Abstract
Sulfonyl hydrazides are viewed as alternatives to sulfinic acids and their salts or sulfonyl halides, which are broadly used in organic synthesis or work as active pharmaceutical substances. Generally, sulfonyl hydrazides are considered good building blocks and show powerful value in a diverse range of reactions to construct C–S bonds or C–C bonds, and even C–N bonds as sulfur, carbon, or nitrogen sources, respectively. As a profound synthetic tool, the electrosynthesis method was recently used to achieve efficient and green applications of sulfonyl hydrazides. Interestingly, many unique and novel electrochemical syntheses using sulfonyl hydrazides as radical precursors have been developed, including cascade reactions, functionalization of heterocycles, as well as a continuous flow method combining with electrochemical synthesis since 2017. Accordingly, it is necessary to specifically summarize the recent developments of electrosynthesis with only sulfonyl hydrazides as radical precursors to more deeply understand and better design novel electrochemical synthesis reactions. Herein, electrosynthesis research using sulfonyl hydrazides as radical precursors since 2017 is reviewed in detail based on the chemical structures of products and reaction mechanisms.
1. Introduction
The application of sulfonyl hydrazides in organic chemistry has witnessed a long history.1 In the Shapiro reaction2,3 and variation of the Wolff–Kishner–Huang Minlon reduction reaction,4 TsNHNH2 was chosen to reduce the carbonyl group (C=O) to the methylene (CH2) (Scheme 1). In chemical reactions, sulfonyl hydrazides are viewed as alternatives to sulfinic acids5 and their salts6,7 or sulfonyl halides,8−11 and are broadly used in organic transformations12,13 or syntheses of active pharmaceutical substances.14 Compared with other sulfonic derivatives or sulfonyl halides, sulfonyl hydrazides are easy to handle, stable, moisture-compatible, and noncorrosive. Thus, sulfonyl hydrazides are good building blocks and provide a powerful value in a diverse range of chemical reactions.15 They could work as sulfur sources to introduce various C–S bonds under oxidative conditions16 or take part in cross-coupling reactions to form C–C or C–S bonds,17 and even donate nitrogen atoms serving as nitrogen sources, to form C–N bonds.18,19 In some cases, sulfonyl hydrazides were used as reductants.20
Scheme 1. Application of Sulfonyl Hydrazides in the Shapiro Reaction (a) and Variation of the Wolff–Kishner–Huang Minlon Reduction Reaction (b).
Because of its attractive properties, how to utilize sulfonyl hydrazides has drawn much attention from organic chemists and pharmaceutical scientists. Photoredox catalyzed radical sulfonylation21 and traditional synthetic strategies usually consist of transition-metal catalyzed reactions,22,23 and iodide catalyzed sulfonylation with molecular or ionic iodide16,24,25 are common methods in the application of sulfonyl hydrazides. However, there are many unavoidable problems with these common methods, such as the consumption of stoichiometric amounts of chemical oxidants, the use of metal catalysts sensitive to moisture or oxygen, as well as the output of a substantial number of environmentally hazardous wastes. These unfavorable disadvantages have limited the further applications of these synthetic methods. Therefore, green and practical usage with sulfonyl hydrazides has always been a challenge to be settled. Recently, several reports on the electrochemical application of sulfonyl hydrazides indicated that electrosynthesis could be one of the solutions to this problem. As known to all, the use of external oxidants or reductants could be limited to a certain extent in electrochemical reactions, and there are many advantages for electrosynthesis over traditional methods.26−28 Therefore, electrosynthesis is green, efficient, and environmental-friendly, and has been widely applied in synthetic methodology development29−33 and natural product synthesis.34 As a profound synthetic tool, it is indisputable that electrosynthesis has a hopeful future.35,36
Due its the important role, there have been several reviews about sulfonyl hydrazides focusing on different aspects.15,37 In 2017, Tian et al. reviewed the synthesis of sulfone compounds using sulfonyl hydrazides as sulfonyl sources,15 and reactions were classified according to the scope of substrates in this review. A recent summary of sulfonyl hydrazides in organic chemistry was prepared by Huang and co-workers in 2020,37 in which reactions were classified by crucial intermediates.25 Although electrochemical applications of sulfonyl hydrazides have been mentioned in several reviews,8,11,38,39 it is still necessary to make a systematic and clear summary of the recent development of electrosynthesis with only sulfonyl hydrazides as radical precursors to gain a deeper understanding of their role in electrochemical transformations and design novel electrochemical reactions more effectively. Herein, the electrosynthesis using sulfonyl hydrazides as radical precursors since 2017 is reviewed in detail based on the chemical structures of products and reaction mechanisms.
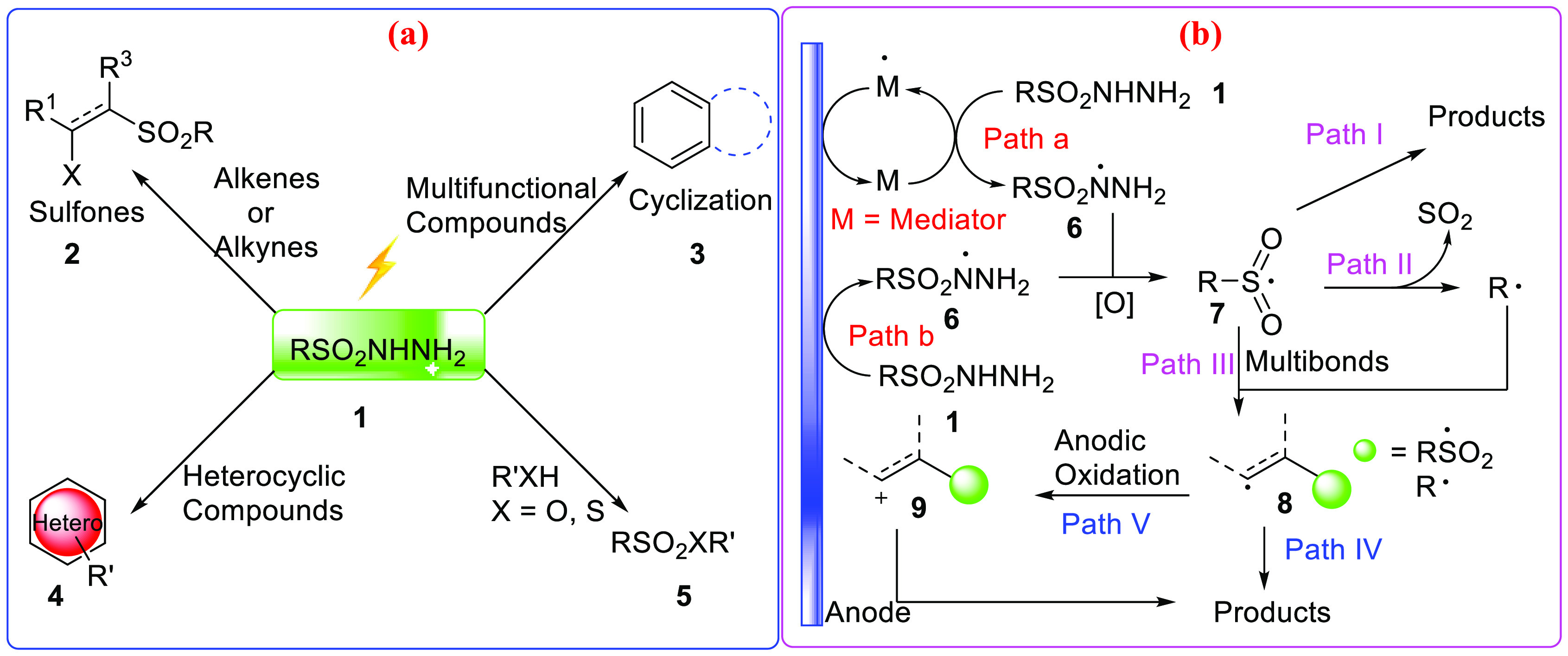
Sulfonyl hydrazides could be applied in a broad range of fields; we classified these cases into four sections: in section I, sulfonyl hydrazides were used in the syntheses of sulfones, mainly including β-substituted and α,β-unsaturated sulfones through the addition to multibonds; in section II, sulfonyl hydrazides participated in reactions with multifunctional compounds involving radical-triggered cyclization; examples in section III are mainly about the functionalization of heterocyclic compounds with sulfonyl hydrazides, and in the last part, syntheses of sulfonyl derivatives with sulfonyl hydrazides are summarized.
When adopted in electrosynthesis, sulfonyl hydrazides have usually taken part in transformation as radicals, and the generation of sulfonyl radicals was viewed as a key step. To the best of our knowledge, sulfonyl hydrazides could be converted into radicals (7) under electrochemical conditions in two ways, i.e., activated by mediators (path a) or oxidized directly at the anode. Subsequently, sulfonyl radicals were transformed mainly through three routes: they could undergo radical coupling and form products such as sulfonyl derivatives (path I); sometimes they might lose SO2 and form alkyl radicals, and then add to multibonds (path II) to give carbo-radical species, or they can add to multibonds (path III) and produce carbo-radical species (8). Carbo-radical species (8) could form products through radical coupling (path IV) or undergo oxidation to form carbo-cation (9) and afford products via the elimination process or the combination with nucleophiles (path V).
2. Sulfonylation of Alkenes and Alkynes
Sulfones have important applications in organic chemistry or medicinal research, and are involved in a number of reactions and could be used as starting materials to synthesize organic molecules12,40−42 or as medicines directly.41,43,44 When treated with electrochemical methods, sulfonyl hydrazides might be converted into sulfonyl radicals, which could undergo sequential addition to C=C double bonds or C≡C triple bonds and generate β-carbon radical species.45 These radical species could be converted into β-functionalized sulfones through various pathways as shown in Scheme 2b. Meanwhile, α, β-unsaturated sulfones which play an important role in organic chemistry46,47 also could be obtained via these pathways.
Scheme 2. Brief Introduction to the Application of Sulfonyl Hydrazides in Electrosynthesis (a) and the General Reaction Procedure of Electrosynthesis with Sulfonyl Hydrazides (b).
β-Alkoxy sulfones are important intermediates in organic synthesis; in 2018, the Lei group reported a green and efficient synthetic method of the alkoxysulfonylation of alkenes using sulfonyl hydrazides and alcohols by electrochemical anodic oxidation, and this was the first report on such type of reaction (Scheme 3).48 In the research, a three-component reaction was carried out in an undivided electrolytic cell charged with a carbon rod anode and a nickel plate cathode with a constant current of 12 mA at room temperature using CH3CN as solvents and nBu4NBF4 (0.1 mmol) as an electrolyte. Under the reaction conditions, sulfonyl hydrazides (10) reacted with alkenes (11) and alcohols (12) to generate β-alkoxyl-substituted sulfones (13) with good yields, giving hydrogen and nitrogen gas as the byproducts. Various conditions were attempted when exploring the research, researchers employed a current of different levels, electrode materials, electrolytes, solvents, and amount of methanol; finally, the reported standard conditions were found to be optimal, and nBu4NBF4 was found to be the key factor.
Scheme 3. Alkoxysulfonylation of Alkenes.
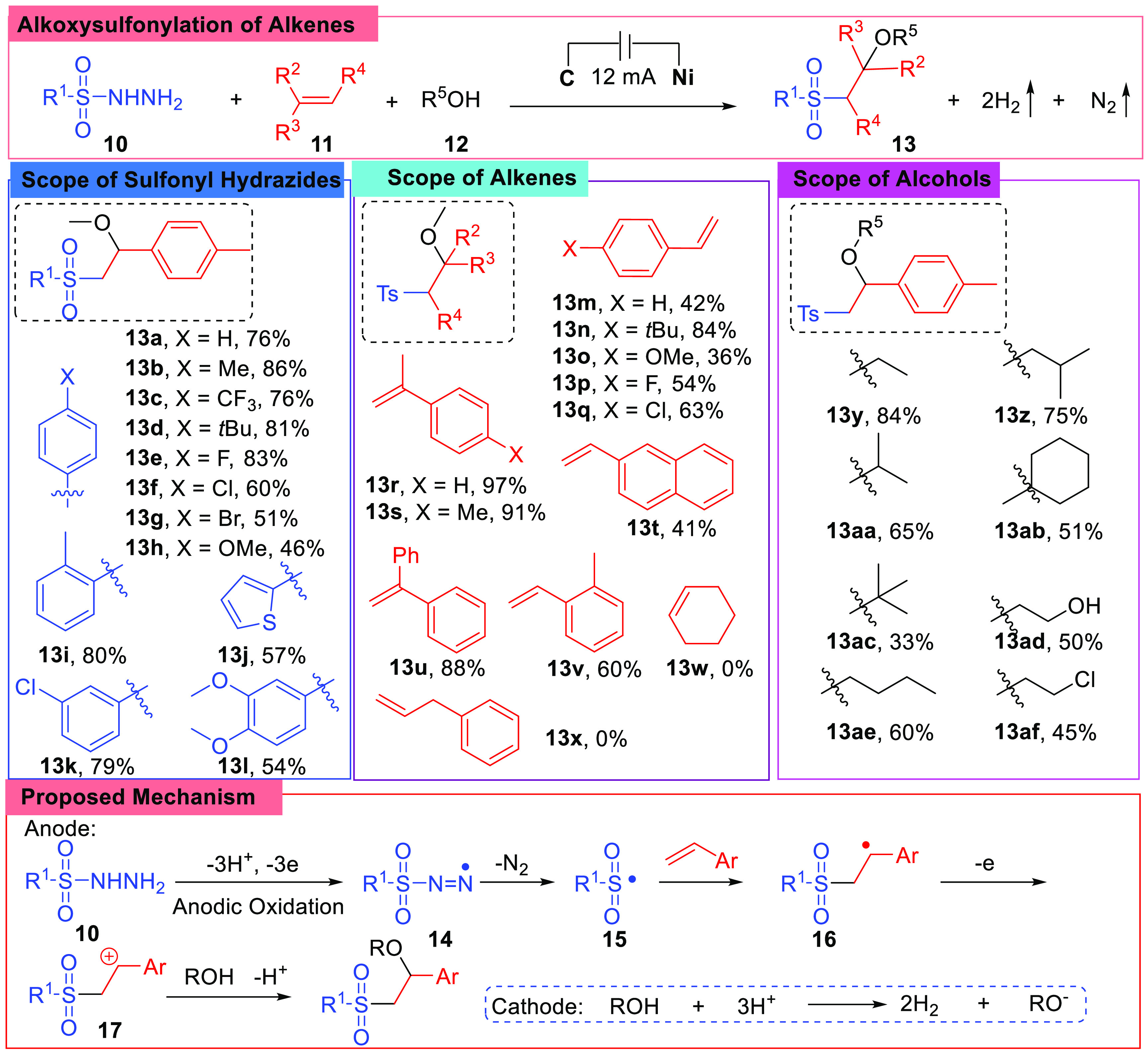
Furthermore, the substrates scope and mechanism were also studied. A range of arylsulfonyl hydrazides were tested. It was found that when arylsulfonyl hydrazides with either electron-withdrawing (e.g., 13c) or electron-donating (e.g., 13b, 13i) groups on the benzene rings were employed, the reactions worked well under the conditions. However, a styrene moiety was necessary for the alkenes; aliphatic alkenes gave none of the desired products (13w, 13x). Finally, products were obtained with middle to high yields when conducted with primary, secondary, or tertiary alcohols.
The researchers proposed a mechanism based on cyclic voltammetry (CV) experiments and radical trap experiments, indicating that the sulfonyl hydrazide (10) was first oxidized at the anode, and the N-centered radical (14) was subsequently formed. Then the radical (14) underwent the loss of nitrogen and gave a sulfonyl radical (15), which added to the C=C double bond in the following step and generated the carbocation intermediate (16). The carbocation intermediate (16) combined with an alcohol and lost the proton subsequently. Then the desired product was produced. Meanwhile, reduction of alcohol with the presence of H+ occurred at the cathode.
Alkenes bearing a trifluoromethyl group, especially at the α-site, have been widely used in chemical transformation.49−52 Due to the strong electron-withdrawing ability of the trifluoromethyl group, application of α-trifluoromethyl alkene in organic synthesis became a challenge, and several electrochemical transformations were introduced recently.53−55 In 2021, Yang and co-workers established a protocol for α-trifluoromethyl β-sulfonyl tertiary alcohols, a class of compounds bearing the β-functionalized sulfone moiety and a neighboring −CF3 group (Scheme 4).56 To achieve the products, sulfonyl hydrazides (18) were treated with α-trifluoromethyl alkenes (19), and a reaction initiated by iodine radical which was produced in situ at the anode was designed. The reaction was performed in an undivided cell under a constant voltage of 4 V at room temperature, using a C anode and a Pt cathode.
Scheme 4. Hydroxysulfonylation of α-CF3 Alkenes.
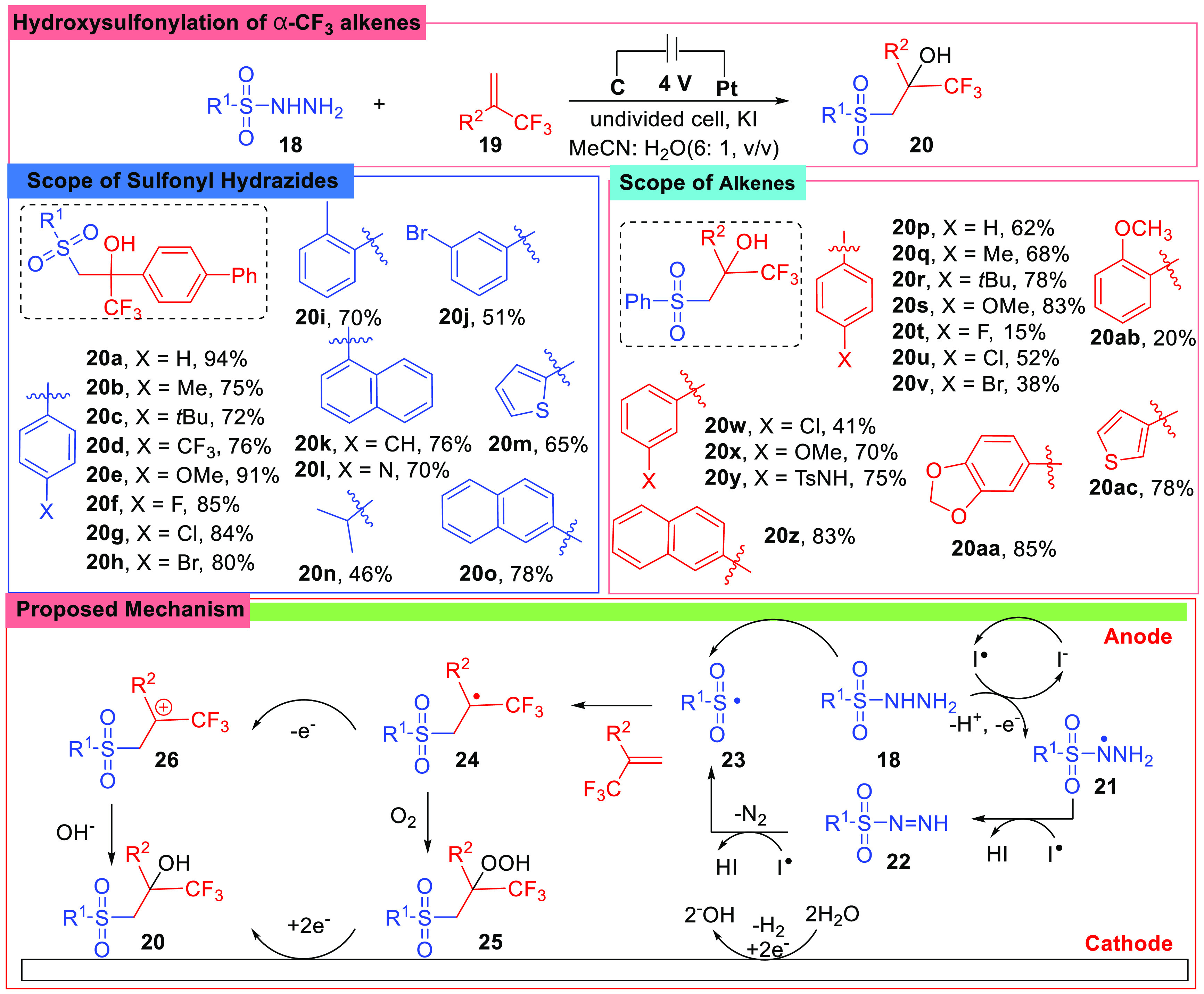
In order to gain a practical method, the researchers studied various factors, showing that when the Pt cathode was replaced with other materials, the yield dropped greatly, indicating that the Pt cathode was crucial to this method. The following investigation of the substrate scope gave a pleasant result showing that sulfonyl hydrazides (18) with either electron-poor or -rich groups on aryl rings worked well under the optimal conditions, while para-substituted hydrazides (20a–20h) worked better than other substrates. When different alkenes were adopted, electron-withdrawing groups (20t–20w) on the phenyl rings showed negative impacts on the yields.
The reaction mechanism was well studied, and various kinds of experiments were conducted. Cyclic voltammetry (CV) experiments on both substrates and KI demonstrated that the oxidation of KI took place first and initiated the transformation. What is more, O18-labeling experiments and D2O exchange proved that water in the system was not the only source of OH group in the product. Combined with radical trap experiments, a mechanism involving the generation of the iodide radical and sulfonyl radical at the anode was proposed. At the beginning, the sulfonyl hydrazide (18) reacted with an iodine radical, and the sulfonyl radical (23) was generated along with molecular N2 release at the same time, and then the sulfonyl radical (23) added to the alkene (19) yielded a carbon radical (24). In the following step, the carbon radical (24) could be converted into the desired compound through two pathways: (i) carbon radical reacted with the O2 and produced a peroxide species (25), which was then reduced at the cathode; (ii) the radical was oxidized at the anode and yielded a carbocation (26), which was trapped by the OH– from the water. Hence, the α-CF3 alkenes were transformed into α-trifluoromethyl-β-sulfonyl tertiary alcohols by a electrochemical technique for the first time.
In pharmaceutical chemistry, fluorination is an important strategy to optimize molecule structure, and it has always been an attractive topic.57,58 The Ye group reported the fluorosulfonylation of styrenes (28) with sulfonyl hydrazides (27) in 2021 (Scheme 5).59 They utilized the electrochemical method and chose Et3N·3HF as the fluorine source. Et3N·3HF also worked as an electrolyte together with (NH4)2HPO4. The reaction was carried out in an undivided cell charged with a carbon cloth anode and a platinum cathode accompanied by a constant current of 10 mA in CH3CN/CH2Cl2 (4:1, v/v). A subsequent substrates scope screen showed this system was effective with most common sulfonyl hydrazides and alkenes, and the reactions charging sulfonyl hydrazides with various substituents or styrenes bearing different groups on the aryl ring or adjacent to the double bond all gave desired products with medium to high yields, and an example exhibited feasibility with alkyl sulfonyl hydrazide (29i).
Scheme 5. Fluorosulfonylation of Styrenes.
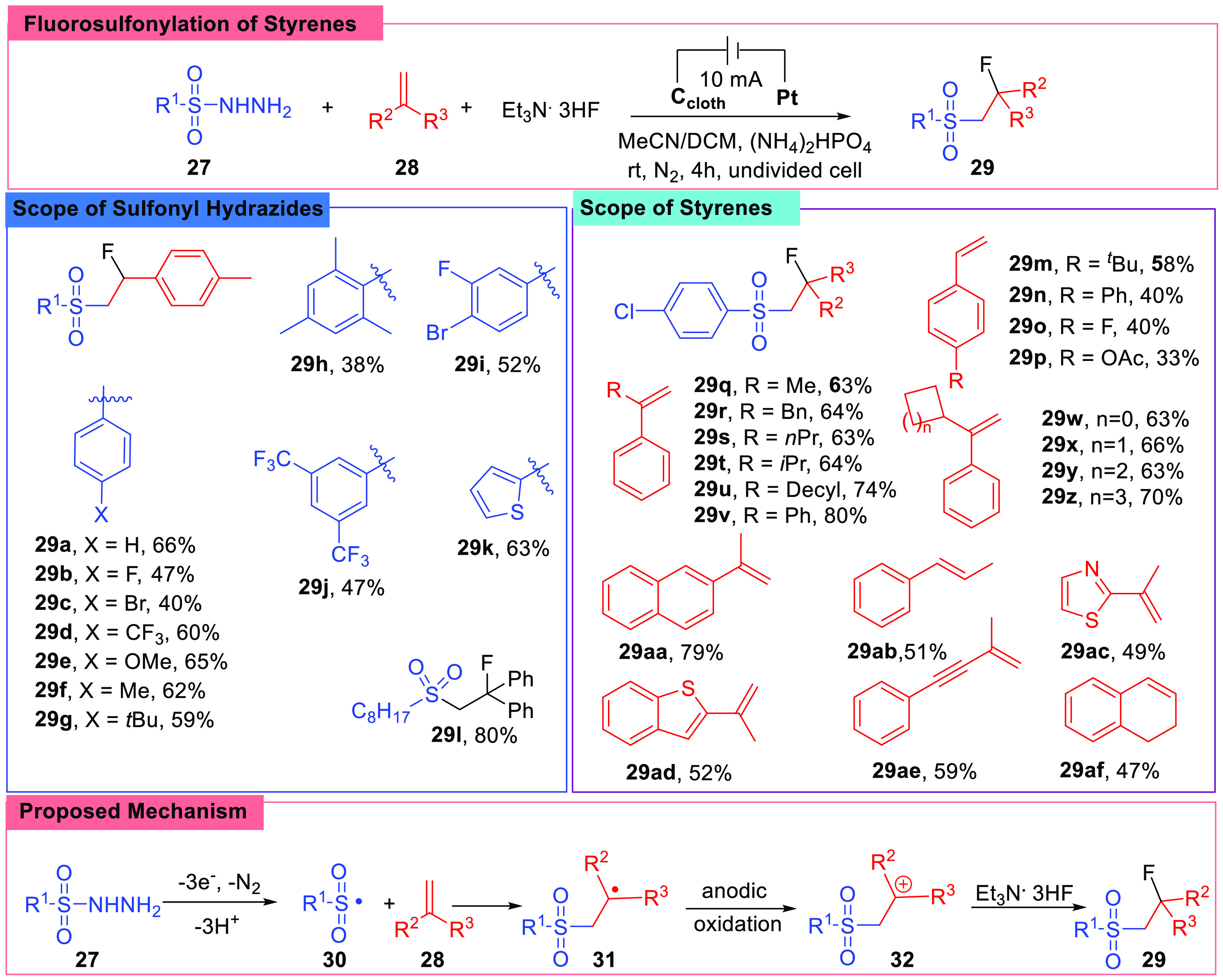
The authors designed several control experiments and conducted CV experiments to investigate the mechanism. Products of the radical rearrangement experiments and radical trap experiments confirmed the generation of sulfonyl radicals. Lastly, according to the CV experiments, it was suggested that the presence of (NH4)2HPO4 kept the styrene from being oxidized. Based on above results, the reaction procedure was concluded as shown in Scheme 5, the sulfonyl hydrazide (27) was activated at the anode, and the key intermediate was a carbon cation species (32).
Among sulfone-type compounds, β-keto-sulfones are a class of valuable chemical intermediates.60 In 2020, the Xu group reported the oxysulfonylation of alkenes toward β-ketosulfones (35) using sulfonyl hydrazides (33) and styrenes (34) with an electrochemical method (Scheme 6).61 To obtain the desired products, an undivided cell equipped with an RVC anode and a Pt cathode was employed. The reaction was conducted in CH3CN at room temperature under an air atmosphere, and nBu4NBF4 was used as the electrolyte. The substrates screen showed that the reaction system could work well with a number of styrenes, except those with electron-withdrawing groups (35j–35l). When styrenes with electron-withdrawing groups were charged, an atmosphere of oxygen was necessary instead of air (35j–35l).
Scheme 6. Oxysulfonylation of Alkenes Towards β-Ketosulfones.
The mechanism study showed a similar result with Yang et al.’s research,56 the reaction started with the generation of sulfonyl radicals (37), which underwent addition to styrene (38) double bonds. By analyzing the reaction conditions and results from substrates screen reactions, it indicated that the radical (39) produced in the addition step was then oxidized with oxygen to generate β-keto-sulfones (41) through a peroxyl radical (40) as the intermediate. Thus, a plausible mechanism was proposed as depicted in Scheme 6.
Radicals’ addition to alkynes is an important method to synthesize β-keto sulfones.62,63 Compared with traditional methods, electrochemical synthesis of β-keto sulfones might be efficient and green.62 In 2021, He and co-workers developed an oxysulfonylation of alkynes using sulfonyl hydrazides (42) and alkynes (43) (Scheme 7).64
Scheme 7. Oxysulfonylation of Alkynes.
The reaction was carried out in a CH3CN-H2O system in an undivided cell charged with a graphite rod anode and a Pt plate cathode, and nBu4NBF4 was selected as an electrolyte. The conditions were compatible with a broad substrate scope, and β-keto-sulfones could be produced with acceptable yields when choosing phenylacetylene-type compounds. It could be noted that phenylacetylene with electron-donating groups (44m–44p) on the benzene ring gave better results than those with electron-withdrawing groups (44q–44s), and aliphatic alkynes (44af, 44ag) were not suitable substrates for this reaction. On the other side, the reaction worked well with various kinds of sulfonyl hydrazides, either aryl or alkyl (44j) compounds.
The researchers conducted radical trap experiments, isotope labeling experiments, as well as CV experiments, to study the reaction mechanism. In the radical trap experiments, TEMPO, BHT and 1,1-diphenylethylene were used, and both sulfonyl radical and N-centered radical species were detected. Moreover, the isotope labeling experiments with H218O proved the source of the oxygen atom in the product, while the CV experiments explained the reaction procedure. On the basis of the above work, a plausible mechanism was reasonably proposed (Scheme 7).
Similarly, another example of alkoxysulfonylation of alkynes was reported by Zhang and co-workers, using alcohols rather than water (Scheme 8) as a nucleophilic reagent.65 They performed the reaction in an undivided cell and selected two graphite felt electrodes with a constant current of 5 mA and chose Et4NPF6 in CH3NO2 as the electrolyte system. The reaction system showed a robust application scope with various sulfonyl hydrazides (54a–54k), alkynes(54l–54aa), and alcohols (54ab–54aj). To gain a further understanding of the reaction, control experiments including sulfonyl radicals and vinyl radical intermediates trap experiments, and CV experiments were carried out. Combined with the DFT calculations, a mechanism involving the generation of sulfonyl radical (57) and the vinyl radical intermediate (58) was proposed as depicted in Scheme 8.
Scheme 8. Alkoxysulfonylation of Alkynes.

Meanwhile, an approach to the electrochemical oxidative selenosulfonylation of alkynes was also involved in the same report by Xu et al. (Scheme 9).61 In this protocol, sulfonyl hydrazides (62), alkynes (63), and diphenyl diselenide (64) were treated in an undivided cell charging a graphite anode and a Pt cathode, using nBu4NBF4 as a supporting electrolyte. The reaction had a broad scope of substrates, and most of the selected alkynes and sulfonyl hydrazides with aryl groups bearing either electron-donating or electron-withdrawing substituents were tolerated. In addition, aliphatic alkynes (65u, 65v) and sulfonyl hydrazides (65ag) were suitable substrates, although the aliphatic alkynes (65u, 65v) gave the desired products in lower yields. In the proposed mechanism, an alkenyl radical (68) was believed to be the key intermediate, which was converted into the product with two possible routes as is shown in Scheme 9, an atom transfer with the diphenyl diselenide (path a) or a radical coupling with the phenylselenium radical (70) (path b).
Scheme 9. Selenosulfonylation of Alkynes.

In 2021, Wang reported an electrochemical route to the iodosulfonylation of alkynes via a sulfonyl radicals’ addition to alkynes (Scheme 10).66 To achieve the target, the reaction was carried out in an undivided cell with two platinum-plate electrodes (10 mm × 10 mm × 0.1 mm) and constant current of 40 mA in a CHCl3-H2O (3:1, v/v) solution. The authors tried a broad scope of substrates, and it was found that reactions with most of the selected alkynes and sulfonyl hydrazides gave the desired products with good yields, except those bearing bulky (75g) or alkyl (75v) groups. With control experiments at hand, a mechanism involving a sulfonyl radical’s addition to a C≡C triple bond was proposed. The mechanism started with the generation of I radical at the anode, and then the sulfonyl hydrazide (73) was activated to generate a sulfonyl radical (78). After the sulfonyl radical’s addition to an alkyne (74), a carbon radical (79) was formed and then gotoxidated at the anode. Lastly, the carbon radical (79) was captured by an iodine radical, and the desired β-iodide sulfone (75) was achieved.
Scheme 10. Iodosulfonylation of Alkynes.

In 2019, Lee reported an electrochemical synthetic protocol for the sulfonylation of tertiary amines toward β-amido-vinyl sulfones, using sulfonyl hydrazides (81) and tertiary amines (82) as substrates (Scheme 11).67 To realize the protocol, the substrates were treated in an undivided cell equipped with a graphite anode and a Pt cathode with a constant current of 5 mA (6.2 F mol–1) in DMSO under an acidic condition (acetic acid). nBu4NBF4 (0.1 M) was selected as an electrolyte. In this research, some aryl hydrazides bearing various substituents including the electron-withdrawing groups (83e–83i) were tested and gave the desired products with good yields. In addition, dealkylation of tertiary amines was a competitive reaction.
Scheme 11. Sulfonylation of Tertiary Amines Towards β-Amido-vinyl Sulfones.

A plausible mechanism was proposed with results from CV experiments and control reactions. It was indicated that the key steps in this reaction involved sulfonyl radical’s addition to alkene, which was generated by the electro-oxidation of tertiary amines in situ. At the early stage, sulfonyl hydrazides (81) and tertiary amines (82) were oxidized at the anode and gave sulfonyl radicals (84) and alkenes (87) respectively, and then the addition took place and yielded carbon radical intermediates (88). Then the intermediates (88) underwent with an oxidation and elimination sequence and afforded the target compounds.
α-Substituted-vinyl-azides were widely used in organic synthesis and could be converted into various compounds.68 In 2020, the Terent’ev group reported the sulfonylation of vinyl azides, converting α-substituted vinyl azides (91) into sulfonyl enamines (92) by reacting with sulfonyl hydrazides (90) under electrochemical conditions (Scheme 12).69 The reaction was conducted in DMSO/THF (1:1, v/v) under constant current conditions (3.5 F/mol 1, I = 60 mA, j = 20 mA/cm2), and the authors chose NH4I as an electrolyte, a graphite anode, and a stainless steel cathode. To study the substrates scope, they tried different vinyl azides and sulfonyl hydrazides. It was found that vinyl azides with either electron-withdrawing or electron-donating substituents on the aryl rings could afford the desired compounds, and all tried sulfonyl hydrazides also showed good results. However, when substrates with electron-withdrawing groups were chosen, the yields dropped on different levels (92h, 92i, 92s). Moreover, the reaction conditions worked well with the methanesulfonyl hydrazide (92k).
Scheme 12. Sulfonylation of Vinyl Azides.
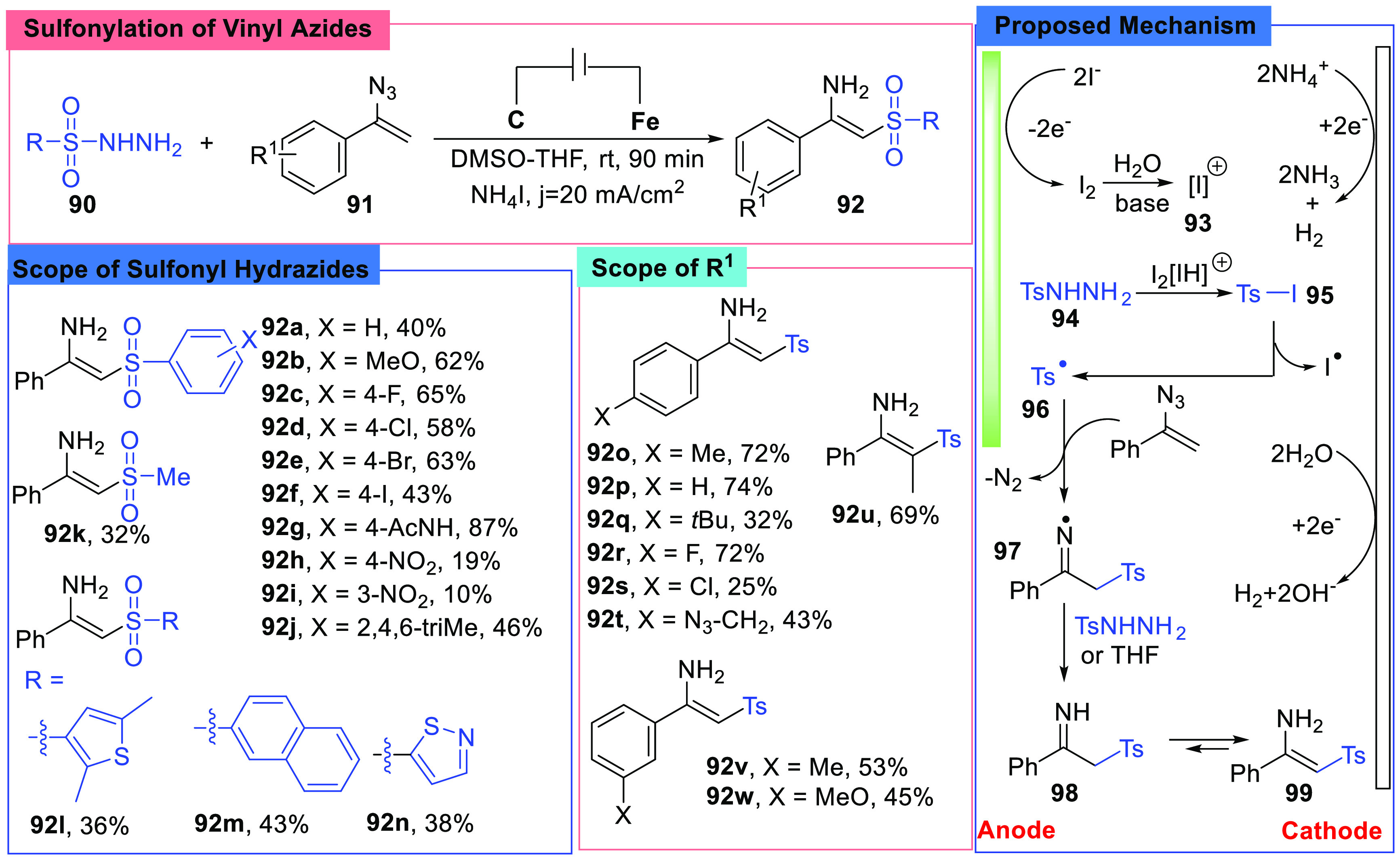
It was quite interesting to learn how the azide group was reduced. To study the mechanism, control experiments and CV experiments were conducted. From those experiments, it was found that the sulfonyl hydrazide (95) was activated by an iodide radical which was generated in situ at the anode, and then the azide group was reduced and an N-centered radical (97) was formed when the sulfonyl radical (96) added to the double bond. At the same time, hydrogen gas was produced at the cathode (Scheme 12).
A similar synthesis of sulfonyl enamines was reported by Chen in 2022 (Scheme 13).70 This sulfonylation of enamines was conducted under a constant current of 10 mA with LiClO4 as the electrolyte in a mixed solvent of CH3CN/H2O (10:1, v/v), using a carbon anode and a Pt cathode. Substrates with either electron-donating (102a, 102b, 102e, 102f) or electron-withdrawing groups (102c, 102e, 102g–102i, 102n–102s) on the aryl rings all gave corresponding products in medium yields. Meaningfully, products of this reaction were solvent-oriented; when AcOH/H2O (1:1, v/v) was used instead of CH3CN/H2O (10:1, v/v), β-ketosulfones were obtained as products. When conducted in CH3CN/H2O (10:1, v/v), aliphatic sulfonyl hydrazides (102u–102y) were also suitable for this protocol.
Scheme 13. Sulfonylation of Enamines.

The reaction was believed to be triggered by the sulfonyl radical (105), and a key intermediate of the carbon radical (107) was generated by the sulfonyl radical’s addition to a C=C double bond. Subsequently, the generated carbon radical (107) was oxidized at the anode, and then a sulfonyl enamine (108) was obtained if the cation was trapped by H2O. Otherwise, a β-ketosulfone (109) was obtained with loss of H+. This proposed mechanism was supported by CV experiments and control reactions.
In 2017, Terent’ev et al. synthesized vinyl sulfones via the sulfonylation of alkenes, choosing alkenes and sulfonyl hydrazides as substrates, in an undivided cell equipped with a graphite anode and an Fe cathode (Scheme 14).71 The reaction was performed in a THF-H2O (1:1, v/v) system at 30 °C under a constant current density of 60 mA/cm2. KI was selected as an electrolyte. To study the substrates scope, various kinds of sulfonyl hydrazides (112a–112f) and styrenes (112g–112u) were tested, and it was found that compounds containing either electron-donating or electron-withdrawing groups on the aryl rings could give the desired products.
Scheme 14. Sulfonylation of Alkenes Towards Vinyl Sulfones.
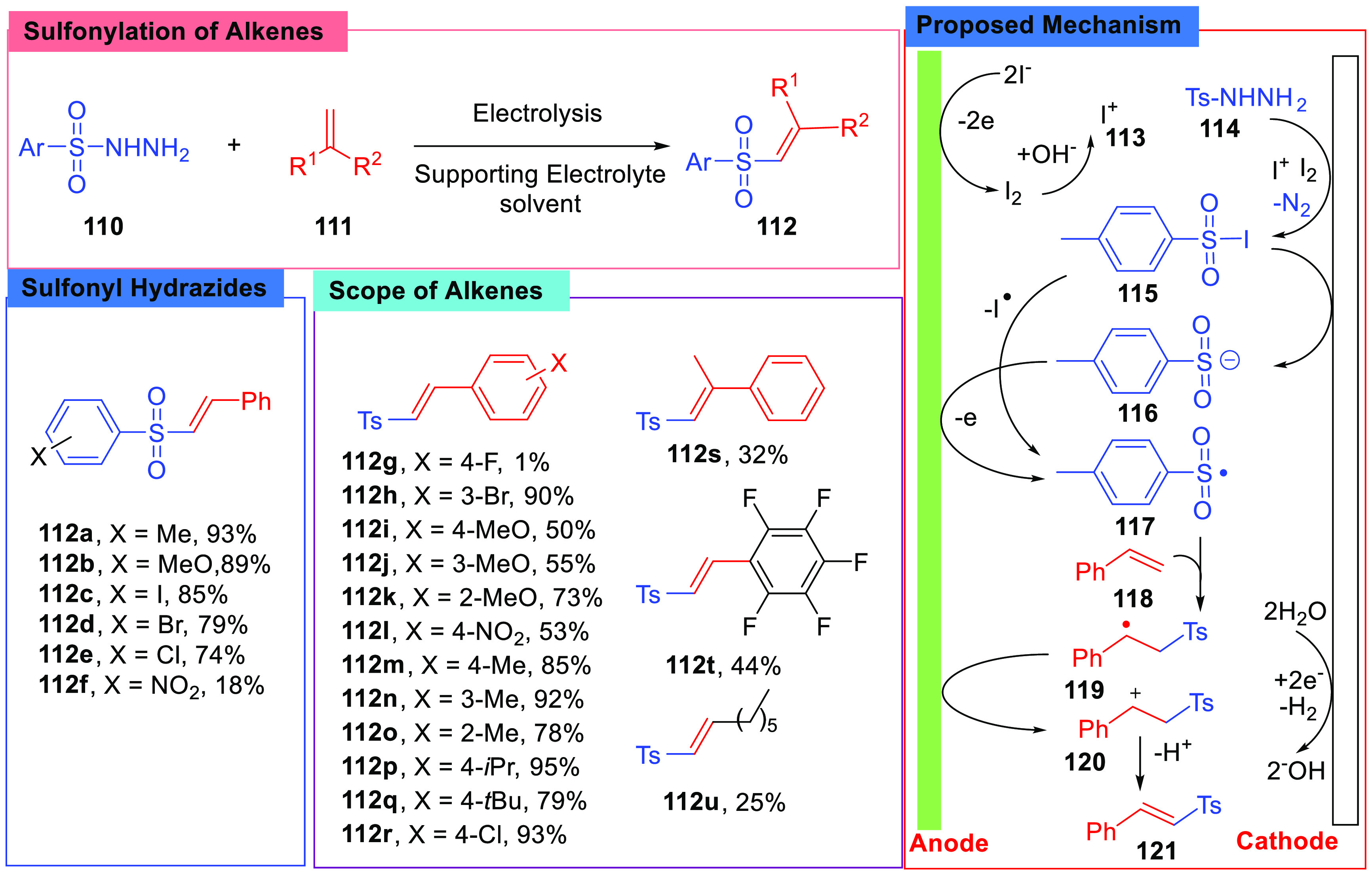
CV experiments showed that the C=C double bond in the product was formed via an addition–oxidation–elimination procedure. At the beginning, sulfonyl hydrazide (114) was activated at the anode by an iodide cation and yielded a radical species (117) at the anode; in this phase, the sulfonyl iodide (115) was proven to be a key intermediate. The radical (117) also could be gained from the sulfonyl iodide (115) via a sulfonyl anion intermediate (116). Then the radical (117) reacted with an alkene and gave a radical intermediate (119) which was then oxidized and produced a carbon cation species (120). The cation (120) might react with I– to give an iodide compound, which proceeded with the elimination of HI or loss of the H+ to produce vinyl sulfones.
Similar to the report of Terent’ev et al., a sulfonylation of alkenes in water was reported by Liao et al. in 2020 (Scheme 15).72 This reaction was conducted under a constant current of 40 mA in an undivided cell in a saturated (NH4)2CO3 solution, using Pt foils (1.0 × 1.5 cm2) as the anode and cathode, and 10 mol % n-Bu4NI was chosen as the catalyst. By screening a number of substrates, it was found that the reaction was compatible with a broad range of sulfonyl hydrazides (124a–124k) and styrenes (124l–124z) in good E selectivity (E/Z > 99:1). A gram-scale experiment was carried out, and the result showed a potential utility of this reaction.
Scheme 15. Sulfonylation of Alkenes in Water.
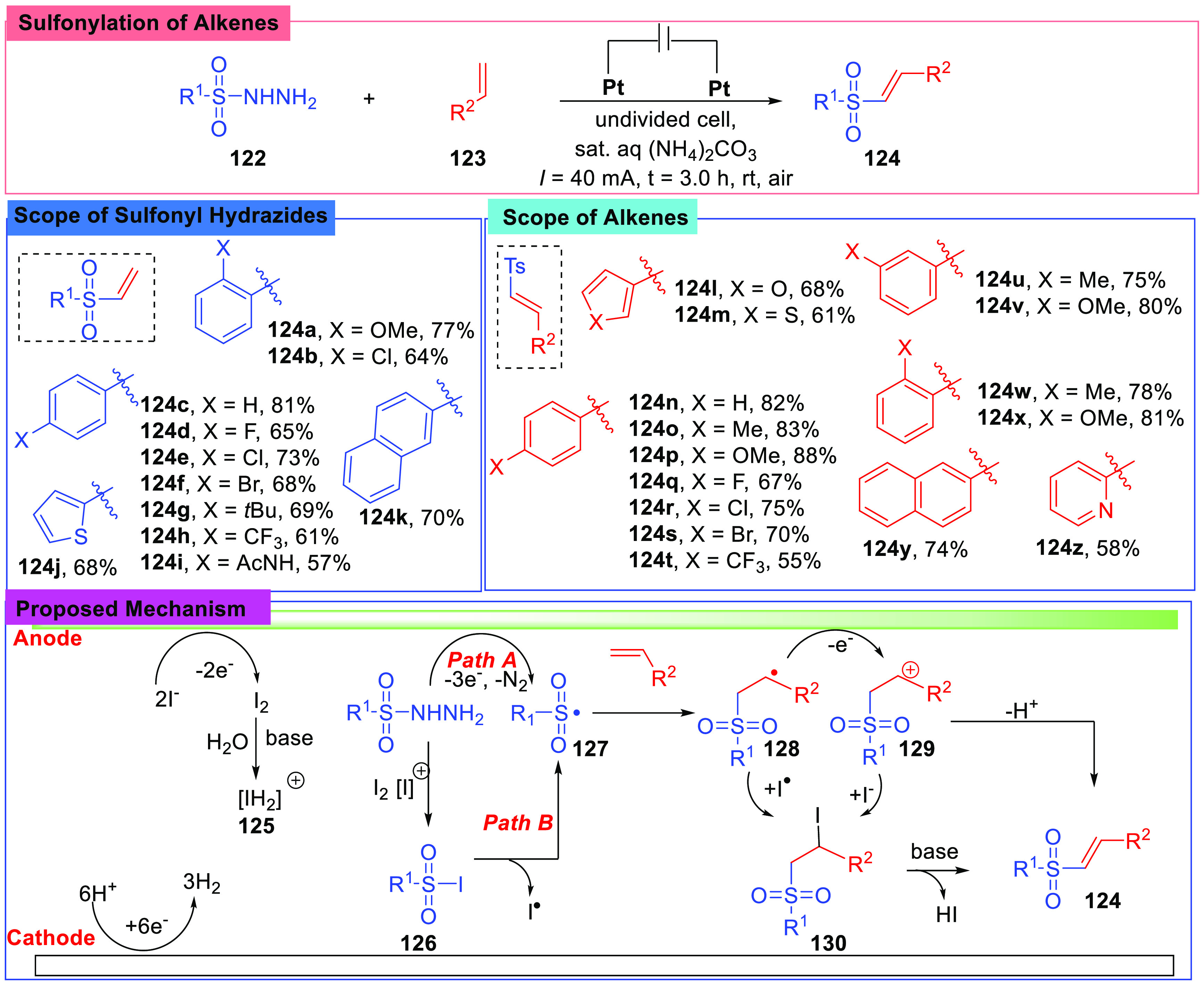
A mechanism was proposed based on the results of control experiments and CV experiments. In control experiments, the authors added radical inhibitors (BHT or TEMPO) to the system or chose presumed intermediates as reactants. Combined with CV experiments, a mechanism consisting of the generation of radical, C=C bond addition, free radical oxidation, and elimination was proposed. Sulfonyl hydrazides and styrenes were converted into vinyl sulfones via two paths. In the carbon cation path, sulfonyl hydrazides (122) were first activated by the base to give sulfonyl radicals (127), and then the generated sulfonyl radicals (127) added to styrenes; therefore, carbon radical intermediates (128) were yielded. Subsequently, the radical intermediates (128) got oxidized and underwent the loss of H+ or HI; in the free radical path, the sulfonyl hydrazides were oxidized by I2 to give sulfonyl radicals (127); after that, the generated sulfonyl radicals (127) combined with iodide free radicals and yielded species (130), and then the elimination of HI took place, and the target product was afforded.
Decarboxylation of carboxylic acids could be realized by electrochemical methods.73 Decarboxylative sulfonylation was an efficient strategy for the synthesis of vinyl sulfones when charging cinnamic acids as a starting material, and an electrochemical mode of such application was reported by Huang in 2017 (Scheme 16).74
Scheme 16. Decarboxylative Sulfonylation of Carboxylic Acids.
The reaction was performed with two Pt foil electrodes, nBu4NBF4 as the electrolyte in a solvent of DMSO under a constant current of 3 mA. In this reaction, t-BuOLi played an important role in facilitating the oxidation of sulfonyl hydrazides, and this was confirmed by CV experiments. Further studies on the reaction procedure with CV experiments showed that the sulfonyl radical (136) was generated with the aid of a base. It was also noted that the radical (136) added to a double bond at the neighbor position, yielding a carbon radical (138) as the key intermediate. The carbon radical (138) then underwent the oxidation followed by the release of CO2.
Alkynyl sulfones which showed excellent bioactivity in various fields could be obtained by the sulfonylation of alkynes.75,76 In 2019, Tang and co-workers reported a sulfonylation of alkynes using sulfonyl hydrazides (139) and terminal alkynes (140) as substrates (Scheme 17).77 The substrates reacted in an undivided cell equipped with a RVC anode (100 PPI, 1 cm × 1 cm × 1.2 cm) and a Pt plate cathode (1 cm × 1 cm) with a constant potential of 1.2 V (vs Ag/AgCl) under an O2 atmosphere, TBAI was selected as an electrolyte, and K2CO3 was used as an additive. By investigating various substrates, it was found that the reactions using most of the selected substrates gave desired products with acceptable yields except for alkyl sulfonyl hydrazides (141k) and alkynes with α-hydrogen atom (141v).
Scheme 17. Sulfonylation of Alkynes.
The authors performed control experiments [CV experiments and electron paramagnetic resonance (EPR) experiments] to study the reaction mechanism. Based on results from these experiments, it was concluded that alkynyl sulfones (141) could be produced through two pathways, and the reaction procedure involved the generation of sulfonyl radicals (146), addition to alkynes and the elimination of iodide. Besides, oxygen did play an important role in this mechanism (path A). Moreover, the authors also evaluated antitumor activity of several compounds (141f, 141n, 141r, 141t).
3. Application in Cyclization Reactions
Cyclization involving cascade reactions is widely used in the synthesis of compounds with complicate structures, and it has been used in various reactions.78,79 Radical-triggered cascade reactions80 were common in cascade reactions, and sulfonyl hydrazides have been good sources of free radicals when adopted under electrochemical conditions. In recent years, several annulation reactions using sulfonyl hydrazides and multifunctional arenes were reported.
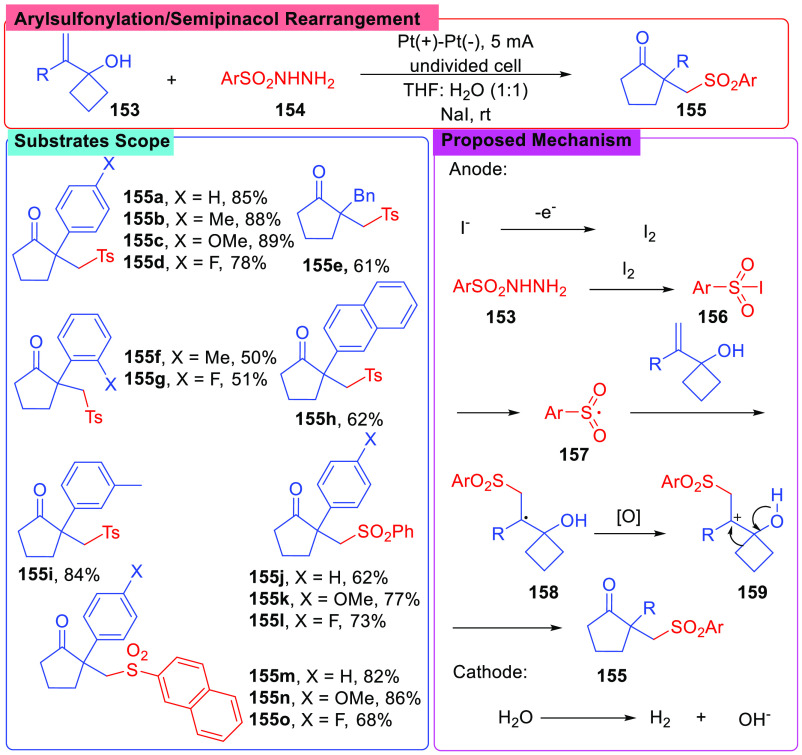
In 2019, an electrosynthesis involving radical arylsulfonylation/semipinacol rearrangement was reported by Kim and Kim81 (Scheme 18). In this method, alkenylcyclobutanols (153) and sulfonyl hydrazides (154) were used as substrates, and the reactions were conducted under a constant current of 5 mA with two Pt electrodes in a mixed solvent of THF/H2O (1:1, v/v), employing NaI as an electrolyte. A substrates screen showed that the reaction conditions were compatible with most of the selected compounds, containing either electron-donating or electron-withdrawing groups.
Scheme 18. Arylsulfonylation/Semipinacol Rearrangement of Alkenylcyclobutanols.
To gain a further understanding of the ring expansion, CV experiments and controlled reactions were conducted. These experiments showed that sulfonyl hydrazides were activated by I2, which was produced by anodic oxidation of I–, and a sulfonyl iodide (156) was formed as the key intermediate. Then a sulfonyl radical (157) was formed via the cleavage of S–I bond. After that, the sulfonyl radical (157) underwent the addition to a C=C bond, and a carbo-radical (158) was generated. It was presumed that the carbo-radical was oxidated to a carbo-cation (159) followed by a semipinacol rearrangement. Thus, the product was afforded through a ring expansion procedure.
Compounds with indolo-[2,1-a]-isoquinolines structures showed excellent bioactivity and attracted much attention from synthetic chemists.82−84 In 2020, Xia and co-workers developed an electrosynthesis route of indole derivatives choosing sulfonyl hydrazides (160) and 2-aryl-N-acryloyl indole derivatives (161) as substrates (Scheme 19).85 To achieve the goal, the reactions were carried out in an undivided cell charged with a Pt anode and a Pt cathode under a constant current of 10 mA in a mixed solvent of THF/H2O (3:1, v/v), and KI was used as an electrolyte. The reactions were conducted at room temperature under an air atmosphere. Reactions with compounds (162o–162v) bearing different groups including either electron-donating or electron-withdrawing ones at C-5 of the indole ring all gave desired compounds. In addition, substituents bearing various groups at C-2 of indoles (162w–162ac) could be transformed under the reaction conditions; alkyl sulfonyl hydrazides (162m, 162n) were suitable starting materials, too. Moreover, when KBr was used instead of KI, the corresponding brominated products (162ai–162am) were obtained.
Scheme 19. Cyclization of 2-Aryl-N-acryloyl Indoles.

It was predicted that the reaction proceeded with an addition–addition–oxidation–elimination process. To prove that, control experiments and CV experiments were carried out. With those experiments, potential intermediates were determined, and a plausible mechanism was proposed. In this procedure, a sulfonyl radical (164) was first generated at the anode and added to the benzene ring to form a multicyclic structure as a carbo-radical (167) through a 6-endo-trig cyclization. Then the radical intermediate (167) was transformed into a cation species at the anode (168). Lastly, a multicyclic compound was formed with the release of H+.
In 2020, an annulation-halosulfonylation of 1,6-enynes toward the synthesis of 1-indanones was reported by Jiang et al. (Scheme 20).86,87 To construct the pentane core, the Jiang group selected 1,6-enynes (170) and sulfonyl hydrazides (171) as substrates. This reaction was carried out in an undivided cell charged with a Pt anode and a Pt cathode under a constant current of 10 mA at room temperature in an air atmosphere, and it was found that a mixed solvent of THF/H2O was essential. Both NaI and NaBr were good electrolytes, and iodo- or bromo- groups were introduced, respectively. To study the effect of substituents on the aryl ring or other sites, a variety of compounds were tried under the conditions, and all reactions gave 1-indanone derivatives with acceptable yields and good E/Z selectivity.
Scheme 20. Annulation-Halosulfonylation of 1,6-Enynes.
The mechanism of this reaction was also investigated. Results of control reactions showed that the sulfonyl radical (174) which triggered the reaction was produced by the oxidation of sulfonyl hydrazides by I+, and the I+ was generated from an I– by the anodic oxidation. The sulfonyl radical added to the double bond and formed a radical species (177), which underwent addition to the triple bond. Thus, the indanone structure was formed as a vinyl radical (178), and then the radical (178) was oxidized and captured by I– or reacted with Ts-I to give the product.
Using 1,5-enyne derivatives instead of 1,6-enynes, Jiang’s group developed annulation-iodosulfonylation of 1,5-enynes (180) toward (E)-spiro-indenes in 2020 (Scheme 21).88 1,5-Enyne derivatives (180) bearing para-quinone methide were treated with sulfonyl hydrazides (181) under electrochemical conditions, and (E)-spiroindenes were obtained. The authors chose an undivided cell equipped with two Pt electrodes and employed KI as an electrolyte. Moreover, the reactions were conducted with a constant current of 20 mA in the open air. By charging different 1,5-enynes with various groups adjacent to the triple bond, it was found that the aryl substituents were necessary for this reaction. However, aliphatic groups (182y, 182z) could not give desired products.
Scheme 21. Annulation-Iodosulfonylation of 1,5-Enynes Towards (E)-Spiro-indenes.
It was proposed that the sulfonyl hydrazide was activated by I+ which was generated by anodic oxidation of I– and yielded a sulfonyl radical (185). Then the radical (185) was added to the double bond and gave a para-quinone radical (187, 188), which was reduced to a phenol anion (189). Next, the anion (189) reacted with I+ to form an iodonium ion (190), which underwent an intramolecular cyclization subsequently through a 5-exo-dig mode, which determined the E/Z selectivity.
Benzoxazines are usually synthesized by transition metal catalysis, Lewis base catalysis, or photoinduced cyclization, and chemists have tried to apply electrochemical methods.89−91 In 2020, Huang et al. developed a cascade protocol for the synthesis of benzoxazines (Scheme 22).92 Sulfonyl hydrazides (192) and acetyl amino-styrenes (193) were chosen to react in an undivided cell equipped with a carbon rod (ϕ = 5 mm, immersion length: 1.5 cm) anode and a Pt foil (1 × 1.5 cm2) cathode with janode = 0.19 mA cm–2. This reaction was conducted in a CH3CN solution with nBu4NBF4 as an electrolyte at room temperature under N2 atmosphere. Various styrenes and sulfonyl hydrazides were tested, and it was found that sulfonyl hydrazides bearing hydroxy (194l) or amino (194m) groups on the aryl ring were not suitable for this reaction, and other selected compounds, including those bearing either electron-donating or electron-withdrawing groups on the acyl sites, all afforded the desired product.
Scheme 22. Annulation-Sulfonylation of Acetyl Amino-styrenes.

This protocol was believed to involve a free radical triggered cascade process, which was confirmed by control reactions including the addition of a radical inhibitor (BHT) to the system. Combined with results from CV experiments, a plausible mechanism involving an addition–oxidation–cyclization–elimination procedure was proposed. The sulfonyl hydrazide (195) was converted into a sulfonyl radical (197) at the anode, and then added to the double bond on the styrene, and a radical intermediate (198) was formed. Next step was the formation of a carbon cation (199) by oxidation of the radical (198) mentioned above at the anode, and the cation (199) then underwent a cyclization and followed by the elimination of H+. Thus, the benzoxazine was constructed in one pot with an efficient and green method.
In some cases, compounds containing the 2-vinylbenzoic acid motif were good building blocks and could be transformed into many meaningful molecules.93 Recently, in 2022, Chen et al. reported an annulation-sulfonylation of 2-vinylbenzoic acids (202) under electrochemical conditions via a lactonization process (Scheme 23).94 In contrast to previous reports with sodium sulfonates as the sulfur source, sulfonyl hydrazides were used here. The reaction was performed in an undivided cell with two electrodes with a constant current of 12 mA in a solvent of CH3CN/H2O (10/1, v/v) under an N2 atmosphere, LiClO4 was selected as the electrolyte, and AcOH was chosen as the additive. To investigate the substrates scope, the effects of groups adjacent to the C=C double bonds were studied, and aromatic substrates all worked well. However, aliphatic acid derivatives (203aa, 203ab) did not work. Sulfonyl hydrazides were also tested, and it was found that bulky groups (203m, 203n) on the aryl ring might inhibit the reaction.
Scheme 23. Sulfonylation-Lactonization of 2-Vinylbenzoic Acids.
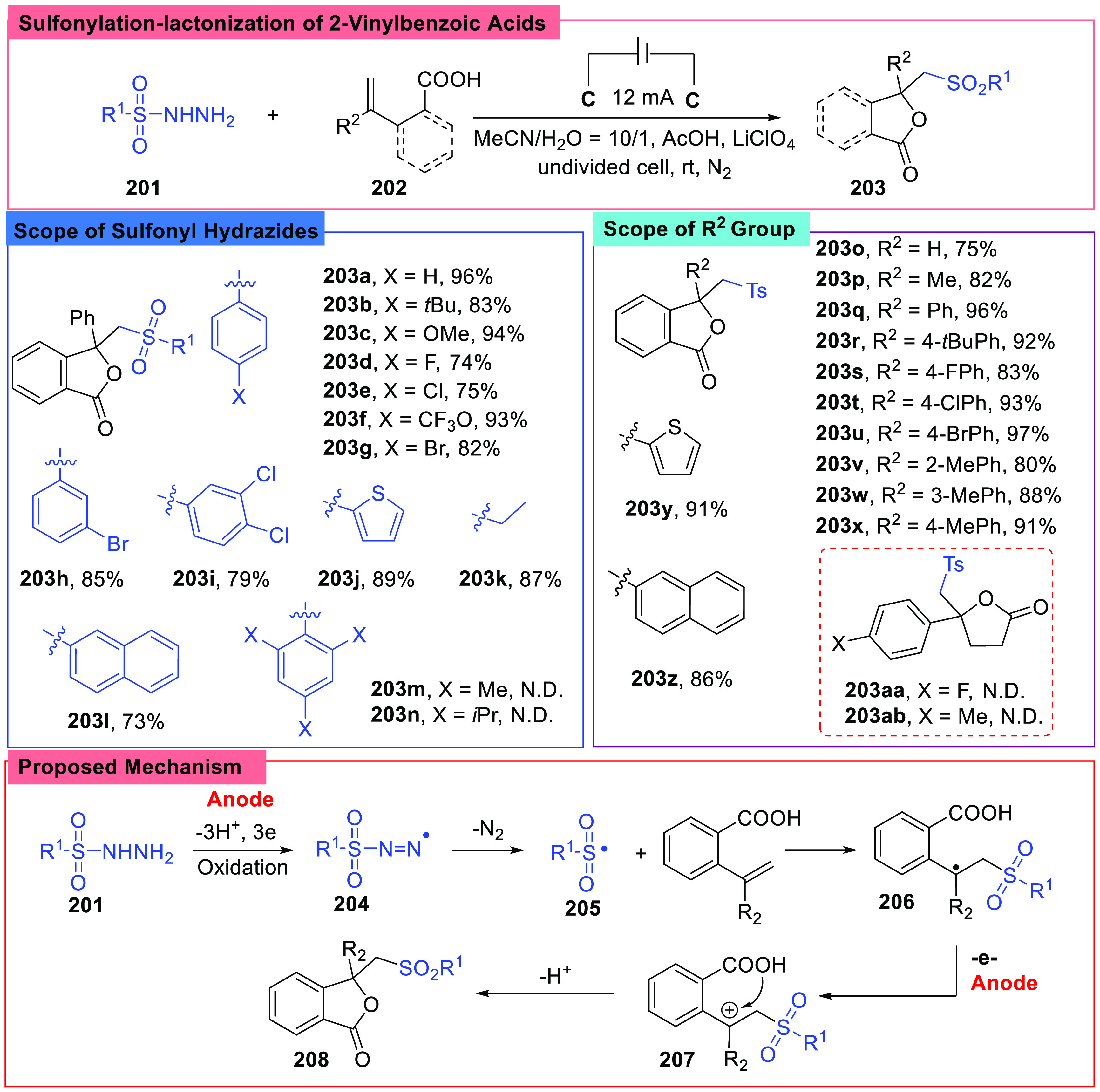
Control reactions and CV experiments indicated that sulfonyl hydrazide was directly oxidized at the anode and generated a sulfonyl radical (205), and then the radical was added to a double bond and gave a radical species (206). The radical (206) was converted into a cation (207), which was then captured by the −OH group and formed a phthalide with the elimination of H+.
The synthesis of heterocycles has always been a popular topic, and it was efficient and applicable to prepare them with cascade reactions.95,96 Synthesizing quinolines with electrochemical methods has attracted the attention of chemists.97 Very recently, in 2022, Zhang and co-workers reported a synthesis of sulfonylated quinolines under electrochemical conditions with sulfonyl hydrazides through a cascade process (Scheme 24).98
Scheme 24. Electrosynthesis of Sulfonylated Quinolines.
The two substrates were treated in an undivided cell equipped with two graphite felt electrodes, Et4NPF6 and nBu4NBF4 as mixed electrolytes under a cell voltage of 3.9 V. The authors chose CoCl2 as an additive and HFIP as a solvent, and this was considered to promote the transformation. The reaction conditions were compatible with a broad range of substrates, and some sensitive groups (211p, 211q) were also tolerated.
A plausible mechanism was proposed based on CV experiments, control reactions, and DFT calculations. The reaction was believed to start with the activation of sulfonyl hydrazides, and then the sulfonyl radical (214) was added to the triple bond and gave a vinyl radical (216), which was then oxidized and generated a carbocation (217). The cation (217) was trapped intramolecularly by the nitrogen atom with the loss of H+ and yielded a cyclic intermediate (218), which then underwent a ring opening step accompanied by the release of CO2 and gave the desired product (219).
Continuous-flow reactions have been applied in synthetic chemistry for a long time, and the nature of electrochemical synthesis makes it suitable to be conducted in a continuous-flow mode and has shown advantages over reactions in batch mode.99−102 A continuous electrochemical synthesis of sulfonylated isoquinoline-1,3(2H,4H)-dione compounds was reported by Guo and co-workers in 2020 (Scheme 25),103 using a flow electrolytic cell charged with a graphite plate anode and a platinum plate cathode under a constant current of 15 mA. Compared with reactions in batch mode, the flow mode required a lower load of sulfonyl hydrazides and shortened the reaction time with a residence time of 1 min. Interestingly, when conducted in 5 or 10 mmol scale, the flow mode gave the desired product in higher yields (79% vs 52% in 5 mmol scale, 78% vs 44% in 10 mmol scale). The reaction conditions were compatible with most of the common functional groups on the rings of two substrates, e.g., 4-nitrobenzenesulfonyl hydrazide (222z). Control reactions indicated that the sulfonyl radical (224) was generated at the anode and added to the double bond of the acrylamide (220), and a carbon radical (225) was yielded. The radical (225) center then underwent cyclization with the aryl ring. In the last stage, the desired product was obtained resulting from the loss of electrons and H+.
Scheme 25. Annulation-Sulfonylation of N-Alkyl-N-methacryloyl Benzamides in Flow Mode.
In 2021, an interesting application of sulfonyl hydrazides in the electrochemical synthesis of cinnolines was reported by Guo et al. (Scheme 26).104 Sulfonyl hydrazides adopted here formed the cinnoline core together with alkynes by the release of SO2. The protocol consisting of two steps in one pot was carried out in an organocatalytic electrochemical mode, using phenothiazines (231) as a catalyst. In the first step, the sulfonyl hydrazides condensed with the keto group of alkyne derivatives and yielded hydrazone type intermediates, and then the electrochemical conditions (carbon cloth anode, platinum plate cathode, nBu4NBF4, 10 mA) were introduced.
Scheme 26. Electrosynthesis of Cinnolines.

The authors performed isotope labeling experiments, CV experiments, control reactions, and EPR tests to study the reaction process, especially the electrochemical part. It was proposed that the catalyst was oxidized at the anode first, and then the hydrazone (234) was oxidized by the activated catalyst and yielded an N-centered radical (236), which then added to the triple bond and formed a bicyclic radical (237). The radical (237) then underwent an aryl group migration and got trapped by O2 after the release of SO2, and the O2-trapped species (240) then became an N-centered radical (241) with the loss of an O2. The N-centered radical (241) formed the cinnoline core (242) by an intramolecular annulation and then was transformed into the desired product with the aid of another activated catalyst. The catalyst in this mechanism forms a recycle process between activated and unactivated states at the anode.
4. Sulfonylation of Heterocyclic Compounds
Sulfonylation of heterocyclic compounds is important and common in organic chemistry and is usually achieved by employing sulfinic acids and their derivatives, which usually require transition metals or a large number of oxidants.105,106 However, those reagents may lead to environmental problems and can be hazardous to body health. Hence, chemists have been seeking for more effective methods, and there have been several reports on sulfonylation of heterocyclic compounds with electrochemical methods.107−110 In 2018, Lei’s group developed the sulfonylation of arenes/heteroarenes without the application of external oxidants (Scheme 27),111 and sulfonyl hydrazides (245) and benzofurans (246) were chosen as substrates. These compounds reacted in an undivided cell charged with a graphite rod anode and a nickel plate cathode. This reaction was carried out in a mixed solvent of CH3CN-H2O with nBu4NBF4 as electrolytes and K2CO3 as additives. The subsequent investigation of substrates scope revealed a complex profile of substituent effects, and it was found that groups at the C-3 site were favorable to this reaction. Most of the selected compounds bearing various substituents at the C-3 position afforded desired products. When C-2 substituted compounds were suitable for the conditions, C-3 sulfonylated products (247y) were obtained. The authors also adopted other heterocyclic and aryl compounds, such as electron-rich thiophenes (247ab), pyrroles (247ad), naphthalene (247af), and 1,3,5-trimethoxybenzene (247ag, 247af), and achieved products in acceptable yields. However, indole and its derivatives were not suitable for this reaction.
Scheme 27. Sulfonylation of Arenes/Heteroarenes.

A plausible mechanism was proposed based on data from radical-trapping experiments and CV analysis. Radical-trapping experiments indicated that a free radical was involved in this reaction. It was concluded that the sulfonyl hydrazide was transformed into a radical species (249) at the anode with the presence of a base, and the radical (249) reacted with an aryl ring to give a radical intermediate (251), and then the radical intermediate (251) was oxidized and a carbon cation was formed. Then the cation underwent the loss of H+ and yielded the sulfonylated product.
Heterocyclic N-oxides were widely used in organic reactions,112 and sulfonyl derivatives sometimes could work as alkylation reagents.113,114 In 2019, the Lei group reported an important study on the arylation of quinoline N-oxides at the C-2 site with an electrosynthetic method (Scheme 28).115
Scheme 28. Deoxygenative C2 Arylation of Quinoline N-Oxides.

In contrast with usual electrochemical reactions, the formation of the product involved both anodic oxidation and cathodic reduction in one cell. In this reaction, sulfonyl hydrazides served as aryl free radical precursors and reacted with quinoline N-oxides (254) in an undivided cell under a constant current of 24 mA. The reaction conditions included a graphite anode and a Pt cathode, nBu4NBF4 as electrolytes, and a mixed solvent of CH3CN-HFIP (9:1, v/v). Various sulfonyl hydrazides and quinoline N-oxides were tried, and it was found that aryl sulfonyl hydrazides containing alkyl- (255e, 255f) or halo- (255g–255i) substituents gave desired products in higher yields than other sulfonyl hydrazides. Moreover, sulfonyl hydrazides with 2-thienyl (255b) or 3-pyridyl (255c) groups were compatible with this condition, but the aliphatic compound (255l) did not work. Quinoline N-oxides bearing various groups at different sites all afforded desired compounds with medium yields, however, isoquinoline N-oxide could not give product under the conditions.
Control experiments showed that the oxidation of sulfonyl hydrazides took place at the anode and generated an aryl free radical (257) with the release of N2 and SO2, and then the radical (257) added to the quinoline at the C-2 site, and an N-oxide radical (259) was formed. The radical (259) was reduced at the cathode and yielded an N-oxide anion (260), which then captured a proton and underwent the elimination of H2O.
Functionalization of indazoles could be realized by electrochemical methods.116 De Sarkar and co-workers developed an electrochemical protocol for the sulfonylation of N2-aryl 2H-indazoles (263) at the C-3 site in 2020 (Scheme 29).117
Scheme 29. Regioselective Sulfonylation of N2-Aryl 2H-Indazoles.
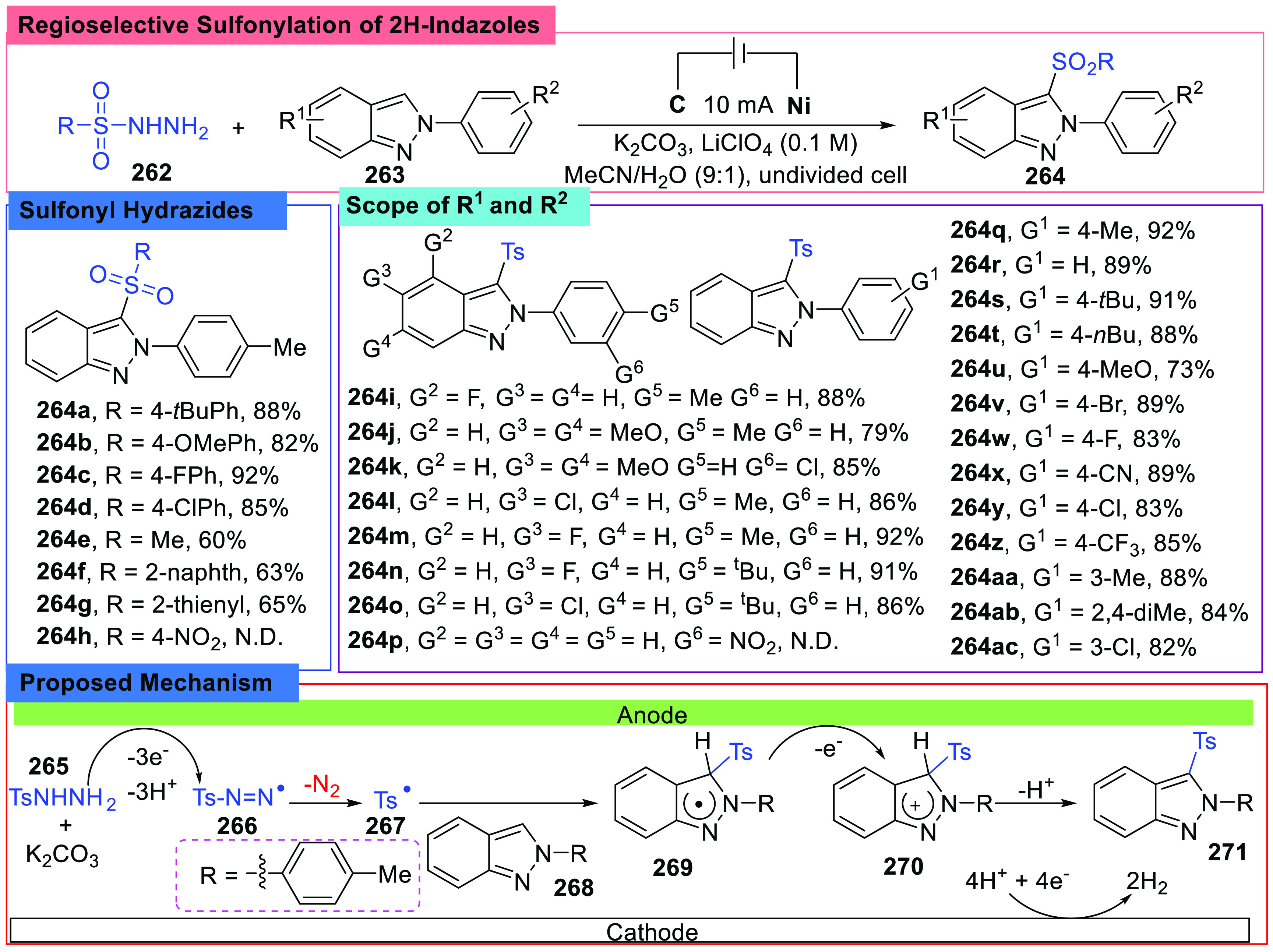
The protocol was realized by employing an undivided cell, using a graphite anode and a Ni foam cathode with a constant current of 10 mA, and LiClO4 was chosen as an electrolyte. This reaction was conducted in a mixed solvent of CH3CN/H2O (9:1, v/v), and K2CO3 was used to provide a basic condition. By adopting various substrates with different substituents, it was found that aryl sulfonyl hydrazides bearing electron-withdrawing or electron-donating groups at the N2-aryl ring or indazole core all afforded desired products. However, compounds with a nitro group (264h, 264p) on the aryl ring at the N2-site did not work under such conditions. Meanwhile, reactions with different sulfonyl hydrazides also gave similar results, and methanesulfonyl hydrazide (264e) was suitable for this reaction, too.
Radical trap reactions and CV experiments were also conducted, and a plausible mechanism was proposed. The key step in this reaction was the addition of a sulfonyl radical (267) to an indazole (268) at the C-3 position, and then oxidation of the addition intermediate (269) occurred, followed by the elimination of H+. In addition, H2 was yielded at the cathode as a byproduct.
Compounds with an indole core are always under focus. However, because of the high activity of the indole ring, it was not easy to handle indole derivatives under some conditions. Oxidative coupling is an effective method of indole functionalizations, especially those under electrochemical conditions. In 2019, an electrochemical coupling of indoles (272) and sulfonyl hydrazides (273) was reported by Pan et al. (Scheme 30).118 A sulfonyl group was introduced to the C3 site, and a sulfonyl hydrazide group was introduced to the C2 site at the same indole ring in one step. To realize this bifunctionalization of the indole ring, the substrates were treated in an undivided cell charged with a Pt anode and a graphite cathode. After testing various materials, NH4Br was found to be optimal in this reaction, working as an electrolyte. The authors tried various sulfonyl hydrazides and indoles bearing several classical groups, and most of the selected examples showed acceptable results and gave desired compounds with from medium to high yields.
Scheme 30. Sulfonylation and Hydrazination of Indoles.
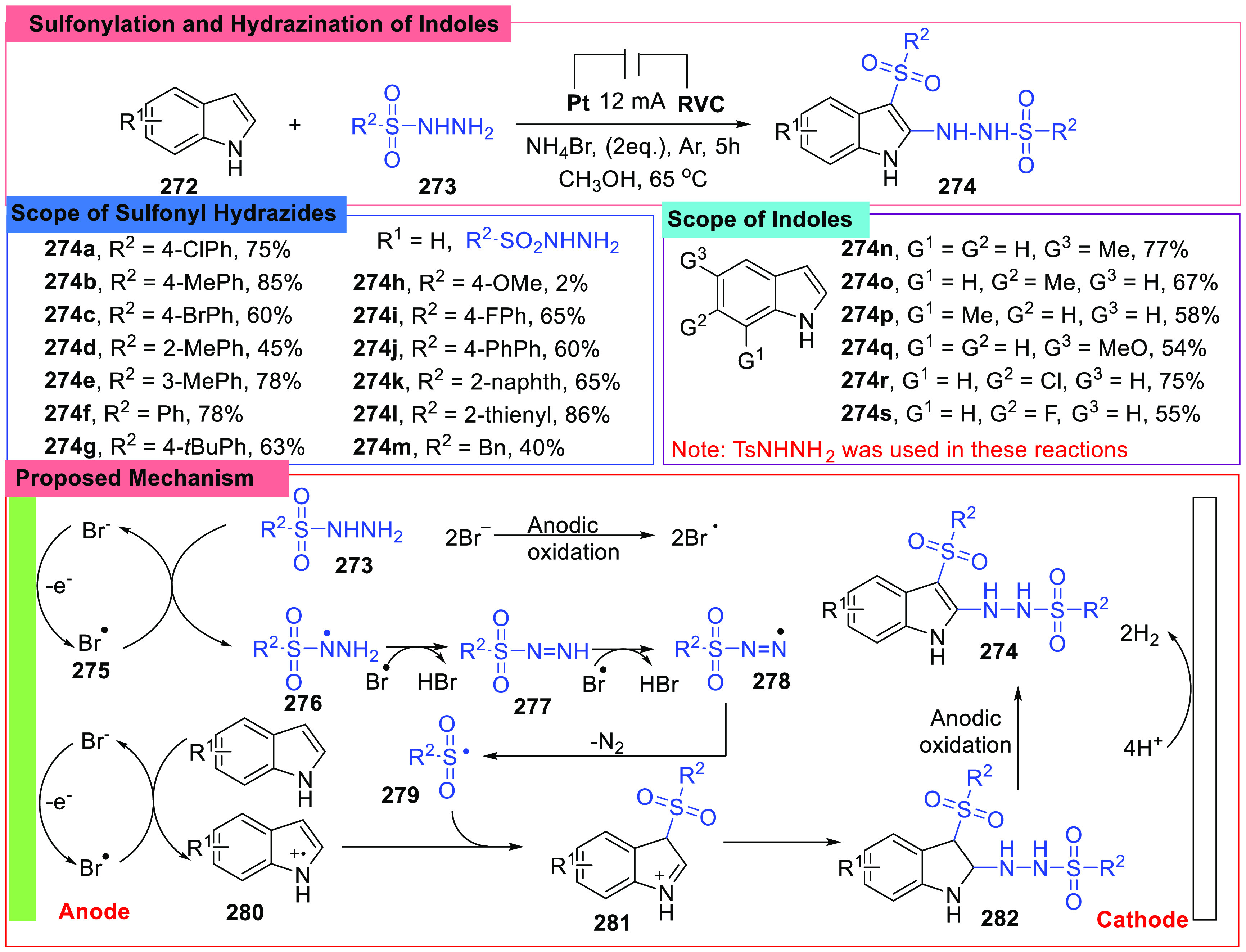
The structure of desired compounds also demonstrated the general mechanism proposed by the authors and other researchers (Scheme 30), especially the process involving the oxidation of sulfonyl hydrazides. Control reactions and CV experiments showed that sulfonyl hydrazide was activated by Br•, which was generated by oxidation of Br– at the anode, and then the hydrazide radical (276) reacted with Br• twice and gave a sulfonyl radical (279). The indole compound was also oxidized by Br•, and a 3-indolyl radical (280) was formed. In the last stage, two active species got coupled, and the desired product was obtained. Meanwhile, H+ was reduced at the cathode.
Due to their unique structure, xanthenes and their derivatives were widely used in chemical research, and recently, they were involved in some C–H activation studies,119,120 especially several electrochemical examples,121−123 and an electrochemical sulfonylaztion of xanthenes (284) at the C-9 position using sulfonyl hydrazides as sulfonating reagent was developed by Mo and co-workers in 2022 (Scheme 31).124 This oxidative C(sp3)-H activation was realized by charging a carbon rod anode and a platinum plate cathode under a constant current of 15 mA, using nBu4NI as an electrolyte, as well as a mediator. To handle the acidic proton at C-9, a base was necessary, and MeONa was found to be optimal. Further study showed that the reaction was compatible with a variety of substrates, and aryl sulfonyl hydrazides bearing different substituents all gave the desired products. It should be noted that some experiments (285f–285i) gave better results using Cs2CO3 instead of MeONa. BnSO2NHNH2 and thiophene-2-sulfonyl hydrazide were also tested, but they did not work. When different xanthenes were adopted, most of the selected substrates worked well.
Scheme 31. Sulfonylation of Xanthene Derivatives.

It was believed that the two substrates were coupled in radical form, and two radicals were generated through different pathways. Xanthenes (289) first lost the C-9 proton by reacting with the base, and then the xanthene inions (290) were oxidized and gave radicals (291) by the loss of electrons. The sulfonyl hydrazides were converted into radicals (288) with the aid of iodine radicals, which were formed in situ at the anode. Then the radicals (288 and 291) combined and afforded products. Moreover, the proposed mechanism was demonstrated by control reactions and CV experiments. Some samples made with this method were tested in research on anticancer bioactivity and gave good results.
5. Formation of Sulfur-Heteroatom Bonds
Sulfonic derivatives such as sulfamides, thiosulfonates, and sulfonic esters are a class of common and important compounds in medicinal chemistry, and these compounds are mainly obtained from sulfonic acids or sulfonic chlorides. Besides, sulfonyl hydrazides were also good building blocks in synthesizing sulfonic esters or sulfamides, and electrochemical methods have been applied in preparing these compounds.
In 2016, O. Terent’ev et al. developed an electrochemical approach to the synthesis of sulfamides by the condensation of sulfonyl hydrazides with amines (Scheme 32).125 This method was realized in an undivided cell equipped with an Fe cathode and a graphite anode in a mode of constant current. To investigate the effect of the electrolyte, a variety of salts were tested, and several of them gave satisfactory results. Among electrolytes (KI, NaI, NH4I, KBr, NaBr, NH4Br, and NH4Cl) that worked well, NH4Br, and NH4Cl gave the best results. Moreover, the researchers found that when using primary amines (295g, 295h), a three-fold excess of amine was crucial to get the desired products.
Scheme 32. Electrosynthesis of Sulfamides.
Amino compounds could be prepared from aryl thiols and sulfonyl hydrazides via many ways, and tertiary amines sometimes could be used to introduce amino groups.126 Application of tertiary amines could promote the tolerance of substrates bearing functional groups sensitive to secondary amines and might avoid some side reactions. In 2017, Sheykhan reported the synthesis of sulfamides, utilizing tertiary amines (297) as sources of amino groups (Scheme 33).127
Scheme 33. Dealkylative Sulfonylation of Tertiary Amines.

The reactions were conducted in an undivided cell charging two graphite electrodes, and Na2SO4 was selected as an electrolyte. It was notable that the material of electrodes used here was the same as used in a pencil and was renewable. The synthetic method was compatible with both two substrates bearing common functional groups. Besides, sulfonyl chlorides were also tested and gave the desired products. Mechanism investigation showed that the tertiary amine (299) was oxidized and gave secondary amine (301) via an iminium ion intermediate 300), and the sulfonyl hydrazide was converted into an active species (302, 303) at the anode.
In 2018, Chen and co-workers reported the synthesis of thiosulfonates with an electrochemical method (Scheme 34).128 The authors used an RVC anode and a Pt cathode in an undivided cell and chose NH4I as the electrolyte, and conducted the reactions in a mode of constant current of 10 mA. With this method, sulfonyl hydrazides (306) and aryl thiols (307) could be converted into thiosulfonates (308), and substrates with various substituents on aryl rings all worked well, giving products in good yields. Furthermore, aliphatic thiols (308z, 308aa) could work well under the conditions, but aliphatic sulfonyl hydrazide (308n) did not. Control reactions and CV experiments indicated that mercaptans and sulfonyl hydrazides were oxidized at the anode and yielded corresponding radicals (313, 314), and then thiosulfonates were formed by the coupling of two radicals.
Scheme 34. Electrosynthesis of Thiosulfonates.

Another synthesis of thiosulfonates was reported by Terent’ev et al. in 2019 (Scheme 35).129 Aryl thiols and sulfonyl hydrazides were treated with electrochemical conditions including two Pt electrodes, an NH4I electrolyte, and a mode of constant current. Further investigation showed that both aryl and aliphatic thiols (317, l317m) worked well with the conditions. Moreover, a mechanism study consisting of control reactions and CV experiments demonstrated that this was a radical-involved procedure.
Scheme 35. Electrosynthesis of Unsymmetrical Thiosulfonate.

Terent’ev et al. also developed an oxidative coupling method between sulfonyl hydrazides and N-hydroxyimides (327a–327l) or N-hydroxybenzotriazoles (327m–327q) in 2019 (Scheme 36), and the NHPI-type products could be used as good radical precursors and widely applied in organic synthesis.130 The authors chose a graphite anode and an Fe cathode, and selected NH4Br as an electrolyte. The reaction has a broad scope of substrate and was compatible with most of the selected substrates. It was found sulfonyl hydrazides containing iodide (327h) or a bulky group (327l) gave desired compounds in lower yields due to the decomposition of starting material or steric effects. Further study showed that sodium sulfonates were also suitable for this reaction. The subsequent control reactions and CV experiments revealed that the S–O bond might be formed in two pathways: (i) radical coupling of sulfonyl (329) and N-hydroxy (332) radicals; or (ii) esterification of sulfonyl bromide (328) and N-hydroxy compounds (331), which was indicated by control experiments that the sulfonyl bromide (328) might be a key intermediate.
Scheme 36. Sulfonylation of N-Hydroxyimides or N-Hydroxybenzotriazoles.

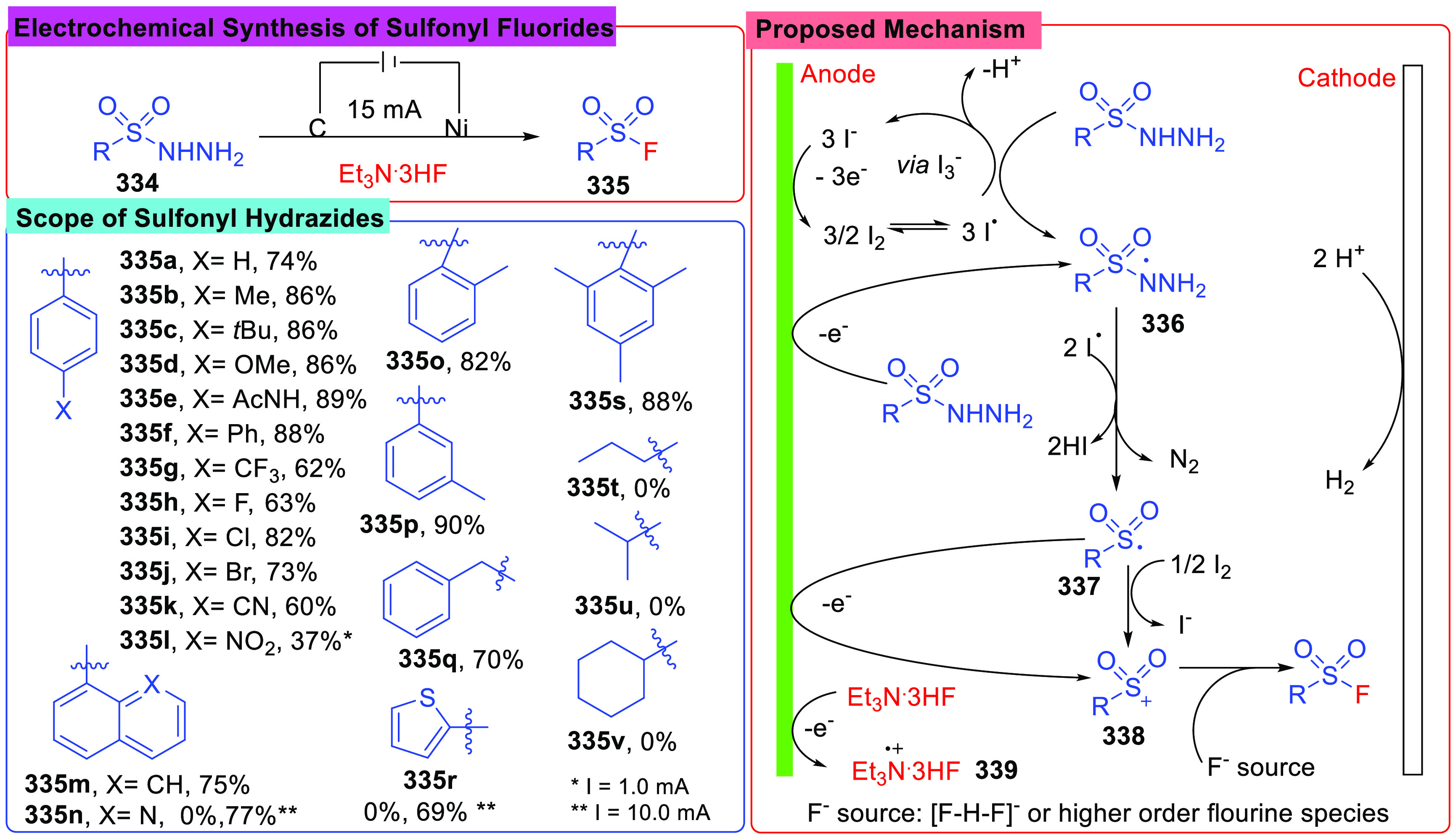
As efficient substrates in the synthesis of sulfur compounds,131,132 sulfonyl hydrazides were widely used in electrosynthesis of sulfonyl halides including sulfonyl fluorides. Recently, in 2022, Lee and Park reported an electrosynthesis of sulfonyl fluorides (335) via the radical pathway under a constant current of 15 mA (Scheme 37).133 The substrates were treated in an undivided cell charged with a graphite anode and a nickel cathode, and nBu4NI was selected as an electrolyte. To introduce the F atom, Et3N·3HF was applied here as a fluoride source. By screening a number of sulfonyl hydrazides bearing various substituents, it was found that aryl sulfonyl hydrazides were suitable for this reaction. However, aliphatic compounds (335t–335v) were not compatible with the conditions. Moreover, when sulfonyl hydrazides bearing sensitive groups such as −NO2 (335l), 8-quinolinyl (335m), and 2-thiophenyl (335r) groups were employed, corresponding products were obtained in good yields under a constant current of 1.0 mA or 10 mA. It was supposed that the product was formed by the combination of a sulfonyl cation (338) and an F– anion. CV experiments and control reactions indicated that the sulfonyl hydrazide was activated by an iodide molecule, which was generated at the anode in situ, and the radical (337) was yielded. Subsequently the radical (337) was oxidized to a sulfonyl cation (338), which was captured by F–.
Scheme 37. Electrochemical Synthesis of Sulfonyl Fluorides.
6. Conclusion
In conclusion, we have summarized the application of sulfonyl hydrazides in electrochemical synthesis in particular, according to the structure of products and the reaction mechanism. Under electrochemical conditions, sulfonyl hydrazides could take part in various kinds of reactions via free radical mechanisms and could introduce sulfonyl groups, or alkyl groups sometimes. It was found that the application of sulfonyl hydrazides in electrochemical methods was efficient and advantageous over other sulfonyl derivatives. We believe that this review may introduce a clear summary of the electrochemical application of sulfonyl hydrazides to promote wide investigation using sulfonyl hydrazides in the future.
Acknowledgments
This study was supported by the doctoral scientific research foundation of Jining Medical University, NSFC cultivation project of Jining Medical University (JYP2019KJ25).
Biographies
Bao-Chen Qian received his Ph.D. degree from University of Chinese Academy of Sciences in 2016, under the supervision of Prof. Chu-Yi Yu. Currently, he is an associate professor at the School of Medical Engineering, Jining Medical University. His research interests include the electrochemical synthesis, synthesis, and bioactivity research of aryl C-glycosides, and DFT computation.
Chao-Zhe Zhu received M.E. and Ph.D. degrees in biomedical engineering from University of Malaya. She is currently an associate professor at the Department of Medical Engineering, Jining Medical University, Jining, China. Her research interests include dielectric properties of determined diseases in the intersection of medical and engineering fields, and microwave systems, diagnostics, dielectric measurements, and the development of electrochemical sensors.
Guang-Bin Shen received his B.S. degree from Ludong University in 2011 and a Ph.D. degree from Nankai University in 2016, under the supervision of Prof. Xiao-Qing Zhu. Currently, he is an associate professor at the School of Medical Engineering, Jining Medical University. His research interests include redox mechanisms, electrochemical synthesis, physical organic chemistry, and computational chemistry.
The authors declare no competing financial interest.
References
- Hunter B. A.; Schoene D. L. Sulfonyl Hydrazide Blowing Agents for Rubber and Plastics. Ind. Eng. Chem. 1952, 44, 119–122. 10.1021/ie50505a036. [DOI] [Google Scholar]
- Adlington R. M.; Barrett A. G. M. Recent Applications of the Shapiro Reaction. Acc. Chem. Res. 1983, 16, 55–59. 10.1021/ar00086a004. [DOI] [Google Scholar]
- Shapiro R. H.; Heath M. J. Tosylhydrazones. V. Reaction of Tosylhydrazones with Alkyllithium Reagents. A New Olefin Synthesis. J. Am. Chem. Soc. 1967, 89, 5734–5735. 10.1021/ja00998a601. [DOI] [Google Scholar]
- Hutchins R. O.; Milewski C. A.; Maryanoff B. E. Selective Deoxygenation of Ketones and Aldehydes Including Hindered Systems with Sodium Cyanoborohydride. J. Am. Chem. Soc. 1973, 95, 3662–3668. 10.1021/ja00792a033. [DOI] [Google Scholar]
- Li L.-Y.; Leng B.-R.; Li J.-Z.; Liu Q.-Q.; Yu J.; Wei P.; Wang D.-C.; Zhu Y.-L. Palladium-catalyzed Regioselective Hydrosulfonylation of Allenes with Sulfinic Acids. RSC Adv. 2022, 12, 8443–8448. 10.1039/D1RA09036D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S.; Zhang Z.-T.; Young D. J.; Chai L.-L.; Wu Q.; Li H.-X. Visible-light Mediated Cross-coupling of Aryl Halides with Sodium Sulfinates via Carbonyl-photoredox/Nickel Dual Catalysis. Org. Chem. Front. 2022, 9, 1437–1444. 10.1039/D1QO01850G. [DOI] [Google Scholar]
- Reddy R. J.; Kumari A. H. Synthesis and Applications Of Sodium Sulfinates (RSO2Na): a Powerful Building Block for The Synthesis of Organosulfur Compounds. RSC Adv. 2021, 11, 9130–9221. 10.1039/D0RA09759D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.; Hao L.; Zhang J.; Zhu T. Progress in the Electrochemical Reactions of Sulfonyl Compounds. ChemSusChem 2022, 15, e202102557 10.1002/cssc.202102557. [DOI] [PubMed] [Google Scholar]
- Zhao F.; Tan Q.; Wang D.; Deng G.-J. Metal- and Solvent-free Direct C-H Thiolation of Aromatic Compounds with Sulfonyl Chlorides. Green Chem. 2020, 22, 427–432. 10.1039/C9GC03384J. [DOI] [Google Scholar]
- Yang R.; Yi D.; Shen K.; Fu Q.; Wei J.; Lu J.; Yang L.; Wang L.; Wei S.; Zhang Z. Indole and Pyrrole Derivatives as Pre-photocatalysts and Substrates in the Sulfonyl Radical-triggered Relay Cyclization Leading to Sulfonylated Heterocycles. Org. Lett. 2022, 24, 2014–2019. 10.1021/acs.orglett.2c00472. [DOI] [PubMed] [Google Scholar]
- Joseph D.; Idris M. A.; Chen J.; Lee S. Recent Advances in the Catalytic Synthesis of Arylsulfonyl Compounds. ACS Catal. 2021, 11, 4169–4204. 10.1021/acscatal.0c05690. [DOI] [Google Scholar]
- Chu X.-Q.; Ge D.; Cui Y.-Y.; Shen Z.-L.; Li C.-J. Desulfonylation via Radical Process: Recent Developments in Organic Synthesis. Chem. Rev. 2021, 121, 12548–12680. 10.1021/acs.chemrev.1c00084. [DOI] [PubMed] [Google Scholar]
- Ge D.; Wang X.; Chu X.-Q. SOMOphilic Alkynylation Using Acetylenic Sulfones as Functional Reagents. Org. Chem. Front. 2021, 8, 5145–5164. 10.1039/D1QO00798J. [DOI] [Google Scholar]
- Lin S.-Y.; Yeh T.-K.; Kuo C.-C.; Song J.-S.; Cheng M.-F.; Liao F.-Y.; Chao M.-W.; Huang H.-L.; Chen Y.-L.; Yang C.-Y.; Wu M.-H.; Hsieh C.-L.; Hsiao W.; Peng Y.-H.; Wu J.-S.; Lin L.-M.; Sun M.; Chao Y.-S.; Shih C.; Wu S.-Y.; Pan S.-L.; Hung M.-S.; Ueng S.-H. Phenyl Benzenesulfonylhydrazides Exhibit Selective Indoleamine 2,3-Dioxygenase Inhibition with Potent in Vivo Pharmacodynamic Activity and Antitumor Efficacy. J. Med. Chem. 2016, 59, 419–430. 10.1021/acs.jmedchem.5b01640. [DOI] [PubMed] [Google Scholar]
- Yang F.-L.; Tian S.-K. Sulfonyl Hydrazides as Sulfonyl Sources in Organic Synthesis. Tetrahedron Lett. 2017, 58, 487–504. 10.1016/j.tetlet.2016.12.058. [DOI] [Google Scholar]
- Yang F.-L.; Tian S.-K. Iodine-Catalyzed Regioselective Sulfenylation of Indoles with Sulfonyl Hydrazides. Angew. Chem., Int. Ed. 2013, 52, 4929–4932. 10.1002/anie.201301437. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Song K. H.; Lee S. Synthesis of S-aryl Thioesters via Palladium-catalyzed Thiocarbonylation of Aryl Iodides and Aryl Sulfonyl Hydrazides. Org. Chem. Front. 2020, 7, 2938–2943. 10.1039/D0QO00748J. [DOI] [Google Scholar]
- Yang K.; Gao J.-J.; Luo S.-H.; Wu H.-Q.; Pang C.-M.; Wang B.-W.; Chen X.-Y.; Wang Z.-Y. Quick Construction of a C-N Bond from Arylsulfonyl Hydrazides and Csp2-X Compounds Promoted by DMAP at room temperature. RSC Adv. 2019, 9, 19917–19923. 10.1039/C9RA03403J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H.-L.; Ma Y.-L.; Man K.-X.; Zhao S.-Y. Transition-Metal-Free Radical-Triggered Hydrosulfonylation and Disulfonylation Reaction of Substituted Maleimides with Sulfonyl Hydrazides. J. Org. Chem. 2022, 87, 3762–3769. 10.1021/acs.joc.1c02816. [DOI] [PubMed] [Google Scholar]
- Zhao Z.; Tian Q.; Chen Y.; Wen S.; Zhang Y.; Cheng G. Base-Promoted Stereoselective Hydrogenation of Ynamides with Sulfonyl Hydrazide to Give Z-Enamides. J. Org. Chem. 2021, 86, 10407–10413. 10.1021/acs.joc.1c01085. [DOI] [PubMed] [Google Scholar]
- Huang A.-X.; Zhu H.-L.; Zeng F.-L.; Chen X.-L.; Huang X.-Q.; Qu L.-B.; Yu B. 1-Acryloyl-2-cyanoindole: A Skeleton for Visible-Light-Induced Cascade Annulation. Org. Lett. 2022, 24, 3014–3018. 10.1021/acs.orglett.2c00927. [DOI] [PubMed] [Google Scholar]
- Li M.-M.; Cheng L.; Xiao L.-J.; Xie J.-H.; Zhou Q.-L. Palladium-Catalyzed Asymmetric Hydrosulfonylation of 1,3-Dienes with Sulfonyl Hydrazides. Angew. Chem., Int. Ed. 2021, 60, 2948–2951. 10.1002/anie.202012485. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Bao Y.; Guan Q.; Sun Q.; Zha Z.; Wang Z. Copper-catalyzed S-methylation of Sulfonyl Hydrazides with TBHP for the Synthesis of Methyl Sulfones in Water. Green Chem. 2017, 19, 112–116. 10.1039/C6GC03142K. [DOI] [Google Scholar]
- Xu Z.-Q.; Wang W.-B.; Zheng L.-C.; Li L.; Duan L.; Li Y.-M. Iodine-mediated Aminosulfonylation of Alkenyl Sulfonamides with Sulfonyl Hydrazides: Synthesis of Sulfonylmethyl Piperidines, Pyrrolidines and Pyrazolines. Org. Biomol. Chem. 2019, 17, 9026–9038. 10.1039/C9OB01847F. [DOI] [PubMed] [Google Scholar]
- Wang F.-X.; Tian S.-K. Cyclization of N-Arylacrylamides via Radical Arylsulfenylation of Carbon-Carbon Double Bonds with Sulfonyl Hydrazides. J. Org. Chem. 2015, 80, 12697–12703. 10.1021/acs.joc.5b02322. [DOI] [PubMed] [Google Scholar]
- Kawamata Y.; Baran P. S. Electrosynthesis: Sustainability Is Not Enough. Joule 2020, 4, 701–704. 10.1016/j.joule.2020.02.002. [DOI] [Google Scholar]
- Kingston C.; Palkowitz M. D.; Takahira Y.; Vantourout J. C.; Peters B. K.; Kawamata Y.; Baran P. S. A Survival Guide for the “Electro-curious. Acc. Chem. Res. 2020, 53, 72–83. 10.1021/acs.accounts.9b00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech M. C.; Lam K. Electrosynthesis Using Carboxylic Acid Derivatives: New Tricks for Old Reactions. Acc. Chem. Res. 2020, 53, 121–134. 10.1021/acs.accounts.9b00586. [DOI] [PubMed] [Google Scholar]
- Shi S.-H.; Liang Y.; Jiao N. Electrochemical Oxidation Induced Selective C-C Bond Cleavage. Chem. Rev. 2021, 121, 485–505. 10.1021/acs.chemrev.0c00335. [DOI] [PubMed] [Google Scholar]
- Waldvogel S. R.; Lips S.; Selt M.; Riehl B.; Kampf C. J. Electrochemical Arylation Reaction. Chem. Rev. 2018, 118, 6706–6765. 10.1021/acs.chemrev.8b00233. [DOI] [PubMed] [Google Scholar]
- Yan M.; Kawamata Y.; Baran P. S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319. 10.1021/acs.chemrev.7b00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.; Yang J.; Lei A. Recent Advances in Electrochemical Oxidative Cross-coupling with Hydrogen Evolution Involving Radicals. Chem. Soc. Rev. 2021, 50, 10058–10086. 10.1039/D1CS00150G. [DOI] [PubMed] [Google Scholar]
- Claraz A.; Masson G. Recent Advances in C(sp3)-C(sp3) and C(sp3)-C(sp2) Bond Formation through Cathodic Reactions: Reductive and Convergent Paired Electrolyses. ACS Organic & Inorganic Au 2022, 2, 126–147. 10.1021/acsorginorgau.1c00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munda M.; Niyogi S.; Shaw K.; Kundu S.; Nandi R.; Bisai A. Electrocatalysis as a Key Strategy for the Total Synthesis of Natural Products. Org. Biomol. Chem. 2022, 20, 727–748. 10.1039/D1OB02115J. [DOI] [PubMed] [Google Scholar]
- Ritter S. K. Electrosynthesis Gives Chemists More Power. C&EN 2017, 95, 23–25. 10.1021/cen-09511-scitech2. [DOI] [Google Scholar]
- Ritter S. Electrosynthesis Got Chemists Charged Up. C&EN 2017, 95, 21. 10.1021/cen-09549-cover2. [DOI] [Google Scholar]
- Zhao S.; Chen K.; Zhang L.; Yang W.; Huang D. Sulfonyl Hydrazides in Organic Synthesis: A Review of Recent Studies. Adv. Synth. Catal. 2020, 362, 3516–3541. 10.1002/adsc.202000466. [DOI] [Google Scholar]
- Yuan Y.; Lei A. Electrochemical Oxidative Cross-Coupling with Hydrogen Evolution Reactions. Acc. Chem. Res. 2019, 52, 3309–3324. 10.1021/acs.accounts.9b00512. [DOI] [PubMed] [Google Scholar]
- Lanfranco A.; Moro R.; Azzi E.; Deagostino A.; Renzi P. Unconventional Approaches for the Introduction of Sulfur-based Functional Groups. Org. Biomol. Chem. 2021, 19, 6926–6957. 10.1039/D1OB01091C. [DOI] [PubMed] [Google Scholar]
- Nambo M.; Maekawa Y.; Crudden C. M. Desulfonylative Transformations of Sulfones by Transition-Metal Catalysis, Photocatalysis, and Organocatalysis. ACS Catal. 2022, 12, 3013–3032. 10.1021/acscatal.1c05608. [DOI] [Google Scholar]
- Colomer I.; Velado M.; Fernández de la Pradilla R.; Viso A. From Allylic Sulfoxides to Allylic Sulfenates: Fifty Years of a Never-Ending [2,3]-Sigmatropic Rearrangement. Chem. Rev. 2017, 117, 14201–14243. 10.1021/acs.chemrev.7b00428. [DOI] [PubMed] [Google Scholar]
- Alba A.-N. R.; Companyó X.; Rios R. Sulfones: New Reagents in Organocatalysis. Chem. Soc. Rev. 2010, 39, 2018–2033. 10.1039/b911852g. [DOI] [PubMed] [Google Scholar]
- Jílková A.; Rubešová P.; Fanfrlík J.; Fajtová P.; R̆ezáčová P.; Brynda J.; Lepšík M.; Mertlíková-Kaiserová H.; Emal C. D.; Renslo A. R.; Roush W. R.; Horn M.; Caffrey C. R.; Mareš M. Druggable Hot Spots in the Schistosomiasis Cathepsin B1 Target Identified by Functional and Binding Mode Analysis of Potent Vinyl Sulfone Inhibitors. ACS Infect. Dis. 2021, 7, 1077–1088. 10.1021/acsinfecdis.0c00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozel V. E. G. Innovative Use of Dapsone. Dermatologic Clinics 2010, 28, 599–610. 10.1016/j.det.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Mulina O. M.; Ilovaisky A. I.; Parshin V. D.; Terent’ev A. O. Oxidative Sulfonylation of Multiple Carbon-Carbon bonds with Sulfonyl Hydrazides, Sulfinic Acids and their Salts. Adv. Synth. Catal. 2020, 362, 4579–4654. 10.1002/adsc.202000708. [DOI] [Google Scholar]
- Jeremias N.; Mohr L.-M.; Bach T. Intermolecular [2 + 2] Photocycloaddition of α,β-Unsaturated Sulfones: Catalyst-Free Reaction and Catalytic Variants. Org. Lett. 2021, 23, 5674–5678. 10.1021/acs.orglett.1c01794. [DOI] [PubMed] [Google Scholar]
- Yue F.; Dong J.; Liu Y.; Wang Q. Visible-Light-Mediated Alkenylation of Alkyl Boronic Acids without an External Lewis Base as an Activator. Org. Lett. 2021, 23, 2477–2481. 10.1021/acs.orglett.1c00399. [DOI] [PubMed] [Google Scholar]
- Yuan Y.; Cao Y.; Lin Y.; Li Y.; Huang Z.; Lei A. Electrochemical Oxidative Alkoxysulfonylation of Alkenes Using Sulfonyl Hydrazines and Alcohols with Hydrogen Evolution. ACS Catal. 2018, 8, 10871–10875. 10.1021/acscatal.8b03302. [DOI] [Google Scholar]
- Yan G.; Qiu K.; Guo M. Recent Aadvance in the C-F Bond Functionalization of Trifluoromethyl-containing Compounds. Org. Chem. Front. 2021, 8, 3915–3942. 10.1039/D1QO00037C. [DOI] [Google Scholar]
- Zhang C.; Wang L.; Shi H.; Lin Z.; Wang C. Iron-Catalyzed Allylic Defluorinative Ketone Olefin Coupling. Org. Lett. 2022, 24, 3211–3216. 10.1021/acs.orglett.2c00979. [DOI] [PubMed] [Google Scholar]
- Luo C.; Zhou Y.; Chen H.; Wang T.; Zhang Z.-B.; Han P.; Jing L.-H. Photoredox Metal-Free Allylic Defluorinative Silylation of α-Trifluoromethylstyrenes with Hydrosilanes. Org. Lett. 2022, 24, 4286–4291. 10.1021/acs.orglett.2c01690. [DOI] [PubMed] [Google Scholar]
- Tian F.; Yan G.; Yu J. Recent Advances in the Synthesis and Applications of α-(trifluoromethyl)styrenes in Organic Synthesis. Chem. Commun. 2019, 55, 13486–13505. 10.1039/C9CC06465F. [DOI] [PubMed] [Google Scholar]
- Luo X.; Wang S.; Lei A. Electrochemical-Induced Hydroxysulfonylation of α-CF3 Alkenes to Access Tertiary β-Hydroxysulfones. Adv. Synth. Catal. 2022, 364, 1016–1022. 10.1002/adsc.202101393. [DOI] [Google Scholar]
- Zhang H.; Liang M.; Zhang X.; He M.-K.; Yang C.; Guo L.; Xia W. Electrochemical Synthesis of Functionalized Gem-difluoroalkenes with Diverse Alkyl Sources via a Defluorinative Alkylation Process. Org. Chem. Front. 2021, 9, 95–101. 10.1039/D1QO01460A. [DOI] [Google Scholar]
- Gao X.-T.; Zhang Z.; Wang X.; Tian J.-S.; Xie S.-L.; Zhou F.; Zhou J. Direct Electrochemical Defluorinative Carboxylation of α-CF3 Alkenes with Carbon Dioxide. Chem. Sci. 2020, 11, 10414–10420. 10.1039/D0SC04091F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z.-P.; Gao J.; Duan X.-Y.; Guan J.-P.; Liu F.; Chen K.; Xiao J.-A.; Xiang H.-Y.; Yang H. Electrochemical Heterodifunctionalization of α-CF3 Alkenes to Access α-Trifluoromethyl β-Sulfonyl Tertiary Alcohols. Chem. Commun. 2021, 57, 8969–8972. 10.1039/D1CC03288G. [DOI] [PubMed] [Google Scholar]
- Kirk K. L. Fluorination in Medicinal Chemistry: Methods, Strategies, and Recent Developments. Org. Process Res. Dev. 2008, 12, 305–321. 10.1021/op700134j. [DOI] [Google Scholar]
- Yerien D. E.; Bonesi S.; Postigo A. Fluorination Methods in Drug Discovery. Org. Biomol. Chem. 2016, 14, 8398–8427. 10.1039/C6OB00764C. [DOI] [PubMed] [Google Scholar]
- Jiang Y.-M.; Yu Y.; Wu S.-F.; Yan H.; Yuan Y.; Ye K.-Y. Electrochemical Fluorosulfonylation of Styrenes. Chem. Commun. 2021, 57, 11481–11484. 10.1039/D1CC04813A. [DOI] [PubMed] [Google Scholar]
- Jannapu Reddy R.; Haritha Kumari A.; Kumar J. J. Recent Advances in the Synthesis and Applications of β-Keto Sulfones: New Prospects for the Synthesis of β-Keto Thiosulfones. Org. Biomol. Chem. 2021, 19, 3087–3118. 10.1039/D1OB00111F. [DOI] [PubMed] [Google Scholar]
- Kong X.; Yu K.; Chen Q.; Xu B. Electrochemical Oxidation-induced Difunctionalization of Alkynes and Alkenes with Sulfonyl Hydrazides: Facile Access to β-Selenovinyl Sulfones and β-Ketosulfones. Asian J. Org. Chem. 2020, 9, 1760–1764. 10.1002/ajoc.202000403. [DOI] [Google Scholar]
- Yavari I.; Shaabanzadeh S. Electrochemical Synthesis of β-Ketosulfones from Switchable Starting Materials. Org. Lett. 2020, 22, 464–467. 10.1021/acs.orglett.9b04221. [DOI] [PubMed] [Google Scholar]
- Wang L.; Lu C.; Yue Y.; Feng C. Visible-Light-Promoted Oxo-Sulfonylation of Ynamides with Sulfonic Acids. Org. Lett. 2019, 21, 3514–3517. 10.1021/acs.orglett.9b00733. [DOI] [PubMed] [Google Scholar]
- Wan H.-L.; Guan Z.; He Y.-H. Electrochemically Promoted Bifunctionalization of Alkynes for the Synthesis of β-Keto Sulfones. Asian J. Org. Chem. 2021, 10, 3406–3410. 10.1002/ajoc.202100620. [DOI] [Google Scholar]
- Du W.-B.; Wang N.-N.; Pan C.; Ni S.-F.; Wen L.-R.; Li M.; Zhang L.-B. Regio- and Stereoselective Electrochemical Synthesis of Sulfonylated Enethers from Alkynes and Sulfonyl Hydrazides. Green Chem. 2021, 23, 2420–2426. 10.1039/D1GC00027F. [DOI] [Google Scholar]
- Zhang X.; Lu D.; Wang Z. Electrochemical Induced Regio- and Stereoselective Difunctionalization of Alkynes: The Synthesis of (E)-β-Iodovinyl Sulfones. Eur. J. Org. Chem. 2021, 2021, 4284–4287. 10.1002/ejoc.202100505. [DOI] [Google Scholar]
- Kim H.-S.; Lee S. Electrochemical Coupling of Arylsulfonyl Hydrazides and Tertiary Amines for the Synthesis of β-Amidovinyl Sulfones. Eur. J. Org. Chem. 2019, 2019, 6951–6955. 10.1002/ejoc.201901277. [DOI] [Google Scholar]
- Fu J.; Zanoni G.; Anderson E. A.; Bi X. α-Substituted Vinyl Azides: an Emerging Functionalized Alkene. Chem. Soc. Rev. 2017, 46, 7208–7228. 10.1039/C7CS00017K. [DOI] [PubMed] [Google Scholar]
- Mulina O. M.; Zhironkina N. V.; Paveliev S. A.; Demchuk D. V.; Terent’ev A. O. Electrochemically Induced Synthesis of Sulfonylated N-Unsubstituted Enamines from Vinyl Azides and Sulfonyl Hydrazides. Org. Lett. 2020, 22, 1818–1824. 10.1021/acs.orglett.0c00139. [DOI] [PubMed] [Google Scholar]
- Qi L.; Xiao L.; Chen X.; Zhang Z.; Shi Q.; Chen J. Oxidant-free C-H Sulfonylation of Enamides: Electrochemical Synthesis of β-Amidovinyl and Carbonyl Sulfones from Sulfonyl Hydrazide and Enamides. Tetrahedron Lett. 2022, 88, 153576. 10.1016/j.tetlet.2021.153576. [DOI] [Google Scholar]
- Terent’ev A. O.; Mulina O. M.; Pirgach D. A.; Ilovaisky A. I.; Syroeshkin M. A.; Kapustina N. I.; Nikishin G. I. Electrosynthesis of Vinyl Sulfones from Alkenes and Sulfonyl Hydrazides Mediated by KI: An Electrochemical Mechanistic Study. Tetrahedron 2017, 73, 6871–6879. 10.1016/j.tet.2017.10.047. [DOI] [Google Scholar]
- Lai Y.-L.; Mo Y.; Yan S.; Zhang S.; Zhu L.; Luo J.; Guo H.; Cai J.; Liao J. Electrochemical Sulfonylation of Aalkenes with Sulfonyl Hydrazides: a Metal- and Oxidant-free Protocol for the Synthesis of (E)-Vinyl Sulfones in Water. RSC Adv. 2020, 10, 33155–33160. 10.1039/D0RA07212E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss V.; Zheng Y.; Shao X.; Tian L.; Wang Y. Advances in Electrochemical Decarboxylative Transformation Reactions. Chem.—Eur. J. 2021, 27, 3213–3228. 10.1002/chem.202001764. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Lai Y.-L.; Du K.-S.; Lin D.-Z.; Huang J.-M. Electrochemical Decarboxylative Sulfonylation of Cinnamic Acids with Aromatic Sulfonylhydrazides to Vinyl Sulfones. J. Org. Chem. 2017, 82, 9655–9661. 10.1021/acs.joc.7b01741. [DOI] [PubMed] [Google Scholar]
- Meng X.; Xu H.; Cao X.; Cai X.-M.; Luo J.; Wang F.; Huang S. Electrochemically Enabled Sulfonylation of Alkynes with Sodium Sulfinates. Org. Lett. 2020, 22, 6827–6831. 10.1021/acs.orglett.0c02341. [DOI] [PubMed] [Google Scholar]
- Meesin J.; Katrun P.; Pareseecharoen C.; Pohmakotr M.; Reutrakul V.; Soorukram D.; Kuhakarn C. Iodine-catalyzed Sulfonylation of Arylacetylenic Acids and Arylacetylenes with Sodium Sulfinates: Synthesis of Arylacetylenic Sulfones. J. Org. Chem. 2016, 81, 2744–2752. 10.1021/acs.joc.5b02810. [DOI] [PubMed] [Google Scholar]
- Mo Z.-Y.; Zhang Y.-Z.; Huang G.-B.; Wang X.-Y.; Pan Y.-M.; Tang H.-T. Electrochemical Sulfonylation of Alkynes with Sulfonyl Hydrazides: A Metal- and Oxidant-Free Protocol for the Synthesis of Alkynyl Sulfones. Adv. Synth. Catal. 2020, 362, 2160–2167. 10.1002/adsc.201901607. [DOI] [Google Scholar]
- Volla C. M. R.; Atodiresei I.; Rueping M. Catalytic C-C Bond-Forming Multi-Component Cascade or Domino Reactions: Pushing the Boundaries of Complexity in Asymmetric Organocatalysis. Chem. Rev. 2014, 114, 2390–2431. 10.1021/cr400215u. [DOI] [PubMed] [Google Scholar]
- Döndas H. A.; Retamosa M. d. G.; Sansano J. M. Recent Development in Palladium-Catalyzed Domino Reactions: Access to Materials and Biologically Important Carbo- and Heterocycles. Organometallics 2019, 38, 1828–1867. 10.1021/acs.organomet.9b00110. [DOI] [Google Scholar]
- Huang H.-M.; Garduño-Castro M. H.; Morrill C.; Procter D. J. Catalytic Cascade Reactions by Radical Relay. Chem. Soc. Rev. 2019, 48, 4626–4638. 10.1039/C8CS00947C. [DOI] [PubMed] [Google Scholar]
- Kim J. Y.; Kim D.-Y. Electrochemical Radical Arylsulfonylation/semipinacol Rearrangement Sequences of Alkenylcyclobutanol: Synthesis of β-Sulfonated Cyclic Ketones. Tetrahedron Lett. 2019, 60, 1287–1290. 10.1016/j.tetlet.2019.04.009. [DOI] [Google Scholar]
- Qin B.; Huang S.; Chen J.-Q.; Xiao W.; Wu J. Metal-Free Synthesis of Sulfonylated Indolo[2,1-a]Isoquinolines from Sulfur Dioxide. Org. Chem. Front. 2022, 9, 3521–3526. 10.1039/D2QO00487A. [DOI] [Google Scholar]
- Chen J.-Q.; Tu X.; Qin B.; Huang S.; Zhang J.; Wu J. Synthesis of Ester-Substituted Indolo[2,1-a]isoquinolines via Photocatalyzed Alkoxycarbonylation/ Cyclization Reactions. Org. Lett. 2022, 24, 642–647. 10.1021/acs.orglett.1c04082. [DOI] [PubMed] [Google Scholar]
- Ambros R.; Schneider M. R.; Von Angerer S. Indolo[2,1-a]isoquinolines. Syntheses, Steroid Hormone Receptor Binding Affinities, and Cytostatic Activity. J. Med. Chem. 1990, 33, 153–160. 10.1021/jm00163a026. [DOI] [PubMed] [Google Scholar]
- Shen Z.-J.; Huang B.; Ma N.; Yao L.; Yang C.; Guo L.; Xia W. Transition Metal-Free Synthesis of Sulfonyl- and Bromo-Substituted Indolo[2,1-α]isoquinoline Derivatives through Electrochemical Radical Cascade Cyclization. Adv. Synth. Catal. 2021, 363, 1944–1954. 10.1002/adsc.202001583. [DOI] [Google Scholar]
- Zhang T.-S.; Hao W.-J.; Wang R.; Wang S.-C.; Tu S.-J.; Jiang B. Electrocatalytic Three-component Annulation-halosulfonylation of 1,6-Enynes toward 1-Indanones Using Sodium Halides as both Halogen Sources and Electrolytes. Green Chem. 2020, 22, 4259–4269. 10.1039/D0GC00771D. [DOI] [Google Scholar]
- Jiang J.; Wang Z.; He W.-M. Electrosynthesis of 1-Indanones. Chin. Chem. Lett. 2021, 32, 1591–1592. 10.1016/j.cclet.2021.02.067. [DOI] [Google Scholar]
- Zuo H.-D.; Hao W.-J.; Zhu C.-F.; Guo C.; Tu S.-J.; Jiang B. Electrochemical Annulation-Iodosulfonylation of 1,5-Enyne-containing para-Quinone Methides (p-QMs) to Access (E)-Spiroindenes. Org. Lett. 2020, 22, 4471–4477. 10.1021/acs.orglett.0c01470. [DOI] [PubMed] [Google Scholar]
- Wu J.; Zong Y.; Zhao C.; Yan Q.; Sun L.; Li Y.; Zhao J.; Ge Y.; Li Z. Silver or Cerium-promoted Free Radical Cascade Difunctionalization of o-Vinylanilides with Sodium Aryl- or Alkylsulfinates. Org. Biomol. Chem. 2019, 17, 794–797. 10.1039/C8OB02964D. [DOI] [PubMed] [Google Scholar]
- Cao R.-F.; Yu L.; Huo Y.-X.; Li Y.; Xue X.-S.; Chen Z.-M. Chiral Lewis Base Catalyzed Enantioselective Selenocyclization of 1,1-Disubstituted Alkenes: Asymmetric Synthesis of Selenium-Containing 4H-3,1-Benzoxazines. Org. Lett. 2022, 24, 4093–4098. 10.1021/acs.orglett.2c01731. [DOI] [PubMed] [Google Scholar]
- Deng Q.-H.; Chen J.-R.; Wei Q.; Zhao Q.-Q.; Lu L.-Q.; Xiao W.-J. Visible-light-induced Photocatalytic Oxytrifluoromethylation of N-allylamides for the Synthesis of CF3-Containing Oxazolines and Benzoxazines. Chem. Commun. 2015, 51, 3537–3540. 10.1039/C4CC10217G. [DOI] [PubMed] [Google Scholar]
- He T.-J.; Zhong W.-Q.; Huang J.-M. The Synthesis of Sulfonated 4H-3,1-Benzoxazines via an Electro-chemical Radical Cascade Cyclization. Chem. Commun. 2020, 56, 2735–2738. 10.1039/C9CC09551A. [DOI] [PubMed] [Google Scholar]
- Xiong Y.-S.; Zhang B.; Yu Y.; Weng J.; Lu G. Construction of Sulfonyl Phthalides via Copper-Catalyzed Oxysulfonylation of 2-Vinylbenzoic Acids with Sodium Sulfinates. J. Org. Chem. 2019, 84, 13465–13472. 10.1021/acs.joc.9b01646. [DOI] [PubMed] [Google Scholar]
- Yang J.; Li G.; Yu K.; Xu B.; Chen Q. Electrochemical Sulfonylation-Induced Lactonization of Alkenes: Synthesis of Sulfonyl Phthalides. J. Org. Chem. 2022, 87, 1208–1217. 10.1021/acs.joc.1c02557. [DOI] [PubMed] [Google Scholar]
- Liao J.; Yang X.; Ouyang L.; Lai Y.; Huang J.; Luo R. Recent Advances in Cascade Radical Cyclization of Radical Acceptors for the Synthesis of Carbo- and Heterocycles. Org. Chem. Front. 2021, 8, 1345–1363. 10.1039/D0QO01453B. [DOI] [Google Scholar]
- Zhang B.; Studer A. Recent Advances in the Synthesis of Nitrogen Heterocycles via Radical Cascade Reactions Using Isonitriles as Radical Acceptors. Chem. Soc. Rev. 2015, 44, 3505–3521. 10.1039/C5CS00083A. [DOI] [PubMed] [Google Scholar]
- Liu J.; Wang M.; Li L.; Wang L. Electrooxidative Tandem Cyclization of N-Propargylanilines with Sulfinic Acids for Rapid Access to 3-Arylsulfonylquinoline Derivatives. Green Chem. 2021, 23, 4733–4740. 10.1039/D1GC00171J. [DOI] [Google Scholar]
- Ma Q.; Li M.; Chen Z.; Ni S.-F.; Wright J. S.; Wen L.-R.; Zhang L.-B. An Approach for the Synthesis of 2-Aryl-3-sulfonyl Substituted Quinolines through an Electrochemical Cascade Annulation Pathway. Green Chem. 2022, 24, 4425–4431. 10.1039/D2GC00151A. [DOI] [Google Scholar]
- Elsherbini M.; Wirth T. Electroorganic Synthesis under Flow Conditions. Acc. Chem. Res. 2019, 52, 3287–3296. 10.1021/acs.accounts.9b00497. [DOI] [PubMed] [Google Scholar]
- Gütz C.; Stenglein A.; Waldvogel S. R. Highly Modular Flow Cell for Electroorganic Synthesis. Org. Process Res. Dev. 2017, 21, 771–778. 10.1021/acs.oprd.7b00123. [DOI] [Google Scholar]
- Bajada M. A.; Sanjosé-Orduna J.; Di Liberto G.; Tosoni S.; Pacchioni G.; Noël T.; Vilé G. Interfacing Single-atom Catalysis with Continuous-flow Organic Electrosynthesis. Chem. Soc. Rev. 2022, 51, 3898–3925. 10.1039/D2CS00100D. [DOI] [PubMed] [Google Scholar]
- Noël T.; Cao Y.; Laudadio G. The Fundamentals Behind the Use of Flow Reactors in Electrochemistry. Acc. Chem. Res. 2019, 52, 2858–2869. 10.1021/acs.accounts.9b00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.; Yang Z.; Hua J.; Lin Y.; Bian M.; Li Y.; Liu C.; He W.; Fang Z.; Guo K. The Continuous-flow Electrosynthesis of 4-(sulfonylmethyl)isoquinoline-1,3(2H,4H)-diones from N-alkyl-N-methacryloyl Benzamides under Metal-free and Oxidant-free Conditions. Org. Chem. Front. 2020, 7, 3223–3228. 10.1039/D0QO00909A. [DOI] [Google Scholar]
- Cai C.; Lu Y.; Yuan C.; Fang Z.; Yang X.; Liu C.; Guo K. Organocatalytic Electrosynthesis of Cinnolines through Cascade Radical Cyclization and Migration. ACS Sustainable Chem. Eng. 2021, 9, 16989–16996. 10.1021/acssuschemeng.1c05810. [DOI] [Google Scholar]
- Karmakar U.; Samanta R. Pd(II)-Catalyzed Direct Sulfonylation of Benzylamines Using Sodium Sulfinates. J. Org. Chem. 2019, 84, 2850–2861. 10.1021/acs.joc.8b03098. [DOI] [PubMed] [Google Scholar]
- Guo Y.-J.; Lu S.; Tian L.-L.; Huang E.-L.; Hao X.-Q.; Zhu X.; Shao T.; Song M.-P. Iodine-Mediated Difunctionalization of Imidazopyridines with Sodium Sulfinates: Synthesis of Sulfones and Sulfides. J. Org. Chem. 2018, 83, 338–349. 10.1021/acs.joc.7b02734. [DOI] [PubMed] [Google Scholar]
- Jiang M.; Yuan Y.; Wang T.; Xiong Y.; Li J.; Guo H.; Lei A. Exogenous-oxidant- and Catalyst-free Electrochemical Deoxygenative C2 Sulfonylation of Quinoline N-oxides. Chem. Commun. 2019, 55, 13852–13855. 10.1039/C9CC07777D. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Chen Z.; He M.; Wang D.; Li L.; Qi J.; Shi R.; Lei A. Metal-free Electrochemical C3-Sulfonylation of Imidazo[1,2-a]pyridines. Org. Chem. Front. 2021, 8, 3815–3819. 10.1039/D1QO00348H. [DOI] [Google Scholar]
- Yin Z.; Yu Y.; Mei H.; Han J. Electrosynthesis of Functionalized Tetrahydrocarbazoles via Sulfonylation Triggered Cyclization Reaction of Indole Derivatives. Green Chem. 2021, 23, 3256–3260. 10.1039/D1GC00609F. [DOI] [Google Scholar]
- Yu Y.; Fang Y.; Tang R.; Xu D.; Dai S.; Zhang W. Electrochemical Oxidative Sulfonylation of N-Arylamides/Amine with Sodium Sulfinates. Asian J. Org. Chem. 2022, 11, e202100805 10.1002/ajoc.202100805. [DOI] [Google Scholar]
- Yuan Y.; Yu Y.; Qiao J.; Liu P.; Yu B.; Zhang W.; Liu H.; He M.; Huang Z.; Lei A. Exogenous-oxidant-free Electrochemical Oxidative C-H Sulfonylation of Arenes/Heteroarenes with Hydrogen Evolution. Chem. Commun. 2018, 54, 11471–11474. 10.1039/C8CC06451B. [DOI] [PubMed] [Google Scholar]
- Singh J.; Patel R. I.; Sharma A. Visible Light Mediated C-2 Functionalization- and Deoxygenative Strategies in Heterocyclic N-Oxides. Adv. Synth. Catal. 2022, 364, 2289–2306. 10.1002/adsc.202200200. [DOI] [Google Scholar]
- Xu Y.-X.; Liang Y.-Q.; Cai Z.-J.; Ji S.-J. Ruthenium(II)-Catalyzed Chelation-Assisted Desulfitative Arylation of Benzo[h]quinolines with Arylsulfonyl Chlorides. Org. Lett. 2022, 24, 2601–2606. 10.1021/acs.orglett.2c00542. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Cai J.; Du J.; Wang Q.; Yang J.; Geng R.; Fang Z.; Guo K. Electrochemical-Oxidation-Promoted Direct N-ortho-Selective Difluoromethylation of Heterocyclic N-Oxides. Org. Lett. 2022, 24, 1434–1438. 10.1021/acs.orglett.1c04241. [DOI] [PubMed] [Google Scholar]
- Yuan Y.; Jiang M.; Wang T.; Xiong Y.; Li J.; Guo H.; Lei A. Synergy of Anodic Oxidation and Cathodic Reduction leads to Electrochemical Deoxygenative C2 Arylation of Quinoline N-oxides. Chem. Commun. 2019, 55, 11091–11094. 10.1039/C9CC05841A. [DOI] [PubMed] [Google Scholar]
- Kim W.; Kim H. Y.; Oh K. Electrochemical Radical-Radical Cross-Coupling Approach between Sodium Sulfinates and 2H-Indazoles to 3-Sulfonylated 2H-Indazoles. Org. Lett. 2020, 22, 6319–6323. 10.1021/acs.orglett.0c02144. [DOI] [PubMed] [Google Scholar]
- Mahanty K.; Maiti D.; De Sarkar S. Regioselective C-H Sulfonylation of 2H-Indazoles by Electrosynthesis. J. Org. Chem. 2020, 85, 3699–3708. 10.1021/acs.joc.9b03330. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-Z.; Mo Z.-Y.; Wang H.-S.; Wen X.-A.; Tang H.-T.; Pan Y.-M. Electrochemically Enabled Chemoselective Sulfonylation and Hydrazination of Indoles. Green Chem. 2019, 21, 3807–3811. 10.1039/C9GC01201J. [DOI] [Google Scholar]
- Song Q.; Zhao H.; Sun Y.; Jiang H.; Zhang M. Direct C(sp3)-H Sulfonylation of Xanthene Derivatives with Sodium Sulfinates by Oxidative Copper Catalysis. Chin. J. Chem. 2022, 40, 371–377. 10.1002/cjoc.202100767. [DOI] [Google Scholar]
- Das S.; Roy S.; Bhowmik A.; Sarkar W.; Mondal I.; Mishra A.; Saha S. J.; Karmakar S.; Deb I. A radical-radical Cross-coupling Reaction of Xanthene with Sulfonyl Hydrazides: Facile Access to Xanthen-9-sulfone Derivatives. Chem. Commun. 2022, 58, 2902–2905. 10.1039/D1CC07143B. [DOI] [PubMed] [Google Scholar]
- Wei B.; Qin J.-H.; Yang Y.-Z.; Xie Y.-X.; Ouyang X.-H.; Song R.-J. Electrochemical Radical C(sp3)-H Arylation of Xanthenes with Electron-rich Arenes. Org. Chem. Front. 2022, 9, 816–821. 10.1039/D1QO01714D. [DOI] [Google Scholar]
- Chen X.; Liu H.; Gao H.; Li P.; Miao T.; Li H. Electrochemical Regioselective Cross-Dehydrogenative Coupling of Indoles with Xanthenes. J. Org. Chem. 2022, 87, 1056–1064. 10.1021/acs.joc.1c02346. [DOI] [PubMed] [Google Scholar]
- Gao H.; Chen X.; Wang P.-L.; Shi M.-M.; Shang L.-L.; Guo H.-Y.; Li H.; Li P. Electrochemical Benzylic C-H Arylation of Xanthenes and Thioxanthenes without a Catalyst and Oxidant. Org. Chem. Front. 2022, 9, 1911–1916. 10.1039/D1QO01925B. [DOI] [Google Scholar]
- Wei W.-J.; Zhong Y.-J.; Feng Y.-F.; Gao L.; Tang H.-T.; Pan Y.-M.; Ma X.-L.; Mo Z.-Y. Electrochemically Mediated Direct C(sp3)-H Sulfonylation of Xanthene Derivatives. Adv. Synth. Catal. 2022, 364, 726–731. 10.1002/adsc.202101289. [DOI] [Google Scholar]
- Terent’ev O. A.; Mulina O. M.; Pirgach D. A.; Syroeshkin M. A.; Glinushkin A. P.; Nikishin G. I. Electrochemical Synthesis of Sulfonamides from Arenesulfonohydrazides or Sodium p-Methylbenzenesulfinate and Amines. Mendeleev Commun. 2016, 26, 538–539. 10.1016/j.mencom.2016.11.027. [DOI] [Google Scholar]
- Deb M. L.; Saikia B. S.; Borpatra P. J.; Baruah P. K. α-C-H Functionalization of Tertiary Amines Catalyzed/Promoted by Molecular Iodine/Derivatives. New J. Chem. 2021, 45, 14345–14359. 10.1039/D1NJ02695J. [DOI] [Google Scholar]
- Sheykhan M.; Khani S.; Abbasnia M.; Shaabanzadeh S.; Joafshan M. an Approach to C-N Activation: Coupling of Arenesulfonyl Hydrazides and Arenesulfonyl Chlorides with Tert-Amines via a Metal-, Oxidant- and Halogen-Free Anodic Oxidation. Green Chem. 2017, 19, 5940–5948. 10.1039/C7GC03141F. [DOI] [Google Scholar]
- Mo Z.-Y.; Swaroop T. R.; Tong W.; Zhang Y.-Z.; Tang H.-T.; Pan Y.-M.; Sun H.-B.; Chen Z.-F. Electrochemical Sulfonylation of T with Sulfonyl Hydrazides: a Metal- and Oxidant-free Protocol for the Synthesis of Thiosulfonates. Green Chem. 2018, 20, 4428–4432. 10.1039/C8GC02143K. [DOI] [Google Scholar]
- Terent’ev A. O.; Mulina O. M.; Ilovaisky A. I.; Kokorekin V. A.; Nikishin G. I. Ammonium Iodide-mediated Electrosynthesis of Unsymmetrical Thiosulfonates from Arenesulfonohydrazides and Thiols. Mendeleev Commun. 2019, 29, 80–82. 10.1016/j.mencom.2019.01.027. [DOI] [Google Scholar]
- Terent’ev A. O.; Mulina O. M.; Parshin V. D.; Kokorekin V. A.; Nikishin G. I. Electrochemically Induced Oxidative S-O Coupling: Synthesis of Sulfonates from Sulfonyl Hydrazides and N-Hydroxyimides or N-Hydroxybenzotriazoles. Org. Biomol. Chem. 2019, 17, 3482–3488. 10.1039/C8OB03162B. [DOI] [PubMed] [Google Scholar]
- Amri N.; Wirth T. Recent Advances in the Electrochemical Synthesis of Organosulfur Compounds. Chem. Rec. 2021, 21, 2526–2537. 10.1002/tcr.202100064. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Cheng X.; Zhou Q.-L. Electrochemical Synthesis of Sulfonyl Fluorides with Triethylamine Hydrofluoride. Chin. J. Chem. 2022, 40, 1687–1692. 10.1002/cjoc.202200112. [DOI] [Google Scholar]
- Park J. K.; Oh J.; Lee S. Electrochemical Synthesis of Sulfonyl Fluorides from Sulfonyl Hydrazides. Org. Chem. Front. 2022, 9, 3407–3413. 10.1039/D2QO00651K. [DOI] [Google Scholar]