Abstract

We show that recently developed quantum Monte Carlo methods, which provide accurate vertical transition energies for single excitations, also successfully treat double excitations. We study the double excitations in medium-sized molecules, some of which are challenging for high-level coupled-cluster calculations to model accurately. Our fixed-node diffusion Monte Carlo excitation energies are in very good agreement with reliable benchmarks, when available, and provide accurate predictions for excitation energies of difficult systems where reference values are lacking.
1. Introduction
An open challenge in computational chemistry is to develop a method which can accurately treat different types of excited states on an equal footing at an affordable cost. An effective way to assess the accuracy of different methods is by comparing vertical transition energies (VTEs) with the theoretical best estimates (TBEs) determined from high-level computational methods. Environmental effects can influence the measured transition energy in the experiment while VTEs calculated using the same molecular geometry are directly comparable.
Conventional single-reference methods, such as time-dependent density functional theory (TD-DFT)1−3 and coupled-cluster (CC) theories (equation-of-motion/linear response4), struggle to model double excitations, that is, excitations whose determinant expansions are dominated by determinants having two orbitals differing from a given reference determinant. The most commonly used, best-scaling, version of TD-DFT uses a linear response formalism which cannot treat multi-electron excitations. A relatively uniform description of singly and doubly excited states relies on workarounds and ad-hoc choices of the exchange–correlation functional.5−10
In theory, CC can capture double excitations, but in practice the excitation level must be truncated. For an M-electron excitation, CC must include at least M + 1 excitations to include a satisfactory amount of correlation,11 but often the M + 2 level theory is needed to obtain reasonable excitation energies.12−16 CC theories needed to treat single and double excitations such as CC317 and CCSDT18 (for singles), and CC419 and CCSDTQ20 (for doubles) scale poorly with system size as N7, N8, N9, and N10, respectively, (with N the number of electrons) limiting their application primarily to small molecules.
Multi-reference methods such as complete active space self-consistent field (CASSCF), CASSCF with a second-order perturbation energy correction (CASPT2),21 and the second-order n-electron valence state perturbation theory (NEVPT2)22 are better suited to treat double excitations. Unfortunately, these methods scale exponentially with the number of orbitals and electrons in the active space, limiting them to small active spaces and system size. CASPT2 tends to underestimate the VTEs of organic molecules, and a shift is generally introduced in the zeroth-order Hamiltonian to provide better global agreement.23 These multi-reference methods also rely on chemical intuition to choose which orbitals to include in the active space, but it is often unintuitive which active space will capture the important determinants of a given state.
Selected configuration interaction (sCI)24,25 methods are capable of obtaining double excitation energies and have recently been shown to reach full-CI (FCI) quality energies for small molecules.26 Among these approaches is the CI perturbatively selected iteratively (CIPSI)24 method in which determinants are selected based on their contribution to the second-order perturbation (PT2) energy, so that the most energetically relevant determinants are included in the determinant expansion first. This selection criterion both circumvents the excitation-ordered exponential expansion of the wave function used by CI and CC methods and removes the dependence on one’s chemical intuition to decide which determinants to include in the expansion.
Quantum Monte Carlo (QMC) methods, specifically, variational (VMC) and fixed-node diffusion Monte Carlo (DMC) are promising first-principles approaches for solving the Schrödinger equation stochastically. These methods scale favorably with system size as O(N4) and naturally parallelize.27−29 In addition, recent improvements in QMC algorithms30−33 allow for fast optimization of trial wave functions with thousands of parameters at a cost per Monte Carlo step of O(N3) + O(Ndet),32 where Ndet is the number of determinants in the wave function. When using multi-determinant expansions, provided by the CIPSI method, fully optimized in the presence of a Jastrow factor as trial wave functions, VMC and DMC have been shown to provide accurate excitation energies.34−36 The role of the Jastrow factor is to account for dynamic correlations allowing for even shorter determinant expansions.37−44
It is shown here that the same VMC and DMC protocols, used previously to calculate single excitations,36 can be used to treat double excitations just as precisely. The pure double excitations of nitroxyl, glyoxal, and tetrazine are calculated using VMC and DMC. In addition, a prediction is made for two excitation energies in cyclopentadienone which have strong double-excitation character in both excited states. The trial wave functions of the ground and excited states are optimized simultaneously, maintaining orthogonality on-the-fly by imposing an overlap penalty.
2. Methods
In the following, we present how we build the trial wave functions for the QMC calculations and how we optimize them in VMC energy minimization using a penalty-based, state-specific scheme for excited states, similar to previous approaches.45−47
2.1. Wave Functions
The QMC trial wave functions take the Jastrow–Slater form
| 1 |
where Ci are spin-adapted configuration state functions (CSFs), cIi are the expansion coefficients, JI are the Jastrow factors, which include electron−electron and electron−nucleus pair correlations, and {ϕ}I are the one-particle orbitals. In all calculations presented here, the ground and excited states have the same symmetry, so the CSFs, Ci , share the same orbital occupation patterns for all states. Note that each state, I, in eq 1, has its own optimal Jastrow factor, set of orbitals, and expansion coefficients. When each state has its own optimizable Jastrow and orbital parameters, the approach is referred to as a state-specific optimization, as opposed to a state-average optimization where all states share a common Jastrow factor and set of orbitals, and only the expansion coefficients are state-dependent.35,37
The expansion coefficients and orbitals are initialized for the VMC optimization by one of the two methods: (i) a Nstate-state-average [SA(Nstate)] CASSCF calculation or (ii) a CIPSI calculation, which builds off of approach (i). In approach (i), the SA-CASSCF wave functions are used as starting determinant components in the VMC optimization. In approach (ii), one only utilizes the orbitals produced by the SA-CASSCF calculation as the molecular orbital basis for a CIPSI expansion; in the VMC optimization, the starting determinant components include the CASSCF orbitals and CIPSI coefficients, cIi.
Alternatively, in approach (ii), the natural orbitals (NO) are calculated from a first CIPSI expansion followed by a second CIPSI calculation in the NO basis. Performing a CIPSI expansion in the NO basis tends to lead to a smoother convergence to lower energies with fewer determinants14 compared to using a basis of SA-CASSCF orbitals. In other words, one can obtain a higher quality wave function with fewer determinants via relaxation of the molecular orbital basis.
Further details on the CASSCF initial wave functions and CIPSI calculations using the NO basis are provided in the Supporting Information.
2.2. Penalty-Based State-Specific Method
When trying to optimize a state which is not the lowest in its symmetry class, the state can collapse to a lower-energy eigenstate. A number of approaches have already been proposed to remedy this issue within QMC, including state-average energy minimization37 and state-specific variance minimization.35 The former requires that all states share a common Jastrow factor and set of orbitals. While the state-average approach has previously been shown to provide accurate results for excited states,36,37 the shared Jastrow factor and orbitals naturally compromise the flexibility of the wave functions. The latter approach48−51 is attractive as it avoids the non-variationality of the excited state energy and the need for extra constraints but, during long optimizations, it was found that one may lose the state of interest due to shallow or no minima in the associated functional.35
Another option to avoid collapse of the excited state is to use state-specific energy minimization with constraints. One way to do this is to impose orthogonality between the higher energy state and all states lower in energy. To this end, we employ a penalty-based state-specific method which requires minimizing the objective function47
| 2 |
where EI is given by
| 3 |
ELI is the local energy, HΨI/ΨI, and ⟨·⟩R∼· denotes the Monte Carlo average of the quantity in brackets over the electron configurations, R, sampled from the indicated distribution (in this case, |ΨI(R)|2). The normalized overlap, SIJ, is given by
| 4 |
In order to simultaneously sample quantities
for multiple states
with comparable efficiency, a guiding function,  , is introduced (see the Supporting Information for the calculation of γI), and the energy of a given state (eq 3) is estimated as a weighted
average
, is introduced (see the Supporting Information for the calculation of γI), and the energy of a given state (eq 3) is estimated as a weighted
average
| 5 |
where now the configurations are sampled from the distribution, ρg = |Ψg|2, and the weight tI is given by tI = |ΨI/Ψg|2. Similar weighted averages are calculated for the derivatives of each state (see the Supporting Information).
The Jastrow, orbital, and CSF parameters of each state are optimized using the stochastic reconfiguration (SR)52,53 method. The gradients used in the SR equations are found by taking the parameter derivatives of eq 2, also derived in the Supporting Information.
In the implementation proposed by Pathak et al.,47 the state being optimized, ΨI, is forced to be orthogonal to fixed, pre-optimized, lower-energy states, or anchor states, ΨJ. Each higher-energy state is then optimized consecutively, imposing orthogonality with all anchor states lower in energy. Each λIJ in eq 2 controls the overlap penalty for the state currently being optimized, ΨI, due to each anchor state, ΨJ. The value of λIJ is suggested to be on the order of, and larger than, the energy spacing between the states.
The method implemented here optimizes all states at the same time with orthogonality imposed on-the-fly. Since all states are changing from one SR step to the next, the overlap penalty must be imposed between all states. Therefore, “≠” is used in the summation in eq 2 instead of “<” so that each state is kept orthogonal to all states, even to those higher in energy. Importantly, in circumstances where states are close in energy, their order may change throughout the optimization and, with “≠” in eq 2, the ordering of the states in energy does not need to be known in advance. Finally, the concomitant optimization of all states leads to a reduced computational cost compared to ref (47) and benefits from the use of correlated sampling in the optimization itself.
2.3. Analysis of λIJ’s Effect on Energies and VTEs
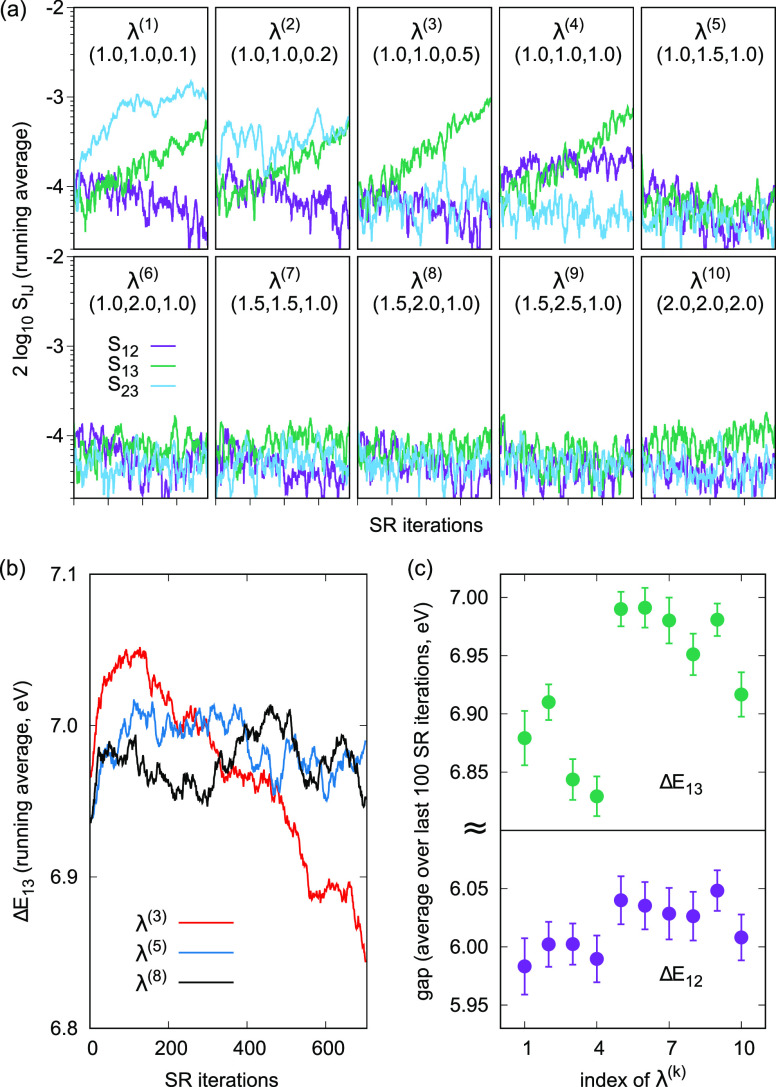
We illustrate the effect of λIJ on the optimization of the three-state cyclopentadienone system with a small CIPSI expansion on CAS orbitals, performing a total of 800 SR iterations for different choices λ(i), each corresponding to a different triplet of values (λ12, λ13, λ23). In general, we expect that if the λIJ are too small, some of the states may lose orthogonality and collapse onto each other; on the other hand, if they are too large, the fluctuations of the overlaps SIJ will be amplified, downgrading the efficiency of the SR minimization because more sampling will be required to pin down the optimal variational parameters at the desired level of statistical precision.
As shown in Figure 1, if all λIJ are set to zero, the excited states collapse toward the ground-state wave function (left panel), whereas they are bounded by the respective eigenstates with the choice of λIJ indicated in the right panel.
Figure 1.
Plot of the VMC energy during optimization of the three-state cyclopentadienone system with a CIPSI wave function with 3036 determinants expanded on CAS orbitals. (Left) Collapse of the excited states to the ground state when λIJ = 0. (Right) Stable optimization using λ12 = 1.5, λ13 = 2.0, and λ23 = 1.0. The energy points in the plot are calculated from a running average of 100 SR iterations.
The selection of suitable values of λIJ is neither difficult nor critical. Figure 2 shows the overlaps |SIJ|2 and excitation energies (ΔEIJ) for various choices of λIJ. In panel (a), for λ(1)–(4), there is a noticeable increase in the overlaps, while the choices of λ(5)–(9) prevent the overlaps from increasing; however, for λ(10), |S13|2 tends to be uniformly larger. Panel (b) shows some examples of the correspondence between increasing overlap and decreasing excitation energy as well as cases where both are stable. Finally, panel (c) shows that all choices λ(5)–λ(9) are equally good in terms of the final estimate of the excitation energies, whereas for λ(1)–λ(4), they (particularly ΔE13) are somewhat smaller and may eventually collapse with more SR iterations. In the case of λ(10), the signal is stable along the iterations, but the excitation energy ΔE13 is somewhat less accurate than that with λ(5)–λ(9); it could be improved with more sampling per SR step, losing, however, efficiency.
Figure 2.
Effect of λIJ on the VMC optimization of the three-state cyclopentadienone system with a CIPSI wave function with 3036 determinants expressed on CAS orbitals. (a) Logarithmic plot of SIJ2 at each SR iteration for different λ(k) = (λ12, λ13, λ23). (b) Running average (100 iterations) of ΔE13 for three different λ(k). (c) Excitations energies averaged over the last 100 SR iterations for each λ(k).
In conclusion, there is a wide range of λIJ (λ(5)–λ(9)) where the estimate of the excitation energies is stable, consistent, and efficient.
In general, the nature of each individual excited state and the trial function itself will impact its sensitivity to the value of λIJ. For instance, in the nitroxyl test case with the two-determinant wave function (see the Supporting Information), even with λIJ = 0, the excited state still cannot collapse completely to the ground state due to orbital symmetries. We also perform an analysis of a three-state optimization of nitroxyl (see the Supporting Information) which reveals little dependence of the VTEs on the choice of λIJ.
As a final note, the number of unique values of λIJ grows as Nstate(Nstate – 1)/2 and, without a formal way to determine appropriate values, the process above can in principle become time-consuming. However, based on the present work, we find that monitoring the overlaps and adjusting λIJ appropriately are effective means to ensure a stable optimization. This can of course also be done in an automated manner.
3. Computational Details
The geometries of nitroxyl, glyoxal, tetrazine, and cyclopentadienone are obtained from ground-state optimizations at the level of CC3/aug-cc-pVTZ.12,13 In all calculations, we utilize scalar-relativistic energy-consistent Hartree Fock (HF) pseudopotentials54,64 with the corresponding aug-cc-pVDZ Gaussian basis sets. The exponents of the diffuse functions are taken from the corresponding all-electron Dunning’s correlation-consistent basis sets.55 For glyoxal, we also tested the use of an aug-cc-pVTZ basis set and found compatible excitation energies both at the VMC and DMC level. All HF and SA-CASSCF calculations are performed in GAMESS(US)56 with equal weights on all states.
CIPSI calculations are performed with the Quantum Package57,58 program. All states of interest are singlets, so determinants are chosen such that the expansions remain eigenstates of Ŝ2 with eigenvalue 0. For each molecule, the ground and excited states of interest have the same symmetry so the same set of orbitals are used in all state’s determinant expansions. States are weighted, and determinants are added to the CIPSI expansion such that the PT2 energy and variance of all states remain similar as this has been shown to give more accurate QMC excitation energies.34−36 The choice of weights in the CIPSI selection criterion is detailed for each molecule in the Supporting Information.
QMC calculations are performed with the CHAMP59 program using the method detailed in Section 2.2. Damping parameters ranging from τSR = 0.05–0.025 a.u. are used in the SR method during VMC optimization. A low-memory conjugate-gradient algorithm60 is used to solve for the wave function parameters in the SR equations. All DMC calculations use a time step of τDMC = 0.02 a.u.
A value of λIJ = 1.0 a.u. is used for all two-state calculations, while multiple values are used for the three-state system, as discussed in the previous section and in the Supporting Information.
4. Results
4.1. Nitroxyl 11A′ → 21A′ Double Excitation
This transition in nitroxyl is a pure double (n, n) → (π*, π*) excitation. The VMC and DMC energies and VTEs are shown in Table 1. There are reliable benchmark calculations for the 11A′ → 21A′ VTE in nitroxyl. In particular, the exFCI/aug-cc-pVQZ (AVQZ) calculation predicts the lowest value for this excitation at 4.32 eV. The latest16 CC4 and CCSDTQ calculations using the aug-cc-pVTZ (AVTZ) basis set somewhat overestimate it at 4.380 and 4.364 eV, respectively.
Table 1. Nitroxyl VMC and DMC Total Energies (a.u.) and Excitation Energies (eV)a.
| VMC |
DMC |
|||||||
|---|---|---|---|---|---|---|---|---|
| WF | # det | # parm | E(11A′) | E(21A′) | ΔE | E(11A′) | E(21A′) | ΔE |
| CIPSI | 321 | 850 | –26.4917(2) | –26.3322(2) | 4.34(1) | –26.5164(2) | –26.3583(2) | 4.30(1) |
| 1573 | 1446 | –26.5001(2) | –26.3415(2) | 4.31(1) | –26.5220(2) | –26.3636(2) | 4.31(1) | |
| 2900 | 2002 | –26.5040(2) | –26.3447(2) | 4.34(1) | –26.5240(2) | –26.3652(2) | 4.32(1) | |
| 7171 | 4124 | –26.5102(2) | –26.3498(2) | 4.36(1) | –26.5267(2) | –26.3677(2) | 4.33(1) | |
| 10,690 | 5712 | –26.5113(2) | –26.3519(2) | 4.34(1) | –26.5273(2) | –26.3686(2) | 4.32(1) | |
| TBEb | 4.33 | |||||||
| CC3/AVQZc | 5.23 | |||||||
| CC4/AVDZd | 4.454 | |||||||
| CC4/AVTZd | 4.380 | |||||||
| CCSDT/AVDZc | 4.756 | |||||||
| CCSDTQ/AVDZc | 4.424 | |||||||
| CCSDTQ/AVTZd | 4.364 | |||||||
| exFCI/AVQZe | 4.32(0) | |||||||
| CASPT2(12,9)/AVQZe | 4.34 | |||||||
| PC-NEVPT2(12,9)/AVQZe | 4.35 | |||||||
CIPSI wave functions are expanded in a CAS orbital basis [SA(2)-CASSCF(12,9)] as well as in an NO basis while matching the PT2 energies and variance. Upon state-specific optimization of the Jastrow-CIPSI wave functions, the VMC and DMC VTEs are very similar for different matching and orbital basis (see Figure 3 and Supporting Information), and all are in very good agreement with benchmarks. In addition, consistency of VMC with the DMC excitation energy is reached at fairly small determinant expansions (∼2000). Due to the fast convergence of the VMC and DMC excitation energies, the use of NOs in the CIPSI expansion is not necessary but still provides similar results.
Figure 3.
Nitroxyl VMC (filled points) and DMC (empty points) excitation energies for different CIPSI wave functions expanded either on starting CAS [SA(2)-CASSCF(12,9)] or NO basis and fully reoptimized in VMC. Horizontal lines represent reference values. TBE: FCI/ATVZ, FCI: exFCI/AVQZ, and CC4: CC4/AVTZ.
Previous results for nitroxyl show that at least CC4 and CCSDTQ are necessary to capture the important correlations of the 11A′ → 21A′ double excitation. There is also a strong basis set dependence on the excitation with CC methods, yet the VMC and DMC calculations, starting from AVDZ with pseudopotentials, reach agreement with expensive quantum chemistry calculations which require the use of at least AVTZ basis set.
4.2. Glyoxal 11Ag → 21Ag Double Excitation
The pure double excitation in glyoxal also corresponds to a (n, n) → (π*, π*) transition. Since the system size is larger compared to nitroxyl, the same level of CC4 and CCSDTQ calculations is not available, and there appears to be a large basis set dependence on the most recent CC4 data.16 A symmetry adapted cluster CI (SAC-CI) calculation of this excitation is available61 (5.66 eV) and agrees well with the recent TBE, which is a basis set corrected FCI/AVDZ value of 5.61 eV.12 These values also agree well with the highest level CCSDTQ estimate for this VTE, 5.670 eV,16 all shown and compared to our QMC results in Table 2.
Table 2. Glyoxal VMC and DMC Total Energies (a.u.) and Excitation Energies (eV)a.
| VMC |
DMC |
|||||||
|---|---|---|---|---|---|---|---|---|
| WF | # det | # parm | E(11Ag) | E(21Ag) | ΔE | E(11Ag) | E(21Ag) | ΔE |
| CIPSI | 3749 | 2908 | –44.6072(2) | –44.3957(2) | 5.76(1) | –44.6564(2) | –44.4482(2) | 5.67(1) |
| 5046 | 3498 | –44.6127(2) | –44.4004(2) | 5.78(1) | –44.6600(2) | –44.4509(2) | 5.69(1) | |
| 7040 | 4500 | –44.6150(2) | –44.4048(2) | 5.72(1) | –44.6613(2) | –44.4534(2) | 5.66(1) | |
| 8642 | 5433 | –44.6181(2) | –44.4098(2) | 5.67(1) | –44.6632(2) | –44.4559(2) | 5.64(1) | |
| 10,010 | 6188 | –44.6205(2) | –44.4114(2) | 5.69(1) | –44.6635(2) | –44.4567(2) | 5.63(1) | |
| 12,132 | 6269 | –44.6237(2) | –44.4145(2) | 5.70(1) | –44.6654(2) | –44.4576(2) | 5.66(1) | |
| 15,349 | 8577 | –44.6276(2) | –44.4180(2) | 5.70(1) | –44.6670(2) | –44.4600(2) | 5.63(1) | |
| TBEb | 5.61 | |||||||
| CC3/AVQZc | 6.76 | |||||||
| CC4/6-31+G*d | 5.699 | |||||||
| CC4/AVDZd | 5.593 | |||||||
| CCSDTQ/6-31+G*d | 5.670 | |||||||
| exFCI/AVDZ | 5.56(11) | |||||||
| SAC-CI/AVDZe | 5.66 | |||||||
| CASPT2(14,12)/AVQZc | 5.43 | |||||||
| PC-NEVPT2(14,12)/AVQZc | 5.52 | |||||||
The number of determinants (# det) and parameters (# parm) in each trial wave function are also presented. The CIPSI expansions are performed on a NO basis and fully reoptimized in VMC.
This value is “safe”, as defined in ref (12), exFCI/AVDZ + (CCSDT/AVTZ-CCSDT/AVDZ).
Ref (13).
Ref (16).
Ref (61), see reference for basis set details.
PT2 and variance-matched CIPSI expansions are generated from the optimal orbitals of a SA(2)-CASSCF(14,12) calculation. As shown in Figure 4, after the optimization of the CIPSI (CAS) wave functions, we observe an increase in the VMC excitation energy as the expansion grows from about 400 to 10,000 determinants. The use of DMC mitigates this increase. The increasing excitation energy is related to the poorer matching at 10,000 determinants than that at smaller expansions for the chosen fixed weights in the CIPSI calculations and is also mirrored in the behavior of the corresponding CIPSI excitations. Using an automated matching algorithm like for cyclopentadienone would resolve the problem (see also the Supporting Information for QMC calculations for glyoxal with different weights). The use of a single set of weights is not an issue for the glyoxal CIPSIs in an NO basis.
Figure 4.
Glyoxal VMC (filled circles) and DMC (empty circles) excitations energies for different CIPSI wave functions expanded either on starting CAS [SA(2)-CASSCF(14,12)] or NO basis and fully reoptimized in VMC. Horizontal lines represent reference values. TBE: FCI/AVDZ + (CCSDT/AVTZ – CCSDT/AVDZ), CC4: CC4/AVDZ, and FCI: exFCI/AVDZ (error bar of ±0.11 eV).
When using NOs built from modest-sized CIPSI expansions (104 determinants) and reperforming the CIPSI expansions, in the presence of the Jastrow factor, the VMC and DMC excitation energies appear to converge nicely and to a value in agreement with available benchmarks. It is possible that still larger CIPSI expansions and more rounds of NO generation could bring the VMC into better alignment with the DMC, which differs at most by 0.05 eV. The DMC value for the largest determinant expansion in the NO basis is 5.63(1) eV, which is ∼0.01 eV from the current TBE value and ∼0.02 eV from the SAC-CI/AVDZ and CC4/AVDZ values. This close agreement and the consistency of the DMC results with different number of determinants in the CIPSI wave functions suggest the value of 5.63(1) eV to be a reasonable prediction for this excitation.
4.3. Tetrazine 11A1g → 21A1g Double Excitation
The tetrazine (s-tetrazine) pure double excitation of interest is another (n, n) → (π*, π*) transition. There are few reliable benchmark calculations available for this transition due to the molecule’s size in addition to its genuine double nature. Although CC3 does not properly treat double excitations, calculations using 6-31+G* up to AVQZ show that there is very little basis set effect, only a change of ∼0.03 eV on the excitation energy. If the CC4/6-31+G* value of 5.06 eV is just as similar for larger basis sets, then this value can be seen as a good guess for the VTE. In any case, without the calculations available to be sure, the VMC and DMC presented in Table 3 suggest a value close to 5.0 eV for the VTE.
Table 3. Tetrazine VMC and DMC Total Energies (a.u.) and Excitation Energies (eV)a.
| VMC |
DMC |
|||||||
|---|---|---|---|---|---|---|---|---|
| WF | # det | # parm | E(11A1g) | E(21A1g) | ΔE | E(11A1g) | E(21A1g) | ΔE |
| CIPSI | 3572 | 2414 | –52.2198(2) | –52.0346(2) | 5.04(1) | –52.2935(3) | –52.1098(3) | 5.00(1) |
| 7025 | 4141 | –52.2300(2) | –52.0451(2) | 5.03(1) | –52.2981(3) | –52.1148(3) | 4.99(1) | |
| 10,723 | 6053 | –52.2379(2) | –52.0528(2) | 5.04(1) | –52.3015(3) | –52.1182(3) | 4.99(1) | |
| TBEb | 4.61 | |||||||
| CC3/6-31+G*c | 6.22 | |||||||
| CC3/AVQZc | 6.19 | |||||||
| CC4/6-31+G*d | 5.06 | |||||||
| CCSDT/AVTZc | 5.96 | |||||||
| exFCI/AVDZ | 5.15(3) | |||||||
| CASPT2(14,10)/AVQZc | 4.68 | |||||||
| PC-NEVPT2(14,10)/AVQZc | 4.60 | |||||||
The VMC optimization of the CAS orbital-based [SA(2)-CASSCF(14,10)] CIPSI expansions tends toward 4.94(1) eV (see the Supporting Information) while the NO-based CIPSI goes to 5.04(1) eV, both converging after about 3600–3700 determinants. While the converged VMC excitation energies are not quite in agreement, their DMC values are consistent, with VTEs of 4.97(1) and 4.99(1) eV at the largest determinant expansions (see Figure 5). Even more than in glyoxal, the consistency of the DMC value for the CIPSI expansions of different lengths and initial MO basis sets suggests that it should be a trusted benchmark for this transition.
Figure 5.
Tetrazine VMC (filled circles) and DMC (empty circles) excitation energies for different CIPSI wave functions expanded either on starting CAS [SA(2)-CASSCF(14,10)] or NO basis and fully reoptimized in VMC. Horizontal lines represent the reference value (CC4: CC4/6-31+G*).
4.4. 3-State VMC Optimization: Cyclopentadienone 11A1 → 21A1 and 11A1 → 31A1 Excitations
To show the capabilities of the VMC optimization method, the three lowest 1A1 states of cyclopentadienone are optimized simultaneously to determine the two lowest excitation energies. One is deemed a double excitation (π, π → π*, π*) with ∼50% T1 and the other a single excitation (π → π*) with ∼74% T1 by CC3 calculations. All results are collected in Table 4.
Table 4. Cyclopentadienone VMC and DMC Excitation Energies (eV)a.
| VMC |
DMC |
|||||||
|---|---|---|---|---|---|---|---|---|
| WF | # det | # parm | ΔE12 | ΔE13 | ΔE23 | ΔE12 | ΔE13 | ΔE23 |
| CIPSI | 1025 | 2403 | 5.94(1) | 6.92(1) | 0.98(1) | 5.90(1) | 6.87(1) | 0.97(1) |
| 3033 | 3645 | 5.95(1) | 6.96(1) | 1.01(1) | 5.87(1) | 6.87(1) | 1.00(1) | |
| 7151 | 5365 | 6.03(1) | 7.05(1) | 1.02(1) | 5.92(1) | 6.91(1) | 0.99(1) | |
| 10,206 | 6574 | 6.04(1) | 7.03(1) | 0.99(1) | 5.90(1) | 6.89(1) | 0.99(1) | |
| TBEb | 6.00c | 6.09d | 0.09 | |||||
| ADC(3)/AVTZ | 4.59e | 6.50e | 1.91 | |||||
| CC2/AVTZ | 6.50e | |||||||
| CC3/AVTZ | 7.10e | 6.21e | –0.89 | |||||
| CCSD/AVTZ | 6.68e | |||||||
| CCSDT-3/AVTZ | 6.33e | |||||||
| exFCI/AVDZ | 5.81(39) | 6.93(24) | 1.10(13) | |||||
The number of determinants (# det) and parameters (# parm) in each trial wave function is also listed. The CIPSI expansions are performed on a NO basis and fully optimized in VMC. Reference values for the double and single excitation are placed in the ΔE12 and ΔE13 columns, respectively, to match the order of the states calculated with VMC and DMC. The VMC optimization of the two smaller CIPSIs used λIJ = (1.5, 2.0, 1.0) and, of the two larger, (1.0, 1.0, 1.0).
This value is “unsafe,” as defined in ref (12).
NEVPT2/AVTZ.
CCSDT/AVDZ + (CC3/AVTZ – CC3/AVDZ).
These values are also “unsafe”.
CC3/AVTZ predicts a value of 7.10 eV for the double excitation and 6.21 eV for the single excitation, which is the opposite of what is found here. The CC3 value for the double excitation is not trustable since it misses quadruple excitations important for describing this type of excitation. Véril et al.(12) determine the CC3 and CCSDT calculations of these excitations to be “unsafe” since they do not agree to within 0.03–0.04 eV. The relatively low T1 of the single excitation could lead to a poor treatment by CC3 and CCSDT. No CC4 or CCSDTQ calculations are currently available for reliable comparison to the present results for the double excitation energy, and all benchmark VTEs in Table 4 are considered unsafe. An exFCI/AVDZ calculation is performed in the present work, although it has fairly large error bars.
All VMC and DMC performed on CIPSI wave functions give roughly the same results (see Table 4, Figure 6, and Supporting Information). The largest CIPSI wave function in the NO basis has DMC ΔE12, ΔE13, and ΔE23 values of 5.90(1), 6.89(1), and 0.99 eV, respectively. We find that the two smaller determinant expansions in Table 4 require larger values of λIJ to stabilize the overlaps (see also the Supporting Information for further discussion).
Figure 6.
Cyclopentadienone VMC (filled circles) and DMC (empty circles) excitation energies for different CIPSI wave functions expanded either on starting CAS [SA(3)-CASSCF(6,6)] or NO basis and fully optimized in VMC. FCI: exFCI/AVDZ (error bar of ±0.13 eV applies to the FCI value for ΔE23).
The present calculations definitively show the ordering of the two lowest 1A1 excited states. The largest CIPSI calculation performed in the CAS orbital basis [SA(3)-CASSCF(6,6)] finds the 21A1 state to have coefficients of 0.70 and 0.46 for the dominant doubly [(π, π) → (π*, π*)] and singly excited (π → π*) CSFs, respectively, while the 31A1 state has coefficients of 0.49 and 0.75. The coefficients show a clear mixing of singly and doubly excited determinants in both states (also suggested by the T1 values), but the 21A1 state is clearly distinguishable as the double excitation and the 31A1 state as the single.
5. Conclusions
With the use of a penalty-based, state-specific optimization scheme, we show that the same QMC method which provides accurate single-excitations energies can also be applied to double excitations. That is, we use PT2 energy and variance-matched CIPSI determinant expansions as the Slater part of the Jastrow–Slater trial function in the VMC optimization. The introduction of an objective function allows a state-specific optimization of multiple states of the same symmetry simultaneously by imposing orthogonality between all eigenstates.
For optimization with two states, it is sufficient to apply a large enough λ12 to stabilize the optimization. With more states, there are wide ranges of the various λIJ which give consistent and stable excitation energies. Good values can be found at a preliminary stage with relatively few iterations.
The pure double excitations in nitroxyl [(4.32(1) eV] and glyoxal [5.63(1) eV] calculated here are in excellent agreement with reliable benchmarks in the literature. We also expect that our VTEs calculated at the level of DMC are reliable benchmarks for molecules and excitation types where CC methods are too costly to do with a large enough basis set. Specifically, we suspect a CC4 calculation with a larger basis set will find a tetrazine double excitation close to our result, 4.99(1) eV, slightly lower than the CC4/6-31G* result. For cyclopentadienone, the 1A1 first excited state (21A1) is predominantly a double excitation at 5.90(1) eV, while the second excited state is predominantly a single excitation at 6.89(1) eV.
The favorable computational scaling of QMC with the system size and its alignment with modern supercomputers make it a serious candidate for applications involving complex excited states, such as long-range charge-transfer excitations, conical intersections, or problems where the ordering between excited states of different characters is unclear.
Acknowledgments
We thank Pierre-François Loos for useful discussions. This work was supported by the European Centre of Excellence in Exascale Computing TREX—Targeting Real Chemical Accuracy at the Exascale. This project has received funding from the European Union’s Horizon 2020—Research and Innovation program—under grant agreement no. 952165. The calculations were carried out on the Dutch national supercomputer Cartesius with the support of SURF Cooperative.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jctc.2c00769.
Choice of input weights for the matching of multiple CIPSI wave functions; CIPSI energies, PT2 energies, and variances for the expansions used in the QMC calculations; additional QMC total and excitation energies obtained with CAS wave functions and with CIPSI determinant components originally expressed in a CAS orbital basis; additional two- and three-state optimization calculations for nitroxyl; and overlap data for cyclopentadienone and different wave functions (PDF)
Author Present Address
∥ Faculty of Chemistry and Food Chemistry, Technische Universität Dresden, 01062 Dresden, Germany
The authors declare no competing financial interest.
Supplementary Material
References
- Chong D. P.Recent Advances in Density Functional Methods; World Scientific, 1995. [Google Scholar]
- Burke K.; Werschnik J.; Gross E. K. U. Time-dependent density functional theory: Past, present, and future. J. Chem. Phys. 2005, 123, 062206. 10.1063/1.1904586. [DOI] [PubMed] [Google Scholar]
- Runge E.; Gross E. K. U. Density-Functional Theory for Time-Dependent Systems. Phys. Rev. Lett. 1984, 52, 997–1000. 10.1103/physrevlett.52.997. [DOI] [Google Scholar]
- Krylov A. I. Equation-of-Motion Coupled-Cluster Methods for Open-Shell and Electronically Excited Species: The Hitchhiker’s Guide to Fock Space. Annu. Rev. Phys. Chem. 2008, 59, 433–462. 10.1146/annurev.physchem.59.032607.093602. [DOI] [PubMed] [Google Scholar]
- Maitra N. T.; Zhang F.; Cave R. J.; Burke K. Double excitations within time-dependent density functional theory linear response. J. Chem. Phys. 2004, 120, 5932–5937. 10.1063/1.1651060. [DOI] [PubMed] [Google Scholar]
- Elliott P.; Goldson S.; Canahui C.; Maitra N. T. Perspectives on double-excitations in TDDFT. Chem. Phys. 2011, 391, 110–119. 10.1016/j.chemphys.2011.03.020. [DOI] [Google Scholar]; Open problems and new solutions in time dependent density functional theory
- Shao Y.; Head-Gordon M.; Krylov A. I. The spin–flip approach within time-dependent density functional theory: Theory and applications to diradicals. J. Chem. Phys. 2003, 118, 4807. 10.1063/1.1545679. [DOI] [Google Scholar]
- Wang F.; Ziegler T. Time-dependent density functional theory based on a noncollinear formulation of the exchange-correlation potential. J. Chem. Phys. 2004, 121, 12191. 10.1063/1.1821494. [DOI] [PubMed] [Google Scholar]
- Wang F.; Ziegler T. The performance of time-dependent density functional theory based on a noncollinear exchange-correlation potential in the calculations of excitation energies. J. Chem. Phys. 2005, 122, 074109. 10.1063/1.1844299. [DOI] [PubMed] [Google Scholar]
- Rinkevicius Z.; Vahtras O.; Ågren H. Spin-flip time dependent density functional theory applied to excited states with single, double, or mixed electron excitation character. J. Chem. Phys. 2010, 133, 114104. 10.1063/1.3479401. [DOI] [PubMed] [Google Scholar]
- Hirata S.; Nooijen M.; Bartlett R. J. High-order determinantal equation-of-motion coupled-cluster calculations for electronic excited states. Chem. Phys. Lett. 2000, 326, 255–262. 10.1016/s0009-2614(00)00772-7. [DOI] [Google Scholar]
- Véril M.; Scemama A.; Caffarel M.; Lipparini F.; Boggio-Pasqua M.; Jacquemin D.; Loos P.-F. QUESTDB: A database of highly accurate excitation energies for the electronic structure community. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2021, 11, e1517 10.1002/wcms.1517. [DOI] [Google Scholar]
- Loos P.-F.; Boggio-Pasqua M.; Scemama A.; Caffarel M.; Jacquemin D. Reference Energies for Double Excitations. J. Chem. Theory Comput. 2019, 15, 1939–1956. 10.1021/acs.jctc.8b01205. [DOI] [PubMed] [Google Scholar]
- Loos P.-F.; Lipparini F.; Boggio-Pasqua M.; Scemama A.; Jacquemin D. A Mountaineering Strategy to Excited States: Highly Accurate Energies and Benchmarks for Medium Sized Molecules. J. Chem. Theory Comput. 2020, 16, 1711–1741. 10.1021/acs.jctc.9b01216. [DOI] [PubMed] [Google Scholar]
- Loos P.-F.; Matthews D. A.; Lipparini F.; Jacquemin D. How accurate are EOM-CC4 vertical excitation energies?. J. Chem. Phys. 2021, 154, 221103. 10.1063/5.0055994. [DOI] [PubMed] [Google Scholar]
- Loos P.-F.; Lipparini F.; Matthews D. A.; Blondel A.; Jacquemin D. A Mountaineering Strategy to Excited States: Revising Reference Values with EOM-CC4. J. Chem. Theory Comput. 2022, 18, 4418–4427. 10.1021/acs.jctc.2c00416. [DOI] [PubMed] [Google Scholar]
- Koch H.; Christiansen O.; Jørgensen P.; Sanchez de Merás A. M.; Helgaker T. The CC3 model: An iterative coupled cluster approach including connected triples. J. Chem. Phys. 1997, 106, 1808–1818. 10.1063/1.473322. [DOI] [Google Scholar]
- Noga J.; Bartlett R. J. The full CCSDT model for molecular electronic structure. J. Chem. Phys. 1987, 86, 7041–7050. 10.1063/1.452353. [DOI] [Google Scholar]
- Kállay M.; Gauss J. Approximate treatment of higher excitations in coupled-cluster theory. J. Chem. Phys. 2005, 123, 214105. 10.1063/1.2121589. [DOI] [PubMed] [Google Scholar]
- Kucharski S. A.; Bartlett R. J. Recursive intermediate factorization and complete computational linearization of the coupled-cluster single, double, triple, and quadruple excitation equations. Theor. Chim. Acta 1991, 80, 387–405. 10.1007/bf01117419. [DOI] [Google Scholar]
- Andersson K.; Malmqvist P. A.; Roos B. O.; Sadlej A. J.; Wolinski K. Second-order perturbation theory with a CASSCF reference function. J. Phys. Chem. 1990, 94, 5483–5488. 10.1021/j100377a012. [DOI] [Google Scholar]
- Angeli C.; Cimiraglia R.; Malrieu J.-P. N-electron valence state perturbation theory: a fast implementation of the strongly contracted variant. Chem. Phys. Lett. 2001, 350, 297–305. 10.1016/s0009-2614(01)01303-3. [DOI] [Google Scholar]
- Zobel J. P.; Nogueira J. J.; González L. The IPEA dilemma in CASPT2. Chem. Sci. 2017, 8, 1482–1499. 10.1039/c6sc03759c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huron B.; Malrieu J. P.; Rancurel P. Iterative perturbation calculations of ground and excited state energies from multiconfigurational zeroth-order wavefunctions. J. Chem. Phys. 1973, 58, 5745–5759. 10.1063/1.1679199. [DOI] [Google Scholar]
- Povill A.; Rubio J.; Illas F. Treating large intermediate spaces in the CIPSI method through a direct selected CI algorithm. Theor. Chim. Acta 1992, 82, 229–238. 10.1007/bf01113255. [DOI] [Google Scholar]
- Zimmerman P. M. Incremental full configuration interaction. J. Chem. Phys. 2017, 146, 104102. 10.1063/1.4977727. [DOI] [PubMed] [Google Scholar]
- Foulkes W. M. C.; Mitas L.; Needs R. J.; Rajagopal G. Quantum Monte Carlo simulations of solids. Rev. Mod. Phys. 2001, 73, 33–83. 10.1103/revmodphys.73.33. [DOI] [Google Scholar]
- Lüchow A. Quantum Monte Carlo methods. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2011, 1, 388–402. 10.1002/wcms.40. [DOI] [Google Scholar]
- Austin B. M.; Zubarev D. Y.; Lester W. A. Quantum Monte Carlo and Related Approaches. Chem. Rev. 2012, 112, 263–288. 10.1021/cr2001564. [DOI] [PubMed] [Google Scholar]
- Sorella S.; Capriotti L. Algorithmic differentiation and the calculation of forces by quantum Monte Carlo. J. Chem. Phys. 2010, 133, 234111. 10.1063/1.3516208. [DOI] [PubMed] [Google Scholar]
- Filippi C.; Assaraf R.; Moroni S. Simple formalism for efficient derivatives and multi-determinant expansions in quantum Monte Carlo. J. Chem. Phys. 2016, 144, 194105. 10.1063/1.4948778. [DOI] [PubMed] [Google Scholar]
- Assaraf R.; Moroni S.; Filippi C. Optimizing the Energy with Quantum Monte Carlo: A Lower Numerical Scaling for Jastrow-Slater Expansions. J. Chem. Theory Comput. 2017, 13, 5273–5281. 10.1021/acs.jctc.7b00648. [DOI] [PubMed] [Google Scholar]
- Ammar A.; Giner E.; Scemama A. Optimization of Large Determinant Expansions in Quantum Monte Carlo. J. Chem. Theory Comput. 2022, 18, 5325–5336. 10.1021/acs.jctc.2c00556. [DOI] [PubMed] [Google Scholar]
- Dash M.; Feldt J.; Moroni S.; Scemama A.; Filippi C. Excited States with Selected Configuration Interaction-Quantum Monte Carlo: Chemically Accurate Excitation Energies and Geometries. J. Chem. Theory Comput. 2019, 15, 4896–4906. 10.1021/acs.jctc.9b00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea A.; Scemama A.; Briels W. J.; Moroni S.; Filippi C. Variational Principles in Quantum Monte Carlo: The Troubled Story of Variance Minimization. J. Chem. Theory Comput. 2020, 16, 4203–4212. 10.1021/acs.jctc.0c00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash M.; Moroni S.; Filippi C.; Scemama A. Tailoring CIPSI Expansions for QMC Calculations of Electronic Excitations: The Case Study of Thiophene. J. Chem. Theory Comput. 2021, 17, 3426–3434. 10.1021/acs.jctc.1c00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi C.; Zaccheddu M.; Buda F. Absorption Spectrum of the Green Fluorescent Protein Chromophore: A Difficult Case for ab Initio Methods?. J. Chem. Theory Comput. 2009, 5, 2074–2087. 10.1021/ct900227j. [DOI] [PubMed] [Google Scholar]
- Zimmerman P. M.; Toulouse J.; Zhang Z.; Musgrave C. B.; Umrigar C. J. Excited states of methylene from quantum Monte Carlo. J. Chem. Phys. 2009, 131, 124103. 10.1063/1.3220671. [DOI] [PubMed] [Google Scholar]
- Valsson O.; Filippi C. Photoisomerization of Model Retinal Chromophores: Insight from Quantum Monte Carlo and Multiconfigurational Perturbation Theory. J. Chem. Theory Comput. 2010, 6, 1275–1292. 10.1021/ct900692y. [DOI] [Google Scholar]
- Send R.; Valsson O.; Filippi C. Electronic Excitations of Simple Cyanine Dyes: Reconciling Density Functional and Wave Function Methods. J. Chem. Theory Comput. 2011, 7, 444–455. 10.1021/ct1006295. [DOI] [PubMed] [Google Scholar]
- Valsson O.; Campomanes P.; Tavernelli I.; Rothlisberger U.; Filippi C. Rhodopsin Absorption from First Principles: Bypassing Common Pitfalls. J. Chem. Theory Comput. 2013, 9, 2441–2454. 10.1021/ct3010408. [DOI] [PubMed] [Google Scholar]
- Guareschi R.; Zulfikri H.; Daday C.; Floris F. M.; Amovilli C.; Mennucci B.; Filippi C. Introducing QMC/MMpol: Quantum Monte Carlo in Polarizable Force Fields for Excited States. J. Chem. Theory Comput. 2016, 12, 1674–1683. 10.1021/acs.jctc.6b00044. [DOI] [PubMed] [Google Scholar]
- Hunt R. J.; Szyniszewski M.; Prayogo G. I.; Maezono R.; Drummond N. D. Quantum Monte Carlo calculations of energy gaps from first principles. Phys. Rev. B 2018, 98, 075122. 10.1103/physrevb.98.075122. [DOI] [Google Scholar]
- Blunt N. S.; Neuscamman E. Excited-State Diffusion Monte Carlo Calculations: A Simple and Efficient Two-Determinant Ansatz. J. Chem. Theory Comput. 2019, 15, 178–189. 10.1021/acs.jctc.8b00879. [DOI] [PubMed] [Google Scholar]
- Blunt N. S.; Smart S. D.; Booth G. H.; Alavi A. An excited-state approach within full configuration interaction quantum Monte Carlo. J. Chem. Phys. 2015, 143, 134117. 10.1063/1.4932595. [DOI] [PubMed] [Google Scholar]
- Choo K.; Carleo G.; Regnault N.; Neupert T. Symmetries and Many-Body Excitations with Neural-Network Quantum States. Phys. Rev. Lett. 2018, 121, 167204. 10.1103/physrevlett.121.167204. [DOI] [PubMed] [Google Scholar]
- Pathak S.; Busemeyer B.; Rodrigues J. N. B.; Wagner L. K. Excited states in variational Monte Carlo using a penalty method. J. Chem. Phys. 2021, 154, 034101. 10.1063/5.0030949. [DOI] [PubMed] [Google Scholar]
- Umrigar C. J.; Wilson K. G.; Wilkins J. W. Optimized trial wave functions for quantum Monte Carlo calculations. Phys. Rev. Lett. 1988, 60, 1719–1722. 10.1103/physrevlett.60.1719. [DOI] [PubMed] [Google Scholar]
- Umrigar C. J.; Filippi C. Energy and Variance Optimization of Many-Body Wave Functions. Phys. Rev. Lett. 2005, 94, 150201. 10.1103/physrevlett.94.150201. [DOI] [PubMed] [Google Scholar]
- Shea J. A. R.; Neuscamman E. Size Consistent Excited States via Algorithmic Transformations between Variational Principles. J. Chem. Theory Comput. 2017, 13, 6078–6088. 10.1021/acs.jctc.7b00923. [DOI] [PubMed] [Google Scholar]
- Pineda Flores S. D.; Neuscamman E. Excited State Specific Multi-Slater Jastrow Wave Functions. J. Phys. Chem. A 2019, 123, 1487–1497. 10.1021/acs.jpca.8b10671. [DOI] [PubMed] [Google Scholar]
- Sorella S. Generalized Lanczos algorithm for variational quantum Monte Carlo. Phys. Rev. B: Condens. Matter Mater. Phys. 2001, 64, 024512. 10.1103/physrevb.64.024512. [DOI] [Google Scholar]
- Casula M.; Attaccalite C.; Sorella S. Correlated geminal wave function for molecules: An efficient resonating valence bond approach. J. Chem. Phys. 2004, 121, 7110–7126. 10.1063/1.1794632. [DOI] [PubMed] [Google Scholar]
- Burkatzki M.; Filippi C.; Dolg M. Energy-consistent pseudopotentials for quantum Monte Carlo calculations. J. Chem. Phys. 2007, 126, 234105. 10.1063/1.2741534. [DOI] [PubMed] [Google Scholar]
- For the hydrogen atom, we use a more accurate BFD pseudopotential and basis set. Dolg M.; Filippi C., private communication.
- Kendall R. A.; Dunning T. H. Jr.; Harrison R. J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. 10.1063/1.462569. [DOI] [Google Scholar]
- Barca G. M. J.; Bertoni C.; Carrington L.; Datta D.; De Silva N.; Deustua J. E.; Fedorov D. G.; Gour J. R.; Gunina A. O.; Guidez E.; Harville T.; Irle S.; Ivanic J.; Kowalski K.; Leang S. S.; Li H.; Li W.; Lutz J. J.; Magoulas I.; Mato J.; Mironov V.; Nakata H.; Pham B. Q.; Piecuch P.; Poole D.; Pruitt S. R.; Rendell A. P.; Roskop L. B.; Ruedenberg K.; Sattasathuchana T.; Schmidt M. W.; Shen J.; Slipchenko L.; Sosonkina M.; Sundriyal V.; Tiwari A.; Galvez Vallejo J. L.; Westheimer B.; Włoch M.; Xu P.; Zahariev F.; Gordon M. S. Recent developments in the general atomic and molecular electronic structure system. J. Chem. Phys. 2020, 152, 154102. 10.1063/5.0005188. [DOI] [PubMed] [Google Scholar]
- Garniron Y.; Applencourt T.; Gasperich K.; Benali A.; Ferté A.; Paquier J.; Pradines B.; Assaraf R.; Reinhardt P.; Toulouse J.; Barbaresco P.; Renon N.; David G.; Malrieu J.-P.; Véril M.; Caffarel M.; Loos P.-F.; Giner E.; Scemama A. Quantum Package 2.0: An Open-Source Determinant-Driven Suite of Programs. J. Chem. Theory Comput. 2019, 15, 3591–3609. 10.1021/acs.jctc.9b00176. [DOI] [PubMed] [Google Scholar]
- Scemama A.; Caffarel M.; Benali A.; Jacquemin D.; Loos P.-F. Influence of pseudopotentials on excitation energies from selected configuration interaction and diffusion Monte Carlo. Results Chem. 2019, 1, 100002. 10.1016/j.rechem.2019.100002. [DOI] [PubMed] [Google Scholar]
- CHAMP is a quantum Monte Carlo program package written by C. J. Umrigar, C. Filippi, S. Moroni and collaborators.
- Neuscamman E.; Umrigar C. J.; Chan G. K.-L. Optimizing large parameter sets in variational quantum Monte Carlo. Phys. Rev. B: Condens. Matter Mater. Phys. 2012, 85, 045103. 10.1103/physrevb.85.045103. [DOI] [Google Scholar]
- Saha B.; Ehara M.; Nakatsuji H. Singly and doubly excited states of butadiene, acrolein, and glyoxal: Geometries and electronic spectra. J. Chem. Phys. 2006, 125, 014316. 10.1063/1.2200344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.