Abstract
Oral cancer is one of the most common malignancies of the head and neck, and approximately 90% of oral cancers are oral squamous cell carcinomas (OSCCs). The purinergic P2Y2 receptor is upregulated in breast cancer, pancreatic cancer, colorectal cancer, and liver cancer, but its role in OSCC is still unclear. Here, we examined the effects of P2Y2 on the invasion and migration of oral cancer cells (SCC15 and CAL27). The BALB/c mouse model was used to observe the involvement of P2Y2 with tumors in vivo. P2Y2, Src, and EGFR are highly expressed in OSCC tissues and cell lines. Stimulation with ATP significantly enhanced cell invasion and migration in oral cancer cells, and enhanced the activity of Src and EGFR protein kinases, which is mediated by the PI3K/AKT signaling pathway. P2Y2 knockdown attenuated the above ATP-driven events in vitro and in vivo. The PI3K/AKT signaling pathway was blocked by Src or EGFR inhibitor. Extracellular ATP activates the PI3K/AKT pathway through the P2Y2-Src-EGFR axis to promote OSCC invasion and migration, and thus, P2Y2 may be a potential novel target for antimetastasis therapy.
1. Introduction
Oral cancer is one of the most common malignancies of the head and neck. Approximately 90% of oral cancers are oral squamous cell carcinomas (OSCCs). According to the World Health Organization (WHO), more than 260,000 people are newly diagnosed with oral cancer every year, and the incidence in people aged over 65 years accounts for more than 50% of the total population. Despite recent advances in treatment, the rich blood supply and complex anatomical structure of the oral and maxillofacial region are conducive to recurrence and distant migration in approximately one-third of patients treated with conventional surgery or radiotherapy.1,2 Tumor invasion and metastasis are still the main causes of death in OSCC patients, and therefore, new antitumor methods are required for more effective clinical treatment of OSCC.
Adenosine 5′-O-triphosphate (ATP) has long been considered as the body’s most direct source of energy. In 1972, Geoff Burnstock put forward the “purinergic hypothesis” of neurotransmission, and the concept of ATP as an extracellular signaling molecule was formalized as a scientific hypothesis.3 Studies have shown that ATP (P2 receptors) is an important transmitter of various biological effects mediated by purinergic receptors, including cell proliferation, differentiation, and death.4−7 ATP may be crucial in promoting or preventing malignant metastasis.8 Under normal conditions, the extracellular ATP concentration (mmol/L) is much lower than the intracellular concentration (3–5 mmol/L), and it remains balanced. In a tumor microenvironment, the ATP concentration (about 100 μmol/L) is higher than that in the normal extracellular environment.4 ATP invasive transfer activity was first reported in prostate cancer.9 However, the pathogenesis of OSCC is still unclear. Purinergic receptors are divided into P1 and P2 receptors; the natural ligand of the P1 receptor is adenosine. P2 receptors are divided further into the following two categories: P2X and P2Y receptors. The P2X receptor is a ligand-gated ion channel receptor, with seven currently known subtypes (P2X1-P2X7), activated by extracellular ATP to release cation flow.10 The P2Y receptor is a G-protein-coupled receptor (GPCR) that plays an important role in a variety of signaling pathways. Currently, eight functional mammalian P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14) have been cloned and identified as GPCRs.11
P2Y2 is a functional receptor. Its first ligands are ATP and uridine triphosphate (UTP).12 P2Y2 can activate a variety of signaling pathways, with the typical path being a Gqα signaling path. Thus, when it is coupled with IP3 to promote the release of Ca2+ from the endoplasmic reticulum calcium store, it increases the intracellular Ca2+ concentration and activates protein kinase C(PKC).9,13,14 P2Y2 acts at the c-terminus of cells, and it activates the mitogen-activated protein kinase (MAPK) pathway by activating nonreceptor tyrosine protein kinase (Src).15,16 P2Y2 activates the matrix metalloproteinases ADAM10 and ADAM17, and the catalytic film binds to the growth factor to activate the epidermal growth factor receptor (EGFR).17 Although P2Y2 is upregulated in breast cancer, pancreatic cancer, colorectal cancer, and liver cancer, and is activated in cell proliferation, invasion, and migration,18−21 the role of P2Y2 in OSCC is still unclear and requires further research.
In this study, we used CAL27 and SCC15 oral squamous cell lines to explore the reaction of P2Y2 to cellular and associated mechanisms of extracellular nucleotide induction.
2. Materials and Methods
2.1. Reagents and Antibodies
P2Y2 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). p-AKT, AKT, p-PI3K, PI3K, Src, p-Src, EGFR (D38B1), and phospho-EGFR Y1068 (D7A5) antibodies were purchased from Abcam (MA). Anti-GAPDH was purchased from Protein Tech (Wuhan, China). ATP was purchased from GLPBIO (Shanghai, China), and siRNA was purchased from RIBOBIO (Guangzhou, China). AG1478 and Dasatinib were purchased from MCE (Shanghai, China).
2.2. Tissue Samples
OSCC tissues were collected from patients (n = 6) who underwent radical surgery between January 2019 and January 2020 at Shandong University (Jinan, China) with informed consent obtained concerning the use of surgically resected specimens for research purposes. All of the patients agreed and signed the informed consent. All human tissue and sample experiments were approved by the Ethics Committee of the School of Stomatology, Shandong University (ref Med. No. 20210802; 10 August 2021). The experiments conformed to the guidelines set by the Declaration of Helsinki. The patients did not receive any form of adjuvant therapy before surgery.
2.3. Tumor Cell Lines and Cell Culture
Human OSCC cell lines (CAL27 and SCC15) were obtained from the Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM-F12, Hyclone) containing 10% fetal bovine serum (Gibco, Grand Island, NY) and 1% penicillin-streptomycin, in a 37 °C, 95% humidified air and 5% CO2.
2.4. Cell Viability Assay
CAL27 and SCC15 cells were seeded in a 96-well culture plate at a density of 1 × 103 cells/well. CAL27 and SCC15 cells were treated with ATP, and after 24, 48, and 72 h of incubation, cell viability was determined by the cell counting kit assay (CCK8, Soleibao, Beijing, China). Subsequently, an enzyme-labeling instrument (iMark, Bio-Rad Laboratories, Inc.) was used to detect the absorbance to determine cell viability.
2.5. P2Y2-siRNA Transfection in OSCC Cells
The sequence of a single small interfering RNA (siRNA)1 was CCCGTGCTCTACTTTGTCA; single siRNA2 was GTAGCGAGAACACTAAGGA, all siRNA single strands are synthesized in vitro, at Guangzhou Borui Co, Ltd. Division (Guangzhou, China). An appropriate number of cells were seeded onto six-well cell culture plates 24 h before transfection, allowing the cell density to reach 30–50%. A transfection complex solution was prepared in accordance with the manufacturer’s protocol. Subsequently, 50 nM siRNAs were mixed with the siRNA Transfection Reagent. CAL27 and SCC15 cells were then transfected for 48 h. Subsequently, quantitative real-time polymerase chain reaction (qPCR) and western blotting were used to confirm the transfection of cell lines, and the siP2Y2 with the highest transfection efficiency was selected. Transfected cells as the experimental group were treated with 100 μM ATP for 24 h, and the expression of related genes and proteins was detected.
2.6. Cell Migration Assay
The migration ability of CAL27 and SCC15 cells was tested via scratch assay. The two types of cells were inoculated into six-well plates at a density of 5 × 105 cells/well, adhere to 80% density, and then replace the serum-free α-DMEM-F12 cell culture medium for culture. Subsequently, the bottom of the plate was scraped vertically with the tip of a 200 μL liquid pipette and washed with PBS. Then, the cells were treated with 100 μM ATP for 24 and 48 h in serum-free medium. Finally, take pictures under an inverted microscope (BX53; Olympus, Japan) at ×100 magnification at 0, 24, and 48 h. Image-Pro Plus 6.0 software (Media Controlnetics, Inc., Rockville, Maryland) was used to calculate the width of the healing area in the cell monolayer Learn analysis.
2.7. Cell Invasion Assay
The effect of P2Y2 on the invasion ability of CAL27 and SCC15 cells was evaluated by the transwell chamber test. A small chamber with 8 μM pore size was placed into a 24-well plate. The upper chamber was filled with 60–80 μl Matrigel (BD, Franklin Lakes, NJ), inoculated with 3.5 × 103 cells in 200 ml of culture medium in the upper chamber, experimental group cells were treated with 100 μM ATP, the lower chamber was filled with 750 mL of α-DMEM containing 10% serum after culturing the F12 medium at 37 °C for 24 h, and then the cells were removed. A cotton swab was used to gently remove the cells in the upper chamber. The cells were fixed with 4% methanol, stained with 0.1% crystal violet, photographed under an optical microscope, counted, and statistically analyzed.
2.8. Cell Colony Assay
CAL27 and SCC15 cells were seeded in a six-well plate at a density of 400 cells per well. The cells were treated with 100 μM ATP cultured with α-DMEM-F12 for 14 days, and when they grew into a colony of 50 cells, the cells were washed with PBS and fixed with 4% methanol. Then, the cells were stained with 0.1% crystal violet and scanned. The number of colonies greater than 50 cells was counted, and statistical analysis was performed.
2.9. Quantitative Real-Time PCR Analysis
CAL27 and SCC15 cell RNA was extracted from the cells using Trizol reagent (AG21102, Accurate Biotechnology Co., Ltd., China). cDNA was synthesized using the Evo M-MLV RT Reverse Transcription kit II (AG11711, Accurate Biotechnology). QPCR was performed using the SYBR Green Pro Taq HS premixed qPCR kit (AG11701, Accurate Biotechnology) in an RT fluorescence quantitative PCR system (Light Cycler 96 SW 1.1, Roche Ltd, Switzerland). The parameters required for denaturation, annealing, and extension were as follows: 95 °C for 30 s, 45 cycles at 95 °C for 5 s, and 60 °C for 20 s. The primer sequences are shown in Table 1. All data were normalized to GAPDH expression. Quantification of the qRT-PCR results was performed by the 2–ΔΔCT method.
Table 1. Primer Sequences for qRT-PCR.
| primer
sequence: (5′-3′) |
||
|---|---|---|
| gene name | forward | reverse |
| GAPDH | CCTGCACCACCAACTGCTTA | GGCCATCCACAGTCTTCTGAG |
| MMP2 | TGCTGGAGACAAATTCTGGA | TTGGTTCTCCAGCTTCAGGT |
| MMP9 | TCTATGGTCCTCGCCCTGAA | CATCGTCCACCGGACTCAAA |
| E-cadherin | AGTCACTGACACCAACGATAAT | ATCGTTGTTCACTGGATTTGTG |
| Vimentin | GACGCCATCAACACCGAGTT | CTTTGTCGTTGGTTAGCTGGT |
| P2Y2 | GGTGTCTGGGCGTCTTACG | TGGTGGTGACAAAGTAGAGCA |
| Snail | CCTTCGTCCTTCTCCTCTACTT | GCTTCGGATGTGCATCTTGA |
| Src | CAAGCAGACATAGAAGAGCCAAGA | TGAAATGCCACGGGACAAAGTA |
| EGFR | ATCATACGCGGCAGGACCA | TCTGACCGGAGGTCCCAAAC |
2.10. Western Blotting
CAL27 and SCC15 cells were washed three times with precooled PBS, RIPA lysate was added to lyse the cells and then collected, and the protein concentration was detected with the BCA protein detection kit. The same amount of total protein (10 μg) was separated by 10% sodium salt-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a poly(vinylidene fluoride) (PVDF) membrane. After being blocked with 5% bovine serum albumin (BSA)/tris-buffered solution with Tween (TBST) for 1 h, the PVDF membrane was incubated with the P2Y2 antibody (concentration 1:2000), Src/p-Src antibody (concentration 1:2000), EGFR/p-EGFR antibody (concentration 1:2000), PI3K/p-PI3K antibody (concentration 1:500), AKT/p-AKT antibody (concentration 1:500), and GAPDH (concentration 1:10 000), at 4 °C overnight. Subsequently, the proteins were washed three times in TBST and then incubated in horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2000) for 1 h. After washing in TBST, immune reaction zones were determined with the ECL detection system and then captured by the gel imaging system (Amersham Imager 600; General Electric Company).
2.11. Mouse Xenograft Tumor Model
Athymic nude BALB/c female mice (aged: 4 weeks, n = 10) were purchased from Jinan Peng Yue Laboratory Animal Breeding Co. Ltd. They were housed in a specific pathogen-free environment under the condition of a 12-h light/12-h dark cycle as well as free access to food and water. The mice were randomly divided into two groups (n = 5), and 2 × 106 CAL27 cells were subcutaneously injected into the back of the right upper limb of each mouse. First, 2 × 106 CAL27 cells and siRNA CAL27 cells were subcutaneously injected into the back of the right upper limb of each mouse. Tumor size was detected every 3 days using a slide caliper, and the tumor volume was calculated using the following formula: A × B2/2, where A is the length of the tumor and B is the width. After 30 days, the mice were euthanized and the tumors were isolated, weighed, photographed, and fixed immediately with 4% paraformaldehyde for subsequent analysis. The animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Shandong University. Animal study and euthanasia were carried out following the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Department of School and Hospital of Stomatology, Shandong University (ref Med. No. 20210803; 10 August 2021).
2.12. Statistical Analysis
GraphPad Prism 6.0 software (GraphPad Software, Inc.) was used for statistical analysis. Paired Student’s t-test was used for comparison between the two groups, and the significance level was adjusted according to the number of tests in multiple comparisons. The cells of the experimental group and control group were tested by independent sample t-test. The differences between three or more groups were tested by one-way analysis of variance (ANOVA). All experiments are repeated at least three times unless otherwise stated. All results are expressed as mean values ± standard deviation. p < 0.05 was considered statistically significant.
3. Results
3.1. P2Y2 Was Highly Expressed in OSCC Tissues and Cells, and Was Closely Correlated with Src and EGFR
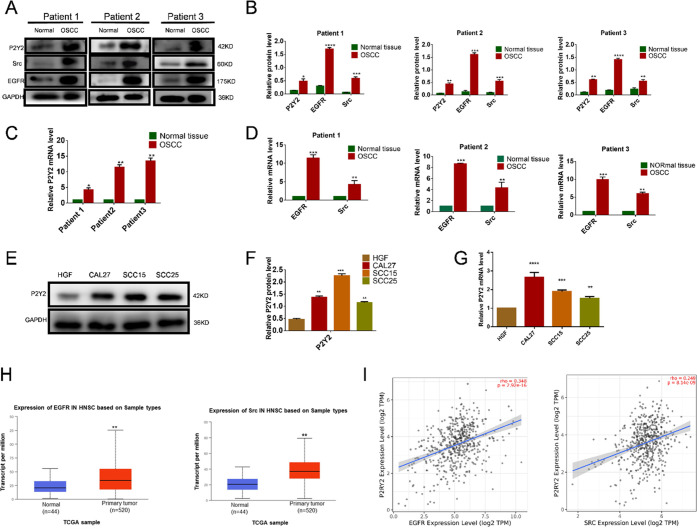
To investigate the expression and role of P2Y2 in OSCC, we collected three surgical specimens of OSCC. Western blotting and qRT-PCR showed that P2Y2 expression was significantly higher in tumor tissue than in adjacent noncancerous tissues (Figure 1A–C). P2Y2 was significantly expressed in all OSCC cells (Figure 1E–G). Furthermore, Src and EGFR were also highly expressed in OSCC (Figure 1A,B,D). To further study the role of P2Y2 receptors in human cancer and explore their clinical significance, we used TCAG database analysis to display that Src and EGFR were highly expressed in head and neck squamous cell carcinoma (HNSCC) (Figure 1H) and that the expression of P2Y2 was highly correlated with that of Src and EGFR (Figure 1I).
Figure 1.
P2Y2 is highly expressed in OSCC tissues and the expression of P2Y2 was highly correlated with that of Src and EGFR. (A–D) P2Y2, EGFR, and Src highly expressed in OSCC tissues were analyzed by western blotting and qRT-PCR. (E–G) P2Y2 high expression in OSCC cells analyzed by western blotting and qRT-PCR. (H) TCGA (http://ualcan.path.uab.edu/) database analysis displays Src and EGFR, which was highly expressed in HNSCC. The boxplot indicates that Src in the HNSCC samples is higher than normal. The boxplot indicates that EGFR in the HNSCC samples is higher than normal. (I) Analysis of the TIMER (https://cistrome.shinyapps.io/timer) dataset illustrated a significant difference in P2Y2-Src-EGFR signaling-related genes between OSCC and normal mucosa tissues. Data were given as mean ± SD from three independent experiments (error bars indicate SD, n = 3); t-test was employed for testing the difference between the two groups. ANOVA for testing the distinctions of the groups. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.2. Extracellular ATP Activated P2Y2 and Increased Src and EGFR Phosphorylation in CAL27 and SCC15 Cells
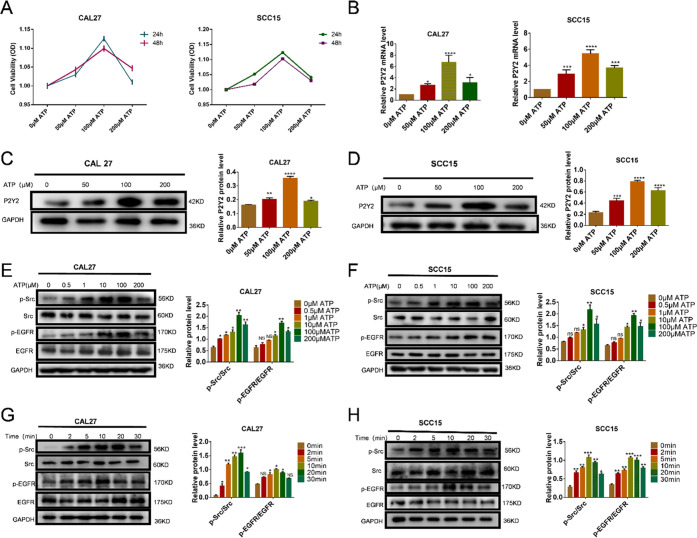
To determine whether OSCC cell lines express functional P2Y2, we used Cell Counting Kit-8 (CCK-8) to ascertain the optimal stimulating concentration of ATP. With increasing ATP concentration, cellular activity was also increased, but at a concentration of 200 μM, the cellular activity was significantly decreased (Figure 2A). Western blotting and qRT-PCR were used to verify the CCK8 results. According to these results in combination, the optimal ATP concentration for stimulation was determined to be 100 μM (Figure 2B–D). The Western blot analysis showed that ATP stimulated the phosphorylation of Src and EGFR in a dose- and time-dependent manner and reached peak activation at 100 μM ATP within 10–20 min (Figure 2E–H).
Figure 2.
Extracellular ATP activated P2Y2 and increased Src and EGFR phosphorylation. (A) CCK8 was used to measure cell viability with different concentrations. (B–D) P2Y2 expression increased in a dose-dependent manner in CAL27 and SCC15 cells, which was detected by western blotting and qRT-PCR. (E–H) ATP activates Src and EGFR kinases in CAL27 (A and C) and SCC15 cells in a time-and dose-dependent manner. Results were demonstrated by histograms to quantify the expression levels. Data were presented as mean ± SD (error bars indicate SD). Three independent experiments were performed (n = 3); t-test was employed for testing the difference between the two groups. ANOVA for testing the distinctions of the groups. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs control.
3.3. Extracellular ATP Regulated the Invasion and Migration of OSCC Cells by Activating the PI3K/AKT Signaling Pathway
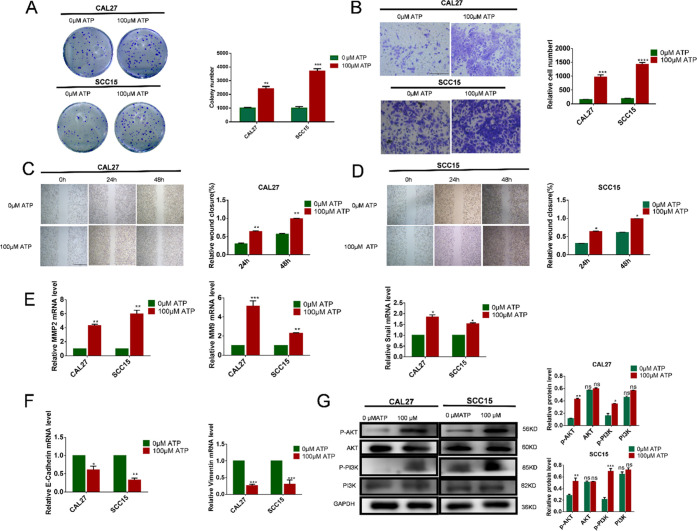
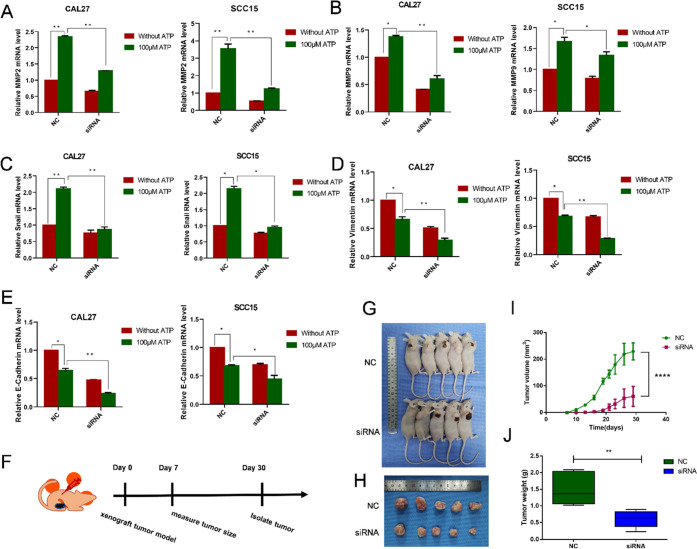
The OSCC cell lines were treated with the extracellular nucleotide ATP. In cell colony analysis, CAL27 and SCC15 cells were treated with 100 μM ATP for 14 days. The number of cell colonies was increased in comparison to that in the control group (Figure 3A). The scratch test and transwell test showed that CAL27 and SCC15 cell migration and invasion were significantly promoted compared to those in the control group (Figure 3B–D). To investigate whether extracellular ATP can enhance the invasion ability of OSCC cells, we used qRT-PCR assay to analyze the gene expression. In CAL27 and SCC15 cells stimulated with 100 μM ATP for 24 h, the expression of Zinc finger protein SNAI1 (Snail), matrix metalloproteinase (MMP)2, and MMP9 genes increased. At the same time, expression of E-cadherin and Vimentin decreased, and these results suggested that ATP induced the invasion and migration of OSCC cells and that P2Y2 receptor activation may play a major role in mediating the expression of genes related to invasion and migration (Figure 3E,F). To investigate whether the PI3K/AKT signaling pathway is involved in the regulation of ATP-induced invasion and migration of OSCC cells, we treated CAL27 and SCC15 cells with 100 μM ATP for 24 h. Compared with the control group, the expression of p-PI3K and p-AKT was significantly increased after 24 h of ATP treatment (Figure 3G).
Figure 3.
Effect of ATP on in vitro proliferation, migration, and invasion of OSCC cells. (A) Colony formation assay was used to evaluate the effect of proliferation after incubation with 100 μM ATP for 14 days. (B) Transwell invasion assays evaluated the effect on in vitro invasion after incubation with 100 μM ATP for 24 h. The scale bar is 500 μm. (C, D) Wound healing evaluated the effect on in vitro migration after incubation with 100 μM ATP for 24 and 48 h. The scale bar is 1 mm. (E, F) qRT-PCR was used to observe changes in migration and invasion-related genes MMP2, MMP9, Vimentin, Snail, and E-cadherin. (G) Expression of AKT and PI3K proteins was detected by western blotting after incubation with 100 μM ATP treatment for 24 h. Results were demonstrated by histograms to quantify the expression levels. Data were presented as mean ± SD (error bars indicate SD). Three independent experiments were performed (n = 3); t-test was employed for testing the difference between the two groups. ANOVA for testing the distinctions of the groups. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs control.
3.4. P2Y2 Was Involved in the Invasion and Migration of OSCC Cells Promoted by ATP
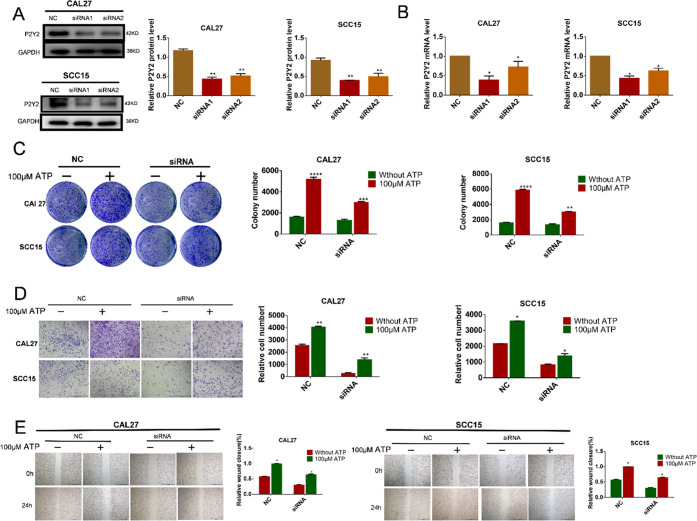
To demonstrate the association between P2Y2 and the invasion and migration ability of OSCC cells, P2Y2 was knocked down by siRNA. CAL27 and SCC15 cells were transfected with 50 nM siRNA for 48 h, and the optimal siRNA was determined using real-time PCR and western blotting. The most effective siRNA of P2Y2-siRNA1 was selected and used in the experiment (Figure 4A,B). In the cell colony analysis, the cells were treated with ATP (100 μM) for 14 days, and after that, the number of cell colonies was lower compared with the P2Y2 knockdown group (Figure 4C). In the in vitro invasion test, P2Y2-siRNA cells were treated with 100 μM ATP for 24 h. The number of CAL27 and SCC15 cells in the P2Y2 knockdown group was significantly lower than that of the control group, indicating that P2Y2 may promote the invasion of CAL27 and SCC15 cells (Figure 4D). The wound-healing assay showed the same result in that after treatment with ATP (100 μM) for 24 h, the wound gap between CAL27 and SCC15 cells was significantly reduced compared with that in the control group, suggesting that P2Y2 is involved in ATP-promoted migration and invasion of OSCC cells (Figure 4E).
Figure 4.
Effects of P2Y2 receptor knockdown on ATP-mediated in vitro invasion and migration. (A, B) CAL27 and SCC15 cells were transfected with three different P2Y2-siRNAs (siRNA1 and siRNA2) and a control siRNA (NC). Western blotting and qRT-PCR were used to evaluate the knockdown efficiency. (C) Colony formation assay was used to evaluate the effect of P2Y2 receptor knockdown on in vitro proliferation after incubation with ATP for 14 days. (D) Transwell invasion assays evaluated the effect of P2Y2 receptor knockdown on in vitro invasion after incubation with ATP for 24 h. The scale bar is 500 μm. (E) Wound healing evaluated the effect of P2Y2 receptor knockdown on in vitro migration after incubation with ATP for 24 h. The scale bar is 1 mm. Data were presented as mean ± SD error bars indicate SD. Three independent experiments were performed (n = 3); t-test was employed for testing the difference between the two groups. ANOVA for testing the distinctions of the groups. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs control.
3.5. Silencing P2Y2 Inhibited the Invasion and Migration of OSCC Cells In Vivo and In Vitro
To study the downstream effect of invasion driven by ATP-P2Y2, we first focused on the genes related to invasion and migration. After P2Y2 was transfected, OSCC cells were stimulated with 100 μM ATP for 24 h. PCR detection showed that the ATP-mediated expression of Snail, MMP2, and MMP9 genes was inhibited, and the expression of E-cadherin and Vimentin was activated (Figure 5A–E). Nude mice were subcutaneously implanted with CAL27 cells from the NC and siRNA groups to study the anticancer effect of P2Y2 in vivo. The average tumor volume in the siRNA group was significantly smaller than that in the control group. The average tumor weight in the P2Y2-siRNA group was also significantly smaller than that in the control group (Figure 5F,G). These data support the hypothesis that P2Y2 receptors play an important role in ATP-mediated invasion and migration in vivo and in vitro.
Figure 5.
Silencing of P2Y2 inhibited the invasion and migration of OSCC cells. (A–E) P2Y2 receptor was involved in the ATP-mediated expression of MMP2, MMP9, Vimentin, Snail and CAL27, and SCC15 cells. The cells were treated with 100 μM ATP for 24 h, and qRT-PCR was used to observe changes in invasion and migration-related genes MMP2, MMP9, Vimentin, Snail, and E-cadherin. (F) Technical roadmap of tumor formation experimental procedures in nude mice. (G) Representative photograph of the tumors in BALB/c nude mice injected with control cells or P2Y2-silenced cells. (H) Tumors derived from BALB/c mice are shown. (I) Tumor volume after inoculation with different groups of CAL27 cells for 30 days. and shown by a histogram. (J) Weight of tumors during the 30 days of treatment. Data were presented as mean ± SD (error bars indicate SD). Three independent experiments were performed (n = 3); t-test was employed for testing the difference between the two groups. ANOVA for testing the distinctions of the groups. *p < 0.05, **p < 0.01, ***p < 0.001 vs control.
3.6. Inhibiting P2Y2 or Src Attenuated the Expression of EGFR Induced by ATP in OSCC Cells
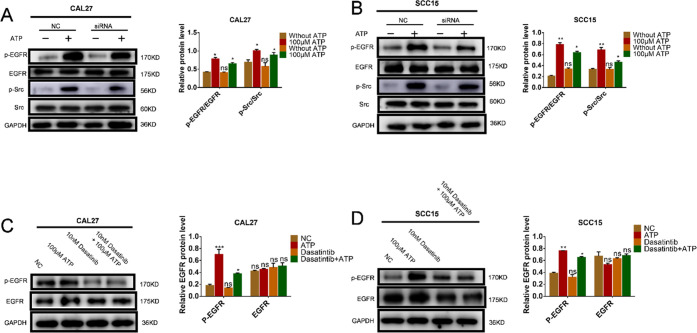
To explore how P2Y2 regulates the Src and EGFR signaling pathways through extracellular ATP, we performed P2Y2 silencing. After P2Y2 silencing, CAL27 and SCC15 cells were stimulated with 100 μM ATP for 20 min. Western blotting showed that the phosphorylation of Src and EGFR was significantly reduced (Figure 6A,B). Following the application of Dasatinib and AG1478 inhibitors for 1 h, CAL27 and SCC15 cells were stimulated with 100 μM ATP for 20 min, and the phosphorylation of EGFR was found to be significantly reduced (Figure 6C,D).
Figure 6.
P2Y2 knockdown and Dasatintib attenuated the expression of EGFR and Src in OSCC cells induced by ATP. (A, B) CAL27 and SCC15 cells were transfected with P2Y2-siRNA or a negative control siRNA (NC) with or without ATP (100 μM), western blotting results showed the expressions of p-EGFR in P2Y2 knockdown CAL27 and SCC15 cells. (C, D) CAL27 and SCC15 cells were pretreated with Dasatinib (10 nM, 1 h), with or without ATP (100 μM) for 20 min, and western blotting was used to detect the expression of p-EGFR. Data were presented as mean ± SD (error bars indicate SD). Three independent experiments were performed (n = 3); t-test was employed for testing the difference between the two groups. ANOVA for testing the distinctions of the groups. *p < 0.05, **p < 0.01, ***p < 0.001 vs control.
3.7. ATP Activated the PI3K/AKT Pathway through the P2Y2-Src-EGFR Signaling Pathway
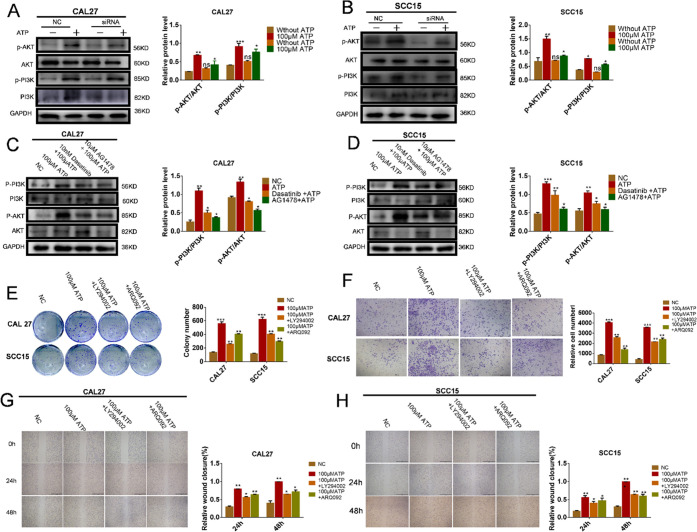
To further investigate whether P2Y2 activates the PI3K/AKT signaling pathway through the Src-EGFR axis, CAL27 and SCC15 cells that have knocked down P2Y2 were treated with ATP (100 μM) for 24 h. Compared with the control group, the expression of p-PI3K and p-AKT was significantly decreased (Figure 7A,B). The cells were further pretreated with Dasatinib and AG1478 for 1 h and then stimulated with ATP. The expression of p-PI3K and p-AKT was decreased (Figure 7C,D). In the cell colony analysis, the cells were treated with PI3K/AKT inhibitors for 14 days, after which the number of cell colonies was lower compared with the ATP (100 μM) group (Figure 7E). In the in vitro invasion test, the cells were treated with PI3K/AKT inhibitors for 24 h. The number of CAL27 and SCC15 cells in the PI3K/AKT inhibitors group was significantly lower than that of the control group, indicating that PI3K/AKT may promote the invasion of CAL27 and SCC15 cells (Figure 7F). The wound-healing assay showed the same result; after treatment with PI3K/AKT inhibitors for 24 and 48 h, the wound gap between CAL27 and SCC15 cells was significantly reduced compared with that in the control group, suggesting that PI3K/AKT is involved in ATP-promoted migration and invasion of OSCC cells (Figure 7G,H).
Figure 7.
P2Y2 activates the PI3K/AKT signaling pathway through the Src-EGFR axis. (A, B) P2Y2-silenced cells and control cells were treated with 100 μM ATP for 20 min. Western blotting was performed to detect the expression of p-PI3K. (C, D) Cells were further pretreated with Dasatinib and AG1478 for 1 h, and then adding ATP (100 μM) for 24 h, western blotting results showed that further reduced the expressions of p-PI3K and p-AKT. (E) Colony formation assay was used to evaluate the effect of PI3K/AKT inhibitors on in vitro proliferation after incubation with ATP for 14 days. (F) Transwell invasion assays evaluated the effect of PI3K/AKT inhibitors on in vitro invasion after incubation with ATP for 24 h. The scale bar is 500 μm. (G, H) Wound healing evaluated the effect of PI3K/AKT inhibitors on in vitro migration after incubation with ATP for 24 and 48 h. The scale bar is 1 mm. Data were presented as mean ± SD (error bars indicate SD). Three independent experiments were performed (n = 3); t-test was employed for testing the difference between the two groups. ANOVA for testing the distinctions of the groups. *p < 0.05, **p < 0.01, ***p < 0.001 vs control.
4. Discussion
The tumor microenvironment (TME) is a dynamic environment, and its biochemistry and cellular composition play a crucial role in the regulation of tumor cell metabolism, proliferation, and motility.16,22 The transdifferentiation of epithelial cells into motile mesenchymal cells, a process known since the 1980s as epithelial-mesenchymal transition (EMT), was first observed by Elizabeth Hay, who described epithelial to mesenchymal phenotype changes in the primitive streak of chick embryos.23 EMT is an indispensable part of the developmental process, and its underlying process is reactivated during wound healing, fibrosis, and cancer progression.24 During the development of cancer, the cytoplasmic damage caused by inflammation and hypoxia and the tissue destruction caused by tumor invasion result in an increased concentration of extracellular ATP, which plays a key role as an extracellular messenger.25
In 1980, P2Y2 suggested that specific plasma membrane receptors for extracellular ATP were expressed by inflammatory and cancer cells; P2Y2 is the first selected ligand of ATP. The role of ATP in the TME is multifaceted, as it regulates the permeability of cell connections by mobilizing intracellular Ca2+ storage, leading to tumor cell invasion and metastasis.26 In cancer, EMT is highly deregulated, and EMT-transcription factors exert important roles in all cancer stages, including initiation, primary tumor growth, invasion, dissemination, metastasis, colonization, and therapy resistance as well.27 Research findings in different models have demonstrated the participation of the P2Y2 receptor in inducing migration or the EMT process.24 In ovarian cancer, ATP induces EMT of ovarian cancer cells through the P2Y2 receptor-dependent activity of EGFR.28 In gastric cancer, purinergic P2Y2 and P2X4 receptors are involved in changes in the expression of EMT and related genes in gastric cancer cell lines.29 It is generally understood that ATP and other nucleotides, and their plasma membrane receptors play a central role in tumor cell proliferation and immune cell regulation. For this reason, an in-depth understanding of purinergic signals in the TME may provide new therapeutic prospects.
Extracellular ATP acts on tumors via specific plasma membrane receptors. Almost all cancer and immune cells express P2 receptors and are sensitive to extracellular ATP. P2Y2, a member of the purine P2 receptor family, is a G-protein-coupled receptor (GPCR). P2Y2 was first cloned from mouse NG108-15 neuroblastoma,30 and it induces a variety of cancer cell responses through its unique structure, including cell proliferation, migration, and invasion. In pancreatic ductal cancer cells, P2Y2 activation induces cell proliferation dependent on the activation of platelet-derived growth factor receptor-β (PDGFR-β) and PI3K/AKT.19,30 P2Y2 is highly expressed in prostate cancer and promotes the invasion and migration of prostate cancer cells in vivo.31 The expression of P2Y2 in human hepatocellular carcinoma cells is higher than that in normal hepatocytes.32 ATP has been shown to promote the proliferation of gastric adenocarcinoma cells, which is blocked by specific purinergic antagonists,33 and ATP treatment can induce proliferation of different glioma cell lines after 24 and 48 h.34 ATP and UTP activation of P2Y2R can induce migration and proliferation of MDA-MB231 and MCF-7 breast cancer cells, and it is associated with inflammation cascade activation.35 ATP and UTP also support cancer cell growth in A-549 human lung cancer cells.36 However, the effect of P2Y2 on most other tumors is still unknown, and the association between P2Y2 and OSCC has been rarely studied.
In our study, P2Y2 was found to be overexpressed in the OSCC cell line and to promote the growth, invasion, and migration of tumors. In clinical samples, the expression of P2Y2 in OSCC tissues was significantly higher than that in normal tissues. In addition, stimulation with the P2Y2 agonist ATP significantly increased P2Y2 expression in OSCC cells, increased the expression of Snail, MMP2, and MMP9 genes, and decreased the expression of E-cadherin and Vimentin; the invasion and migration ability of tumor cells was enhanced. After the knockdown of the P2Y2 receptor, the expression changes of genes related to intracellular invasion and migration regulated by ATP were also weakened, and the invasion and migration of tumor cells were weakened. These results are also consistent with other findings demonstrating that P2Y2 receptors have the potential for transformative use in both in vitro and in vivo.31
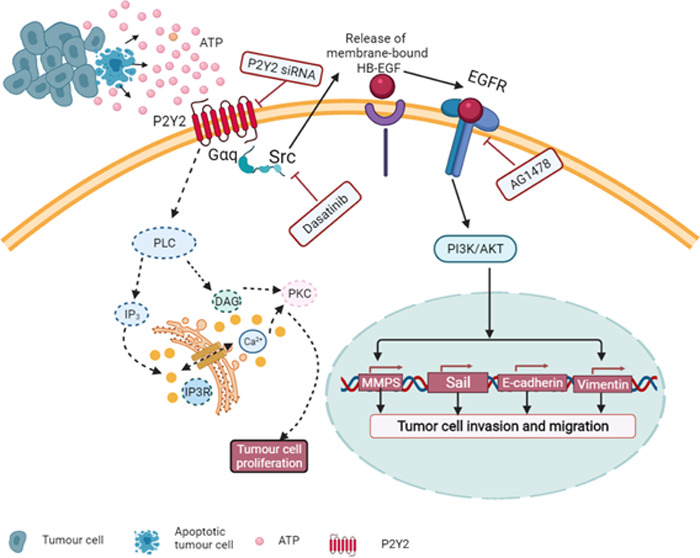
Extracellular ATP can activate many signaling pathways, and selectively modulate Src and EGFR. Based on analysis using the TCGA database data, it was shown that Src and EGFR are highly expressed in squamous cell carcinoma tissue and also that Src and EGRF are closely correlated with P2Y2. Src, a nonreceptor tyrosine kinase, can cause phosphorylation of tyrosine residues by substrate, serving as a signal transducer of the cell surface receptor.37,38 Studies have shown that Src is excessively expressed and highly activated in OSCC and is a cancer protein that drives OSCC progression. Furthermore, Src has been shown to have a close relationship with the progression, migration, and prognosis of solid tumors,39 The Src family protein kinase has been demonstrated to mediate epidermal growth factor receptor (EGFR)-dependent and nondependent passages, and can even act as an upstream activator of EGFR.40,41 EGFR is considered to be the main goal of a new treatment for OSCC, as it is overexpressed in the advanced stage of the disease and prognosis in OSCC patients.42,43 It has been confirmed that GPCR-induced cell migration requires the participation of EGFR. The mechanism may be that GPCR activates MMP to release heparin-binding epidermal growth factor (HB-EGF), which is originally bound to the cell surface or extracellular matrix, and subsequently interacts with EGFR. The P2Y receptor is activated by attracting nonreceptor tyrosine protein kinase Src phosphorylated EGFR.44,45
The phosphatidylinositol-3-kinase (PI3K)/AKT signaling pathway is involved in the regulation of various cell activities, and the activation of the PI3K/AKT signaling pathway can regulate the growth, proliferation, apoptosis, and energy metabolism of tumor cells.46,47 Many studies have confirmed that AKT can increase the glycolysis level of tumor cells and promote the production of ATP without affecting aerobic oxidation, thus providing sufficient substances for biosynthesis. Abnormal activation of PI3K/AKT signaling has been found in a variety of tumors.48 It was found that ATP promotes MCF-7 cell proliferation through the PI3K/AKT signaling pathway.49 The PI3K/AKT pathway also induces stem-cell-like properties in gastric cancer cells.50 The Src inhibitor PP2 has been shown to inhibit the PI3K activity of colonic cancer cells,51 and Src has been reported to play a role in the upstream modulation of PI3K. Therefore, investigations into blocking this signaling pathway as a potential therapeutic mechanism have been undertaken. The biological function of the PI3K/AKT signaling pathway in tumor progression has been well established, but the role of P2Y2 in its regulation of the PI3K/AKT pathway remains poorly understood. Our study found that Src and EGFR increase in a time-and dose-dependent manner with ATP. ATP upregulates Src and EGFR through P2Y2 expression and then activates the PI3K/AKT pathway. After silencing P2Y2, the expression of Src and EGFR was downregulated; the PI3K/AKT pathway was weakened; and tumor growth, invasion, and migration were significantly inhibited. After adding Src and EGFR inhibitors, the expression of EGFR and PI3K/AKT was significantly inhibited. By considering these results in the context of previous studies, we concluded that P2Y2 promotes the invasion and migration of OSCC by activating the PI3K/AKT signaling pathway through the Src-EGFR axis. P2Y2 is an active regulator in tumor progression.
We found that ATP was involved in tumor metabolism through the P2Y2 receptor. Also, ATP promoted the invasion and migration of OSCC cells through the Src-EGFR axis activation of the PI3K/AKT signaling pathway. These findings provide important new insight into the occurrence and development of OSCC and deliver evidence that P2Y2 has the potential to be a new therapeutic target for OSCC.
Acknowledgments
This research was funded by National Nature Science Foundation of China (nos. 81972072; 81800982), and the Construction Engineering Special Fund of “Taishan Scholars” of Shandong Province (no. tsqn202103177). The authors thank Dr. Rongjian Su for help in designing this research.
Author Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Q.Z., G.X.Z., and M.Q.L.; R.J.S., S.S.L., and Y.Y.K.; and P.P.Y., H.R.L., and T.H. The first draft of the manuscript was written by Q.Z., G.X.Z., and M.Q.L. All authors have made substantial contributions to the conceptualization and design of the study. All authors reviewed and approved the final manuscript.
The authors declare no competing financial interest.
Notes
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Notes
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- Huang Q.; Hsueh C.; Shen Y.; Guo Y.; Huang J.; Zhang Y.; Li J.; Gong H.; Zhou L. Small extracellular vesicle-packaged TGFβ1 promotes the reprogramming of normal fibroblasts into cancer-associated fibroblasts by regulating fibronectin in head and neck squamous cell carcinoma. Cancer Lett. 2021, 10.1016/j.canlet.2021.05.017. [DOI] [PubMed] [Google Scholar]
- Siegel R. L.; Miller K. D.; Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- Purinergic nerves. Lancet 1977, 21331–1332.. 10.1016/s0140-6736(77)90373-7. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F.; Sarti A. C.; Falzoni S.; De Marchi E.; Adinolfi E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat. Rev. Cancer 2018, 18, 601–618. 10.1038/s41568-018-0037-0. [DOI] [PubMed] [Google Scholar]
- Zhao S.; Torres A.; Henry R. A.; Trefely S.; Wallace M.; Lee J. V.; Carrer A.; Sengupta A.; Campbell S. L.; Kuo Y. M.; Frey A. J.; Meurs N.; Viola J. M.; Blair I. A.; Weljie A. M.; Metallo C. M.; Snyder N. W.; Andrews A. J.; Wellen K. E. ATP-Citrate Lyase Controls a Glucose-to-Acetate Metabolic Switch. Cell Rep. 2016, 17, 1037–1052. 10.1016/j.celrep.2016.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Techatharatip O.; Nowwarote N.; Taebunpakul S.; Pavasant P. Biphasic Effect of ATP on In Vitro Mineralization of Dental Pulp Cells. J. Cell. Biochem. 2018, 119, 488–498. 10.1002/jcb.26206. [DOI] [PubMed] [Google Scholar]
- Vultaggio-Poma V.; Sarti A. C.; Di Virgilio F. Extracellular ATP: A Feasible Target for Cancer Therapy. Cells 2020, 9, 2496. 10.3390/cells9112496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol. Ther. 2006, 110, 433–454. 10.1016/j.pharmthera.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Fang W. G.; Pirnia F.; Bang Y. J.; Myers C. E.; Trepel J. B. P2-purinergic receptor agonists inhibit the growth of androgen-independent prostate carcinoma cells. J. Clin. Invest. 1992, 89, 191–196. 10.1172/JCI115562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake A. J.; Wagenbach M. J.; Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature 1994, 371, 519–523. 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Jacobson K. A.; Muller C. E. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 2016, 104, 31–49. 10.1016/j.neuropharm.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F. Purines, purinergic receptors, and cancer. Cancer Res. 2012, 72, 5441–5447. 10.1158/0008-5472.CAN-12-1600. [DOI] [PubMed] [Google Scholar]
- Ratchford A. M.; Baker O. J.; Camden J. M.; Rikka S.; Petris M. J.; Seye C. I.; Erb L.; Weisman G. A. P2Y2 nucleotide receptors mediate metalloprotease-dependent phosphorylation of epidermal growth factor receptor and ErbB3 in human salivary gland cells. J. Biol. Chem. 2010, 285, 7545–7555. 10.1074/jbc.M109.078170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltoff S. P. Related adhesion focal tyrosine kinase and the epidermal growth factor receptor mediate the stimulation of mitogen-activated protein kinase by the G-protein-coupled P2Y2 receptor. Phorbol ester or [Ca2+]i elevation can substitute for receptor activation. J. Biol. Chem. 1998, 273, 23110–23117. 10.1074/jbc.273.36.23110. [DOI] [PubMed] [Google Scholar]
- Li W. H.; Qiu Y.; Zhang H. Q.; Tian X. X.; Fang W. G. P2Y2 Receptor and EGFR Cooperate to Promote Prostate Cancer Cell Invasion via ERK1/2 Pathway. PLoS One 2015, 10, e0133165 10.1371/journal.pone.0133165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G.; Di Virgilio F. Purinergic signalling and cancer. Purinergic Signalling 2013, 9, 491–540. 10.1007/s11302-013-9372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreymueller D.; Pruessmeyer J.; Groth E.; Ludwig A. The role of ADAM-mediated shedding in vascular biology. Eur. J. Cell Biol. 2012, 91, 472–485. 10.1016/j.ejcb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Qiu Y.; Liu Y.; Li W. H.; Zhang H. Q.; Tian X. X.; Fang W. G. P2Y2 receptor promotes the migration and invasion of breast cancer cells via EMT-related genes Snail and E-cadherin. Oncol. Rep. 2018, 39, 138–150. 10.3892/or.2017.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylund G.; Hultman L.; Nordgren S.; Delbro D. S. P2Y2- and P2Y4 purinergic receptors are over-expressed in human colon cancer. Auton Auta. Pharm. 2007, 27, 79–84. 10.1111/j.1474-8673.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- Tak E.; Jun D. Y.; Kim S. H.; Park G. C.; Lee J.; Hwang S.; Song G. W.; Lee S. G. Upregulation of P2Y2 nucleotide receptor in human hepatocellular carcinoma cells. J. Int. Med. Res. 2016, 44, 1234–1247. 10.1177/0300060516662135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L. P.; Zhang X. X.; Jiang S. H.; Tao L. Y.; Li Q.; Zhu L. L.; Yang M. W.; Huo Y. M.; Jiang Y. S.; Tian G. A.; Cao X. Y.; Zhang Y. L.; Yang Q.; Yang X. M.; Wang Y. H.; Li J.; Xiao G. G.; Sun Y. W.; Zhang Z. G. Targeting Purinergic Receptor P2Y2 Prevents the Growth of Pancreatic Ductal Adenocarcinoma by Inhibiting Cancer Cell Glycolysis. Clin. Cancer Res. 2019, 25, 1318–1330. 10.1158/1078-0432.CCR-18-2297. [DOI] [PubMed] [Google Scholar]
- Forrester T. A case of serendipity*. Purinergic Signalling 2008, 4, 93–100. 10.1007/s11302-007-9090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay E. D. An overview of epithelio-mesenchymal transformation. Cells Tissues Organs 1995, 154, 8–20. 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- Lamouille S.; Xu J.; Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Pathophysiology and therapeutic potential of Purinergic Signalling. Pharmacol. Rev. 2006, 58, 58–86. 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- Weisman G. A.; Camden J. M.; Peterson T. S.; Ajit D.; Woods L. T.; Erb L. P2 receptors for extracellular nucleotides in the central nervous system: role of P2X7 and P2Y(2) receptor interactions in neuroinflammation. Mol. Neurobiol. 2012, 46, 96–113. 10.1007/s12035-012-8263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T.; Kalluri R.; Nieto M. A.; Weinberg R. A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. 10.1038/nrc.2017.118. [DOI] [PubMed] [Google Scholar]
- Martínez-Ramírez A.; Garay E.; Garcia-Carranca A.; Vazquez-Cuevas F. G. The P2RY2 Receptor Induces Carcinoma Cell Migration and EMT Through Cross-Talk With Epidermal Growth Factor Receptor. J. Cell. Biochem. 2016, 117, 1016–1026. 10.1002/jcb.25390. [DOI] [PubMed] [Google Scholar]
- Reyna-Jeldes M.; De la Fuente-Ortega E.; Cerda D.; Velazquez-Miranda E.; Pinto K.; Vazquez-Cuevas F. G.; Coddou C. Purinergic P2Y2 and P2X4 Receptors Are Involved in the Epithelial-Mesenchymal Transition and Metastatic Potential of Gastric Cancer Derived Cell Lines. Pharmaceutics 2021, 13, 1234. 10.3390/pharmaceutics13081234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzi N.; Boland R.; Russo de Boland A. Signal transduction pathways associated with ATP-induced proliferation of colon adenocarcinoma cells. Biochim. Biophys. Acta 2010, 1800, 946–955. 10.1016/j.bbagen.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Li W. H.; Qiu Y.; Zhang H. Q.; Liu Y.; You J. F.; Tian X. X.; Fang W. G. P2Y2 receptor promotes cell invasion and metastasis in prostate cancer cells. Br. J. Cancer 2013, 109, 1666–1675. 10.1038/bjc.2013.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R.; Xu J.; Wen G.; Jin H.; Liu X.; Yang Y.; Ji B.; Jiang Y.; Song P.; Dong H.; Tuo B. The P2Y2 nucleotide receptor mediates the proliferation and migration of human hepatocellular carcinoma cells induced by ATP. J. Biol. Chem. 2014, 289, 19137–19149. 10.1074/jbc.M113.540047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevia M. J.; Castro P.; Pinto K.; Reyna-Jeldes M.; Rodriguez-Tirado F.; Robles-Planells C.; Ramirez-Rivera S.; Madariaga J. A.; Gutierrez F.; Lopez J.; Barra M.; De la Fuente-Ortega E.; Bernal G.; Coddou C. Differential Effects of Purinergic Signaling in Gastric Cancer-Derived Cells Through P2Y and P2X Receptors. Front. Pharmacol. 2019, 10, 612. 10.3389/fphar.2019.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone F. B.; Jacques-Silva M. C.; Horn A. P.; Bernardi A.; Schwartsmann G.; Rodnight R.; Lenz G. Extracellular nucleotides and nucleosides induce proliferation and increase nucleoside transport in human glioma cell lines. J. Neurooncol. 2003, 64, 211–218. 10.1023/A:1025699932270. [DOI] [PubMed] [Google Scholar]
- Yang H.; Geng Y. H.; Wang P.; Zhou Y. T.; Yang H.; Huo Y. F.; Zhang H. Q.; Li Y.; He H. Y.; Tian X. X.; Fang W. G. Extracellular ATP promotes breast cancer invasion and epithelial-mesenchymal transition via hypoxia-inducible factor 2α signaling. Cancer Sci. 2019, 110, 2456–2470. 10.1111/cas.14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer R.; Sedehizade F.; Welte T.; Reiser G. ATP- and UTP-activated P2Y receptors differently regulate proliferation of human lung epithelial tumor cells. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2003, 285, L376–85. 10.1152/ajplung.00447.2002. [DOI] [PubMed] [Google Scholar]
- Martin G. S. The hunting of the Src. Nat. Rev. Mol. Cell Biol. 2001, 2, 467–475. 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- Lang L.; Shay C.; Xiong Y.; Thakkar P.; Chemmalakuzhy R.; Wang X.; Teng Y. Combating head and neck cancer metastases by targeting Src using multifunctional nanoparticle-based saracatinib. J. Hematol. Oncol. 2018, 11, 85. 10.1186/s13045-018-0623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irby R. B.; Yeatman T. J. Role of Src expression and activation in human cancer. Oncogene 2000, 19, 5636–5642. 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- Bauman J. E.; Duvvuri U.; Gooding W. E.; Rath T. J.; Gross N. D.; Song J.; Jimeno A.; Yarbrough W. G.; Johnson F. M.; Wang L.; Chiosea S.; Sen M.; Kass J.; Johnson J. T.; Ferris R. L.; Kim S.; Hirsch F. R.; Ellison K.; Flaherty J. T.; Mills G. B.; Grandis J. R. Randomized, placebo-controlled window trial of EGFR, Src, or combined blockade in head and neck cancer. JCI Insight 2017, 2, e90449 10.1172/jci.insight.90449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen B.; Johnson F. M. Regulation of SRC family kinases in human cancers. J. Signal Transduct. 2011, 2011, 865819 10.1155/2011/865819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santuray R. T.; Johnson D. E.; Grandis J. R. New Therapies in Head and Neck Cancer. Trends Cancer 2018, 4, 385–396. 10.1016/j.trecan.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byeon H. K.; Ku M.; Yang J. Beyond EGFR inhibition: multilateral combat strategies to stop the progression of head and neck cancer. Exp. Mol. Med. 2019, 51, 1–14. 10.1038/s12276-018-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norambuena A.; Palma F.; Poblete M. I.; Donoso M. V.; Pardo E.; Gonzalez A.; Huidobro-Toro J. P. UTP controls cell surface distribution and vasomotor activity of the human P2Y2 receptor through an epidermal growth factor receptor-transregulated mechanism. J. Biol. Chem. 2010, 285, 2940–2950. 10.1074/jbc.M109.081166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods L. T.; Jasmer K. J.; Munoz Forti K.; Shanbhag V. C.; Camden J. M.; Erb L.; Petris M. J.; Weisman G. A. P2Y2 receptors mediate nucleotide-induced EGFR phosphorylation and stimulate proliferation and tumorigenesis of head and neck squamous cell carcinoma cell lines. Oral Oncol. 2020, 109, 104808 10.1016/j.oraloncology.2020.104808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S.; Ayala V.; Santillan G.; Boland R. Activation of the PI3K/Akt signaling pathway through P2Y(2) receptors by extracellular ATP is involved in osteoblastic cell proliferation. Arch. Biochem. Biophys. 2011, 513, 144–152. 10.1016/j.abb.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Xu Z. X.; Tan J. W.; Xu H.; Hill C. J.; Ostrovskaya O.; Martemyanov K. A.; Xu B. Caspase-2 promotes AMPA receptor internalization and cognitive flexibility via mTORC2-AKT-GSK3beta signaling. Nat. Commun. 2019, 10, 3622 10.1038/s41467-019-11575-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Shao C.; Lu W.; Yan C.; Yao Q.; Zhu M.; Chen P.; Gu P.; Fu Y.; Fan X. Adenosine triphosphate-induced rabbit corneal endothelial cell proliferation in vitro via the P2Y2-PI3K/Akt signaling axis. Cells Tissues Organs 2014, 199, 131–139. 10.1159/000365654. [DOI] [PubMed] [Google Scholar]
- Scodelaro Bilbao P.; Boland R. Extracellular ATP regulates FoxO family of transcription factors and cell cycle progression through PI3K/Akt in MCF-7 cells. Biochim. Biophys. Acta 2013, 1830, 4456–4469. 10.1016/j.bbagen.2013.05.034. [DOI] [PubMed] [Google Scholar]
- Xu L.; Chen J.; Jia L.; Chen X.; Awaleh Moumin F.; Cai J. SLC1A3 promotes gastric cancer progression via the PI3K/AKT signalling pathway. J. Cell Mol. Med. 2020, 24, 14392–14404. 10.1111/jcmm.16060. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Thamilselvan V.; Craig D. H.; Basson M. D. FAK association with multiple signal proteins mediates pressure-induced colon cancer cell adhesion via a Src-dependent PI3K/Akt pathway. FASEB J. 2007, 21, 1730–1741. 10.1096/fj.06-6545com. [DOI] [PubMed] [Google Scholar]