Abstract
A hybrid protein [Met-Ala-(His)6OprF190–342-OprI21–83] consisting of the mature outer membrane protein I (OprI) and amino acids 190 to 342 of OprF of Pseudomonas aeruginosa was expressed in Escherichia coli and purified by Ni2+ chelate-affinity chromatography. After safety and pyrogenicity evaluations in animals, four groups of eight adult human volunteers were vaccinated intramuscularly three times at 4-week intervals and revaccinated 6 months later with either 500, 100, 50, or 20 μg of OprF-OprI adsorbed onto A1(OH)3. All vaccinations were well tolerated. After the first vaccination, a significant rise of antibody titers against P. aeruginosa OprF and OprI was measured in volunteers receiving the 100- or the 500-μg dose. After the second vaccination, significant antibody titers were measured for all groups. Elevated antibody titers against OprF and OprI could still be measured 6 months after the third vaccination. The capacity of the elicited antibodies to promote complement binding and opsonization could be demonstrated by a C1q-binding assay and by the in vitro opsonophagocytic uptake of P. aeruginosa bacteria. These data support the continued development of an OprF-OprI vaccine for use in humans.
Pseudomonas aeruginosa is a leading cause of nosocomial infections and pneumonia in hospitals (15, 21, 28). The pathogen affects mainly immunocompromised patients, such as patients with large burns (36, 44, 45), or patients undergoing immunosuppressive or cytostatic therapy for the prevention of rejection after organ transplantation (33) or for cancer treatment (22, 51). Eradication of Pseudomonas infections is hampered, since strains isolated in hospitals are highly resistant to antibiotics (23, 24, 31, 47, 49, 56).
The effectiveness of vaccination against P. aeruginosa infection in burn patients was demonstrated 20 years ago (1, 32, 37). However, the polyvalent vaccine, which was based on isolated lipopolysaccharides (LPS) of P. aeruginosa serotypes, was not approved for routine clinical use because of the toxicity associated with the lipid A portion of the LPS. Subunit vaccines based on oligosaccharides purified from LPS conjugated to P. aeruginosa exotoxin (5–7) or mucoid exopolysaccharide (alginate) of P. aeruginosa (40–43) were shown to be less toxic and have been used successfully to elicit antibodies in a number of volunteers and groups of patients (6, 7, 40, 43). However, currently no clinical vaccine against P. aeruginosa for which safety and efficacy have been shown in clinical trials with patients from one of the major risk groups for nosocomial P. aeruginosa infection is available for routine use. Our research during the last decade has been focused on the development of a vaccine against P. aeruginosa based on its outer membrane proteins (OPRs). A vaccine based on OPRs may have several advantages. OPRs, which induce cross-protective immunity among all 17 known P. aeruginosa serotypes (38), can be produced by recombinant DNA technology free of contaminating P. aeruginosa LPS. Additionally, cloned genes of OPRs would be applicable for naked DNA immunization (4, 8) or could be transfected into special vectors such as nonpathogenic Salmonella strains to induce a mucosal immune response (34, 50). The efficacy of OPRs as a vaccine candidate was shown by us and other research groups (12, 13, 18, 19, 35, 52, 53) in various animal models. We have cloned the major OPRs, outer membrane protein F (OprF) (9) and OprI (10). Recombinant OprI was expressed in Escherichia coli and used to vaccinate human volunteers (54). Vaccination was well tolerated. In addition, the elicited antibodies against P. aeruginosa promoted complement-dependent opsonization of P. aeruginosa. Compared to LPS antigens, OprI represents a rather small target for protective antibodies on the bacterial surface. We have therefore recently generated a recombinant hybrid protein consisting of the entire OprI molecule fused to the carboxy-terminal sequence (amino acids 190 to 342) of OprF (53). The presence of the main known protective epitopes (14, 16, 20, 25) of both proteins was demonstrated in the hybrid protein. This hybrid protein could be expressed as a glutathione S-transferase (GST)-linked fusion protein (GST-OprF190–342-OprI21–83) in E. coli. In two different models involving P. aeruginosa infection of immunocompromised mice, the vaccine proved to be highly protective (53). The use of GST as a constituent of a clinical vaccine in humans, however, cannot be approved because of the induction of a high GST-specific, nonvaccine-related immune response, which may lead to cross-reacting autoantibodies. We therefore directed our attention toward the cloning of an OprF-OprI hybrid protein which can be expressed in E. coli without a fusion component. Because the expression of OprF190–342-OprI21–83 without a fusion protein in E. coli was not successful due to rapid degradation of the hybrid protein, modifications with various extensions of the hybrid protein were tested (14). Finally, two recombinant vaccine candidates could be expressed as histidine-tagged fusion proteins and tested in immunosuppressed mice (55). One of them, Met-Ala-(His)6OprF190–342-OprI21–83, was found to be partly soluble and was found in the pellet as well as in the supernatant of ruptured E. coli bacteria. Therefore, this protein could be purified under native conditions from the supernatant as well as from the inclusion bodies by solubilization under denaturing conditions with 6 M urea, followed by subsequent renaturation. The second candidate, OprF179–342-OprI21–83(His)6, remained totally soluble when expressed in E. coli. Therefore, the entire process of isolation and purification could be performed under native conditions.
Only one of the vaccine candidates, Met-Ala-(His)6 OprF190–342-OprI21–83 isolated and purified under native conditions, showed significant protection against experimental P. aeruginosa infection in mice (55). We now present data demonstrating that Met-Ala-(His)6OprF190–342-OprI21–83 was isolated and purified from E. coli to yield a clinically applicable vaccine that was successfully used without any apparent side effects for the vaccination of human volunteers against P. aeruginosa.
MATERIALS AND METHODS
Materials.
Ni-nitrilotriacetic acid (NTA)-agarose was obtained from Qiagen (Hilden, Germany). For ultrafiltration, the DIAFLO YM10 membrane was used in a 50-ml Amicon stirred cell under pressure (Millipore GmbH, Eschborn, Germany). The substrate for enhanced chemiluminescence was from Amersham (Braunschweig, Germany). All other chemicals were obtained from standard chemical suppliers.
Culture medium and conditions.
Recombinant cells of E. coli were grown in phosphate-buffered Luria broth (LB) (1% tryptone, 0.5% yeast extract, 1% NaCl, 50 mM Na-K-phosphate (pH 7.4) with or without ampicillin (100 μg/ml) at 37°C. When the bacterial cell density reached a reading at A600 of 0.7 to 0.8, expression of recombinant proteins was induced by adding IPTG (isopropyl-β-d-thiogalactopyranoside) to a concentration of 0.4 to 1 mM for 3 h. After being harvested, the cells were washed once with the corresponding equilibration buffer.
Vector.
The recombinant vector pTrc-His-F-I, carrying the hybrid gene encoding parts of OprF and OprI from P. aeruginosa, was constructed as described previously (14). The CprF and CprI genes were cloned from P. aeruginosa serogroup 6 (ATCC 33354) as published before (9, 10). The vector was transformed into E. coli XL-1 Blue bacteria by using standard procedures (2).
Production.
E. coli XL-1 Blue, which carries the plasmid pTrcHis-F-I, was inoculated into 1 liter of LB medium containing 0.2% glucose and shaken at 37°C for 12 h. This culture was used to inoculate 10 liters of medium composed of 2% (wt/vol) yeast extract, 0.15% (wt/vol) glucose, 0.78% (wt/vol) potassium dihydrogen phosphate, 0.22% (wt/vol) magnesium sulfate, 0.10% (wt/vol) tri-sodium citrate dihydrate, 0.02% (wt/vol) ammonium hydrogen phosphate, 0.01% (vol/vol) trace elements, 0.36 parts per million (ppm) (wt/vol) ferric citrate, and 0.5 ppm (wt/vol) thiamine. The fermentor involved batch-fed cultivation at 37°C with an aeration rate of 3 air volumes per culture volume per min. Feeding with a 50% glucose solution at a constant rate of 2 ml/min was started 7 h after inoculation. One hour later, expression was induced by adding IPTG to a final concentration of 0.025% (wt/vol). Cells were harvested after 27 h of cultivation by centrifugation, washed once with ice-cold buffer B1 (0.2 M Tris-HCl [pH 8.5], 0.137 M NaCl), and frozen in aliquots in liquid nitrogen.
Purification.
Forty grams of wet cell mass was resuspended in 200 ml of buffer B1 (see above) and lysed by one passage through a Gaulin press at 1,200 lb/in2. The cell extract was clarified at 48,000 × g for 90 min at 4°C and passed through a 0.45-μm-pore-size filter. The crude extract was loaded onto a Ni-NTA superflow column (Qiagen) equilibrated with buffer B2 (0.1 M NaH2PO4 monohydrate, 0.5 M NaCl, 0.02 M imidazole [pH 8.0]) (27). Unbound material was eluted by washing the column with buffer B3 (0.1 M NaH2PO4 monohydrate, 0.5 M NaCl, 0.05 M imidazole [pH 8.0]). The specific protein was eluted with buffer B4 (0.1 M NaH2PO4 monohydrate, 0.5 M NaCl, 0.5 M imidazole [pH 8.0]). The eluted product was applied onto a Sephadex G-25 column equilibrated with buffer B5 (0.02 M NaH2PO4 monohydrate [pH 6.5]). The specific eluate was concentrated by centrifugation in MACROSEP 10 units by a factor of 3. The pH of this eluate was lowered to 5.9 by adding buffer B6 (0.02 M NaH2PO4 monohydrate [pH 3.0]), incubated at 4°C overnight, and the eluate was then clarified for 10 min at 4°C and 5,000 × g to precipitate the LPS. The pH was retitrated to 7.0 to 7.2 by adding a 0.1 N NaOH solution dropwise. The neutralized protein solution was filter (0.22-μm pore size) sterilized and stored at 4°C overnight. Finally, the purified protein was concentrated to about 1 mg/ml by ultrafiltration by using a stirred Amicon cell and a YM10 membrane and then extensively dialyzed against sterile, pyrogen-free phosphate-buffered saline (PBS) at 6°C for 20 h. After each run, the columns were sanitized by washing with at least 2 column volumes of 0.5 M sodium hydroxide and reequilibrated with the corresponding buffers.
SDS-polyacrylamide gel electrophoresis and Western blotting.
Proteins were separated in sodium dodecyl sulfate (SDS)-polyacrylamide gels as described in detail elsewhere (46) and electroblotted onto a nitrocellulose membrane according to standard procedures at 0.8 mA/cm2. Proteins were visualized by staining with Coomassie brilliant blue G250, by silver staining (Silver Xpress; Novex, San Diego, Calif.), or by immunodetection with rabbit anti-OprF αD1 antibody diluted 1:500, anti-OprF mouse monoclonal antibodies against epitopes D1 (1:10,000), D2 (1:5,000), D5 (1:7,500), or D6 (1:5,000) or mouse monoclonal anti-OprI antibodies (2A1 and 6A4) diluted 1:10,000 (13, 46). E. coli impurities were detected with rabbit anti-E. coli 11179 diluted 1:500. Specifically, membranes were blocked in freshly prepared 25 mM sodium phosphate, 150 mM NaCl, 0.05% Tween-20 (PBST), plus 5% low-fat milk powder (MP) (pH 7.5) for 1 h at room temperature (RT). After a short wash, specific antisera prepared in 1% MP in PBST were added for 1 to 2 h at RT or overnight at 4°C followed again by a short washing. Then anti-mouse or anti-rabbit horseradish peroxidase-conjugated antisera were added at a dilution of 1:5,000 in the same buffer as the sera described above for 1 h. The blot was developed by enhanced chemiluminescence substrate and exposure to a standard X-ray film. Western blot analysis of native P. aeruginosa OprF and OprI with sera from vaccinated volunteers was carried out as described previously (13).
Vaccine preparation.
Recombinant OprF-OprI was adsorbed to A1(OH)3 (Alhydrogel; Superfos, Vedbaek, Denmark), and thimerosal (Caesar & Lorenz, Hilden, Germany) was added as a preservative. A thimerosal stock solution was prepared with a sterile, pyrogen-free physiological saline solution. For the 1 mg of vaccine/ml preparation, a dispersion of 3% (wt/vol) of A1(OH)3 was mixed with the OprF-OprI solution and the thimerosal stock solution to yield the following final concentrations: OprF-OprI, 1 mg/ml; A1(OH)3, 3 mg/ml; and thimerosal, 0.05 mg/ml. A1(OH)3 and the OprF-OprI solution were mixed and stirred for 30 min, and the thimerosal solution was then added, followed by additional stirring for 10 min. For the 0.1 mg/ml OprF-OprI vaccine preparation, pyrogen-free physiological saline solution was added to yield final concentrations of 0.1 mg of OprF-OprI/ml, 0.3 mg of A1(OH)3/ml, and 0.05 mg of thimerosal/ml. Aliquots (1 ml each) were aseptically introduced into sterile pyrogen-free glass vials, and the vials were stoppered and sealed.
Quality assessment.
Ten percent of the samples of both vaccine preparations were sent for routine testing for sterility. Five samples of the 1 mg/ml preparation were analyzed at the Chemische Landesuntersuchungsanstalt (Freiburg, Germany) by atom adsorption spectrometry for nickel, mercury, and aluminum content.
Extraction of DNA from the recombinant OprF-OprI vaccine.
Five hundred microliters of the recombinant vaccine corresponding to 500 μg of protein was extracted by chloropane according to standard protocols. To enhance the precipitation of low DNA contents, the protein solution was supplemented with 500 ng of yeast tRNA prior to extraction. Nucleic acids were precipitated by isopropanol. The extraction method was validated with the original vector pTrcHisFI used as a control plasmid.
PCR.
Aliquots of the isolated nucleic acids were used in a PCR containing 5 μl of template, 0.1 μM primer FINfor (5′-GCTCCGGCTCCGGAACC-3′), 0.1 μM primer FINrev (5′-CTTGCGGCTGGCTTTTTCC-3′), 0.2 μM each deoxynucleoside triphosphate, 1.5 mM MgCl2, and 2 U of Taq polymerase (Qiagen) in 50 μl of Taq buffer. PCR was carried out with 40 cycles at 96°C for 30 s, 64°C for 30 s, and 72°C for 1 min. PCR products were analyzed by agarose gel electrophoresis and detected by ethidium bromide staining.
The LPS content in three samples was measured by the Limulus lysate assay (Limusate; Labtech, St. Louis, Mo.).
The pyrogenicity of the OprF-OprI preparation was evaluated in three samples, each taken before the addition of A1(OH)3 and thimerosal. One milliliter of the OprF-OprI solution, diluted to 1 mg/ml, was injected into the ear veins of each of three New Zealand White rabbits. The body temperature of the animals was continuously monitored for 3 h with a rectal temperature recorder.
Local tolerance of the vaccine was assessed in Wistar rats. Eight rats (250 g each, four male, four female) were anesthetized with ether, and each animal was injected with 0.25 ml of vaccine (1 mg/ml) in the left rectus femoris and 0.5 ml of vaccine in the right rectus femoris. Four control animals received injections of equal volumes of sterile saline solution. The injection sites were shaved and disinfected beforehand. After 13 days, the animals were killed by CO2 inhalation, and after macroscopic inspection, both quadriceps were fixed in formalin and sent for histological examination.
Vaccination study.
Thirty-two healthy volunteers (16 men, 16 women; >18 years of age) gave their informed consent in accordance with institutional review board-approved protocols. As specified by the German regulations for vaccination studies, protocols concerning the preparation of the vaccine and the laboratory and animal safety testing of the vaccine were deposited at the Paul Ehrlich Institute, Langen, Germany. Volunteers were randomly assigned to four groups, and each received in the deltoid muscle of the left arm three injections of either 20 μg (0.2 ml of 100 μg/ml), 50 μg (0.5 ml of 100 μg/ml), 100 μg (0.1 ml of 1 mg/ml), or 500 μg (0.5 ml of 1 mg/ml) of OprF-OprI at 4-week intervals. Six months after the third vaccination, a booster vaccination was given. All volunteers underwent a physical examination, and their histories were taken to rule out any conditions necessitating exclusion from the study. Before each vaccination and 2 and 14 days after each vaccination, blood samples were taken and sent to the clinical laboratory for a complete blood count and evaluation of liver-specific enzymes, creatinine, and urea. Reactions to the vaccine were assessed for 3 consecutive days and documented by the volunteers. Local and systemic responses were graded with a subjective scale from 0 to 3, with scores representing absent, mild, moderate, and severe reactions, respectively. Vaccinees were instructed to take their temperature before vaccination and 12, 24, 48, and 72 h afterward. In addition, each volunteer underwent a physical examination 2 days after vaccination.
For the determination of OprF- and OprI-specific antibodies, venous blood samples were taken on day 0 (prior to immunization) and 2 weeks after each vaccination. Sera were stored in aliquots at −20°C.
Analysis of immune response.
An enzyme-linked immunosorbent assay (ELISA) was used for analysis of antibodies of the immunoglobulin G (IgG) class and IgG subclasses elicited in response to OprF-OprI. Briefly, 96-well microtiter plates (module U8 BL 7217786 B-type; Nunc Immunology) were coated with 125 μl of an OprF-OprI preparation per well, diluted at 10 μg/ml in PBS (pH 7.5), and incubated at RT for 18 h. The plates were washed and blocked with 200 μl of 0.2% bovine serum albumin (BSA), 50 mM Tris-HCl (pH 7.4), 50 mM citric acid, 100 mM NaOH. One hundred microliters of the sera diluted at 1:100 in 800 mM Tris-HCl (pH 7.4), 800 mM NaCl, 0.1% Haemaccel, 5% Boviserin, and 0.1% Synperonic F68 was added in duplicate, and twofold serial dilutions were performed. Plates were incubated for 1 h at 37°C and subsequently washed with washing buffer (OSEW 96; Dade Behring).
Binding was visualized with peroxidase-conjugated, IgG-specific rabbit anti-human secondary antibodies (63AP011; Dade Behring), diluted 1:10,000 in dilution buffer (50 mM Tris-HCl [pH 7.2], 1.15 M NaCl, 2% Tween, 0.1% BSA, 10% glycerin), with tetramethylbenzidine (TMB) as chromogen. The plate was incubated with the conjugate for 1 h at 37°C and washed again. One hundred microliters of substrate (1 ml of chromogen TMB Behring OUVG 925 and 10 ml of dilution buffer TMB Behring OUVG 945) was added to each well. After 30 min of incubation at RT, the reaction was stopped with 1 M sulphuric acid. Washing, addition of the conjugate, and reading of the plates (A4501650) were done with the Behring ELISA processor II. The titer was calculated with ELDAN1.3 software. ELISA titers were specified as the last dilution of the sample whose absorbance was above the threefold background value.
To detect IgG subclasses, the assay was performed essentially as described above with secondary antibodies against IgG1 to IgG4 from CLB (Amsterdam, The Netherlands). Pure human IgG subclasses (Calbiochem) were used to adjust the appropriate dilutions.
Western blot analysis of the reaction of recombinant OprF-OprI, OprI, and OprF and native P. aeruginosa OPR with the sera of vaccinated volunteers was performed as described above.
C1q-binding assay.
C1q binding was assayed as previously described (11, 54). Briefly, microtiter plates were coated with recombinant OprF-OprI as described above. After nonspecific binding sites were blocked, 50 μl of heat-inactivated 1.4-diluted serum and 50 μl of complement source were added. Human AB serum from an unvaccinated blood donor tested for a low titer of OprF-OprI antibodies was used as the complement source. The optimal concentrations of complement source and serum dilution had been evaluated before by serial dilutions. After incubation for 1 h at 37°C, the plates were washed and 50 μl of diluted peroxidase-linked anti-C1q antibodies (The Binding Site, Birmingham, England) was added. Binding was visualized as described previously, with OPD as a substrate (54).
Opsonic phagocytosis by PMNs.
The ability of the sera to mediate the opsonophagocytic uptake of P. aeruginosa by human polymorphonuclear leukocytes (PMNs) was assessed as described previously (17). All sera were heated at 56°C for 30 min to inactivate complement. Cultures of strain ATCC 27313, which is a Fisher-Devlin immunotype 2 strain (16), were diluted to a cell density of 108 cells/ml for use in the assay. The reaction mixtures included 50 μl of bacterial culture plus 50 μl of serum. These mixtures were incubated for 30 min at 37°C with shaking. Then, 100 μl of human venous blood was added to each mixture, and this final combination was incubated for 30 min at 37°C with shaking. Blood smears were prepared from each of the reaction mixtures and stained with Giemsa stain. Each smear was examined microscopically, and the number of bacterial cells per slide contained within the first 25 isolated, intact PMNs encountered was determined. Two different slides for each serum were examined in this fashion. Three repeat experiments were performed, so that 150 PMNs were examined for each serum. The mean number and standard deviation of bacterial cells associated per PMN were calculated for each reaction mixture.
Statistics.
The Kruskal-Wallis rank test was used for intragroup comparison of immune responses, and the Mann-Whitney U test was used for intergroup comparison of immune responses. A paired, two-tailed Student’s t test was used for comparison of the mean numbers of bacteria associated with PMNs in the opsonophagocytic assay. A P value of <0.05 was considered significant.
RESULTS
Characterization of the vaccine preparation.
At the end of the fermentation process, 1.7 kg of wet cell mass of E. coli was harvested from a 10-1 fermentor. The cells were aliquoted into 130- to 150-g portions and stored at −80°C. The presence of OprF-OprI was checked by SDS-polyacrylamide gel electrophoresis and Western blotting, and the protein content of the soluble hybrid antigen fraction was estimated to be approximately 1 mg per ml of culture. About 25 to 30% of the recombinant protein Met-Ala-(His)6OprF-OprI expressed in E. coli was found in the supernatant of ruptured E. coli cells, whereas the rest remained insoluble in the cells. The described purification procedure focused only on the purification of the soluble material. In summary, for the production of approximately 100 mg of native Met-Ala-(His)6OprF-OprI hybrid protein, 85 g of cell mass was needed.
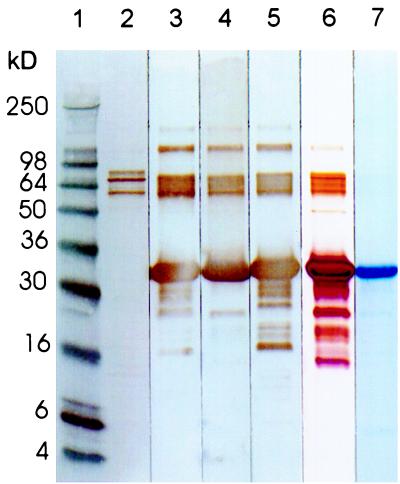
The soluble fraction of recombinant Met-Ala-(His)6 OprF190–342-OprI21–83 protein was purified by Ni2+-chelate-affinity chromatography (27, 48). Contaminating E. coli LPS could be precipitated at pH 5.9. The purity of the final vaccine protein and the presence of its antigenic epitopes were determined by SDS-polyacrylamide gel electrophoresis and Western blotting. Coomassie staining after electrophoresis (Fig. 1, lane 7) showed one main band at 33 kDa. After silver staining (Fig. 1, lane 6), additional low- and high-molecular-weight bands were visualized. Three high-molecular-mass bands (60 to 90 kDa) were identified by Western blotting with an E. coli-specific antibody to represent E. coli impurities (Fig. 1, lane 2). All other bands reacted with either OprI- or OprF-specific antibodies and thus appear to be degradation fragments or aggregates (trimers) of OprF-OprI (Fig. 1, lanes 3 to 5, 100-kDa band). The correct expression of OprI- and OprF-relevant antigenic epitopes could be demonstrated by Western blotting with a panel of monoclonal antibodies against epitopes D1 and D5 (Fig. 1a, lanes 3 and 4) and D2 and D6 (data not shown) of OprF, and against the protective epitope of OprI (13) recognized by monoclonal antibody 2A1 (Fig. 1, lane 5). To estimate the amount of E. coli impurities, increasing amounts of OprF-OprI (1.4, 5.7, and 14 μg) were electrophoretically separated and the bands were visualized by silver staining. BSA (5 to 100 ng) was used as a standard. The intensity of the E. coli-positive bands was compared with those of the standard dilutions (data not shown). From this it was calculated that each of the three bands at about 70 kDa, which cannot be attributed to OprF or OprI, represents less than 2/1,000 of the total OprF-OprI protein preparation. The purity of the vaccine protein was thus >98%. The endotoxin content of the final product was determined with the Limulus assay to be 25 endotoxin units/mg of product.
FIG. 1.
Lanes 2 to 7: SDS-polyacrylamide gel electrophoresis under reducing conditions of 8 μl (8 μg) of OprF-OprI, purified from E. coli. Lanes: 1, 8 μl marker (Novex; blue); 6, silver staining; 7, Coomassie blue staining; 2, Western blotting with antibody 11179 against E. coli 1:500; 3, Western blotting with antibody 944/5 against OprF epitope D5 1:7,500; 4, Western blotting with antibody 948-12 against OprF epitope D1 1:10,000; 5, Western blotting with antibody 2-A1 against Opr-I 1:10,000.
By PCR amplification, no more than 500 pg of DNA per mg of OprF-OprI protein could be detected. The sensitivity of the PCR method under the described conditions was found to be 0.5 pg of plasmid pTrcHisFI. The highest dose of vaccine (500 μg of protein) therefore contained no more than 250 pg of DNA, and the 100-μg dose contained no more than 50 pg of DNA.
The nickel content was below the detection limit of the assay (0.2 mg/kg). The amounts of mercury and aluminum measured were found to correspond to the amounts added to the vaccine. None of the samples assessed for sterility yielded microbial growth.
Local tolerance of the vaccine was evaluated in rats after intramuscular injection of 0.25 and 0.5 ml of vaccine (1 mg of OprF-OprI/ml). Histological examination of the injection sites showed signs of inflammation with focal infiltration of lymphocytes due to the alum adjuvant. No rise in body temperature was measured after injection of 1 mg of OprF-OprI protein into the ear veins of three rabbits.
Response to vaccination in human volunteers.
Four groups of eight volunteers each were vaccinated three times at 4-week intervals with either 20 (group 1), 50 (group 2), 100 (group 3), or 500 (group 4) μg of OprF-OprI protein adsorbed onto A1(OH)3. Those of the 32 vaccinated volunteers who had received the 0.1- and 0.5-ml doses of the 1 mg/ml-concentration (groups 3 and 4) complained of mild pain at the injection site, which disappeared after 3 days. No local side effects, such as an inflammatory response, were observed. Systemic reactions, such as a rise in body temperature, headache, or general illness were not observed after vaccination. During the safety clinical laboratory evaluations, four volunteers from group 4 showed increased levels of the C reactive protein (1.0, 1.3, 3.0, and 4.2 mg/dl; normal range, 0 to 0.8 mg/dl). The leukocyte counts of these volunteers were measured in the normal range, and the alterations were observed only once in each of the patients and after different vaccination time points. Six months after the third vaccination, one volunteer (group 1, no. 8) was excluded from the study in accordance with protocol because of arthritis in his left knee joint, which became apparent 3 months after the third vaccination.
Analysis of the immune response.
Before each vaccination and 2 weeks after each vaccination, antibody titers against OprI, OprF, and OprF-OprI were determined by ELISA.
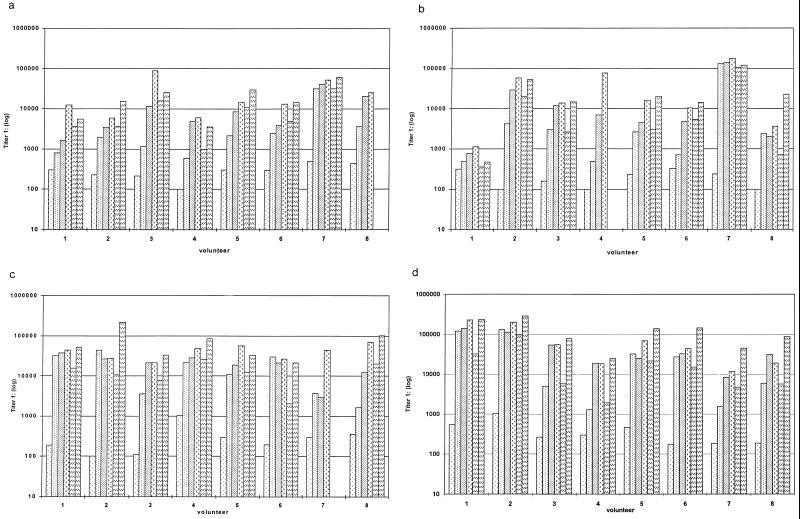
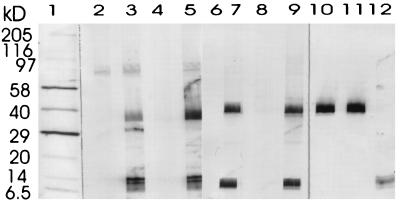
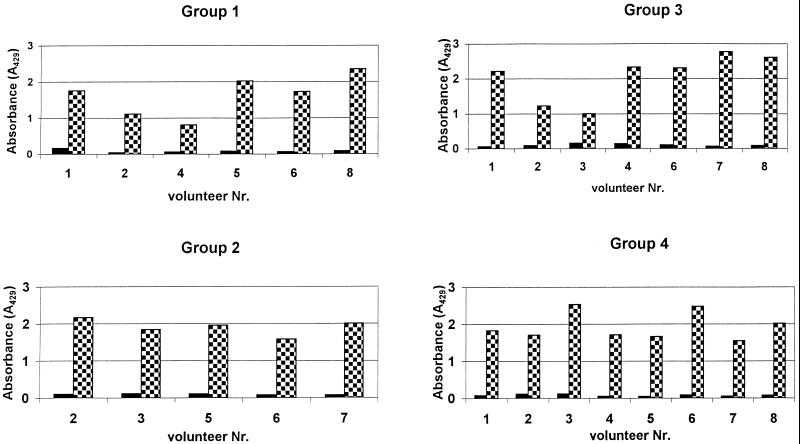
As shown in Fig. 2a to d, there was a significant increase in antibody titers within all the different dosage groups. The specificity of the antibodies against native P. aeruginosa OprF and OprI was confirmed by Western blotting (Fig. 3). Wild-type OprI (6 kDa) and OprF (33 kDa) were both recognized by the immunosera. Two low responders, subjects 1 and 8 in group 2, were observed (Fig. 2b). Considerable differences were observed between dosage groups and between volunteers in the same dosage group.
FIG. 2.
Serum anti-OprF-OprI IgG ELISA titers measured in sera from volunteers. Groups of volunteers were vaccinated intramuscularly three times with 20 μg (a), 50 μg (b), 100 μg (c), or 500 μg (d) of OprF-OprI at 4-week intervals and 6 months after the third vaccination. Blood was taken before each vaccination (day 0) and 2 weeks after each vaccination. Key: 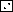 , day 0;
, day 0; 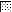 , day 14;
, day 14; 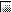 , day 42;
, day 42;  , day 70;
, day 70;  , day 240;
, day 240;  , day 254.
, day 254.
FIG. 3.
Western immunoblot analysis of antibodies against P. aeruginosa serogroup 1. Cell extracts were prepared from P. aeruginosa ATCC 33348. Blots were developed with alkaline phosphatase-conjugated monoclonal antihuman antibodies (Sigma A-2064) or with alkaline-phosphatase-conjugated rabbit anti-mouse antibodies (Zymed no. 61-6522). Lanes: 1, marker (Sigma wide range, B-2787); 2 and 3, preimmune serum and serum obtained from volunteer 4 in group 2 after the third vaccination; 4 and 5, preimmune serum and serum obtained from volunteer 4 in group 3 after the third vaccination; 6 and 7, preimmune serum and serum obtained from volunteer 3 in group 4 after the third vaccination; 8 and 9, preimmune serum and serum obtained from volunteer 5 in group 4 after the third vaccination; 10 and 11, mouse monoclonal antibodies against OprF (epitope D5); 12, mouse monoclonal antibodies against OprI (2-A1).
Statistical analysis showed that after only one vaccination, a maximal response was observed for groups which received the 100- or 500-μg dose (Table 1). No statistically significant increase of specific antibody titer was measured in these groups after the first and second revaccinations. After vaccination with the 20-μg OprF-OprI dose, a significant antibody response was measured only after revaccination. However, individual volunteers (no. 7, group 1) already showed a maximal response after the first dose.
TABLE 1.
OprF-I-specific IgG antibody titer determined by ELISA
| Time point and statistic | Titer for dose of:
|
|||
|---|---|---|---|---|
| 20 μg | 50 μg | 100 μg | 500 μg | |
| Day 0 | ||||
| Mean | 294 | 195 | 318 | 391 |
| Median | 295 | 194 | 240 | 280 |
| SD | 125 | 94 | 297 | 291 |
| Minimum/maximum | 100/494 | 100/321 | 100/1,020 | 172/1,030 |
| Day 14 | ||||
| Mean | 5,580 | 18,375 | 18,516 | 40,258 |
| Median | 1,995 | 2,530 | 16,350 | 16,510 |
| SD | 10,721 | 46,335 | 15,844 | 53,359 |
| Minimum/maximum | 581/32,000 | 492/133,000 | 1,620/43,600 | 1,290/130,000 |
| Day 42 | ||||
| Mean | 11,778 | 24,965 | 21,262 | 52,539 |
| Median | 6,650 | 5,870 | 21,250 | 31,800 |
| SD | 12,948 | 47,332 | 10,546 | 47,404 |
| Minimum/maximum | 1,630/40,200 | 773/140,000 | 3,000/38,100 | 8,310/139,000 |
| Day 70 | ||||
| Mean | 27,262 | 44,318 | 42,412 | 80,456 |
| Median | 13,800 | 15,125 | 43,975 | 49,450 |
| SD | 28,998 | 58,733 | 16,680 | 84,806 |
| Minimum/maximum | 5,790/88,650 | 1,130/107,000 | 21,100/70,150 | 11,595/228,000 |
| After 6 mo | ||||
| Mean | 10,146 | 19,922 | 13,573 | 22,774 |
| Median | 4,890 | 3,040 | 12,400 | 10,365 |
| SD | 10,599 | 39,007 | 7,963 | 31,256 |
| Minimum/maximum | 985/31,300 | 351/107,000 | 2,050/26,300 | 1,920/95,900 |
| Booster day 14 | ||||
| Mean | 22,290 | 34,910 | 78,014 | 129,388 |
| Median | 15,500 | 19,800 | 51,200 | 112,100 |
| SD | 19,686 | 40,370 | 69,138 | 91,281 |
| Minimum/maximum | 3,500/61,200 | 471/119,000 | 21,600/220,000 | 24,900/286,000 |
Six months after the third vaccination, antibody titers against OprF-OprI were still significantly elevated in all groups. After the booster vaccination a 3- to 10-fold increase of specific antibody titers was measured. One volunteer in each of groups 2 to 4 had relocated and was unavailable for this booster vaccination.
Protection against P. aeruginosa is mediated in humans by specific IgG1 antibody and both antibody-mediated and complement-mediated phagocytosis (30). To address the question of whether the vaccine would be protective in patients, IgG subclasses of antibodies against OprF-OprI were determined. In all groups a significant increase in IgG1 antibodies was observed (Fig. 4). This increase occurred after the first vaccination in group 4 (the 500-μg dose) but after the second boost in the other groups. The increases in the other IgG subclasses, IgG2, IgG3, and IgG4, were only marginal (1:100 to 1:1,000) after the third vaccination (data not shown).
FIG. 4.
IgG1-specific antibody response in sera of volunteers as shown in Fig. 2 measured by ELISA with 1:100 diluted mouse human IgG1 as described in Materials and Methods. Key: □, day 0; ▨, day 14;  , day 42;
, day 42; 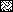 , day 70;
, day 70; 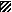 , day 240;
, day 240; 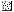 , day 254.
, day 254.
C1q-binding assay.
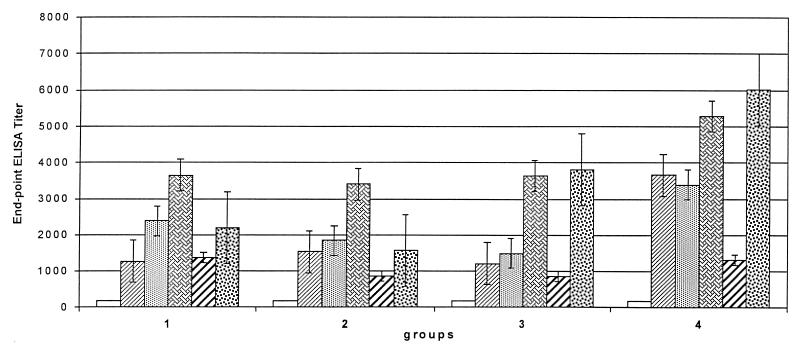
To determine whether the elicited antibodies had the potential for promoting antibody-mediated complement-dependent opsonization, we measured by a C1q-binding ELISA (11) the C1q-binding capacity of the sera before immunization and after the third vaccination. As shown in Fig. 5, a significant increase of C1q binding to antibodies was detected after the third vaccination in all 26 serum samples tested.
FIG. 5.
The binding of C1q, a subcomponent of the complement classical pathway component C1, was measured by ELISA as reported previously (11, 54). The sera were tested before ■ and after 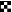 the third vaccination. Plates were coated with OprF-OprI and incubated with 50 μl of the respective 1:4-diluted serum and 50 μl of complement source. Binding was measured with peroxidase-linked anti-C1q antibodies, and ortho-phenylen-diamine was used as the substrate.
the third vaccination. Plates were coated with OprF-OprI and incubated with 50 μl of the respective 1:4-diluted serum and 50 μl of complement source. Binding was measured with peroxidase-linked anti-C1q antibodies, and ortho-phenylen-diamine was used as the substrate.
Analysis of the opsonophagocytic efficacy of the various sera.
Of the 11 antiserum samples (two to three randomly chosen samples from each group) selected for analysis after the third vaccination, 8 had a statistically significant increase in opsonic activity compared to their paired preimmune sera (Table 2). These eight volunteers appeared to be responding to the vaccine preparation and not to a possible previous exposure to P. aeruginosa, since in all eight cases the first vaccination alone failed to boost opsonic activity. Five of the eight positive antiserum samples had a mean for their preimmune sera similar to the mean obtained with normal, unimmunized human sera. These five antiserum samples had opsonic ratios (Table 2) that exceeded the ratio obtained for the protein F antisera (the positive control). The remaining three positive antiserum samples had ratios of 1.3 to 1.4. However, the means for the preimmune sera for all three individuals were approximately double that of the normal human sera, indicating that these individuals had a higher than normal opsonic activity against P. aeruginosa prior to immunization. Vaccination was still successful in boosting the opsonic activity of the antisera of these individuals after three immunizations. The OprF-OprI hybrid protein vaccine demonstrated the ability to boost the opsonophagocytic activity of the antisera obtained from 73% of the volunteers tested by this assay.
TABLE 2.
Quantitation of phagocytic uptake by human PMNs of P. aeruginosa exposed to various sera
| Test seruma | Mean no.b of bacteria/PMN ± SD | Ratioc | P valued |
|---|---|---|---|
| 1-3-0 | 5.9 ± 9.2 | — | |
| 1-3-1 | 5.8 ± 9.2 | 0.92 | |
| 1-3-3 | 11.6 ± 18.4 | 2.0 | <0.001* |
| 1-7-0 | 7.3 ± 11.0 | — | |
| 1-7-1 | 9.1 ± 13.2 | 0.156 | |
| 1-7-3 | 14.1 ± 15.0 | 1.9 | <0.001* |
| 2-1-0 | 13.5 ± 14.3 | — | |
| 2-1-1 | 13.4 ± 16.2 | 0.990 | |
| 2-1-3 | 17.1 ± 18.8 | 1.3 | 0.033* |
| 2-4-0 | 6.1 ± 8.3 | — | |
| 2-4-1 | 8.0 ± 14.1 | 0.154 | |
| 2-4-3 | 13.2 ± 16.3 | 2.2 | <0.001* |
| 2-8-0 | 5.1 ± 9.1 | — | |
| 2-8-1 | 3.0 ± 6.4 | 0.025* | |
| 2-8-3 | 8.4 ± 14.1 | 1.7 | 0.012* |
| 3-4-0 | 14.5 ± 14.2 | — | |
| 3-4-1 | 15.2 ± 14.6 | 0.646 | |
| 3-4-3 | 20.9 ± 19.6 | 1.4 | <0.001* |
| 3-5-0 | 13.2 ± 14.1 | — | |
| 3-5-1 | 13.5 ± 16.5 | 0.864 | |
| 3-5-3 | 18.3 ± 18.5 | 1.4 | 0.005* |
| 3-7-0 | 8.0 ± 11.6 | — | |
| 3-7-1 | 5.1 ± 9.0 | 0.009* | |
| 3-7-3 | 6.4 ± 11.2 | 0.8 | 0.150 |
| 4-1-0 | 5.9 ± 9.1 | — | |
| 4-1-1 | 7.1 ± 11.3 | 0.318 | |
| 4-1-3 | 5.5 ± 9.6 | 0.9 | 0.695 |
| 4-2-0 | 9.1 ± 11.3 | — | |
| 4-2-1 | 9.0 ± 13.0 | 0.957 | |
| 4-2-3 | 15.2 ± 21.3 | 1.7 | 0.0020* |
| 4-5-0 | 6.2 ± 10.0 | — | |
| 4-5-1 | 9.2 ± 15.1 | 0.024* | |
| 4-5-3 | 6.2 ± 9.6 | 1.0 | 0.961 |
| Protein F | 18.9 ± 13.4 | 1.6 | <0.001* |
| Normal mouse | 11.5 ± 9.6 | ||
| Normal human | 6.9 ± 10.2 |
Sera from human volunteers are identified as follows: group number-individual number within group-number of vaccinations received. Protein F sera were collected from mice immunized with purified, intact OprF. Normal sera were collected from unimmunized mice or humans.
Mean for total of 150 PMNs counted.
Ratio represents the mean number of bacteria associated per PMN in the sera from the volunteers after the third immunization divided by the mean number of bacteria per PMN for the sera before immunization of that individual.
P values determined by paired, two-tail Student’s t test. Values denoted by an asterisk are statistically significant.
DISCUSSION
Improvements in the treatment of severe burns which allow victims to survive the initial shock phase, aggressive treatment of cancer patients with cytostatic drugs and surgery, and the increasing number of elderly polymorbidic patients make nosocomial infections and sepsis the leading cause of death in surgical intensive care units. The frequency of P. aeruginosa among other gram-negative bacterial infections varies to a large extent at different medical centers (3, 15, 22, 29, 33, 36, 44). However, the mortality rate of P. aeruginosa septicemia is very high at all medical centers. P. aeruginosa is the main cause of nosocomial pneumonia in the United States (39). Jones et al. showed in a clinical trial with burn patients immunized with a polyvalent P. aeruginosa LPS-based vaccine that overall survival of patients can be improved by vaccination immediately after a burn (32). This observation and the fact that the majority of patients in other risk groups such as transplant patients or patients undergoing major surgery can be easily vaccinated before the onset of treatment make vaccination against P. aeruginosa, as well as against other nosocomial pathogens, a promising tool to reduce the high incidence of infections and decrease the high costs of intensive care treatment.
We have produced and tested a new vaccine against P. aeruginosa based on a recombinant hybrid protein which expresses the main known protective epitopes of OprF and OprI. After appropriate quality assessment according to our previous trial in volunteers with a recombinant OprI vaccine (54), three doses of 20, 50, 100, and 500 μg of the vaccine were administered at 4-week intervals. No systemic side effects, such as a rise in body temperature, headache, or general illness, were observed. The alterations of the C-reactive protein levels observed in four volunteers of group 4 were not attributed to vaccination, because the leukocyte counts remained in the normal range and the alterations occurred only once. Because the arthritis in the left knee joint of volunteer 8 in group 1 appeared 3 months after the third vaccination, following an extended biking tour, this illness was not attributed to vaccination either. This volunteer had had arthritis in his left knee 4 years previously, also after a biking tour. Local effects were limited to a feeling of pressure after the injection in some of the volunteers who received 100- and 500-μg doses. This result can be explained by the fact that these doses contained a 10-fold concentration of A1(OH)3 compared to those in the lower doses received by groups 1 and 2. No sign of inflammation was observed. With all four different vaccine doses used, a significant increase in P. aeruginosa-specific antibody was observed. A maximal response was observed after the first vaccination for the higher doses of 100 or 500 μg. A single-shot vaccination leading to protective antibody levels would be advantageous especially for burn patients. In order to investigate the longevity of the induced antibody response, blood was taken from the volunteers 6 months after the third vaccination. In addition, volunteers received a fourth vaccination to investigate the booster response. As shown in Fig. 2, the vaccine-specific antibody titers were still detectable after a 6-month period, and a significant increase in antibody titers was measurable following the boost. An important question which remains to be answered concerns the protective efficacy of the vaccine under clinical conditions. In experimental animal models, the efficacy of GST-OprF-OprI for protection against P. aeruginosa infection was clearly demonstrated (53). In mice the protective epitope D5 of OprF and the protective epitope of OprI defined by the monoclonal antibody 2A1 could be identified (16, 53). Using the appropriate monoclonal antibodies, we were able to prove that these epitopes (Fig. 1) as well as the surface-localized epitope D2 (25) (data not shown) are expressed by the recombinant Met-Ala-(His)6-OprF190–342 OprI21–83 vaccine preparation. Analysis of the sera of the volunteers indicated that the antibodies recognize mainly the epitope D2 (data not shown). However, in addition to these peptide-defined linear epitopes, antibody-defined conformational epitopes have been associated with protection in mice (25).
To gain further evidence for the protective efficacy of the vaccine in vitro, two different in vitro assays were performed. Binding of the first complement component to the Fc portion of antibodies bound to the bacterial target is a prerequisite for optimal opsonic phagocytosis. Sera of volunteers before vaccination and after the third vaccination were tested by the C1q-binding assay (Fig. 5). Immunization with the hybrid OprF-OprI vaccine resulted in an increase in complement-binding capacity in the sera of all volunteers tested by this assay. The relevance of C1q binding for protection has been demonstrated before (11, 30). During previous investigations in mice, we showed that the protective ability of monoclonal antibodies against P. aeruginosa OPRs can be predicted by the C1q-binding assay (11). Further evidence for the protective potential of the vaccine-induced antibody response was indicated by counting the phagocytized Pseudomonas bacteria after incubation of whole blood phagocytes supplemented with either preimmune serum or serum postvaccination (Table 2). The OprF-OprI vaccine elicited antibodies with significant enhanced opsonic activity in 73% of the volunteers tested. Of the sera from five volunteers, who were tested by both in vitro assays, only two (subject 4 in group 3 and subject 2 in group 4) showed identical positive behaviors. The other three (subject 7 in group 3 and subjects 1 and 2 in group 4) showed significant C1q binding but a lack of opsonophagocytic activity. The differences between the results obtained by the C1q-binding assay and those obtained by the in vitro opsonophagocytosis assay may be explained by the fact that the OprF-OprI protein, and not intact bacteria, was used for C1q-binding evaluation. Thus, the C1q-binding assay reflects only the capacity of the antibodies to bind C1q in the presence of a high density of antigen and not the situation with intact bacteria.
A clinical vaccine against P. aeruginosa has to meet several criteria. The vaccine should be protective, well tolerated, and cheap to produce, and because of the need for swift therapy for patients, a single-shot vaccine would be preferable. At least 17 different serotypes of P. aeruginosa can be identified by the International Antigenic Typing Scheme, which is based on the oligosaccharide expression of O side chains. The vaccine therefore should induce cross-protective immunity against all serotypes. Because the OPRs are highly conserved among all known serotypes (38), an OPR vaccine could provide such cross-protective immunity, which serotype-specific LPS O-polysaccharide vaccines have been unable to do. Polysaccharide vaccines are also unlikely to be able to provide either protection upon a single immunizing dose or the desired long-term memory responses. The data from the current clinical trial in volunteers show that the OprF-OprI hybrid vaccine meets most of the desired criteria.
A leading polysaccharide vaccine researcher has recently (26) expressed doubts that OPR vaccines elicit antibodies of a potency sufficient to protect humans against infection. Data from one animal study (35) were cited to support this position; in the burned mouse model used, an anti-O-antigen vaccine provided a much higher level of protection against the homologous O-antigen strain than that provided by a protein F vaccine against each of the seven heterologous Fisher-Devlin immunotype strains. However, in that study no effort was made to determine the maximal level of protection that could have been provided by the OprF vaccine. The vaccine was administered without adjuvant, and only two immunizations were given, with the result that on the day of challenge, the IgG antibody titer to protein F was only 640 and only 160 to cell envelopes of several of the heterologous strains. Still, significant cross-protection was observed against all heterologous strains. One cannot accurately conclude that the level of protection in humans in which an OPR vaccine is administered under conditions that maximize the protective response will be only as high as that observed in the mice in this study. OPR vaccines have been shown to afford significant protection in animal models of systemic infection and burn injury and in acute and chronic pulmonary infections with P. aeruginosa. These vaccines have been shown to elicit antibodies capable of affording passive protection against P. aeruginosa infection as do monoclonal antibodies against OPRs. Upon passive administration of OprF-OprI-antibodies in SCID mice, protection values were obtained, which were comparable to protection values obtained with serotype-specific LPS-antibodies (53). Antibodies elicited by OPR vaccines are opsonic and bind complement, as confirmed again for humans by the present study. Furthermore, in certain clinical situations, such as in the immunotherapy of pulmonary infections in children with cystic fibrosis, OPR vaccines appear to have much greater potential for successful use than LPS O-antigenic vaccines. The emergence of LPS rough strains in the lung with cystic fibrosis that would make LPS vaccines ineffective should increase the accessibility of antibodies to OPRs to their surface protein targets. The preponderance of available evidence strongly suggests that OPR vaccines will be successful for use in humans. A definitive answer to this question can be obtained only from a clinical trial in patients. A phase 3 trial in burn patients was therefore begun at the end of 1998.
ACKNOWLEDGMENTS
This work was supported by grant 01KI8910/4 from the Bundesministerium für Forschung und Technologie to H. Domdey and B.-U. von Specht and by grant E/B41G/L0407/L5921 from the Bundesministerium für Verteidigung to B.-U. von Specht.
We thank G. Köhler for performing the histological examinations.
REFERENCES
- 1.Alexander J W, Fisher M W. Immunization against Pseudomonas infection after thermal injury. J Infect Dis. 1974;130:152–158. doi: 10.1093/infdis/130.supplement.s152. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K, editors. Current protocols in molecular biology 1994–1997. New York, N.Y: John Wiley and Sons; 1997. High efficiency transformation by electroporation; pp. 1.8.4–1.8.6. [Google Scholar]
- 3.Benerjee S S, Emori T G, Culver D H, Gaynes R P, Jarvis W R, Horan T, Edwards J R, Henderson T, Martone W J. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. Am J Med. 1991;91:86S–89S. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J. Naked DNA points way to vaccines. Science. 1993;259:1691–1692. doi: 10.1126/science.8456293. [DOI] [PubMed] [Google Scholar]
- 5.Cryz S J, Jr, Sadoff J C, Fürer E. Octavalent Pseudomonas aeruginosa-O-polysaccharide-toxin A conjugate vaccine. Microb Pathog. 1987;6:75–80. doi: 10.1016/0882-4010(89)90010-7. [DOI] [PubMed] [Google Scholar]
- 6.Cryz S J, Jr, Fürer E, Cross A S, Wegmann A, Germanier R, Sadoff J C. Safety and immunogenicity of Pseudomonas aeruginosa O-polysaccharide-toxin A conjugate vaccine in humans. J Clin Investig. 1987;80:51–56. doi: 10.1172/JCI113062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cryz S J, Jr, Sadoff C, Ohmann D, Fürer E. Characterization of the human immune response to a Pseudomonas aeruginosa O-polysaccharide-toxin A conjugate vaccine. J Lab Clin Med. 1988;111:701–707. [PubMed] [Google Scholar]
- 8.Donnelly J J, Ulmer J B, Liu M A. Immunization with polynucleotides. Immunologist. 1994;2:20–26. [Google Scholar]
- 9.Duchêne M, Schweizer A, Lottspeich F, Krauss G, Marget M, Vogel K, von Specht B-U, Domdey H. Sequence and transcriptional start site of the Pseudomonas aeruginosa outer membrane porin protein F gene. J Bacteriol. 1988;170:155–162. doi: 10.1128/jb.170.1.155-162.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duchêne M, Barron C, Schweizer A, von Specht B-U, Domdey H. Pseudomonas aeruginosa outer membrane lipoprotein I gene: molecular cloning sequence, and expression in Escherichia coli. J Bacteriol. 1989;171:4130–4137. doi: 10.1128/jb.171.8.4130-4137.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckhardt A, Heiss M M, Ehret E, Permanetter W, Duchêne M, Domdey H, von Specht B-U. Evaluation of protective mAbs against Pseudomonas aeruginosa outer membrane protein I by C1q binding assay. Zentbl Bakteriol. 1991;275:100–111. doi: 10.1016/s0934-8840(11)80773-5. [DOI] [PubMed] [Google Scholar]
- 12.Finke M, Duchêne M, Eckhardt A, Domdey H, von Specht B-U. Protection against experimental Pseudomonas aeruginosa infection by recombinant P. aeruginosa lipoprotein I expressed in Escherichia coli. Infect Immun. 1990;58:2241–2244. doi: 10.1128/iai.58.7.2241-2244.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finke M, Muth G, Reichhelm T, Thoma M, Duchêne M, Hungerer K-D, Domdey H, von Specht B-U. Protection of immunosuppressed mice against infection with Pseudomonas aeruginosa by recombinant P. aeruginosa lipoprotein I and lipoprotein I-specific monoclonal antibodies. Infect Immun. 1991;59:1251–1254. doi: 10.1128/iai.59.4.1251-1254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabelsberger J, Knapp B, Bauersachs S, Lenz U, von Specht B-U, Domdey H. A hybrid outer membrane protein antigen for vaccination against Pseudomonas aeruginosa. Behring Inst Mitt. 1997;98:302–314. [PubMed] [Google Scholar]
- 15.Gallagher P G, Watanakunakorn C. Pseudomonas bacteremia in a community teaching hospital, 1980–1984. Rev Infect Dis. 1989;11:846–852. doi: 10.1093/clinids/11.6.846. [DOI] [PubMed] [Google Scholar]
- 16.Gilleland H E, Jr, Hughes E E, Gilleland L B, Matthews-Greer J M, Staczek J. Use of synthetic peptides to identify surface-exposed, linear B-cell epitopes within outer membrane protein F of Pseudomonas aeruginosa. Curr Microbiol. 1995;31:279–286. doi: 10.1007/BF00314580. [DOI] [PubMed] [Google Scholar]
- 17.Gilleland H E, Jr, Gilleland L B, Hughes E E, Matthews-Greer J M. Recombinant outer membrane protein F of Pseudomonas aeruginosa elicits antibodies that mediated opsonophagocytic killing, but not complement-mediated bacteriolysis, of various strains of P. aeruginosa. Curr Microbiol. 1992;24:1–7. [Google Scholar]
- 18.Gilleland H E, Jr, Gilleland L B, Matthews-Greer J M. Outer membrane protein F preparation of Pseudomonas aeruginosa as a vaccine against chronic pulmonary infection with heterologous immunotype strains in rats. Infect Immun. 1988;56:1017–1022. doi: 10.1128/iai.56.5.1017-1022.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilleland H E, Jr, Parker M G, Matthews J M, Berg R D. Use of purified outer membrane protein F (porin) of Pseudomonas aeruginosa as a protective vaccine in mice. Infect Immun. 1984;44:49–54. doi: 10.1128/iai.44.1.49-54.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilleland L B, Gilleland H E., Jr Synthetic peptides representing two protective, linear B cell epitopes of outer membrane protein F of Pseudomonas aeruginosa elicit whole-cell-reactive antibodies that are functionally pseudomonad specific. Infect Immun. 1995;63:2347–2351. doi: 10.1128/iai.63.6.2347-2351.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon S M, Serke J M, Keys T F, Ryan T, Fatica C A, Schmitt S K, Borsh J A, Cosgrove III D M, Yared J P. Secular trends in nosocomial bloodstream infections in a 55-bed cardiothoracic intensive care unit. Ann Thorac Surg. 1998;65:95–100. doi: 10.1016/s0003-4975(97)01039-4. [DOI] [PubMed] [Google Scholar]
- 22.Griffith S J, Nathan C, Selander R K, Chamberlin W, Gordon S, Kabins S, Weinstein R A. The epidemiology of Pseudomonas aeruginosa in oncology patients in a general hospital. J Infect Dis. 1989;160:1030–1036. doi: 10.1093/infdis/160.6.1030. [DOI] [PubMed] [Google Scholar]
- 23.Hanberger H, Hoffmann M, Lindgren S, Nilson L E. High incidence of antibiotic resistance among bacteria in 4 intensive care units at a university hospital in Sweden. Scand J Infect Dis. 1997;29:602–614. doi: 10.3109/00365549709035904. [DOI] [PubMed] [Google Scholar]
- 24.Hancock R E W. Intrinsic antibiotic resistance of Pseudomonas aeruginosa. J Antimicrob Chemother. 1986;18:653–656. doi: 10.1093/jac/18.6.653. [DOI] [PubMed] [Google Scholar]
- 25.Hancock R E W, Wong R. Potential of protein OprF of Pseudomonas in bivalent vaccines. Behring Inst Mitt. 1997;98:283–290. [PubMed] [Google Scholar]
- 26.Hatano K, Pier G B. Complex serology and immune response of mice to variant high-Molecular-weight O polysaccharides isolated from Pseudomonas aeruginosa serogroup O2 strains. Infect Immun. 1998;66:3719–3726. doi: 10.1128/iai.66.8.3719-3726.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hochuli E, Bannwarth W, Döbeli H, Gentz R, Stüber D. Genetic approach to facilitate purification of recombinant proteins with novel metal chelate adsorbent. Bio/Technology. 1998;6:1325–1341. [Google Scholar]
- 28.Holder I A. Pseudomonas immunotherapy. Serodiagn Immunother. 1988;2:7–16. [Google Scholar]
- 29.Holzheimer R G, Quoika P, Pätzmann D, Füssle R. Nosocomial infections in general surgery: surveillance report from a German University Clinic. Infection. 1990;18:219–225. doi: 10.1007/BF01643391. [DOI] [PubMed] [Google Scholar]
- 30.Hong Y Q, Ghebrehiwet B. Effect of Pseudomonas aeruginosa elastase and alkaline protease on serum complement and isolated components C1q and C3. Clin Immunol Immunopathol. 1992;62:1333–1338. doi: 10.1016/0090-1229(92)90065-v. [DOI] [PubMed] [Google Scholar]
- 31.Hsueh P R, Teng L J, Chen Y C, Ho S W, Luh K T. Persistence of a multidrug-resistance Pseudomonas aeruginosa clone in an intensive care burn unit. J Clin Microbiol. 1998;2:1347–1351. doi: 10.1128/jcm.36.5.1347-1351.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones R J, Roe E A, Gupta J L. Controlled trials of a polyvalent Pseudomonas vaccine in burns. Lancet. 1979;ii:977–983. doi: 10.1016/s0140-6736(79)92559-5. [DOI] [PubMed] [Google Scholar]
- 33.Korvick J A, Marsh J W, Starzl T E, Yu V L. Pseudomonas aeruginosa bacteremia in patients undergoing liver transplantation: an emerging problem. Surgery. 1991;109:62–68. [PMC free article] [PubMed] [Google Scholar]
- 34.Kraehenbuhl J P, Neutra M R. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev. 1992;72:853–879. doi: 10.1152/physrev.1992.72.4.853. [DOI] [PubMed] [Google Scholar]
- 35.Matthews-Greer J M, Gilleland H E., Jr Outer membrane protein F (porin) preparation of Pseudomonas aeruginosa as a protective vaccine against heterologous immunotype strains in a burned mouse model. J Infect Dis. 1987;155:1282–1291. doi: 10.1093/infdis/155.6.1282. [DOI] [PubMed] [Google Scholar]
- 36.McManus A T, Mason A D, Jr, McManus W F, Pruitt B A., Jr Twenty-five year review of Pseudomonas aeruginosa bacteremia in a burn center. Eur J Clin Microbiol. 1985;4:219–223. doi: 10.1007/BF02013601. [DOI] [PubMed] [Google Scholar]
- 37.Miller J M, Spilsbury J F, Jones R J, Roe E A, Lowbury E J L. A new polyvalent Pseudomonas vaccine. J Med Microbiol. 1977;10:19–27. doi: 10.1099/00222615-10-1-19. [DOI] [PubMed] [Google Scholar]
- 38.Mutharia L M, Nicas T I, Hancock R E W. Outer membrane proteins of P. aeruginosa serotype strains. J Infect Dis. 1982;146:770–779. doi: 10.1093/infdis/146.6.770. [DOI] [PubMed] [Google Scholar]
- 39.Pennington J E. Pseudomonas aeruginosa pneumonia and other respiratory tract infections. In: Baltch A, editor. Pseudomonas aeruginosa infections and treatment. New York, N.Y: Marcel Dekker Inc.; 1994. pp. 159–181. [Google Scholar]
- 40.Pier G B. Safety and immunogenicity of high-molecular weight polysaccharide vaccine from immunotype 1 Pseudomonas aeruginosa. J Clin Investig. 1982;69:303–308. doi: 10.1172/JCI110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pier G B. Pseudomonas aeruginosa surface polysaccharide vaccines. New therapeutic approaches from basic research. In: Speert D P, Hancock R E W, editors. Pseudomonas aeruginosa: new therapeutic approaches from basic research. Vol. 36. Basel, Switzerland: S. Karger; 1985. pp. 157–167. [PubMed] [Google Scholar]
- 42.Pier G B, Des Jardin D, Grout M, Garner C, Bennet S E, Pekoe G. Human immune response to Pseudomonas aeruginosa mucoid exopolysaccharide (alginate) vaccine. Infect Immun. 1994;62:3972–3979. doi: 10.1128/iai.62.9.3972-3979.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pier G B. Rational for development of immunotherapies that target mucoid Pseudomonas aeruginosa infection in cystic fibrosis patients. Behring Inst Mitt. 1997;98:350–360. [PubMed] [Google Scholar]
- 44.Pruitt B A, Jr, Colonel M C, McManus A T. Opportunistic infections in severely burned patients. Am J Med. 1984;30:146–154. doi: 10.1016/0002-9343(84)90334-6. [DOI] [PubMed] [Google Scholar]
- 45.Pruitt B A, Jr, McManus A T, Kim S H, Goodwin C W. Burn wound infections: current status. World J Surg. 1998;22:135–145. doi: 10.1007/s002689900361. [DOI] [PubMed] [Google Scholar]
- 46.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kD. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 47.Srikumar R, Kon T, Gotoh N, Poole K. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in a multidrug-sensitive Escherichia coli strain. 1998. Antimicrob Agents Chemother. 1998;42:65–71. doi: 10.1128/aac.42.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takacs B J, Girard M F. Preparation of clinical grade proteins produced by recombinant DNA technologies. J Immunol Methods. 1991;143:231–240. doi: 10.1016/0022-1759(91)90048-k. [DOI] [PubMed] [Google Scholar]
- 49.Tassios P T, Gennimata V, Maniatis A N, Fock C, Legakis N J the Greek Pseudomonas aeruginosa Study Group. Emergence of multidrug resistance in ubiquitous and dominant Pseudomonas aeruginosa serogroup O:11. J Clin Microbiol. 1998;36:897–901. doi: 10.1128/jcm.36.4.897-901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toth A, Schödel F, Duchêne M, Massarrat K, Blum B, Schmitt A, Domdey H, von Specht B-U. Protection of immunosuppressed mice against translocation of Pseudomonas aeruginosa from the gut by oral immunization with recombinant Pseudomonas aeruginosa outer membrane protein I expressing Salmonella dublin. Vaccine. 1994;12:1215–1221. doi: 10.1016/0264-410x(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 51.Velasco E L, Thuler C, Martins C A, Dias L M, Goncalves V M. Nosocomial infections in an oncology intensive care unit. Am J Infect Control. 1997;25:458–462. doi: 10.1016/s0196-6553(97)90067-5. [DOI] [PubMed] [Google Scholar]
- 52.von Specht B-U, Strigl G, Ehret W, Brendel W. Protective effect of an outer membrane vaccine against Pseudomonas aeruginosa infection. Infection. 1987;15:408–412. doi: 10.1007/BF01647755. [DOI] [PubMed] [Google Scholar]
- 53.von Specht B-U, Knapp B, Muth G, Bröker M, Hungerer K-D, Diehl K-D, Massarrat K, Seeemann A, Domdey H. Protection of immunocompromised mice against lethal infection with P. aeruginosa by active or passive immunization with recombinant P. aeruginosa outer membrane protein F and outer membrane protein I fusion proteins. Infect Immun. 1995;63:1855–1861. doi: 10.1128/iai.63.5.1855-1862.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Specht B-U, Lücking H C, Blum B, Schmitt A, Hungerer K-D, Domdey H. Safety and immunogenicity of a Pseudomonas aeruginosa outer membrane protein I vaccine in human volunteers. Vaccine. 1996;14:1111–1117. doi: 10.1016/0264-410x(96)00054-0. [DOI] [PubMed] [Google Scholar]
- 55.von Specht, B.-U., J. Gabelsberger, B. Knapp, E. Hundt, H. Schmidt-Pilger, S. Bauernsachs, U. Lenz, and H. Domdey. Immunogenic efficacy of differently produced recombinant vaccines candidates against Pseudomonas aeruginosa infections. J. Biotechnol., in press. [DOI] [PubMed]
- 56.Zak O. Antibiotics and Pseudomonas aeruginosa. In: Sabath L D, et al., editors. Pseudomonas aeruginosa International Symposium. Bern, Switzerland: Huber; 1980. pp. 133–159. [Google Scholar]