Abstract
Introduction:
Remdesivir, an antiviral drug, received an emergency use authorization for treating coronavirus disease 2019 (COVID-19) patients. Though many studies have reported the safety aspects of this antiviral agent, most of them were observed in severely ill COVID-19 patients, making very less data available in the moderately ill patients. The present study was conducted with an objective of finding the adverse events (AEs) associated with remdesivir in moderately ill COVID-19 patients.
Methodology:
A retrospective observational study was conducted by collecting data of demographic details and details of remdesivir, laboratory investigations, and AEs from the patient medical records from May to July 2021 and analyzed by using the appropriate statistics.
Results:
Out of the 160 COVID-19 patients, 32 were moderately ill (males: 29, females: 03) and were treated with remdesivir along with steroids and low molecular weight heparin (LMW) heparin. The average number of administered remdesivir doses was 4, with a loading dose of 200 mg and a maintenance dose of 100 mg. A total of 41 AEs were observed out of which 17 were adverse drug reactions (ADRs) (a significant increase in the alanine transaminase (ALT) [P < 0.001]) and 23 AEs (a significant rise in random blood sugars, RBS [one of the AEs] [P = 0.007]). The AEs were more commonly seen in the hypertensive patients. An increased oxygen requirement was a major serious AE observed in four patients.
Conclusion:
Remdesivir caused a significant increase in the liver enzymes. Increased blood sugar levels were the most common AE and increased oxygen requirement was the major serious AE observed.
Keywords: Adverse events, antiviral, moderately ill COVID-19, remdesivir
Background
The COVID-19 pandemic is continuing its spread with more than 26 crores of confirmed cases and more than 5 million deaths globally, while there are more than 3.4 crores of confirmed cases and 4.7 lac deaths in India as of on December 1, 2021.[1] The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus responsible for this highly contagious disease, which first started in Wuhan, China, and eventually, spread all over the world affecting millions of people. Once a person gets infected with this virus, based on the immune system’s capacity to fight against the same, some will end up in a condition called acute respiratory distress syndrome (ARDS), which may further lead to respiratory compromise and death.[2] Ever since its origin and impact on public health, scientists have been focusing on finding novel drugs and vaccines for SARS-CoV-2, yet there is no certain treatment for it. A few antiviral drugs such as oseltamivir, favipiravir, umifenovir, lopinavir, remdesivir, and antimicrobials like azithromycin are mostly used as off-label drugs for treating these patients. In addition, anti-inflammatory agents have critical roles to inhibit lung injury and multisystem organ dysfunction and to prevent cytokine storm which is the main reason of deaths related to SARS-CoV-2. Low-molecular-weight heparin (LMWH) along with corticosteroids, when necessary, is also considered.[3] Remdesivir is a nucleotide prodrug which after getting converted to its active metabolite interferes with an enzyme called ribonucleic acid-dependent RNA polymerase, which is responsible for the multiplication of the virus in the human body.[4]
Remdesivir, because of its wide spectrum of coverage in terms of antiviral activity, has become a potential candidate in treating the COVID-19-infected patients. After showing promising results in the early phases of drug development, it got an emergency-use authorization on October 22, 2020, by the US food and drug administration (FDA) to treat severe COVID-19 patients, which is available either as a solution or a freeze-dried formulation to be dissolved and infused. The drug is given as a loading dose of 200 mg followed by a 100 mg maintenance dose over 5–10 days after evaluating for the normal renal and hepatic parameters.[5,6] The known adverse drug reactions, as per the information provided in the drug’s monographs, are an elevation in the liver enzymes, skin rash, increase in prothrombin time, gastrointestinal upset, and hypersensitivity reactions.[7] Its wide range of usage ever since its approval has become a concern to the health care professionals because of its potential adverse effects. An updated review has been published regarding the safety profile of this antiviral drug in COVID-19 in terms of the hepatotoxicity, gastrointestinal symptoms, respiratory toxicity, cardiovascular toxicity, and nephrotoxicity.[8] A study conducted by Grein et al.[9] has reported AEs in 60% of the patients in which hepatotoxicity was indicated by an increase in the liver enzymes (23% of the recipients), gastrointestinal events like loose stools (10%), cardiovascular toxicity in the form of atrial arrhythmias (6%), and nephrotoxicity as acute renal failure (6%). A multicentric randomized study done by Wang et al.[10] had shown 66% (102/155) of remdesivir recipients developing various adverse events (AEs) out of which 12% (18/155) of the recipients discontinued the drug due to the same reason. A study conducted by Singh et al. had also shown the same findings of liver injury in the form of elevated liver enzymes, cardiovascular events, and acute renal impairment after administering this antiviral drug to the patients.[11]
Though a good number of studies have been reported, the safety profile of remdesivir, in terms of AEs and outcomes, is observed in severely ill patients and as a part of randomized controlled trials, making less data available on the observational studies conducted in moderately ill patients. Hence, our study is going to be one of the very few studies available on the AEs among the moderately ill COVID-19 patients receiving the remdesivir injection, and thereby, throwing a light, to some extent, on how to use this drug with discretion by the primary care physicians while treating the COVID-19 patients of the same category.
The objectives of the study were:
To identify the AEs following remdesivir in moderately ill COVID-19 patients.
To correlate the AEs in the remdesivir-treated patients with co-morbidities.
To evaluate the severity of the AEs.
Methodology
A retrospective observational study was conducted by collecting data from medical records from a teaching hospital after getting approval from the Institutional Research Committee (IRC) and Institutional Ethics Committee (IEC).
The medical record data related to moderately ill COVID-19 patients, and as per the inclusion and exclusion criteria, were collected from 01.05.21 to 22.07.21.
A moderately ill COVID-19 patient is a case with clinical signs of pneumonia (fever, cough, dyspnea, fast breathing) but no signs of severe pneumonia, including SpO2 ≥90% on room air.[12,13]
Inclusion criteria
Patients with moderate COVID-19 illness.
Patients who received the remdesivir injection.
Patients above 18 years of age.
Patients of both sexes.
Patients with controlled diabetes and hypertension.
Exclusion criteria
Patients with end-stage renal disease and acute/chronic liver disease.
Patients with a history of drug hypersensitivity.
Patients with uncontrolled metabolic disorders.
Those who were on other anti-viral drugs.
Those with severe or critically ill COVID-19 disease.
The following data were collected at the time of admission: data regarding the socio-demographic details of the patients, diagnosis, co-morbidities, medication history, and vital parameters including the blood oxygen saturation levels (SpO2). During the hospital stay, the following data were collected: The patient’s daily average SpO2 levels, daily average vital parameters, laboratory investigations such as liver function tests (LFTs), renal function tests (RFTs), remdesivir dose, frequency, the total number of doses administered, data regarding the completion of the planned remdesivir course, the reason for discontinuation if any; concomitant medications, their doses, and frequencies; any noted adverse drug reactions, AEs and their seriousness; condition of the patient such as recovered or not at the time of hospital discharge and duration of the hospital stay.
Statistical analysis
The data were entered and analyzed using Microsoft Excel from the case report form (CRF) (Annexure) that was used to collect the data from the patient medical records. The average parameters were calculated for multiple values of the same parameter. Descriptive statistics were applied to analyze the data and the results were expressed in the form of mean, median, and percentages.
Results
A total of 160 COVID-19 patients’ medical records were screened during the above-mentioned time and 32 patients were found to be moderately ill of which 29 were males and 3 were females, with the average age group being 41.8 years. The most common presenting symptom was shortness of breath (SOB) (28; 87.5%), followed by cough (26; 81.2%). The demographic details, presenting symptoms, and co-morbidities are depicted in [Table 1 and Figures 1 and 2].
Table 1.
Demographic and baseline data
| Parameter | Observation (n=32) |
|---|---|
| Average age group in years (range) | 41.8 (25-80) |
| Male | 29 (90.7%) |
| Female | 03 (9.3%) |
| Average duration of hospital stay (in days) | 7.375 |
| Average time lag between symptom onset and visit to hospital (in days) | 5.58 1–7 Days) |
| Average vital parameters at the time of admission | |
| Systolic blood pressure in mmHg (range) | 129 (110-150) |
| Diastolic blood pressure in mmHg (range) | 85.3 (70-110) |
| Pulse rate beats/minute (range) | 95 (90-120) |
| Respiratory rate/minute | 33.8 (24-38) |
| SpO2 % (on room air) | 91.3% (85-97%) |
| Temperature | Afebrile |
Figure 1.
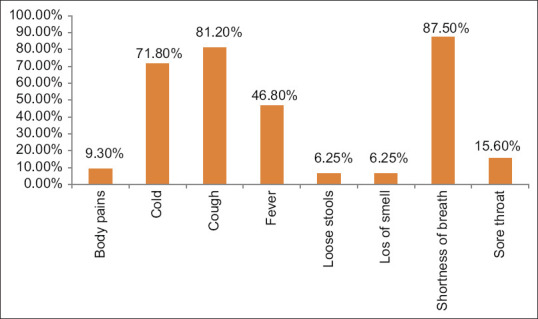
Presenting symptoms in percentage
Figure 2.
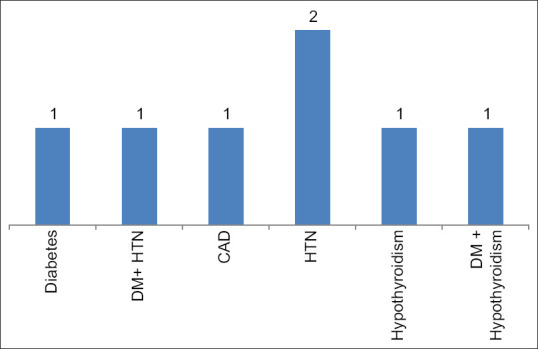
Co-morbidities observed among the moderately ill COVID-19 patients Note: Total co-morbid patients -7
All the moderately ill patients were administered remdesivir with a loading dose of 200 mg followed by 100 mg of maintenance dose; each patient receiving four doses on average. Out of the 32 patients, 28 (87.5%) were able to tolerate well and could finish the actual advised remdesivir course while the rest were referred to the higher COVID care centers due to increased oxygen requirement (3; 9.3%) and hemoptysis (1; 3.1%) [Table 2].
Table 2.
Details of remdesivir administered to the patients
| Parameter | Observation (n=32) |
|---|---|
| Loading dose | 200 mg |
| Maintenance dose | 100 mg |
| Average number of doses administered to each patient | 4.37 (1-5) |
| Number of patients completed the full course advised | 28 (87.5%) |
| Not completed the full treatment course | 4 (12.5%) |
| Reason for discontinuation | Referral to higher COVID care lefts Due to increased O2 requirement (3; 9.3%) Due to hemoptysis (1; 3.1%) |
The average laboratory parameters before and after finishing at least two doses of remdesivir showed a significant increase in the alanine transaminase (ALT) and random blood sugars (RBS) [Table 3]. All the average vital parameters during remdesivir administration were found to be within the normal limits with oxygen supplementation at a required flow rate [Table 4]. The details of concomitant medication are shown in [Table 5].
Table 3.
Average laboratory parameters before and after remdesivir therapy
| Parameter | At the time of admission | On the 3rd day of remdesivir therapy | Two-tailed P |
|---|---|---|---|
| Liver function tests | |||
| ALT (IU) | 43.04±30.89 | 71.21±70.23 | <0.001 |
| AST (IU) | 46.6±20.16 | 50.78±38.8 | 0.9672 |
| ALP (IU) | 80±31.19 | 100.4±118.54 | 0.2522 |
| Albumin (mg/dL) | 3.58±0.44 | 3.45±0.45 | 0.0029 |
| Globulin (mg/dL) | 3.41±0.47 | 3.44±0.30 | >0.999 |
| Total protein (mg/dL) | 6.84±0.52 | 6.89±0.47 | 0.1327 |
| Total bilirubin (mg/dL) | 0.87±0.49 | 0.59±0.18 | <0.001 |
| Renal function tests | |||
| Serum creatinine (mg/dL) | 0.82±0.20 | 0.70±0.11 | <0.001 |
| Blood urea (mg/dL) | 39.64±17.90 | 41.82±12.90 | 0.0089 |
| WBC count | 5631±3089 | 5962±3610 | 0.189 |
| Platelet count | 2.37±0.60 | 2.54±0.65 | <0.001 |
| Random blood sugars (RBS) mg/dL | 177.12±91.86 | 226.26±87.15 | 0.0071 |
P<0.05 significant
Table 4.
Average vital parameters during remdesivir administration
| Parameter | Average value |
|---|---|
| Systolic blood pressure in mmHg | 123.3 |
| Diastolic blood pressure in mmHg | 77 |
| Pulse rate beats/min | 89.9 (80-100) |
| Respiratory rate/min | 26.4 (20-28) |
| SpO2 % (with O2 supplementation) | 96.8 (95-98) |
| Temperature | Afebrile |
Table 5.
Concomitant medications
| Medication group | Drug details |
|---|---|
| Anti-hyperglycemic agents | Human actrapid insulin, metformin, glargine insulin |
| Nutraceuticals | Oral formulations of zinc and vitamin-C |
| Steroids | Dexamethasone 6 mg (oral/parenteral formulations) |
| Anticoagulants | Low molecular weight heparin (LMWH) |
| Antibiotics | Azithromycin |
| Antipyretics | Paracetamol |
| Anti-hypertensives | Telmisartan, hydrochlorothiazide |
Adverse events
A total of 17 adverse drug reactions due to remdesivir (elevated ALT: 53.12%; P < 0.001) and 23 AEs (elevated blood glucose levels [56.25%; P = 0.0071], increased oxygen requirement: 12.5%; hemoptysis: 3.1%) were noted among the moderately ill COVID-19 patients during the remdesivir therapy [Table 6]. The AEs were more commonly seen in the hypertensive patients (28.57%). The association between the co-morbidities and the incidence of AEs is shown in [Table 7].
Table 6.
Adverse events among the moderately ill COVID-19 patients during remdesivir therapy percentage
| Type of adverse event/Reaction | Attributable to | Percentage (n=32) |
|---|---|---|
| Elevated liver enzymes -ALT | Remdesivir | 17 (53.12%) |
| Elevated blood sugar levels | Steroid (Dexamethasone) | 18 (56.25%) |
| Increased oxygen requirement | Disease process | 4 (12.5%) |
| Hemoptysis | 1 (3.1%) |
Table 7.
Co-morbidities and adverse events expressed in percentage
| Co-morbidity (Number of patients; %) | Total number of co-morbid patients: 7 | ||
|---|---|---|---|
|
| |||
| Elevated liver enzymes (3; 42.85%) | Elevated blood sugar levels (4; 57.14%) | Increased oxygen requirement (2; 28.57%) | |
| Hypothyroidism (1) | 1 (14.28%) | 1 (14.28%) | - |
| DM + HTN (1) | 1 (14.28%) | - | - |
| HTN alone (2) | 1 (14.28%) | 2 (28.57%) | - |
| DM alone (1) | - | 1 (14.28%) | |
| DM + Hypothyroidism (1) | - | - | 1 (14.28%) |
| Coronary artery disease (CAD) (1) | - | - | 1 (14.28%) |
Serious adverse events
A total of five patients experienced serious adverse events (SAE) (increased oxygen requirement: 4; hemoptysis: 1) due to which they were referred to the higher COVID care centers for further management.
Discussion
The coronavirus disease 2019 (COVID-19)—a super spreading illness caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—showed its dreadful effects on human health across the globe. So far, four variants of the virus: alpha, beta, gamma, and delta have shown to cause illness manifestations ranging from cold, cough, fever to acute respiratory distress syndrome.[14] Remdesivir is a broad-spectrum antiviral drug which received emergency use authorization to treat patients suffering from severe COVID-19, which is given intravenously over 5–10 days. The treatment with the same is commonly associated with a temporary, mild-to-moderate rise in the liver enzymes.[15] The majority of data available on the AEs of remdesivir is extracted from the studies conducted among the severely ill COVID-19 patients’ AE. An AE is defined as any untoward medical occurrence in a participant which does not necessarily have a causal relationship with the trial intervention.[16]
Very few studies have been reported addressing the AEs in the moderately ill COVID-19-treated patients with remdesivir, hence, this study was conducted to add something to the existing knowledge on this antiviral agent among the same patient group. A total of 40 AEs were observed in our study of which 17 patients showed a significant increase in the liver enzymes (raised ALT; P < 0.001), which was similar to the findings reported by Spinner et al. and Zampino et al.[4,17] Our study showed more increase in ALT than AST, which was contrary to the study reported by Van Laar et al.[18] A good number of studies including case reports, case series, and randomized controlled studies have also reported liver injury following remdesivir administration in COVID-19 patients.[19] The probable mechanism involving liver injury could be due to the inflammatory changes caused by the virus leading to hepatic hypoxia and injury[20] and evidence shows that liver injury is manifested in the form of mild-to-moderate ALT and AST elevation after 1–5 days of starting the therapy.[21] The evidence shows that remdesivir along with steroids like dexamethasone improves the survival rate in severely ill patients and causes hyperglycemic status during the treatment course, though increased blood sugar is not a well-reported adverse drug reaction to this antiviral agent.[22]
The meta-analysis done by Shrestha et al.[23] also showed that COVID-19 induces a hyperglycemic status in the body through angiotensin-converting enzyme-2 (ACE-2) receptor-mediated mechanisms. Our study observed hyperglycemia as the major AE (53%), which was similar to the findings reported by Beigel et al.,[24] but contrary to the findings by the Gilead reports from phase 3 trials, which showed gastrointestinal upset, such as nausea and diarrhea, being the most common AE.[25] An increased oxygen requirement was the major serious AE observed in our study which was similar to the findings by Grein et al. and Beigel et al.[9,24] One patient experienced hemoptysis which might have been due to further disease progression. All the patients who had SAE were referred to higher COVID care centers for further management. It is a well-known fact that the incidence of AEs related to drugs is associated with many factors, such as patient-related, drug-related, metabolic disorders, liver and kidney disorders, etc., and the co-existence of other chronic illnesses increases their incidence.[26]
From our study, it has been observed that the incidence of AEs, which may or may not be attributable to remdesivir, was more among the hypertensive patients (28.57%), and this finding was comparable to the findings by Falcao et al.,[27] who had reported hypertension being the third most common co-morbidity after chronic kidney disease, HIV, and diabetes for a high incidence of AEs among the patients treated with remdesivir.
The limitations of our study: 1) Retrospective analysis 2) Small sample size
Key points
All the moderately ill COVID-19 patients in our study (n = 32) were administered remdesivir, out of which 28 patients tolerated the treatment well.
Elevated liver enzymes (ALT) was the major adverse drug reaction associated with remdesivir administration and elevated blood sugar levels was the major AE observed.
A higher incidence of AEs was observed in COVID-19 patients with hypertension.
Increased oxygen requirement was the major serious AE causing referral of the patients to higher COVID care centers.
Conclusion
From our study, we observed that the elevation of liver enzymes, increased oxygen requirement, and hyperglycemia were common AEs in moderately ill COVID-19 patients treated with remdesivir. The raised liver enzymes could be attributable to remdesivir and the increased blood sugar levels could be due to the concomitant steroid use whereas the increased oxygen requirement could be due to disease progression.
Take home message
As the above-mentioned AEs due to remdesivir are well-reported by various studies, which were observed in our study as well, it is prudent to be a little cautious while prescribing this drug to treat moderately ill COVID-19 patients with comorbidities like liver disorders and hypertension.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.WHO Corona virus (COVID19) dashboard. Available from: https://covid19.who.int/region/searo/country/in .
- 2.Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection:Origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–8. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kivrak A, Ulaş B, Kivrak H. A comparative analysis for anti-viral drugs:Their efficiency against SARS-CoV-2. Int Immunopharmacol. 2021;90:107232. doi: 10.1016/j.intimp.2020.107232. doi: 10.1016/j.intimp. 2020.107232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19:A randomized clinical trial. JAMA. 2020;324:1048–57. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamb YN. Remdesivir:First approval. Drugs. 2020;80:1355–63. doi: 10.1007/s40265-020-01378-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FDA NEWS RELEASE. FDA Approves First Treatment for COVID-19. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 .
- 7.Remdesivir: Drug information. Available from: https://www.uptodate.com/contents/remdesivir-drug-information .
- 8.Fan Q, Zhang B, Ma J, Zhang S. Safety profile of the antiviral drug remdesivir: An update. Biomed Pharmacother. 2020;130:110532. doi: 10.1016/j.biopha.2020.110532. doi: 10.1016/j.biopha. 2020.110532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of remdesivir for patients with severe covid-19. N Engl J Med. 2020;382:2327–36. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID- 19:A randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–78. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh A, Kamath A. Assessment of adverse events associated with remdesivir use for coronavirus disease 2019 using real-world data. Expert Opin Drug Saf. 2021;20:1559–64. doi: 10.1080/14740338.2021.1962846. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Clinical management of COVID-19. Interim guidance. 2020 [Google Scholar]
- 13.Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19) [Updated 2021 Sep 02] In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554776/. LiverTox: Clinical and Research Informationon Drug-Induced Liver Injury . Available from: https://www.ncbi.nlm.nih.gov/books/NBK564049/ [PubMed] [Google Scholar]
- 14.World Health Organization. (2012). IMAI district clinician manual: hospital care adolescents and adults : guidelines for the management of illnessess with limited-resources. World Health Organization. [Last accessed on 2020 May 13]. Available from: https://apps.who.int/iris/bitstream/handle/10665/77751/9789241548290_Vol2_eng.pdf?sequence=3 .
- 15.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Available from: https://www. ncbi.nlm.nih.gov/books/NBK564049/ [PubMed]
- 16.Aspden P, Corrigan JM, Wolcott J, Erickson SM. Institute of Medicine (US) Committee on Data Standards for Patient Safety. Patient Safety: Achieving a New Standard for Care. Washington (DC): National Academies Press (US); 2004. [PubMed] [Google Scholar]
- 17.Zampino R, Mele F, Lucia Florio L, Bertolino L, Andini R, Galdo M, et al. Liver injury in remdesivir-treated COVID-19 patients. Hepatol Int. 2020;28:881–3. doi: 10.1007/s12072-020-10077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Laar SA, de Boer MGJ, Gombert-Handoko KB, Guchelaar HJ, Zwaveling J, et al. Liver and kidney function in patients with COVID-19 treated with remdesivir. Br J Clin Pharmacol. 2021;87:4450–4. doi: 10.1111/bcp.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sodeifian F, Seyedalhosseini ZS, Kian N, Eftekhari M, Najari S, Mirsaeidi M, et al. Drug-induced liver injury in COVID-19 patients:A systematic review. Front Med (Lausanne) 2021;8:731436. doi: 10.3389/fmed.2021.731436. doi:10.3389/fmed. 2021.731436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olry A, Meunier L, Délire B, Larrey D, Horsmans Y, Le Louët H. Drug-induced liver injury and COVID-19 infection:The rules remain the same. Drug Saf. 2020;43:615–7. doi: 10.1007/s40264-020-00954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xavier Ortiz GX, Lenhart G, Becker MW, Schwambach KH, Tovo CV, Blatt CR. Drug-induced liver injury and COVID-19:A review for clinical practice. World J Hepatol. 2021;13:1143–53. doi: 10.4254/wjh.v13.i9.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negahdaripour M. Post-COVID-19 hyperglycemia:A concern in selection of therapeutic regimens. Iran J Med Sci. 2021;46:235–6. doi: 10.30476/ijms.2021.47666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrestha DB, Budhathoki P, Raut S, Adhikari S, Ghimire P, Thapaliya S, et al. New-onset diabetes in COVID-19 and clinical outcomes:A systematic review and meta-analysis. World J Virol. 2021;10:275–87. doi: 10.5501/wjv.v10.i5.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of covid-19-Final report. N Engl J Med. 2020;383:1813–26. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilead reports remdesivir helped moderately ill COVID-19 patients. Phase 3 clinical trial. Clinical Trials & Research News. Available form: https://pharmanewsintel.com/news/gilead-reports-remdesivir-helped-moderatelyill-covid-19-patients .
- 26.Alomar MJ. Factors affecting the development of adverse drug reactions (Review article) Saudi Pharm J. 2014;22:83–94. doi: 10.1016/j.jsps.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falcao F, Viegas E, Carmo I, Soares J, Falcao M, Solano M, et al. A prospective, observational study to evaluate adverse drug reactions in patients with COVID-19 treated with remdesivir or hydroxychloroquine:A preliminary report. Eur J Hosp Pharm. 2021;28:248–53. doi: 10.1136/ejhpharm-2020-002613. [DOI] [PMC free article] [PubMed] [Google Scholar]


