Abstract
BACKGROUND AND OBJECTIVES
Multiple strategies are used to identify newborn infants at high risk of culture-confirmed early-onset sepsis (EOS). Delivery characteristics have been used to identify preterm infants at lowest risk of infection to guide initiation of empirical antibiotics. Our objectives were to identify term and preterm infants at lowest risk of EOS using delivery characteristics and to determine antibiotic use among them.
METHODS
This was a retrospective cohort study of term and preterm infants born January 1, 2009 to December 31, 2014, with blood culture with or without cerebrospinal fluid culture obtained ≤72 hours after birth. Criteria for determining low EOS risk included: cesarean delivery, without labor or membrane rupture before delivery, and no antepartum concern for intraamniotic infection or nonreassuring fetal status. We determined the association between these characteristics, incidence of EOS, and antibiotic duration among infants without EOS.
RESULTS
Among 53 575 births, 7549 infants (14.1%) were evaluated and 41 (0.5%) of those evaluated had EOS. Low-risk delivery characteristics were present for 1121 (14.8%) evaluated infants, and none had EOS. Whereas antibiotics were initiated in a lower proportion of these infants (80.4% vs 91.0%, P < .001), duration of antibiotics administered to infants born with and without low-risk characteristics was not different (adjusted difference 0.6 hours, 95% CI [−3.8, 5.1]).
CONCLUSIONS
Risk of EOS among infants with low-risk delivery characteristics is extremely low. Despite this, a substantial proportion of these infants are administered antibiotics. Delivery characteristics should inform empirical antibiotic management decisions among infants born at all gestational ages.
What’s Known on This Subject:
Low-risk delivery characteristics, including cesarean delivery, no labor, no membrane rupture before delivery, and no concern for intraamniotic infection or nonreassuring fetal status, can identify preterm newborns at lowest risk of infection to guide initiation of empirical antibiotics.
What This Study Adds:
The risk of EOS among infants of all gestational ages born in the setting of low-risk delivery characteristics is extremely low. Delivery characteristics should inform empirical antibiotic management decisions at birth among infants of all gestational ages.
Newborn infants are at risk for early-onset sepsis (EOS), defined as isolation of a pathogen from blood or cerebrospinal fluid (CSF) culture obtained at ≤72 hours after birth. EOS causes significant morbidity and mortality.1 Predicting which infants will develop EOS is challenging for neonatal care providers and leads to relatively higher rates of antibiotic use compared to the proportion infected.2 Multiple studies suggest that intrapartum, neonatal, and especially prolonged neonatal antibiotic exposures are associated with adverse outcomes among preterm infants and longer-term morbidities among term infants, underscoring the need for refined infection risk assessment among this population.3–10
The pathogenesis of EOS is most commonly that of ascending colonization and infection of the fetus and newborn with maternal gastrointestinal and genitourinary bacteria.11,12 Therefore, delivery characteristics may help providers predict which infants are at lowest risk of EOS and assist in determining whether empirical antibiotic therapy is indicated. For instance, among extremely preterm infants, the combination of cesarean delivery, rupture of membranes at delivery, and absence of intraamniotic infection identifies infants with a 5- to 12-fold lower relative risk of EOS.13,14 Implementation of an algorithm to manage such infants without initiating antibiotics at birth was found to decrease antibiotic use in 1 recent study without increasing adverse events.15 For term infants, risk prediction models have been developed to refine the approach to EOS risk assessment and have substantially impacted antibiotic use just after birth. However, these models do not incorporate labor or distinguish infants at the lowest risk of EOS.16 Additionally, studies of preterm infants investigating low-risk delivery criteria often exclude moderately preterm and late preterm infants.15 Applying low-risk delivery criteria to infants across all gestations may strengthen prediction models in term infants, improve risk categorization among preterm infants, and lead to improved antibiotic utilization in both these populations.
A robust estimate of EOS risk in relation to delivery characteristics among infants of all gestational ages at birth could significantly contribute to newborn clinical management by identifying newborns unlikely to benefit from empirical antibiotic therapy. Therefore, the objectives of this study were to: (1) determine if delivery characteristics used to identify extremely preterm newborns at lower risk for EOS apply to infants born at all gestational ages; and (2) determine if such characteristics correlate with empirical antibiotic administration and duration. We hypothesized that infants born with specified low-risk delivery characteristics are at lowest risk of infection, but this differential risk does not translate to proportionately lower antibiotic use.
Methods
Data Source and Study Population
This was a retrospective cohort study. The study setting was 2 high-risk perinatal units at academic birth hospitals in Philadelphia, Pennsylvania: Pennsylvania Hospital and Hospital of the University of Pennsylvania. We included all infants born between January 1, 2009 and December 31, 2014, with at least 1 blood culture obtained at ≤72 hours of age with or without CSF culture. We excluded infants born outside of the study centers. Birth census and delivery characteristics for all deliveries were extracted from hospital birth logs. Clinical diagnoses, laboratory data, and medication details were obtained from clinical data repositories. We conducted medical chart review to obtain data missing from data repositories. This study was approved by the institutional review board at the University of Pennsylvania with waiver of informed consent.
Study Definitions
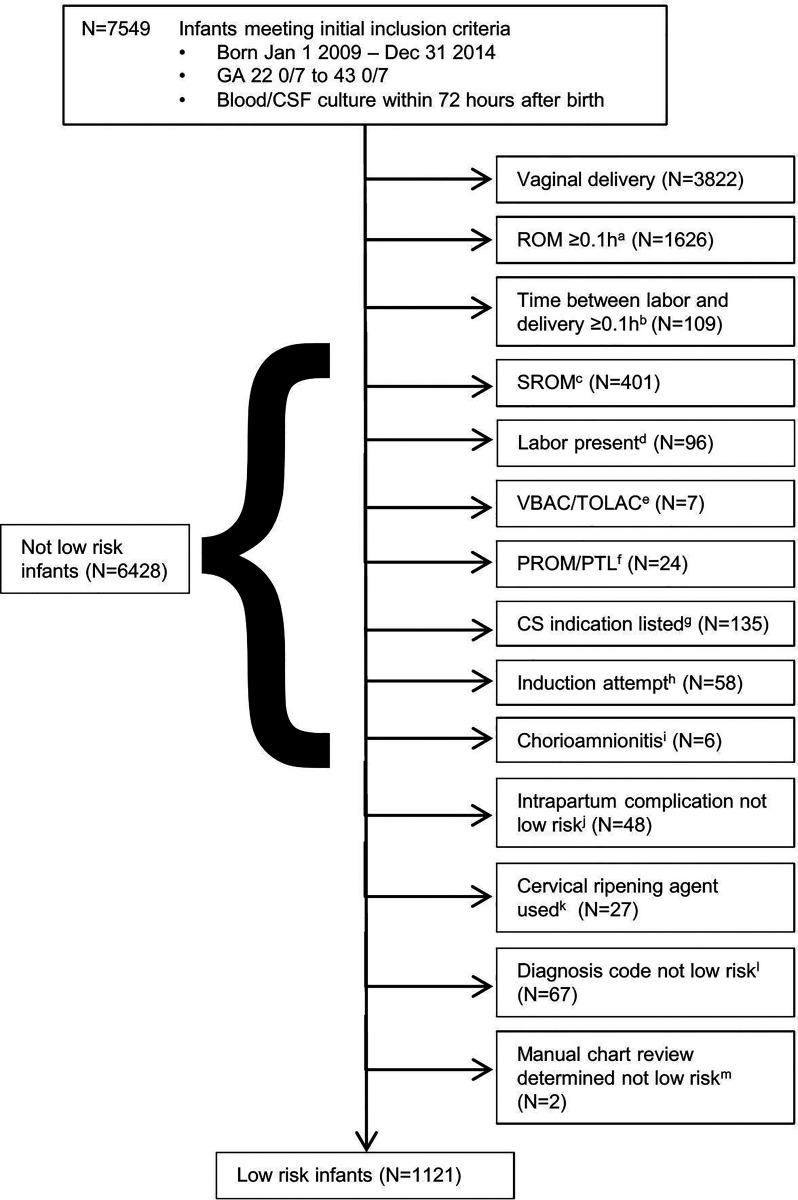
Hypothesized low-risk delivery characteristics were defined as in previous studies13,14 as the presence of all of the following: birth by cesarean delivery; rupture of amniotic membranes at delivery; absence of labor or attempts to induce labor; absence of suspected or confirmed maternal intraamniotic infection; and absence of acute unexplained nonreassuring fetal status. We identified infants as low-risk or not, using a stepwise algorithmic approach based on expected reliability and commonality of the variables of interest, because not all variables were available for all infants (Fig 1). We performed detailed chart review for all cases of EOS.
FIGURE 1.
Algorithmic approach for low-risk delivery characteristics determination. A, ROM ≥0.1 hours and ≤24 hours. B, If ROM <0.1 hours but duration between onset of labor and delivery ≥0.1 hours (6 minutes), then classified as not low-risk. If ROM missing, >24 hours, or implausible (ie, negative number), then classified as not low-risk in the presence of any labor (duration between labor and delivery >0). C, SROM indicated among subjects with ROM negative, missing, or >24 hours. D, Labor and delivery admission reason indicating labor, preterm labor, or ROM. E, Attempt to VBAC or TOLAC. F, PTL and/or PROM indicated; G, cesarean delivery indicating labor, arrest of labor, arrest or failure of descent, secondary arrest of dilation, prolonged labor, failure to progress, failure of induction, failure of vacuum, attempt to VBAC, failure of VBAC, ROM, placental abruption, or cord prolapse. H, Induction of labor indicated; I, Chorioamnionitis present, diagnosed by obstetric provider. J, Intrapartum complication indicating placental abruption, placenta previa with hemorrhage, labor, PROM, chorioamnionitis, or maternal fever. K, Cervical ripening agents (Cervidil, Cytotec, Foley Bulb, or Misoprostol) used indicating trial of vaginal delivery. L, Diagnosis code from delivery encounter indicating labor, PROM, prolonged ROM, placental abruption, chorioamnionitis, maternal fever, or failed induction. M, Chart review only performed for infants with culture-confirmed early-onset sepsis; evaluated for any previous criteria for not low-risk including contractions and nonreassuring fetal status. CSF (cerebrospinal fluid); GA (gestational age); PROM (premature rupture of membranes); PTL (preterm labor); CS (cesarean delivery); ROM (rupture of membranes); SROM (spontaneous rupture of membranes); TOLAC (trial of labor after cesarean delivery); VBAC (vaginal birth after cesarean delivery).
The primary outcome of interest was EOS, defined as at least 1 blood and/or CSF culture obtained ≤72 hours after birth growing a pathogen. Cultures growing coagulase-negative Staphylococci or known commensal organisms were considered contaminants. The secondary outcomes of interest were initiation of empirical antibiotics (antibiotic therapy beginning at ≤72 hours after birth) and duration of antibiotic administration among infants administered empirical antibiotics, for whom cultures were ultimately sterile. We analyzed duration of antibiotics both as a continuous variable (duration in hours) as well as a binary variable (prolonged antibiotic administration, defined as antibiotic therapy beginning at ≤72 hours of age and continued for >72 hours).
Statistical Analysis
Standard descriptive analyses of the demographic and clinical data were performed by using Student’s t test, Mann-Whitney U test, or chi-squared test (χ2) test as appropriate. We determined how many infants with EOS were born in the setting of low-risk delivery characteristics and described the demographics and clinical characteristics of infants with EOS and the causative organisms. The association between delivery characteristics and antibiotic initiation and duration were determined by using linear and logistic regression models. The following covariates were assessed as predictors of antibiotic duration in addition to delivery characteristics: birth weight (BW), gestational age (GA), sex, 5-minute Apgar score, maternal race and ethnicity, and maternal age. The regression models were adjusted for maternal and infant characteristics associated with the study outcome in bivariable testing, as well as sex and center. Two-sided tests were conducted and P < .05 was considered statistically significant. Statistical analyses were performed by using Stata statistical software version 14.2 (StataCorp, College Station, TX).
Results
Characteristics of Study Cohort
Of 53 575 live births during the study period, 7549 infants (14.1%) had a blood culture drawn, of which 1091 (14.5%) also had CSF obtained for culture. Blood cultures were drawn at a median time of 1.4 hours after birth (IQR 0.7–2.8). Demographics and clinical characteristics of the study infants are shown in Table 1. Forty-one infants had culture-confirmed EOS (0.77 cases per 1000 live births; 0.5% of those with blood cultures drawn); all EOS cases were defined by bacteremia, and 1 infant had both bacteremia and meningitis with the same organism. Contaminant species were isolated from blood cultures in 15 additional infants. In no case was a pathogen isolated from CSF culture when the accompanying blood culture was sterile. Escherichia coli (16 of 41, 39.0%) and group B Streptococcus (16 of 41, 39.0%) were the most frequently isolated pathogens (Table 2). Infants with EOS had a median GA of 35 weeks (interquartile range [IQR] 28–39), median BW of 2415 g (IQR 1040–3000), and 28 of 41 (68.3%) were female. All 41 infants with EOS were administered empirical antibiotics. Of the 7508 infants without EOS who had at least 1 blood culture obtained at ≤72 hours of age, 6713 (89.4%) were started on antibiotics, with median duration of antibiotic therapy of 39 hours (IQR 37–59) (Table 1).
TABLE 1.
Demographics and Clinical Characteristics of the Study Infants
| Characteristica | All Infants N = 7549 | Low-Risk N = 1121 | Non-Low-Risk N = 6428 | P |
|---|---|---|---|---|
| GA (completed weeks), median, IQR | 37 (33–39) | 34 (30–37) | 38 (34–40) | <.001 |
| GA category (completed weeks), n, % | <.001 | |||
| <28 | 462 (6.1) | 113 (10.1) | 349 (5.4) | |
| 28–30 | 540 (7.2) | 170 (15.1) | 370 (5.8) | |
| 31–33 | 938 (12.4) | 232 (20.7) | 706 (11.0) | |
| 34–36 | 1480 (19.6) | 299 (26.7) | 1181 (18.4) | |
| ≥37 | 4123 (54.6) | 307 (27.3) | 3816 (59.4) | |
| BW (g), median, IQR | 2859 (2000–3440) | 2067 (1310–2910) | 2970 (2160–3490) | <.001 |
| BW category (g), n, % | <.001 | |||
| <1000 | 497 (6.6) | 150 (13.4) | 347 (5.4) | |
| 1000–1999 | 1379 (18.3) | 382 (34.0) | 997 (15.5) | |
| 2000–2999 | 2256 (29.9) | 323 (28.8) | 1933 (30.1) | |
| ≥3000 | 3412 (45.2) | 266 (23.7) | 3146 (48.9) | |
| Female sex, n, % | 3313 (43.9) | 520 (46.4) | 2793 (43.5) | .068 |
| Maternal race/ethnicity, n, % | <.001 | |||
| African American | 4136 (54.8) | 548 (48.9) | 3588 (55.8) | |
| White | 2186 (29.0) | 438 (39.1) | 1748 (27.2) | |
| Hispanic | 435 (5.8) | 56 (5.0) | 379 (5.9) | |
| Other/unknown | 792 (10.5) | 79 (7.1) | 713 (11.1) | |
| Maternal age (y), median, IQR | 28 (22–33) | 30 (25–35) | 27 (22–32) | <.001 |
| 5-min Apgar score <5, n, % | 331 (4.4) | 65 (5.8) | 266 (4.1) | .004 |
| Length of stay (d), median, IQR | 6 (3–18) | 15 (5–40) | 5 (3–15) | <.001 |
| Early-onset sepsis, n, % | 41 (0.54) | 0 | 41 (0.64) | .003 |
| Antibiotics in first 72 h, n, % | 6754 (89.5) | 901 (80.4) | 5853 (91.1) | <.001 |
| Antibiotic duration, hours, median, IQR | 39 (37–59)b | 47 (37–62)c | 38 (37–51)d | <.001 |
| Prolonged antibiotics, n, % | 1241 (18.5)b | 203 (22.5)c | 1038 (17.9)d | .001 |
BW, birth weight; g, grams; GA, gestational age; IQR, interquartile range.
Missing data are present for the following characteristics: gestational age (n = 6), birth wt (n = 5), maternal age (n = 214), 5-min Apgar score (n = 74).
Denominator (6713) includes infants started on antibiotics in the first 72 h without EOS.
Denominator (901) includes infants started on antibiotics in the first 72 h without EOS.
Denominator (5812) includes infants started on antibiotics in the first 72 h without EOS.
TABLE 2.
Positive Blood Culture Results (n = 56)
| Organism Category | n (%) |
|---|---|
| Pathogenic Species | 41 (73.2) |
| Group B Streptococcusa | 16 (39.0) |
| Other Streptococcia | 4 (9.8) |
| Escherichia colia | 16 (39.0) |
| Haemophilus influenzaea | 1 (2.4) |
| Citrobacter koseria | 1 (2.4) |
| Klebsiella pneumoniaa | 1 (2.4) |
| Morganella morganiia | 1 (2.4) |
| Candida albicansa | 1 (2.4) |
| Contaminant speciesa | 15 (26.8) |
Only blood culture data are shown. One infant had a positive cerebrospinal fluid culture during the study period, with the same organism identified in the blood (group B Streptococcus).
Denominator includes only pathogenic species (n = 41).
Delivery Characteristics and Risk of EOS
Of the 7549 infants with a blood culture drawn, 1121 (14.8%) were born in the setting of the hypothesized low-risk delivery characteristics and 6428 (85.2%) were not (Fig 1). Multiple clinical characteristics differed among those with and without low-risk delivery characteristics, primarily because of the larger proportion of infants with low-risk delivery characteristics who were born at <37 weeks’ gestation (Table 1). All 41 cases of EOS were among the 6428 infants without low-risk delivery characteristics. In 2 of 41 cases, all low-risk delivery characteristics were met except for the presence of acute unexplained nonreassuring fetal status. In one of these cases, delivery was by emergent cesarean delivery at 36 weeks’ gestation because of the absence of fetal heart rate. The infant required delivery room resuscitation with Apgar scores of 0 at 1 minute and 0 at 5 minutes, and was subsequently treated with mechanical ventilation, pressor medications, and therapeutic hypothermia; the blood culture grew group B Streptococcus. In the second case, delivery was by emergent cesarean delivery at 35 weeks’ gestation because of concern for placental abruption with severe maternal abdominal pain, contractions, and nonreassuring fetal heart tracing. The infant was well-appearing at birth, but blood culture grew α-hemolytic Streptococcus (not group D).
Delivery Characteristics and Empirical Antibiotic Administration Among Infants Without EOS
Of the 7508 infants without EOS, 6713 (89.4%) were started on antibiotics (901 of 1121 [80.4%] in the low-risk group and 5812 of 6387 [91.0%] in the not low-risk group). Low-risk infants without EOS who were started on antibiotics were more likely to have prolonged antibiotic duration (>72 hours) than non-low-risk infants without EOS (203 of 901 [22.5%] vs 1038 of 5812 [17.9%]) (Table 1). The unadjusted median duration of antibiotic therapy among uninfected infants who were started on antibiotics was significantly longer among those with low-risk delivery characteristics compared to those without (Table 1). After adjustment for GA, sex, maternal race and ethnicity, maternal age, 5-minute Apgar, and center, low-risk infants had lower odds of antibiotic initiation (Table 3). Among uninfected infants started on antibiotics, there was no difference in either the adjusted odds of prolonged antibiotic duration or antibiotic duration in hours by the presence or absence of low-risk delivery characteristics (Table 3).
TABLE 3.
Bivariable and Multivariable Regression of Antibiotic Initiation and Duration and Low-Risk Delivery Characteristics Among Infants Without Early-Onset Sepsis
| Low-Risk | Not Low-Risk | Low-Risk Compared to Not Low-Risk | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | OR (95% CI) | P | aOR (95% CI)d | P | ||
| Antibiotic initiationa | 901 | 80.4 | 5812 | 91.0 | 0.4 (0.3, 0.5) | <.001 | 0.5 (0.4, 0.6) | <.001 | |
| Prolonged antibioticsb,c | 203 | 22.5 | 1038 | 17.9 | 1.3 (1.1, 1.6) | .001 | 1.1 (0.9, 1.3) | ||
| Median | IQR | Median | IQR | Unadjusted difference (95% CI) | P | Adjusted difference (95% CI)e | P | ||
| Antibiotic duration (hours)b | 47 | 37–62 | 38 | 37–51 | 9.3 (4.8, 13.8) | <.001 | 0.6 (−3.8, 5.1) | .79 | |
aOR, adjusted odds ratio; CI, confidence interval; IQR, interquartile range; OR, odds ratio.
Includes infants who did not have early-onset sepsis (n = 7508).
Includes infants started on antibiotics in first 72 h after birth who did not have early-onset sepsis (n = 6713).
Prolonged antibiotics defined as antibiotics continued for more than 72 h after initiation.
Logistic regression (complete case) adjusted for center, gestational age, sex, 5-min Apgar, maternal race/ethnicity, and maternal age.
Linear regression (complete case) adjusted for center, gestational age, sex, 5-min Apgar, maternal race/ethnicity, and maternal age.
To assess if rate of antibiotic use was proportionate to the incidence of EOS in each group, we calculated proportion of infants initiated on antibiotics for each case of culture-confirmed EOS identified. Among infants outside of low-risk delivery criteria, 157 infants were started on antibiotics for each case of EOS. Because no cases of EOS were identified in the low-risk group, this proportion could not be calculated but suggests that antibiotic exposure in this group was disproportionately higher for incidence of EOS.
Discussion
Over a 6-year period among 53 575 newborns of all gestational ages at 2 academic centers, no infant with culture-confirmed EOS was born in the setting of low-risk delivery characteristics. In addition, 80% of such infants were empirically treated with antibiotics, and these characteristics were not associated with a shorter duration of treatment, suggesting that clinicians did not appreciate or account for the lower risk of infection. These findings have important implications for EOS risk assessment among term and late preterm infants; for EOS risk assessment among preterm infants; and for antibiotic management decisions among infants with sterile cultures born across the gestational age spectrum.
Given the risks of morbidity and mortality with EOS, it is not surprising that newborns are often administered empirical antibiotic therapy. This is especially true for preterm infants, who have a relatively higher prior probability of EOS along with inherent clinical instability at birth. Rates of empirical antibiotic therapy for preterm infants are alarmingly high: ∼90% of extremely preterm infants are started on antibiotics at birth.17 This is aligned with long-recommended practice, because guidance has historically advocated for the use of antibiotics in all preterm newborns with critical illness.18 More recently, evidence suggests that EOS risk assessment strategies for preterm infants could be based on delivery characteristics and/or placental pathologic findings that correlate with culture-confirmed infection.13,14 One study of infants born at 22 to 28 weeks’ gestation in Neonatal Research Network centers found that risk factors established at birth could be used to identify a subset of premature infants at lower risk of EOS. Investigators defined “low-risk” characteristics as cesarean delivery, with rupture of membranes at delivery, and absence of clinical chorioamnionitis.13 On the basis of limitations of the Neonatal Research Network data collection, the study limited the question to extremely preterm infants and important delivery characteristics were not included such as labor or attempts to induce labor. Our center recently reported on the prospective use of delivery characteristics to identify preterm infants at lowest risk of EOS. We observed significant reduction of antibiotic use among very preterm infants without short-term safety concerns.15 Our findings support the American Academy of Pediatrics Committee on the Fetus and Newborn EOS guideline published in 2018, which advocates that EOS risk assessment among preterm infants be based on delivery characteristics and enhances these recommendations by providing data for moderately preterm infants.19
In the United States, an estimated 400 000 uninfected term infants receive empirical antibiotics at birth every year, although rates of antibiotic exposure vary widely by center.20–22 Assessing the risk of EOS in term and late preterm infants has historically been based on published algorithms by using established risk factors in dichotomous fashion, as well as subjective assessment of risk. More recently, alternative strategies have emerged, such as the use of multivariate models that include objective maternal risk factor data and the evolving newborn condition to provide a quantitative estimate of risk.23 These models were used to develop the neonatal early-onset sepsis calculator, which provides infant-specific EOS risk estimates for term and late preterm infants.24 Prospective validation of these models demonstrate that centers using these for newborn management significantly and safely decrease the number of EOS evaluations and decrease empirical antibiotic exposure compared to previous approaches.25,26 The components of the multivariable model include GA at delivery, duration of rupture of amniotic membranes, group B Streptococcus colonization status, highest intrapartum maternal temperature, and intrapartum antibiotic administration. Given the limitations of the data set used to create the calculator, absence or presence of labor or attempts to induce labor were not included. We addressed this limitation using an algorithmic approach and detailed chart review to identify labor, attempts to induce labor, and nonreassuring fetal status among a large birth cohort. We were not able to calculate sepsis risk scores for the infants in this study, although the risk estimate at birth is likely to be very low for a term infant born to an afebrile mother with rupture of membranes at the moment of delivery. The neonatal early-onset sepsis calculator takes a Bayesian approach to estimating the risk of EOS for an individual infant, beginning with the prior probability of infection. One possible implication of our current study would be to determine that term infants born in the setting of low-risk delivery characteristics have a near-zero prior probability of EOS, and to exempt them in most circumstances from EOS consideration. Such a management approach would be similar to the approach currently recommended by the American Academy of Pediatrics for risk assessment among preterm infants.19
Newborn infants started on empirical antibiotic therapy are often continued on such therapy for varying durations despite sterile cultures.27 Kiser et al reported in 2014 at their institution that 24.2% of term and late preterm infants at risk for EOS because of maternal chorioamnionitis received antibiotics for ≥7 days.28 Furthermore, other studies demonstrate that more than half of very preterm infants receive prolonged antibiotic therapy (more than 3 to 4 days) despite negative cultures.6,29,30 Recent evidence suggests that both early and prolonged neonatal antibiotic exposure are associated with adverse outcomes among preterm infants, including necrotizing enterocolitis, invasive fungal infection, dysbiosis, and subsequent infection and colonization with resistant organisms, without longer-term survival or neurodevelopmental advantage.5,6,8,13,31 Accordingly, recommendations regarding duration of antibiotic therapy for suspected EOS continue to evolve.32,33 We found no evidence that infants with and without low-risk characteristics differed with respect to use of antibiotics for prolonged durations in the setting of sterile cultures. If clinicians are uncomfortable not starting antibiotics, recognition of differential risk may at least provide support for stopping such treatments when cultures fail to identify an infecting pathogen.
A strength of our study is the inclusion of moderately preterm infants, for whom EOS risk assessment has rarely been studied, despite the fact that these infants make up a large portion of NICU admissions.34,35 Previous studies have focused on infants born either close to term or those born at the earliest gestational ages.13,14,16,20 The algorithmic criteria we applied in this study can be readily translated into algorithms with clinical utility to distinguish infants with low-risk delivery characteristics. Our study does have limitations. The study was performed at 2 centers in 1 health system and may not be widely generalizable, and the study period was from 2009 to 2014, before the implementation of more refined EOS risk assessment strategies. We were unable to assess the impact of laboratory results on antibiotic initiation and continuation, and the centers did not routinely obtain anaerobic blood cultures during the study period. We were not able to uniformly delineate the exact indication for delivery. There were 2 EOS cases that required further detailed chart review to determine that delivery circumstances were not low-risk, emphasizing the importance of real-time attention to clinical detail. In addition, we did not perform detailed chart review for uninfected infants which could lead to a misclassification bias. We do not suggest that application of our findings to data derived from a medical record alone would be adequate to determine individual infant risk of EOS. Risk assessment tools should not make decisions; they should instead be used by experienced clinicians to make informed choices.
Conclusions
A well-defined subset of infants born at all gestational ages are at low-risk of EOS and may be spared empirical and/or prolonged antibiotic exposure. The implications of our study include potential adjustments to sepsis risk assessment in term infants, and confirmation and enhancement of previous studies that identify a subset of lower risk preterm infants.
Glossary
- BW
birth weight
- CSF
cerebrospinal fluid
- EOS
early-onset sepsis
- GA
gestational age
Footnotes
Dr Flannery conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript; Dr Mukhopadhyay contributed to the study design and analytic plan, assisted with data acquisition and cleaning, and reviewed and revised the manuscript; Dr Dhudasia assisted with data acquisition and cleaning, and reviewed and revised the manuscript; Ms Passarella contributed to the study design and analytic plan, and reviewed and revised the manuscript; Dr Morales contributed to the study design and analytic plan, and reviewed and revised the manuscript; Dr Gerber contributed to the study design and analytic plan, and reviewed and revised the manuscript; Dr Puopolo conceptualized and designed the study and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FUNDING: Dr Flannery reports receiving research funding from the Agency for Healthcare Research and Quality (grant K08HS027468), from 2 contracts with the Centers for Disease Control and Prevention, and from the Children’s Hospital of Philadelphia. Dr Mukhopadhyay reports receiving research funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant K23HD088753) and from the Children’s Hospital of Philadelphia. Dr Puopolo reports receiving research funding from the National Institutes of Health (grants 5UG1HD068244 and 5R01AI121383), from 2 contracts with the Centers for Disease Control and Prevention, and from the Children’s Hospital of Philadelphia. The other authors received no external funding. The funders and sponsors had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscripts; or decision to submit the manuscript for publication.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2021-054221.
References
- 1. Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27(1): 21–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taylor JA, Opel DJ. Choriophobia: a 1-act play. Pediatrics. 2012;130(2):342–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cotten CM, McDonald S, Stoll B, Goldberg RN, Poole K, Benjamin DK Jr; National Institute for Child Health and Human Development Neonatal Research Network . The association of third- generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics. 2006;118(2):717–722 [DOI] [PubMed] [Google Scholar]
- 4. Cotten CM. Adverse consequences of neonatal antibiotic exposure. Curr Opin Pediatr. 2016;28(2):141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159(5):720–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ting JY, Synnes A, Roberts A, et al. ; Canadian Neonatal Network Investigators . Association between antibiotic use and neonatal mortality and morbidities in very low-birth-weight infants without culture-proven sepsis or necrotizing enterocolitis. JAMA Pediatr. 2016; 170(12):1181–1187 [DOI] [PubMed] [Google Scholar]
- 7. Cantey JB, Huffman LW, Subramanian A, et al. Antibiotic exposure and risk for death or bronchopulmonary dysplasia in very low birth weight infants. J Pediatr. 2017;181:289–293.e1 [DOI] [PubMed] [Google Scholar]
- 8. Mukhopadhyay S, Puopolo KM, Hansen NI, et al. Impact of early-onset sepsis and antibiotic use on death or survival with neurodevelopmental impairment at 2 years of age among extremely preterm infants. J Pediatr. 2020; 221:39–46.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koebnick C, Sidell MA, Getahun D, et al. Intrapartum antibiotic exposure and body mass index in children. Clin Infect Dis. 2021;73(4):e938–e946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mukhopadhyay S, Lieberman ES, Puopolo KM, Riley LE, Johnson LC. Effect of early-onset sepsis evaluations on in-hospital breastfeeding practices among asymptomatic term neonates. Hosp Pediatr. 2015;5(4):203–210 [DOI] [PubMed] [Google Scholar]
- 11. Baker CJ, Barrett FF. Transmission of group B streptococci among parturient women and their neonates. J Pediatr. 1973;83(6):919–925 [DOI] [PubMed] [Google Scholar]
- 12. Benirschke K. Routes and types of infection in the fetus and the newborn. AMA J Dis Child. 1960;99:714–721 [DOI] [PubMed] [Google Scholar]
- 13. Puopolo KM, Mukhopadhyay S, Hansen NI, et al. ; NICHD Neonatal Research Network . Identification of extremely premature infants at low risk for early-onset sepsis. Pediatrics. 2017;140(5): e20170925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mukhopadhyay S, Puopolo KM. Clinical and microbiologic characteristics of early-onset sepsis among very low birth weight infants: opportunities for antibiotic stewardship. Pediatr Infect Dis J. 2017;36(5):477–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garber SJ, Dhudasia MB, Flannery DD, Passarella MR, Puopolo KM, Mukhopadhyay S. Delivery-based criteria for empiric antibiotic administration among preterm infants. J Perinatol. 2021;41(2): 255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puopolo KM, Draper D, Wi S, et al. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. Pediatrics. 2011;128(5):e1155–e1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flannery DD, Ross RK, Mukhopadhyay S, Tribble AC, Puopolo KM, Gerber JS. Temporal trends and center variation in early antibiotic use among premature infants. JAMA Netw Open. 2018;1(1):e180164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Polin RA; Committee on Fetus and Newborn . Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012;129(5):1006–1015 [DOI] [PubMed] [Google Scholar]
- 19. Puopolo KM, Benitz WE, Zaoutis TE; Committee on Fetus and Newborn; Committee on Infectious Diseases . Management of neonates born at ≤34 6/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics. 2018;142(6):e20182896. [DOI] [PubMed] [Google Scholar]
- 20. Escobar GJ, Puopolo KM, Wi S, et al. Stratification of risk of early-onset sepsis in newborns ≥ 34 weeks’ gestation. Pediatrics. 2014;133(1):30–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oliver EA, Reagan PB, Slaughter JL, Buhimschi CS, Buhimschi IA. Patterns of empiric antibiotic administration for presumed early-onset neonatal sepsis in neonatal intensive care units in the United States. Am J Perinatol. 2017;34(7):640–647 [DOI] [PubMed] [Google Scholar]
- 22. Mukhopadhyay S, Taylor JA, Von Kohorn I, et al. Variation in sepsis evaluation across a national network of nurseries. Pediatrics. 2017;139(3):e20162845. [DOI] [PubMed] [Google Scholar]
- 23. Wortham JM, Hansen NI, Schrag SJ, et al. Early-onset sepsis: a predictive model based on maternal risk factors. Pediatrics. 2016;6(2):690–695 [Google Scholar]
- 24. Puopolo KM, Escobar GJ. Early-onset sepsis: a predictive model based on maternal risk factors. Curr Opin Pediatr 2013;25(2):161–166. [DOI] [PubMed] [Google Scholar]
- 25. Dhudasia MB, Mukhopadhyay S, Puopolo KM. Implementation of the sepsis risk calculator at an academic birth hospital. Hosp Pediatr. 2018;8(5):243–250 [DOI] [PubMed] [Google Scholar]
- 26. Kuzniewicz MW, Puopolo KM, Fischer A, et al. A Quantitative, Risk-Based Approach to the Management of Neonatal Early-Onset Sepsis. JAMA Pediatr. 2017;171(4):365–371 [DOI] [PubMed] [Google Scholar]
- 27. Ting JY, Roberts A, Sherlock R, et al. ; Canadian Neonatal Network Investigators . Duration of initial empirical antibiotic therapy and outcomes in very low birth weight infants. Pediatrics. 2019;143(3):e20182286. [DOI] [PubMed] [Google Scholar]
- 28. Kiser C, Nawab U, McKenna K, Aghai ZH. Role of guidelines on length of therapy in chorioamnionitis and neonatal sepsis. Pediatrics. 2014; 133(6):992–998 [DOI] [PubMed] [Google Scholar]
- 29. Cotten CM, Taylor S, Stoll B, et al. ; NICHD Neonatal Research Network . Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cordero L, Ayers L. Duration of empiric antibiotics for suspected early-onset sepsis in extremely low birth weight infants. Infect Control Hosp Epidemiol. 2018;24(9):662–666 [DOI] [PubMed] [Google Scholar]
- 31. Flannery DD, Puopolo KM. Neonatal antibiotic use: what are we doing and where shall we go? NeoReviews. 2018;19(9):e516–e525 [Google Scholar]
- 32. Wortham JM, Hansen NI, Schrag SJ, et al. ; Eunice Kennedy Shriver NICHD Neonatal Research Network . Chorioamnionitis and culture-confirmed, early-onset neonatal infections. Pediatrics. 2016;137(1):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Higgins RD, Saade G, Polin RA, et al. ; Chorioamnionitis Workshop Participants . Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop. Obstet Gynecol. 2016;127(3):426–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Escobar GJ, McCormick MC, Zupancic JA, et al. Unstudied infants: outcomes of moderately premature infants in the neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed. 2006;91(4):F238–F244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boyle EM, Johnson S, Manktelow B, et al. Neonatal outcomes and delivery of care for infants born late preterm or moderately preterm: a prospective population-based study. Arch Dis Child Fetal Neonatal Ed. 2015;100(6): F479–F485 [DOI] [PMC free article] [PubMed] [Google Scholar]