Abstract
Objective:
Perinatal asphyxia affects different organs of body depending upon the severity of hypoxemia and ischemia. This study was carried out to evaluate severity of hyperbilirubinemia in relation to severity of asphyxia.
Study Design:
A case-control study.
Methodology:
Asphyxiated newborns with Apgar score ≤7 at 1 min. and categorized as severe birth asphyxia according to the WHO classification of diseases (ICD10) were matched with controls without birth asphyxia. All babies were examined twice daily for dermal icterus until start of phototherapy. Babies with congenital heart disease, sepsis, cephalohematoma, blood group incompatibility were excluded. Arterial blood gas analysis was done along with serial TSB measurement as per standard guidelines.
Results:
50 cases and 50 matched controls were enrolled. The average birth weight and gestation in cases was 2427 ± 30.05 g and 35.9 ± 2.5 weeks and among control it was 2633 ± 378.62 g and 37.76 ± 0.116 weeks. Among cases, onset of jaundice was 56.64 ± 20.43 h compared to 63.36 ± 23 h in control group. In the cases, the average pH was 7.31 ± 0.06, CO2 was 41.52 ± 84, O2 was 94.98 ± 14.83, and HCO3 was 18.56 ± 2.04. The rise and peak of serum bilirubin differed between the case and control groups; in the cases, the peak occurred at the 22nd h of life, then plateaued from the 40th to the 78th hour of life, and ultimately fell at the 96th hour of life. In comparison, the rise and peak of serum bilirubin occurred comparatively late in the control group. The rise and peak in the control group occurred at the 80th and 96th h of life, respectively. The multiple linear regression analysis showed CRP, Apgar at 5 min. below 7 and male gender significantly affects the rise of serum bilirubin (P < 0.05).
Conclusion:
The peak serum bilirubin in asphyxiated newborns occurs earlier, and plateau for longer duration compared to normal newborns. Low Apgar at 5 min. has significant correlation to earlier rise of bilirubin.
Keywords: Acidosis, asphyxia, hyperbilirubinemia, newborn
Introduction
WHO defined birth asphyxia as “failure to initiate or sustain breathing at birth.”[1] Every year, in India 0.78 million newborns die, with neonatal mortality rate of 30/1,000 live birth.[2] The major causes of neonatal deaths in India are prematurity, birth asphyxia, and infections.[3] Neonatal hyperbilirubinemia affects 55.2% of term babies and 80% of preterms. Various physiological factors contribute to neonatal hyperbilirubinemia. These include higher bilirubin load, hepatic enzyme immaturity, enterohepatic circulation, and bilirubin excretion.[4,5] The toxic effect of bilirubin is due to deposition of free bilirubin in tissues.[5] Perinatal asphyxia affects various physiological milieus of newborns, one of which is neonatal hyperbilirubinemia. The effect of blood pH in asphyxiated newborn on serum bilirubin remained to be understood. The interaction of unconjugated bilirubin with albumin binding site varies along with changes in blood pH.[6] With blood albumin concentration of 3 gm/100 ml, it will bind 25 mg/dl of unconjugated bilirubin, this gets disturbed in the presence of acidosis.[7] The neurotoxic effect of bilirubin is dependent on ratio of free bilirubin and serum albumin rather than total serum bilirubin.[8] What is the effect of perinatal asphyxia on bilirubin needs to be understood, with very few studies on correlation between both. Hence this study done to see the effect of perinatal asphyxia on the severity of serum bilirubin among newborns.
Methodology
Study setting
The study was conducted in the department of Paediatrics at Govt Medical College, Patiala.
Between August 2004 and July 2006. It was approved by the Institutional Ethical Committee, and informed consent was obtained from the parents before enrolment of each subject.
Study population
Inclusion criteria: Asphyxiated newborn with Apgar score ≤7 at 1 min. were taken as cases and categorized according to the WHO classification of diseases (ICD10). Severe birth asphyxia was defined as Apgar score of ≤3 at 5 min., mild and moderate birth asphyxia was defined as Apgar score of 6 and 4--5 at 5 min.[1] Babies with congenital heart disease, perinatal infection, sepsis, cephalohematoma, blood group incompatibility, or hemodynamically unstable were excluded. For each case, normal newborn without perinatal complication born within 48 h of birth of case with same maturity and sex was selected as control.
Sampling method
Examinations of the newborn was conducted in well lighted room under natural light. All babies were examined twice daily for dermal icterus till baby is placed under phototherapy. 2 ml venous blood was taken in dry test tube avoiding contamination and was allowed to clot for evaluation of jaundice. The Serum was separated and sample was analyzed for total serum bilirubin, hemogram, CRP.ABG was done at same time. Serum bilirubin was estimated by Malloy and Evelyn method.[9]
Statistical analysis
Data was recorded in excel sheet, quantitative data recorded as means ± SD, or in percentage. Analysis was done on Stata 13 version software, means between two groups compared with t-test. Linear regression analysis was performed to check correlation between various variables.
Results
Total 50 cases and 50 matched controls were included. In both cases and control group, 31 males and 19 females were enrolled. Description of variables are mentioned in [Table 1]. The various parameters like day of life (DOL), birth weight, gestation, blood gas analysis were compared with serum bilirubin between asphyxiated group and age matched control and found statistically significant (P<0.05) for birth weight and gestation as shown in [Table 2]. The pattern of rise and peak of serum bilirubin differed among cases and controls. In some cases, the peak of serum bilirubin occurred at 22nd h of life and then plateau was achieved from 40th to 78th h of life, with fall at 98th h of life [Figure 1]. The mean difference of total serum bilirubin between cases and control was 1.5 mg/dl. In control group, rise and peak of serum bilirubin occurred relatively late at 80th h and fall by 100th h of life [Figure 2]. Cases were categorized according as mild, moderate, and severe birth asphyxia; the mean serum bilirubin in each group was 17.26 mg/dl, 16.46 mg/dl, and 17.6 mg/dl, respectively [Table 3]. The average pH in cases was 7.31 ± 0.06, CO2 was 41.5 ± 2.84 and O2 94.98 ± 14.83 and HCO3 was 18.56 ± 2.04. Among mild asphyxia cases, mean pH was 7.323 ± 0.06, in moderate asphyxia it was 7.29 ± 0.6 and 7 ± 0.1 in severe asphyxia. On multiple linear regression CRP, male gender and Apgar at 5 min. ≤7 showed a significant relation (P < 0.05) with rate of rise of serum bilirubin [Table 4].
Table 1.
Description of study subjects
| Variables | Asphyxia Cases | Matched Controls |
|---|---|---|
| Male: Female | 1.63:1 | 1.63:1 |
| Average weight | 2427±30.05g | 2633±378.62 g |
| Average Gestation | 37.9±2.5 week | 37.7±0.1 week |
| Average hours of presentation | 56.64±20.43 | 63.36±23.13 |
| Apgar score (Median, IQR) at 1 min | 5 (6-5) | - |
| Apgar score (Median, IQR) at 5 min | 8 (8-7) | - |
| NNJ Dermal zone II | 3 (6%) | 5 (10%) |
| NNJ Dermal zone III | 19 (38%) | 15 (30%) |
| NNJ Dermal zone IV | 24 (48%) | 23 (46%) |
| NNJ Dermal zone V | 4 (8%) | 7 (14%) |
| Max TSB in mg/dl | 37.76±10.6 | 16.89±3.11 |
| Exchange transfusion done | 4 (8%) | 6 (12%) |
| Phototherapy given | 46 (92%) | 44 (88%) |
| CRP positive | 21 (42%) | 23 (46%) |
Table 2.
Correlation of various parameters with serum bilirubin
| Variables | Correlation coefficient | P | |
|---|---|---|---|
|
| |||
| Asphyxia Cases | Matched Controls | ||
| DOL | -0.127 | 0 | 0.379 |
| Birth weight | 0.350 | 0.397 | 0.013 |
| Gestation | 0.319 | 0.065 | 0.024 |
| pH | 0.006 | - | |
| CO2 | 0.024 | - | |
| O2 | 0.206 | - | |
| Bicarbonate | 0.007 | - | |
P<0.05 is significant
Figure 1.
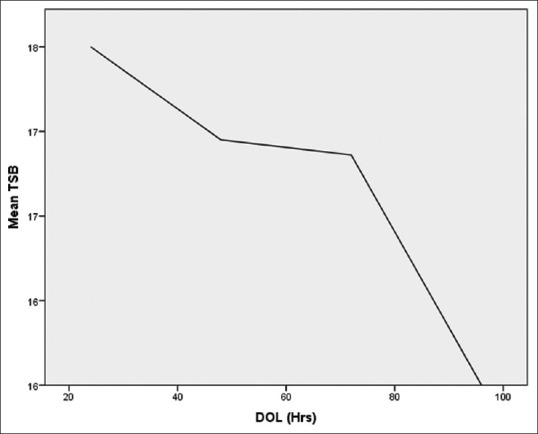
Rise of Serum bilirubin in asphyxiated cases
Figure 2.
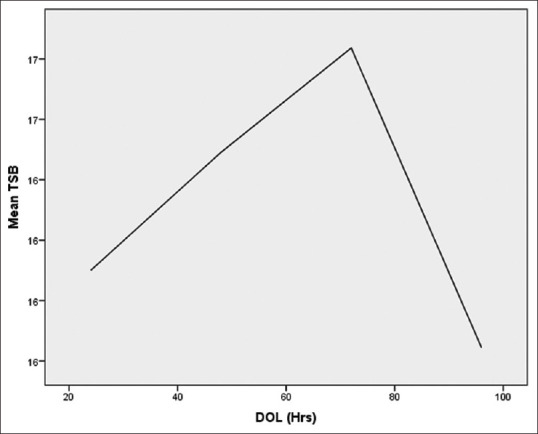
Rise of serum bilirubin in control group
Table 3.
Asphyxia grades and mean serum bilirubin
| Apgar score | Number of cases | PH | Mean serum bilirubin (mg/dl) |
|---|---|---|---|
| MILD (6) | 23 | 7.323±0.0618 | 17.26 |
| MODERATE (4-5) | 24 | 7.29±0.612 | 16.46 |
| SEVERE (0-3) | 7 | 7±0.1 | 17.6 |
Table 4.
Multiple linear regression of serum bilirubin with variables among cases
| Variables | Co-efficient | P | CI (95%) |
|---|---|---|---|
| DOL | 0.446 | 0.319 NS | -0.44-1.33 |
| Apgar 1 min | 0.227 | 0.814 NS | -1.71-2.16 |
| Apgar 5 min | -0.536 | 0.048 S | -1.07 to -0.004 |
| Gender | 2.2 | 0.014 S | 0.58-3.93 |
| Gestation | -1.088 | 0.177 NS | 4.6-0.88 |
| CRP | -2.9 | 0.001 S | 4.5-1.34 |
| Birth weight | 0.003 | 0.174 NS | 0.0012-0.006 |
Discussion
The present study was conducted to assess whether asphyxia induced biochemistry changes affect the course of serum bilirubin in newborns. The rise of serum bilirubin was compared between asphyxia group and controls and it was found that bilirubin started rising early in the asphyxia group, to a higher level compared to the controls. This is in contrast to study done by Fekete et al.[10] where the peak serum bilirubin was attained at around same time by 5th postnatal day both in asphyxia and idiopathic hyperbilirubinemia group.
The blood pH affected the serum bilirubin binding with plasma protein, free bilirubin has deleterious effects on brain and more prone to kernicterus at low bilirubin levels along with other risk factors like sepsis, blood group incompatibility. In our study, the pH in asphyxia group was acidic with mean pH 7.31 ± 0.06. Association between kernicterus and acidosis had also been found in earlier studies,[7,8] explanation given was increase in free fatty acids in asphyxia competing with albumin for binding to albumin sites and increase in free bilirubin and tissue toxicity.[10] In our study, we could not find any correlation between low pH related to asphyxia with higher bilirubin. On the contrary, serum bilirubin in our study showed a significant correlation with birth weight and gestation.
We can therefore say that serum bilirubin in asphyxiated newborns rise earlier with higher peak compared to normal newborns. The pH, CO2, and O2 did not show significant correlation with serum bilirubin. Very few studies are available in literature to show correlation of serum bilirubin with birth asphyxia. The weakness of the study is small sample size; therefore more studies with large sample size are required for further postulating same observations.
The ethical committee approved the study with number BFUHS/2K7/GMC_PTA/TH/3537
Abbreviations
TSB = total serum bilirubin, NNH = neonatal hyperbilirubinemia
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.ICD-10 Version: 2016. [Last accessed on 2021 Oct 09]. Available from: https://icd.who.int/browse10/2016/en .
- 2.National Family Health Survey (NFHS-5) [Last accessed on 2021 May 15]. Available from: http://rchiips.org/nfhs/factsheet_NFHS-5.shtml .
- 3.India Newborn Action Plan (INAP): National Health Mission. [Last accesseed on 2021 Jul 18]. Available from: https://www.nhm.gov.in/index4.php?lang=1&level=0&linkid=153&lid=174 .
- 4.New Born Baby. [Last accessed on 2021 Aug 03]. Available from: https://www.newbornwhocc.org/clinical_proto.html .
- 5.Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316. doi: 10.1542/peds.114.1.297. [DOI] [PubMed] [Google Scholar]
- 6.Brits H, Adendorff J, Huisamen D, Beukes D, Botha K, Herbst H, et al. The prevalence of neonatal jaundice and risk factors in healthy term neonates at National District Hospital in Bloemfontein. Afr J Prim Heal Care Fam Med. 2018;10:e1–6. doi: 10.4102/phcfm.v10i1.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scrafford CG, Mullany LC, Katz J, Khatry SK, LeClerq SC, Darmstadt GL, et al. Incidence of and risk factors for neonatal jaundice among newborns in southern Nepal. Trop Med Int Heal. 2013;18:1317–28. doi: 10.1111/tmi.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahlfors CE, Wennberg RP. Bilirubin—albumin binding and neonatal jaundice. Semin Perinatol. 2004;28:334–9. doi: 10.1053/j.semperi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Malloy HT, Evelyn KA. The determination of bilirubin with the photoelectric colorimeter. J Biol Chem. 1937;119:481–90. [Google Scholar]
- 10.Fekete M, Horváth M, Vincellér M. Perinatal asphyxia and jaundice in newborn infants. Acta Paediatr Acad Sci Hung. 1978;19:17–26. [PubMed] [Google Scholar]


