregnant study groups among the various ratios of PBMCs:target cells evaluated (Figure 4C), indicating that peripheral lymphocytes from both study groups were able to display comparable cytotoxic activity.
FIGURE 4.
Comparison of lymphocyte cytotoxicity between non-pregnant and pregnant women. (A) Peripheral blood samples were collected from non-pregnant (n = 21, indicated in blue) and pregnant (n = 17–20, indicated in red) women to isolate peripheral blood mononuclear cells (PBMCs) for in vitro culturing with CFSE-labeled target cells. (B) Flow cytometry gating strategy used to identify killed target cells (CFSE+7AAD+), live target cells (CFSE+7AAD−), and live PBMCs (CFSE−7AAD−). (C) Percentage of target cells killed (calculated as [CFSE+7AAD+ / (CFSE+7AAD+ + CFSE+7AAD−) * 100]) among ratios of PBMCs:target cells ranging from 0:1 to 50:1 in non-pregnant (blue circles) and pregnant (red circles) women. Data are presented as box-and-whisker plots where midlines indicate medians, boxes indicate interquartile ranges, and whiskers indicate minimum/maximum ranges. Trend lines for each study group are included.
3.4 |. B-cell activation following IgM/IgG stimulation is increased in pregnancy
After evaluating functional differences in peripheral T cells in the context of pregnancy, we next focused on the second cellular component of adaptive immunity, B cells.65 During gestation, B cells are necessary for immune regulation and the promotion of humoral immunity,65 including the production of protective antibodies specific for paternal antigens.91,92 However, the pregnancy-specific cellular responses exhibited by circulating B cells require further investigation. Hence, PBMCs were isolated from pregnant and non-pregnant women to evaluate differences in B-cell phenotypes as well as B-cell functionality (Figure 5A). First, flow cytometry was used to evaluate differences in B-cell phenotypes following the gating strategy presented in Supplemental Figure 2. Several B-cell subsets displayed distinct modulation in pregnant compared to non-pregnant women (Figure 5B). The proportion of memory-like CD27+IgG+ B cells (Figure 5C) was found to be elevated during pregnancy, as was the proportion of B cells with an activated CD38+CD24− phenotype (Figure 5D). By contrast, the proportion of B cells displaying a CD40+CD138− phenotype was increased in non-pregnant women compared to the pregnant study group (Figure 5E). Next, PBMCs were stimulated with anti-human IgM and anti-human IgG, and then flow cytometry was utilized to quantify downstream B-cell receptor activation (Figure 5F). Consistent with the increased proportion of B cells displaying a CD38+ activated phenotype, we found that there was a significantly higher fold-change in activation following anti-human IgM/IgG stimulation by B cells isolated from pregnant compared to non-pregnant women (Figure 5G). This finding suggests that pregnancy enhances circulating B-cell responses.
FIGURE 5.
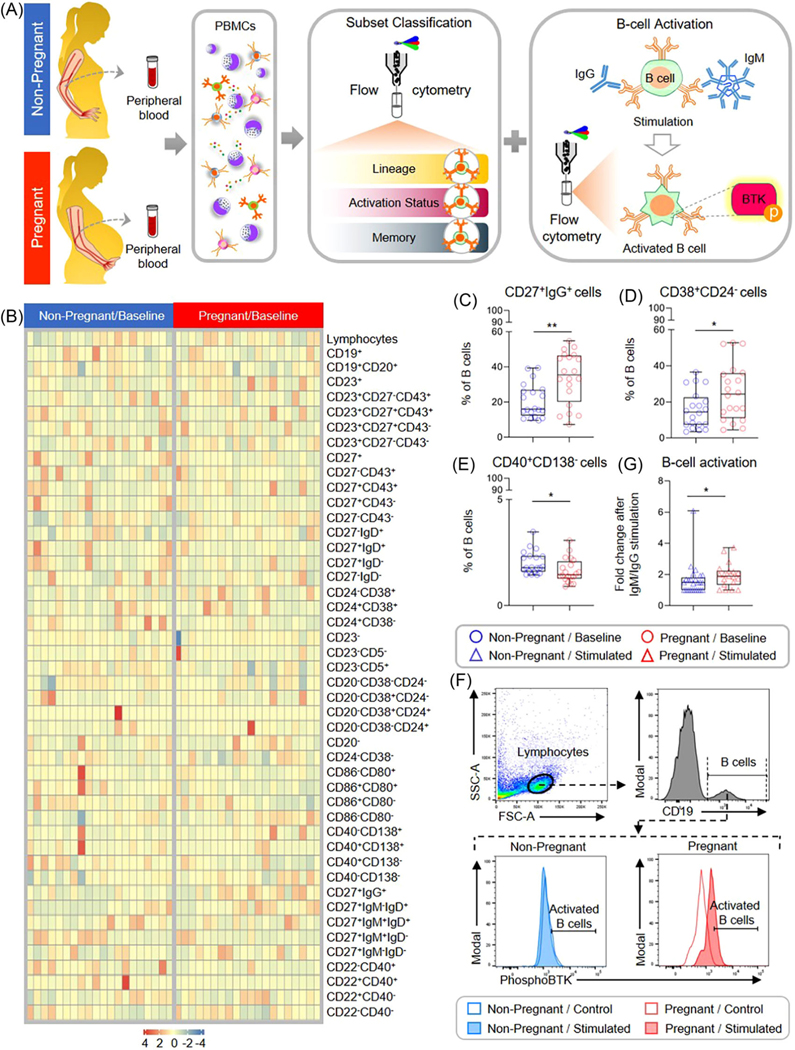
Comparison of B-cell subset composition and activation between non-pregnant and pregnant women. (A) Peripheral blood samples were collected from non-pregnant [n = 20 (phenotyping) - 25 (activation), indicated in blue] and pregnant (n = 19, indicated in red) women to evaluate B-cell phenotypes and activation following anti-human IgM/IgG stimulation. (B) Heatmap representation showing the basal proportion of B cells with various immunophenotypes from non-pregnant (indicated in blue) and pregnant (indicated in red) women. The color key indicates the relative proportion of B cells with the various immunophenotypes considered, which were not compared among each other. (C-E) Proportion of B cells with the phenotype (C) CD19+CD20+CD27+IgG+, (D) CD19+CD20+CD38+CD24−, and (E) CD19+CD20+CD40+CD138− from non-pregnant (red circles) and pregnant (blue circles) women. (F) Representative flow cytometry gating strategy for B-cell activation assay: viable B cells were identified as CD19+, and then B-cell activation in control (open histograms) and stimulated (filled histograms) samples from non-pregnant (indicated in blue) and pregnant (indicated in red) women was determined as described in the Methods. (G) Fold change in B-cell activation in non-pregnant (blue triangles) and pregnant (red triangles) samples after anti-human IgM/IgG stimulation, calculated as the adjusted MFI of IgM/IgG-stimulated samples divided by the adjusted MFI of control samples. Fold changes < 1 were considered as “no change” and assigned a value of 1. Data are presented as box-and-whisker plots where midlines indicate medians, boxes indicate interquartile ranges, and whiskers indicate minimum/maximum ranges. *p < .05; **p < .01
4 |. DISCUSSION
Herein, we evaluated the phenotypes and functions of peripheral T and B cells in pregnant compared to non-pregnant women, as these adaptive immune cells play a critical role in maintaining maternal-fetal tolerance.62,93–98 First, we showed that pregnancy is associated with modestly enhanced basal activation of peripheral CD4+ T cells. Interestingly, both CD4+ and CD8+ T cells derived from pregnant women showed increased activation-induced proliferation; yet, a reduced proportion of these cells expressed markers of activation compared to T cells from non-pregnant women. No differences were observed in peripheral lymphocyte cytotoxicity between the study groups. Finally, a greater proportion of B cells from pregnant women displayed memory-like and activated phenotypes, and such cells exhibited higher activation following stimulation. Taken together, these data indicate generalized T- and B-cell activation in pregnancy, with a restricted T-cell response to stimulation that may foster systemic maternal-fetal tolerance.
We observed a pregnancy-specific increase in the basal proportion of activated peripheral CD4+ T cells, as indicated by the expression of the early activation marker CD69. In line with this finding, a higher baseline proportion of CD4+CD69+, but not CD8+CD69+, T cells has been reported in C57BL/6 mice in late pregnancy relative to non-pregnant mice.99 We also detected a modest pregnancy-specific increase in the proportion of peripheral CD4+ T cells co-expressing CD69 and PD-1, of which the latter is typically regarded as a co-inhibitory receptor.82,100,101 Yet, PD-1 expression is upregulated within 24 – 48 hours of T-cell activation,85 potentially as a mechanism to limit excessive responses and tissue damage.86 Thus, the co-expression of CD69 and PD-1 likely indicates prolonged T-cell activation, as would be expected following chronic antigen exposure. Considering the presence of fetal antigens in the maternal circulation,53,102,103 it is tempting to suggest that the increased proportion of CD4+CD69+PD-1+ T cells in pregnant women may reflect repeated exposure to such fetal antigens.102–105 Indeed, prior studies in mice have demonstrated that innate immune cells in the periphery interact with fetal antigens throughout pregnancy, which was replicated in vitro by using human innate immune cells from the second and third trimesters.103 Furthermore, cell-free fetal DNA (cffDNA) concentrations have been shown to increase in the maternal circulation in late gestation, which coincides with a pro-inflammatory shift in maternal immunity prior to parturition.106–109 Specifically, cffDNA has been demonstrated to stimulate a monocyte response in the third trimester capable of activating bystander T cells.109 Moreover, phenotyping and omics studies have provided evidence of T-cell activation that occurs during labor,19,21,23,110 and T-cell responses in late pregnancy have been associated with the increased expression of activation markers.111–113 In support of these concepts, the samples herein were obtained from pregnant women in late gestation and close to delivery. Of note, parity information was not available for the control/non-pregnant study participants, so analyses accounting for both pregnancy status and parity were not performed. Collectively, these data suggest the possibility that the presence of or increases in the circulating concentrations of fetal antigens and cffDNA may contribute to the modest increase in basal activation of peripheral CD4+ T cells observed in pregnancy.
Herein, we also found that CD4+ and CD8+ T cells from pregnant women displayed greater proliferation in response to in vitro stimulation than those from non-pregnant women. In support of this finding, increased proliferation of CD4+ and CD8+ T cells as a function of pregnancy has also been reported in mice.114 One of the most prominent findings in the current study was that, by contrast with the baseline differences in T-cell subset composition, the proportions of proliferated CD4+ and CD8+ T-cell populations expressing the activation markers CD69 and PD-1 were reduced in pregnant women. In mice, an increase in the proliferation of CD4+ and CD8+ pregnancy-derived T cells following the blockade of PD-1 has been reported114; therefore, the reduced proportion of peripheral T cells expressing PD-1 in pregnant women may have contributed to the higher proliferation of pregnancy-derived CD4+ and CD8+ T cells observed in this study. PD-1 is well-studied for its role in cancer and the therapeutic potential of inhibiting this pathway,115,116 and the co-expression of PD-1 and CD69 has been reported in activated CD4+ and CD8+ T117 cells and in NK118 cells isolated from cancer patients. Of note, CD69 has also been demonstrated to play a role in immune119,120 and metabolic121,122 regulation, indicating it may be more than a marker of activation.123 Yet, additional experiments are needed to define the functional implications of this phenotype in the context of pregnancy.
In this regard, increased expression of CD69 by peripheral T cells has been described in patients with a history of recurrent spontaneous abortion,124,125 and the basal and stimulated CD69 expression was higher in women with miscarriage than in those with normal pregnancy.125 Furthermore, increased CD69 expression by peripheral CD8+ T cells has been reported in patients with cardiac126 and renal127 allograft rejection, and thus proposed as a biomarker for transplant rejection. Together, these studies suggest that the strong upregulation of this activation marker in response to a stimulus can indicate adverse consequences for pregnancy. Indeed, the in vivo activation of T cells with an anti-CD3ε antibody in late and mid pregnancy has been shown to cause systemic inflammation and preterm labor and birth19 as well as pregnancy loss (Gomez-Lopez et al., unpublished data), respectively. In this murine model, the systemic inflammatory response also extended to the amniotic cavity and resulted in fetal growth restriction,19 indicating that the systemic over-activation of maternal T cells in pregnancy can be detrimental to the fetus. Thus, the lower proportion of pregnancy-derived CD69+PD-1+ peripheral T cells following stimulation observed herein may indicate a higher threshold for T-cell activation as a mechanism to preserve systemic immune homeostasis.
In addition to the protective mechanism proposed above, the diminished activation of circulating maternal T cells observed in the current study may also allow them to retain memory and proliferative functions for a longer duration.49,128 This concept is in line with our finding that a lower proportion of terminally differentiated effector memory cells was observed in proliferated T cells from pregnant compared to non-pregnant women. Terminally differentiated CD8+ effector memory T cells display greater effector functions but lower memory and proliferative capabilities and are considered to be short-lived.129–132 The reduced proportion of T cells expressing activation and terminal effector memory phenotypes following stimulation may reflect a more stringent use of effector functions by T cells during pregnancy. That is, we observed fewer T cells to be activated or terminally differentiated following stimulation in the context of pregnancy, which could reflect a diminished tendency to display effector functions by this peripheral T-cell population.
In line with the above concept, it is reasonable to propose that maternal peripheral T-cell responses are controlled in an antigen-specific manner,67 which could be a useful feature for avoiding unnecessary T-cell activation that could adversely affect pregnancy. Herein, we utilized a form of T-cell stimulation that bypasses antigen recognition to directly stimulate the T-cell and costimulatory receptors. Yet, prior in vitro studies evaluating the response to influenza A viral stimulation in PBMCs have demonstrated a pregnancy-specific attenuation of the release of pro-inflammatory cytokines such as IFNα133,134 and IL-2.134 Futhermore, we have recently shown a reduction in the proportions of pro-inflammatory T-cell subsets, such as Th1 and Tc17, in pregnant women with asymptomatic SARS-CoV-2 infection relative to healthy controls.135 On the other hand, in vitro stimulation of PBMCs from pregnant women with SARS-CoV-2 proteins results in enhanced T-cell activation 158. Taken together, these data suggest that stimulation with antigens of differing nature/pathogenicity can elicit distinct T-cell responses in the maternal circulation.
In this study, we observed comparable cytotoxic activity by PBMCs from pregnant and non-pregnant women, as reported previously.136 In the periphery, both T and NK cells are capable of cytotoxic activity,137 and T cells have been reported to be more prevalent in the maternal periphery.138 However, the assay used herein relies on C-type lectin-like receptor NKG2D-mediated cytotoxicity139; although the CD8+ T and NK cells express this activation receptor,140 NKG2D signaling in isolation is only sufficient to activate NK cells, as CD8+ T cells require simultaneous stimulation of the T-cell receptor and by cytokines.139 Despite this limitation, the data indicate comparable peripheral lymphocyte cytotoxicity upon exposure to non-self-antigens between pregnant and non-pregnant women.
While a large body of work has considered the role of T cells in establishing and maintaining maternal-fetal tolerance, the second cellular component of adaptive immunity – B cells – is also critical to establishing and maintaining tolerance throughout gestation.31,65,92,141,142 Prior studies have demonstrated that IgG immunoglobulins contained in maternal serum prevent maternal lymphocytes from mounting a cytotoxic response against cultured trophoblasts.91,143 Indeed, spontaneous recurrent abortions are characterized by a lack of protective maternal antibodies directed toward paternal HLA antigens.144–147 Protective antibodies bind their antigens with high affinity but are unable to initiate downstream immune responses such as complement activation and cytotoxicity.148 By contrast, natural autoantibodies, produced by B1a cells,65,149,150 are associated with a range of obstetrical complications that includesintrauterine fetal demise and preeclampsia.151–154 Accordingly, the circulating proportion of B1, but not B2, cells has been reported to decrease throughout gestation,30 and B-cell subset composition at the maternal-fetal interface is altered by the process of labor, preterm birth, or chronic histologic chorioamnionitis.31 Herein, we considered alterations in peripheral B-cell subset composition as a function of pregnancy itself and reported increased frequencies of memory-like CD27+IgG+ B cells and activated CD38+CD24− B cells in pregnant women. Notably, the latter finding is consistent with the observed greater responses to in vitro IgM/IgG stimulation in pregnancy-derived B cells, given that CD38 ligation has been linked to Bruton tyrosine kinase (BTK) phosphorylation.155 Higher median peripheral concentrations of B-cell activating factor (BAFF) have been reported in pregnant compared to non-pregnant women, suggesting that BAFF may prime B cells and thus contribute to the pregnancy-specific increase in activation displayed by these cells.156 In this study, we showed that peripheral B cells display a heightened response to stimulation during gestation, which could provide a more efficient cellular immune response to insults.
Collectively, the results presented herein indicate that maternal circulating T cells and B cells display specific responses during pregnancy. Pregnancy-derived T cells show modestly higher basal activation and greatly increased proliferative capacity; yet, such proliferated T cells resist signs of prolonged activation displayed by their non-pregnant counterparts. Furthermore, B cells isolated from pregnant women display greater basal proportions of memory-like and activated phenotypes and exhibit higher activation following stimulation. These findings show that maternal circulating T cells and B cells display distinct responses during pregnancy, suggesting that maternal peripheral T cells are capable of responding to potential threats but are more resistant to aberrant activation, thereby preventing a systemic inflammatory response that can lead to adverse perinatal consequences.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS) under Contract No. HHSN275201300006C. Dr. Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors would like to thank the physicians, nurses, and research assistants from the Center for Advanced Obstetrical Care and Research, Intrapartum Unit, and Perinatology Research Branch Clinical Laboratory for help with sample collection.
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Sacks G, Sargent I, Redman C. Innate immunity in pregnancy. Immunol Today. 2000;21(4):200–201. [DOI] [PubMed] [Google Scholar]
- 2.Faas MM, Spaans F, De Vos P. Monocytes and macrophages in pregnancy and pre-eclampsia. Front Immunol. 2014;5:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu-Raya B, Michalski C, Sadarangani M, Lavoie PM. Maternal Immunological Adaptation During Normal Pregnancy. Front Immunol. 2020;11:575197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Efrati P, Presentey B, Margalith M, Rozenszajn L. Leukocytes Of Normal Pregnant Women. Obstet Gynecol. 1964;23:429–432. [PubMed] [Google Scholar]
- 5.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179(1):80–86. [DOI] [PubMed] [Google Scholar]
- 6.Koumandakis E, Koumandaki I, Kaklamani E, Sparos L, Aravantinos D, Trichopoulos D. Enhanced phagocytosis of mononuclear phagocytes in pregnancy. Br J Obstet Gynaecol. 1986;93(11):1150–1154. [DOI] [PubMed] [Google Scholar]
- 7.Shibuya T, Izuchi K, Kuroiwa A, Okabe N, Shirakawa K. Study on nonspecific immunity in pregnant women: increased chemiluminescence response of peripheral blood phagocytes. Am J Reprod Immunol Microbiol. 1987;15(1):19–23. [DOI] [PubMed] [Google Scholar]
- 8.Naccasha N, Gervasi MT, Chaiworapongsa T, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol. 2001;185(5):1118–1123. [DOI] [PubMed] [Google Scholar]
- 9.Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007;178(9):5949–5956. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Shynlova O, Sabra S, Bang A, Briollais L, Lye SJ. Immunophenotyping and activation status of maternal peripheral blood leukocytes during pregnancy and labour, both term and preterm. J Cell Mol Med. 2017;21(10):2386–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farias-Jofre M, Romero R, Galaz J, et al. Pregnancy Tailors Endotoxin-Induced Monocyte and Neutrophil Responses in the Maternal Circulation. Inflamm Res. 2022;71(5–6):653–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitzmiller JL, Stoneburner L, Yelenosky PF, Lucas WE. Serum complement in normal pregnancy and pre-eclampsia. Am J Obstet Gynecol. 1973;117(3):312–315. [DOI] [PubMed] [Google Scholar]
- 13.Baines MG, Millar KG, Mills P. Studies of complement levels in normal human pregnancy. Obstet Gynecol. 1974;43(6):806–810. [PubMed] [Google Scholar]
- 14.Stirling Y, Woolf L, North WR, Seghatchian MJ, Meade TW. Haemostasis in normal pregnancy. Thromb Haemost. 1984;52(2):176–182. [PubMed] [Google Scholar]
- 15.Hopkinson ND, Powell RJ. Classical complement activation induced by pregnancy: implications for management of connective tissue diseases. J Clin Pathol. 1992;45(1):66–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richani K, Soto E, Romero R, et al. Normal pregnancy is characterized by systemic activation of the complement system. J Matern Fetal Neonatal Med. 2005;17(4):239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blazkova J, Gupta S, Liu Y, et al. Multicenter systems analysis of human blood reveals immature neutrophils in males and during pregnancy. J Immunol. 2017;198(6):2479–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aghaeepour N, Ganio EA, McIlwain D, et al. An immune clock of human pregnancy. Sci Immunol. 2017;2(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arenas-Hernandez M, Romero R, Xu Y, et al. Effector and activated T cells induce preterm labor and birth that is prevented by treatment with progesterone. J Immunol. 2019;202(9):2585–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han X, Ghaemi MS, Ando K, et al. Differential dynamics of the maternal immune system in healthy pregnancy and preeclampsia. Front Immunol. 2019;10:1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pique-Regi R, Romero R, Tarca AL, et al. Single cell transcriptional signatures of the human placenta in term and preterm parturition. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stelzer IA, Ghaemi MS, Han X, et al. Integrated trajectories of the maternal metabolome, proteome, and immunome predict labor onset. Sci Transl Med. 2021;13(592). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarca AL, Romero R, Xu Z, et al. Targeted expression profiling by RNA-Seq improves detection of cellular dynamics during pregnancy and identifies a role for T cells in term parturition. Sci Rep. 2019;9(1):848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aghaeepour N, Lehallier B, Baca Q, et al. A proteomic clock of human pregnancy. Am J Obstet Gynecol. 2018;218(3):347 e341–347 e314. [DOI] [PubMed] [Google Scholar]
- 25.Saito S, Umekage H, Sakamoto Y, et al. Increased T-helper-1-type immunity and decreased T-helper-2-type immunity in patients with preeclampsia. Am J Reprod Immunol. 1999;41(5):297–306. [DOI] [PubMed] [Google Scholar]
- 26.Chaiworapongsa T, Gervasi MT, Refuerzo J, et al. Maternal lymphocyte subpopulations (CD45RA+ and CD45RO+) in preeclampsia. Am J Obstet Gynecol. 2002;187(4):889–893. [DOI] [PubMed] [Google Scholar]
- 27.Darmochwal-Kolarz D, Saito S, Rolinski J, et al. Activated T lymphocytes in pre-eclampsia. Am J Reprod Immunol. 2007;58(1):39–45. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki Y, Darmochwal-Kolarz D, Suzuki D, et al. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin Exp Immunol. 2007;149(1):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller D, Motomura K, Galaz J, et al. Cellular immune responses in the pathophysiology of preeclampsia. J Leukoc Biol. 2022;111(1):237–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhat NM, Mithal A, Bieber MM, Herzenberg LA, Teng NN. Human CD5+ B lymphocytes (B-1 cells) decrease in peripheral blood during pregnancy. J Reprod Immunol. 1995;28(1):53–60. [DOI] [PubMed] [Google Scholar]
- 31.Leng Y, Romero R, Xu Y, et al. Are B cells altered in the decidua of women with preterm or term labor? Am J Reprod Immunol. 2019;81(5):e13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14(7):353–356. [DOI] [PubMed] [Google Scholar]
- 33.Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol. 1993;151(9):4562–4573. [PubMed] [Google Scholar]
- 34.Tafuri A, Alferink J, Möller P, Hämmerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270(5236):630–633. [DOI] [PubMed] [Google Scholar]
- 35.Marzi M, Vigano A, Trabattoni D, et al. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106(1):127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekerfelt C, Matthiesen L, Berg G, Ernerudh J. Th2-deviation of fetus-specific T cells. Immunol Today. 1999;20(11):534. [DOI] [PubMed] [Google Scholar]
- 37.Chaouat G, Petitbarat M, Dubanchet S, Rahmati M, Ledee N. Tolerance to the foetal allograft? Am J Reprod Immunol. 2010;63(6):624–636. [DOI] [PubMed] [Google Scholar]
- 38.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339(5):285–291. [DOI] [PubMed] [Google Scholar]
- 39.Buchel E, Van Steenbergen W, Nevens F, Fevery J. Improvement of autoimmune hepatitis during pregnancy followed by flare-up after delivery. Am J Gastroenterol. 2002;97(12):3160–3165. [DOI] [PubMed] [Google Scholar]
- 40.Langer-Gould A, Garren H, Slansky A, Ruiz PJ, Steinman L. Late pregnancy suppresses relapses in experimental autoimmune encephalomyelitis: evidence for a suppressive pregnancy-related serum factor. J Immunol. 2002;169(2):1084–1091. [DOI] [PubMed] [Google Scholar]
- 41.McClain MA, Gatson NN, Powell ND, et al. Pregnancy suppresses experimental autoimmune encephalomyelitis through immunoregulatory cytokine production. J Immunol. 2007;179(12):8146–8152. [DOI] [PubMed] [Google Scholar]
- 42.de Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP, Hazes JM. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum. 2008;59(9):1241–1248. [DOI] [PubMed] [Google Scholar]
- 43.Gatson NN, Williams JL, Powell ND, et al. Induction of pregnancy during established EAE halts progression of CNS autoimmune injury via pregnancy-specific serum factors. J Neuroimmunol. 2011;230(1–2):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engler JB, Kursawe N, Solano ME, et al. Glucocorticoid receptor in T cells mediates protection from autoimmunity in pregnancy. Proc Natl Acad Sci U S A. 2017;114(2):E181–e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koetzier SC, Neuteboom RF, Wierenga-Wolf AF, et al. Effector T Helper Cells Are Selectively Controlled During Pregnancy and Related to a Postpartum Relapse in Multiple Sclerosis. Front Immunol. 2021;12:642038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lockshin MD, Reinitz E, Druzin ML, Murrman M, Estes D. Lupus pregnancy. Case-control prospective study demonstrating absence of lupus exacerbation during or after pregnancy. Am J Med. 1984;77(5):893–898. [DOI] [PubMed] [Google Scholar]
- 47.Wang SC, Li YH, Piao HL, et al. PD-1 and Tim-3 pathways are associated with regulatory CD8+ T-cell function in decidua and maintenance of normal pregnancy. Cell Death Dis. 2015;6(5):e1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Zwan A, Bi K, Norwitz ER, et al. Mixed signature of activation and dysfunction allows human decidual CD8(+) T cells to provide both tolerance and immunity. Proc Natl Acad Sci U S A. 2018;115(2):385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slutsky R, Romero R, Xu Y, et al. Exhausted and Senescent T Cells at the Maternal-Fetal Interface in Preterm and Term Labor. J Immunol Res. 2019;2019:3128010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daya S, Rosenthal KL, Clark DA. Immunosuppressor factor(s) produced by decidua-associated suppressor cells: a proposed mechanism for fetal allograft survival. Am J Obstet Gynecol. 1987;156(2):344–350. [DOI] [PubMed] [Google Scholar]
- 51.Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117(5):1399–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336(6086):1317–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zenclussen AC, Gerlof K, Zenclussen ML, et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166(3):811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Darrasse-Jèze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett. 2006;102(1):106–109. [DOI] [PubMed] [Google Scholar]
- 55.Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci U S A. 2010;107(20):9299–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shima T, Sasaki Y, Itoh M, et al. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol. 2010;85(2):121–129. [DOI] [PubMed] [Google Scholar]
- 57.Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, Way SS. Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe. 2011;10(1):54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schober L, Radnai D, Schmitt E, Mahnke K, Sohn C, Steinborn A. Term and preterm labor: decreased suppressive activity and changes in composition of the regulatory T-cell pool. Immunol Cell Biol. 2012;90(10):935–944. [DOI] [PubMed] [Google Scholar]
- 60.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490(7418):102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen T, Darrasse-Jèze G, Bergot AS, et al. Self-specific memory regulatory T cells protect embryos at implantation in mice. J Immunol. 2013;191(5):2273–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomez-Lopez N, Arenas-Hernandez M, Romero R, et al. Regulatory T Cells Play a Role in a Subset of Idiopathic Preterm Labor/Birth and Adverse Neonatal Outcomes. Cell Rep. 2020;32(1):107874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diao L, Hierweger AM, Wieczorek A, Arck PC, Thiele K. Disruption of Glucocorticoid Action on CD11c(+) Dendritic Cells Favors the Generation of CD4(+) Regulatory T Cells and Improves Fetal Development in Mice. Front Immunol. 2021;12:729742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gomez-Lopez N, Romero R, Hassan SS, et al. The Cellular Transcriptome in the Maternal Circulation During Normal Pregnancy: A Longitudinal Study. Front Immunol. 2019;10:2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muzzio D, Zenclussen AC, Jensen F. The role of B cells in pregnancy: the good and the bad. Am J Reprod Immunol. 2013;69(4):408–412. [DOI] [PubMed] [Google Scholar]
- 66.Bartmann C, Segerer SE, Rieger L, Kapp M, Sutterlin M, Kammerer U. Quantification of the predominant immune cell populations in decidua throughout human pregnancy. Am J Reprod Immunol. 2014;71(2):109–119. [DOI] [PubMed] [Google Scholar]
- 67.Kieffer TEC, Laskewitz A, Scherjon SA, Faas MM, Prins JR. Memory T Cells in Pregnancy. Front Immunol. 2019;10:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Valeff N, Muzzio DO, Matzner F, et al. B cells acquire a unique and differential transcriptomic profile during pregnancy. Genomics. 2021;113(4):2614–2622. [DOI] [PubMed] [Google Scholar]
- 69.Garson D, Dokhelar MC, Wakasugi H, Mishal Z, Tursz T. HLA class-I and class-II antigen expression by human leukemic K562 cells and by Burkitt-K562 hybrids: modulation by differentiation inducers and interferon. Exp Hematol. 1985;13(9):885–890. [PubMed] [Google Scholar]
- 70.Day NE, Ugai H, Yokoyama KK, Ichiki AT. K-562 cells lack MHC class II expression due to an alternatively spliced CIITA transcript with a truncated coding region. Leuk Res. 2003;27(11):1027–1038. [DOI] [PubMed] [Google Scholar]
- 71.Tremblay-McLean A, Coenraads S, Kiani Z, Dupuy FP, Bernard NF. Expression of ligands for activating natural killer cell receptors on cell lines commonly used to assess natural killer cell function. BMC Immunol. 2019;20(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67(1):1–48. [Google Scholar]
- 73.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological). 1995;57:289–300. [Google Scholar]
- 74.Saito S, Nishikawa K, Morii T, Narita N, Enomoto M, Ichijo M. Expression of activation antigens CD69, HLA-DR, interleukin-2 receptor-alpha (IL-2R alpha) and IL-2R beta on T cells of human decidua at an early stage of pregnancy. Immunology. 1992;75(4):710–712. [PMC free article] [PubMed] [Google Scholar]
- 75.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10(5):347–353. [DOI] [PubMed] [Google Scholar]
- 76.Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlström AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod. 2009;80(5):1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu S, Diao L, Huang C, Li Y, Zeng Y, Kwak-Kim JYH. The role of decidual immune cells on human pregnancy. J Reprod Immunol. 2017;124:44–53. [DOI] [PubMed] [Google Scholar]
- 78.Robertson SA, Care AS, Moldenhauer LM. Regulatory T cells in embryo implantation and the immune response to pregnancy. J Clin Invest. 2018;128(10):4224–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang W, Zhao Y, Zhou X, et al. Dynamic changes in regulatory T cells during normal pregnancy, recurrent pregnancy loss, and gestational diabetes. J Reprod Immunol. 2022;150:103492. [DOI] [PubMed] [Google Scholar]
- 80.Hara T, Jung LK, Bjorndahl JM, Fu SM. Human T cell activation. III. Rapid induction of a phosphorylated 28 kD/32 kD disulfide-linked early activation antigen (EA 1) by 12-o-tetradecanoyl phorbol-13acetate, mitogens, and antigens. J Exp Med. 1986;164(6):1988–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Testi R, Phillips JH, Lanier LL. T cell activation via Leu-23 (CD69). J Immunol. 1989;143(4):1123–1128. [PubMed] [Google Scholar]
- 82.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. [DOI] [PubMed] [Google Scholar]
- 84.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8(5):765–772. [DOI] [PubMed] [Google Scholar]
- 86.Datar I, Sanmamed MF, Wang J, et al. Expression Analysis and Significance of PD-1, LAG-3, and TIM-3 in Human Non-Small Cell Lung Cancer Using Spatially Resolved and Multiparametric Single-Cell Analysis. Clin Cancer Res. 2019;25(15):4663–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. [DOI] [PubMed] [Google Scholar]
- 88.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101(11):4260–4266. [DOI] [PubMed] [Google Scholar]
- 89.Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol. 2002;2(6):401–409. [DOI] [PubMed] [Google Scholar]
- 90.Fan Z, Zhang Q. Molecular mechanisms of lymphocyte-mediated cytotoxicity. Cell Mol Immunol. 2005;2(4):259–264. [PubMed] [Google Scholar]
- 91.Taylor PV, Hancock KW. Antigenicity of trophoblast and possible antigen-masking effects during pregnancy. Immunology. 1975;28(5):973–982. [PMC free article] [PubMed] [Google Scholar]
- 92.Nguyen TG, Ward CM, Morris JM. To B or not to B cells-mediate a healthy start to life. Clin Exp Immunol. 2013;171(2):124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miller D, Gershater M, Slutsky R, Romero R, Gomez-Lopez N. Maternal and fetal T cells in term pregnancy and preterm labor. Cell Mol Immunol. 2020;17(7):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1953;7:320–328. [Google Scholar]
- 95.Gomez-Lopez N, Guilbert LJ, Olson DM. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J Leukoc Biol. 2010;88(4):625–633. [DOI] [PubMed] [Google Scholar]
- 96.Bonney EA, Shepard MT, Bizargity P. Transient modification within a pool of CD4 T cells in the maternal spleen. Immunology. 2011;134(3):270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cell Mol Immunol. 2014;11(6):571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bonney EA. Alternative theories: Pregnancy and immune tolerance. J Reprod Immunol. 2017;123:65–71. [DOI] [PubMed] [Google Scholar]
- 99.Elderman M, Hugenholtz F, Belzer C, et al. Changes in intestinal gene expression and microbiota composition during late pregnancy are mouse strain dependent. Sci Rep. 2018;8(1):10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. [DOI] [PubMed] [Google Scholar]
- 101.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moldenhauer LM, Diener KR, Thring DM, Brown MP, Hayball JD, Robertson SA. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol. 2009;182(12):8080–8093. [DOI] [PubMed] [Google Scholar]
- 103.Arenas-Hernandez M, Romero R, Gershater M, et al. Specific innate immune cells uptake fetal antigen and display homeostatic phenotypes in the maternal circulation. J Leukoc Biol. 2022;111(3):519–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schumacher A, Wafula PO, Bertoja AZ, et al. Mechanisms of action of regulatory T cells specific for paternal antigens during pregnancy. Obstet Gynecol. 2007;110(5):1137–1145. [DOI] [PubMed] [Google Scholar]
- 105.Boly TJ, Bermick JR. Maternal-fetal tolerance: Not just a uterine affair. J Leukoc Biol. 2022;111(3):515–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Phillippe M. Cell-Free Fetal DNA, Telomeres, and the Spontaneous Onset of Parturition. Reprod Sci. 2015;22(10):1186–1201. [DOI] [PubMed] [Google Scholar]
- 107.Goldfarb IT, Adeli S, Berk T, Phillippe M. Fetal and Placental DNA Stimulation of TLR9: A Mechanism Possibly Contributing to the Pro-inflammatory Events During Parturition. Reprod Sci. 2018;25(5):788–796. [DOI] [PubMed] [Google Scholar]
- 108.Gomez-Lopez N, Romero R, Schwenkel G, et al. Cell-Free Fetal DNA Increases Prior to Labor at Term and in a Subset of Preterm Births. Reprod Sci. 2020;27(1):218–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yeganeh Kazemi N, Fedyshyn B, Sutor S, Fedyshyn Y, Markovic S, Enninga EAL. Maternal Monocytes Respond to Cell-Free Fetal DNA and Initiate Key Processes of Human Parturition. J Immunol. 2021;207(10):2433–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gomez-Lopez N, Romero R, Galaz J, et al. Transcriptome changes in maternal peripheral blood during term parturition mimic perturbations preceding spontaneous preterm birthdagger. Biol Reprod. 2022;106(1):185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abadía-Molina AC, Ruiz C, Montes MJ, King A, Loke YW, Olivares EG. Immune phenotype and cytotoxic activity of lymphocytes from human term decidua against trophoblast. J Reprod Immunol. 1996;31(1–2):109–123. [DOI] [PubMed] [Google Scholar]
- 112.Sindram-Trujillo A, Scherjon S, Kanhai H, Roelen D, Claas F. Increased T-cell activation in decidua parietalis compared to decidua basalis in uncomplicated human term pregnancy. Am J Reprod Immunol. 2003;49(5):261–268. [DOI] [PubMed] [Google Scholar]
- 113.Shah NM, Herasimtschuk AA, Boasso A, et al. Changes in T Cell and Dendritic Cell Phenotype from Mid to Late Pregnancy Are Indicative of a Shift from Immune Tolerance to Immune Activation. Front Immunol. 2017;8:1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shepard MT, Bonney EA. PD-1 regulates T cell proliferation in a tissue and subset-specific manner during normal mouse pregnancy. Immunol Invest. 2013;42(5):385–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ostrand-Rosenberg S, Horn LA, Haile ST. The programmed death1 immune-suppressive pathway: barrier to antitumor immunity. J Immunol. 2014;193(8):3835–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.LaFleur MW, Muroyama Y, Drake CG, Sharpe AH. Inhibitors of the PD-1 Pathway in Tumor Therapy. J Immunol. 2018;200(2):375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Piersiala K, Farrajota Neves da Silva P, Hjalmarsson E, et al. CD4(+) and CD8(+) T cells in sentinel nodes exhibit distinct pattern of PD-1, CD69, and HLA-DR expression compared to tumor tissue in oral squamous cell carcinoma. Cancer Sci. 2021;112(3):1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Concha-Benavente F, Kansy B, Moskovitz J, Moy J, Chandran U, Ferris RL. PD-L1 Mediates Dysfunction in Activated PD-1(+) NK Cells in Head and Neck Cancer Patients. Cancer Immunol Res. 2018;6(12):1548–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shiow LR, Rosen DB, Brdickova N, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–544. [DOI] [PubMed] [Google Scholar]
- 120.Lin CR, Wei TY, Tsai HY, Wu YT, Wu PY, Chen ST. Glycosylation-dependent interaction between CD69 and S100A8/S100A9 complex is required for regulatory T-cell differentiation. FASEB J. 2015;29(12):5006–5017. [DOI] [PubMed] [Google Scholar]
- 121.Cibrian D, Saiz ML, de la Fuente H, et al. CD69 controls the uptake of L-tryptophan through LAT1-CD98 and AhR-dependent secretion of IL-22 in psoriasis. Nat Immunol. 2016;17(8):985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Labiano S, Melendez-Rodriguez F, Palazon A, et al. CD69 is a direct HIF-1alpha target gene in hypoxia as a mechanism enhancing expression on tumor-infiltrating T lymphocytes. Oncoimmunology. 2017;6(4):e1283468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cibrian D, Sanchez-Madrid F. CD69: from activation marker to metabolic gatekeeper. Eur J Immunol. 2017;47(6):946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Prado-Drayer A, Teppa J, Sanchez P, Camejo MI. Immunophenotype of peripheral T lymphocytes, NK cells and expression of CD69 activation marker in patients with recurrent spontaneous abortions, during the mid-luteal phase. Am J Reprod Immunol. 2008;60(1):66–74. [DOI] [PubMed] [Google Scholar]
- 125.Krechetova LV, Vtorushina VV, Nikolaeva MA, et al. Expression of Early Activation Marker CD69 on Peripheral Blood Lymphocytes from Pregnant Women after First Trimester Alloimmunization. Bull Exp Biol Med. 2016;161(4):529–532. [DOI] [PubMed] [Google Scholar]
- 126.Schowengerdt KO, Fricker FJ, Bahjat KS, Kuntz ST. Increased expression of the lymphocyte early activation marker CD69 in peripheral blood correlates with histologic evidence of cardiac allograft rejection. Transplantation. 2000;69(10):2102–2107. [DOI] [PubMed] [Google Scholar]
- 127.Posselt AM, Vincenti F, Bedolli M, Lantz M, Roberts JP, Hirose R. CD69 expression on peripheral CD8 T cells correlates with acute rejection in renal transplant recipients. Transplantation. 2003;76(1):190–195. [DOI] [PubMed] [Google Scholar]
- 128.Kurachi M. CD8(+) T cell exhaustion. Semin Immunopathol. 2019;41(3):327–337. [DOI] [PubMed] [Google Scholar]
- 129.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205(3):625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Golubovskaya V, Wu L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers (Basel). 2016;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Verma K, Ogonek J, Varanasi PR, et al. Human CD8+ CD57- TEMRA cells: Too young to be called “old”. PLoS One. 2017;12(5):e0177405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Milner JJ, Nguyen H, Omilusik K, et al. Delineation of a molecularly distinct terminally differentiated memory CD8 T cell population. Proc Natl Acad Sci U S A. 2020;117(41):25667–25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Forbes RL, Wark PA, Murphy VE, Gibson PG. Pregnant women have attenuated innate interferon responses to 2009 pandemic influenza A virus subtype H1N1. J Infect Dis. 2012;206(5):646–653. [DOI] [PubMed] [Google Scholar]
- 134.Vanders RL, Gibson PG, Murphy VE, Wark PA. Plasmacytoid dendritic cells and CD8 T cells from pregnant women show altered phenotype and function following H1N1/09 infection. J Infect Dis. 2013;208(7):1062–1070. [DOI] [PubMed] [Google Scholar]
- 135.Garcia-Flores V, Romero R, Xu Y, et al. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat Commun. 2022;13(1):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Siklos P, Nemeth-Csoka A, Bartalits L, Ungar L, Hercz P, Garam T. Cytotoxic activity of peripheral mononuclear cells in normal pregnancy. Haematologia (Budap). 1985;18(4):259–264. [PubMed] [Google Scholar]
- 137.Arancia G, Malorni W, Donelli G. Cellular mechanisms of lymphocyte-mediated lysis of tumor cells. Ann Ist Super Sanita. 1990;26(3–4):369–384. [PubMed] [Google Scholar]
- 138.Kuhnert M, Strohmeier R, Stegmuller M, Halberstadt E. Changes in lymphocyte subsets during normal pregnancy. Eur J Obstet Gynecol Reprod Biol. 1998;76(2):147–151. [DOI] [PubMed] [Google Scholar]
- 139.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17(1):19–29. [DOI] [PubMed] [Google Scholar]
- 140.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285(5428):727–729. [DOI] [PubMed] [Google Scholar]
- 141.Fettke F, Schumacher A, Costa SD, Zenclussen AC. B cells: the old new players in reproductive immunology. Front Immunol. 2014;5:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ziegler KB, Muzzio DO, Matzner F, et al. Human pregnancy is accompanied by modifications in B cell development and immunoglobulin profile. J Reprod Immunol. 2018;129:40–47. [DOI] [PubMed] [Google Scholar]
- 143.Chaouat G, Kolb JP. Immunoactive products of murine placenta. II.–Afferent suppression of maternal cell-mediated immunity by supernatants from short-term cultures of murine trophoblast-enriched cell suspensions. Ann Immunol (Paris). 1984;135C(2):205–218. [PubMed] [Google Scholar]
- 144.Power DA, Catto GR, Mason RJ, et al. The fetus as an allograft: evidence for protective antibodies to HLA-linked paternal antigens. Lancet. 1983;2(8352):701–704. [DOI] [PubMed] [Google Scholar]
- 145.Beard RW, Braude P, Mowbray JF, Underwood JL. Protective antibodies and spontaneous abortion. Lancet. 1983;2(8358):1090. [DOI] [PubMed] [Google Scholar]
- 146.Beer AE, Semprini AE, Zhu XY, Quebbeman JF. Pregnancy outcome in human couples with recurrent spontaneous abortions: HLA antigen profiles; HLA antigen sharing; female serum MLR blocking factors; and paternal leukocyte immunization. Exp Clin Immunogenet. 1985;2(3):137–153. [PubMed] [Google Scholar]
- 147.Agrawal S, Pandey MK, Pandey A. Prevalence of MLR blocking antibodies before and after immunotherapy. J Hematother Stem Cell Res. 2000;9(2):257–262. [DOI] [PubMed] [Google Scholar]
- 148.Margni RA, Paz CB, Cordal ME. Immunochemical behavior of sheep non-precipitating antibodies isolated by immunoadsorption. Immunochemistry. 1976;13(3):209–214. [DOI] [PubMed] [Google Scholar]
- 149.Sthoeger ZM, Wakai M, Tse DB, et al. Production of autoantibodies by CD5-expressing B lymphocytes from patients with chronic lymphocytic leukemia. J Exp Med. 1989;169(1):255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Velasquillo MC, Alcocer-Varela J, Alarcon-Segovia D, Cabiedes J, Sanchez-Guerrero J. Some patients with primary antiphospholipid syndrome have increased circulating CD5+ B cells that correlate with levels of IgM antiphospholipid antibodies. Clin Exp Rheumatol. 1991;9(5):501–505. [PubMed] [Google Scholar]
- 151.Ayres MA, Sulak PJ. Pregnancy complicated by antiphospholipid antibodies. South Med J. 1991;84(2):266–269. [DOI] [PubMed] [Google Scholar]
- 152.Wallukat G, Homuth V, Fischer T, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103(7):945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Erez O, Romero R, Espinoza J, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21(5):279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jensen F, Wallukat G, Herse F, et al. CD19+CD5+ cells as indicators of preeclampsia. Hypertension. 2012;59(4):861–868. [DOI] [PubMed] [Google Scholar]
- 155.Kikuchi Y, Yasue T, Miyake K, Kimoto M, Takatsu K. CD38 ligation induces tyrosine phosphorylation of Bruton tyrosine kinase and enhanced expression of interleukin 5-receptor alpha chain: synergistic effects with interleukin 5. Proc Natl Acad Sci U S A. 1995;92(25):11814–11818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lima J, Cambridge G, Vilas-Boas A, Martins C, Borrego LM, Leandro M. Serum markers of B-cell activation in pregnancy during late gestation, delivery, and the postpartum period. Am J Reprod Immunol. 2019;81(3):e13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gomez-Lopez N, Romero R, Tao L, Gershater M, Leng Y, Zou C, Farias-Jofre M, Galaz J, Miller D, Tarca AL, Arenas-Hernandez M, Bhatti G, Garcia-Flores V, Liu Z, Para R, Kanninen T, Hadaya O, Paredes C, & Xu Y (2022). Distinct Cellular Immune Responses to SARS-CoV-2 in Pregnant Women. The Journal of Immunology, 208(8), 1857–1872. 10.4049/jimmunol.2101123 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.