Abstract
Background:
Many studies have provided evidence for an increased risk of atrial fibrillation among diabetic patients as compared to the nondiabetic population. It is also well known that diabetes predisposes a person to an increased risk of diabetic nephropathy. A few reviews and studies have hinted towards an increased risk of atrial fibrillation among diabetic nephropathy patients; however, there is no concrete evidence at present.
Aim:
To conduct a meta-analysis to explore if there is an association between diabetic nephropathy and atrial fibrillation.
Methods:
The available literature was searched for relevant studies from the period of January 1995 to November 2020. The following quality assessment criteria were considered for study shortlisting: clearly defined comparison groups, same outcome measured in both comparison groups, known confounders addressed, and a sufficiently long and complete (more than 80%) follow-up of patients. Two independent reviewers searched the databases, formed their search strategies, and finalized the studies. The data were analyzed to obtain a summary odds ratio along with a forest plot by Cochrane’s RevMan 5.3.
Results:
Only four studies were found to meet the inclusion criterion for this meta-analysis (total number of study participants: 307330, diabetic nephropathy patients: 22855). Of these, two were retrospective cross-sectional studies, one was a prospective cohort study, and one was a case-control study. Three studies had provided the odds ratio as the measure of effect (two retrospective cross-sectional studies and one case-control study), with the one cohort study reporting the hazards ratio as the measure of effect. Therefore, the meta-analysis was done excluding the cohort study. The summary odds ratio in the present study was 1.32 (0.80–2.18), which was not statistically significant. Due to large heterogeneity among the included studies and their small sample sizes, it was found that the summary estimate shifted towards the null value.
Conclusion:
The present meta-analysis found no significant association between atrial fibrillation and diabetic nephropathy. However, more studies with large sample sizes are required to strengthen the evidence for an association.
Keywords: Atrial fibrillation, diabetes mellitus, diabetic nephropathy, meta-analysis, risk factors
Introduction
Atrial fibrillation (AF), a type of arrhythmia, has been recognized as a leading cause of morbidity and mortality. Around 5 million new cases of AF are estimated to occur every year according to the Global Burden of Diseases Study.[1] AF has been identified as a potential risk factor for stroke and other cardiovascular diseases (CVDs), which are well established globally to result in a high mortality rate.[2] Therefore, AF indirectly leads to an increase in morbidity and mortality caused by CVD, which calls for more attention in research.
The next important step is to control this epidemic. For this, we must understand and recognize the risk factors and predictors associated with AF. The first attempt to acknowledge these independent risk factors was carried out three decades ago; factors such as age, hypertension, diabetes mellitus, coronary and valvular heart diseases, and congestive heart failure were identified as independent predictors of AF in the Framingham Heart Study (one of the largest cohort studies).[3]
Diabetes mellitus is known to raise the risk for AF 1.24 times as compared to the general population without diabetes.[4,5] Furthermore, diabetes mellitus increases susceptibility to micro-and macrovascular complications like nephropathy, neuropathy, retinopathy, and CVDs.[6,7] The presence of microvascular complications can then increase the risk of macrovascular complications among the diabetic population and most of the complicated diabetic patients getting care in primary health care and outpatient clinics. Only a few studies have thus far explored such associations, such as those documenting an association between diabetic nephropathy and retinopathy with stroke, valvular heart diseases, and AF, to name a few.[8,9]
Therefore, many independent reviews and meta-analyses have recognized a link between diabetes mellitus and AF[4,5]; however, few studies have explored additional risk factors for AF among the diabetic population. Age, sex, body mass index (BMI), systolic and diastolic blood pressure, and lipid levels have commonly been identified as risk factors leading to an increased risk of AF among diabetic patients.[2,4,9,10] Only a few studies have explored the association between diabetic nephropathy and AF.[11,12,13,14,15] Hence, it is imperative to examine this association to further understand the burden of morbidity and mortality caused by AF due to nephropathy among diabetic patients.
Considering the lack of knowledge, the objective of this study was to perform a systematic review and meta-analysis to address whether diabetic nephropathy leads to an increased risk of AF compared with diabetic patients without nephropathy. The primary outcome measure was the combined odds ratio (OR) from the studies included in the meta-analysis.
Materials and Methods
Literature search
This meta-analysis was conducted as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[16] The following data sources were searched for all cross-sectional, cohort, and case-control studies: PubMed, EMBASE/ExcerptaMedica, Cochrane Central Register of Controlled Trials, Google Scholar, Reference lists. Search strategies were independently designed and performed by two separate investigators. We used the following MeSH terms or keywords in different combinations and permutations for searching studies from January 1995 to November 2020 in advanced PubMed search: “Atrial fibrillation,” “microalbuminuria,” “macroalbuminuria,” “overt proteinuria,” “diabetic nephropathy,” “diabetes mellitus,” and “risk factors for atrial fibrillation.”
Study selection criteria
The search strategies described above provided a list of studies. The titles and abstracts of all the retrieved studies were screened independently by two authors. The irrelevant studies were discarded in the first attempt. The full-text version of the shortlisted studies was then analyzed for the presence of a measurable outcome variable in terms of incidence, risk ratio (RR), OR, or proportions of AF among diabetic nephropathy patients and diabetic patients without nephropathy, depending upon the study design. Also, we took care not to overestimate the measure of effect between the exposure of interest and the outcome. Therefore, we chose to include studies with a sample size of at least 100 in both groups. We did not pose any restrictions on the language of the articles as most of the articles could be translated by the google translate tool; most of the journals supported language conversion.
Data extraction procedure
Data were extracted independently by the same reviewers using a standard data extraction matrix in excel. The abstracted data pertained to the name of the author along with the year of publication, study design, study period, type of diabetes addressed in the study, age of participants, percentage of male participants, the total number of events of interest, OR/RR of AF among diabetic patients with/without nephropathy, a measure of effect and its 95% confidence interval (CI), and potential confounders addressed in the study. In a few studies, where the full text was not available, and the abstract provided only information on the measure of effect (OR/RR), it was decided to use the data directly in RevMan using the generic inverse variance option for entering data. Hence, such data were also utilized for this analysis. Where more than one publication of one cohort study existed, only the publication with the complete dataset was used for the meta-analysis. Disagreements in data extraction were resolved by discussion among the authors.
Further, we assessed the bias in the studies based on Cochrane’s guidelines.
Statistical analysis
The data were analyzed using RevMan 5.3. The risk of AF was summarized using either the RR with 95% CI for cohort studies or the OR with 95% CI for cross-sectional studies and case-control studies. It was decided to present the results of the cohort and case-control studies separately as they have different measures of effect.
Data from all studies comparing the RR or OR for AF among two groups were pooled in the meta-analyses using the inverse generic variance method with the random-effects model. Heterogeneity of outcome measures between studies was examined using the Cochran Q and I² statistics.
The statistical methods of this study were reviewed by Dr. Mamta Gupta, Medical Director at Alchemist Research and Data Analysis and Project coordinator at Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh (India).
Results
Study selection
The combined literature search identified 3526 studies that contained the MeSH terms either in the title or abstract. After reviewing the title, we included 160 studies for abstract review. Finally, only four studies matched the inclusion criteria. Of these four studies, the full text was available for three studies for which data could be easily extracted.[11,14,15] For one study where only the abstract was available, the related data were available in the abstract, which was utilized for the analysis.[12] The studies that were excluded were rejected on various grounds, which are shown in Figure 1.
Figure 1.
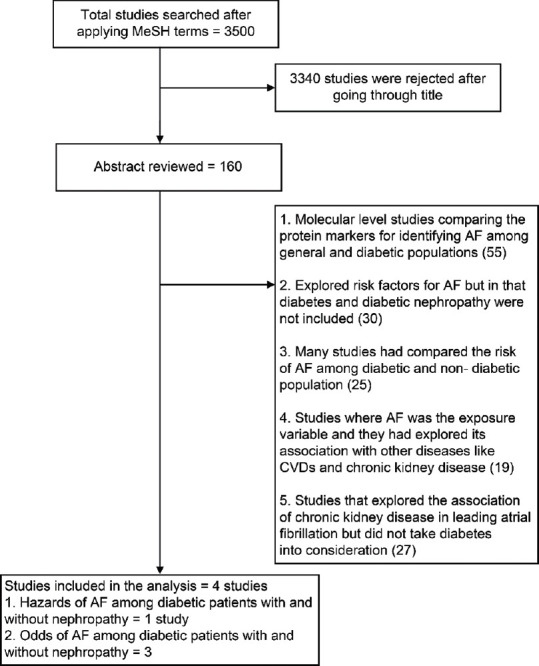
Study selection flow chart
The characteristics of the four shortlisted studies are presented in Table 1. Of these four studies, one was a longitudinal cohort study, which was published as part of the Swedish National Diabetes Registry carried out by Zethelius et al. in 2015.[15] As it is a cohort study, the authors had used Cox Regression to estimate the hazards ratio (HR) of AF among diabetic patients with and without nephropathy. The two studies by Ananthapanyasut et al.[11] and Papa et al.[14] were retrospective cross-sectional studies where the measure of effect used was the OR. The fourth study was a case-control carried out by Dahlqvist et al.[12] in 2017, which reported the OR of AF among the two exposure groups (raw data was not available for this study). None of the studies addressed the bias adequately.
Table 1.
Characteristics of included studies
| First author, year | Country | Design | Diabetes type | Sample size | Age (in years) | Sex (% males) | Study period | No. of total AF events | No. of AF events/No. of diabetic nephropathy patients | No. of AF events/No. of diabetic patients without nephropathy | Odds ratio | 95% CI | Confounders |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ananthapanyasut et al. 2010 | USA | Retrospective cross-sectional | Unspecified | 1010 | 65.7±15 | 52 | NA | 214 | 88/465 | 126/465 | 0.82 | 0.64-1.04 | Age, SBP, race, H/o CHF, left atrium diameter, e GFR |
| Papa G et al. 2014 | Italy | Retrospective cross-sectional | Type 2 | 6920 | 66.4±11.4 | 50.4 | 2008-2014 | 465 | 209/1932 | 256/4988 | 2.11 | 1.77-2.51 | Age, weight, BMI, SBP, DBP, HDL, LDL, HbAlc, creatinine, smoking, medications, h/o CVD |
| Zetheliun et al. 2015* | Swedish National Diabetes Resgistry | Prospective Observational | Type 2 | 83162 | 64.1±9.2 | 57.7 | 6.8 (Mean)- follow up | 4141 | 1449/20458 | 2692/62704 | 1.21 | (1.08-1.38) | Age, sex, BMI, height, obesity, cumulative microalbuminuria, H/o CHF, CHD, SBP |
| Dahlqvist et al. 2017 | Swedish National Diabetes Resgistry | Prospective Observational | Type 1 | Case-36258, controls-179980 | NA | NA | NA | NA | NA | NA | 13 | 1.09-1.48 | Cumulative microalbuminuria vs norm albuminuria addressed |
*This study was excluded from the summary estimate and forest plot. Here, the measure of effect is hazards ratio instead of odds ratio
Study analysis
Keeping in view the different study designs and corresponding measures of effect presented in these studies, we decided to do an analysis of the studies presenting the OR separately from the study where the HR was calculated as the outcome measure. Therefore, we could include only three studies where OR was the measure in the meta-analysis to estimate the summary OR for the overall analysis. As there was only one cohort study, a meta-analysis was not possible; thus, we decided to include the results of this study as such.
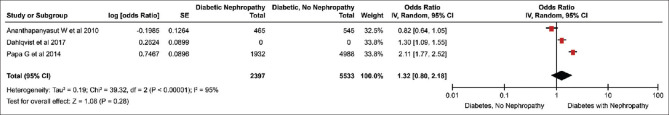
All included studies were conducted after 2009. The two studies by Zethelius et al.[12] and Dahlqvist et al.[15] pertained to the Swedish population. The other two studies represented a sample population from the USA[11] and Italy.[14] The total diabetic population in these four studies was 127620 patients. The total number of reported AF cases in three studies was 4820 (data missing for Dahlqvist et al.). The summary results of the meta-analysis of the three studies are shown in Figure 2.
Figure 2.
Forest Plot. The odds ratio of atrial fibrillation among diabetic patients with and without nephropathy
The summary OR was 1.32 (0.80–2.18), which indicated no statistically significant association between AF and diabetic nephropathy. A significant heterogeneity was present among the included studies (τ2 = 0.19, χ2 = 39.32, df = 2 (P < 0.00001); I2 = 95%) following the random-effects model.
The risk of having AF among diabetic patients with nephropathy was found to be 1.21 times (1.08–1.38) greater as compared to diabetic patients without nephropathy in the study by Zethelius et al.,[12] which was statistically significant.
Discussion
To the best of our knowledge, this is the first meta-analysis to assess the association between AF and diabetic nephropathy. The present study included the findings of three cross-sectional/case-control studies and one cohort study, which reported the OR/RR of AF in diabetic patients with and without nephropathy.[11,12,14,15] These four studies had a combined sample size of 127620 and included both Type 1 and Type 2 diabetes mellitus patients. We have found that after adjusting for various confounders, as mentioned in Table 1, the authors of these studies primarily looked upon the association of AF development among the diabetic population with and without nephropathy.
The summary OR in this meta-analysis was 1.32 (0.80–2.18), which is not statistically significant. This was due to two reasons. First, we could not include one large study by Zethelius et al.[15] in this pooled estimate due to the different study designs and measure of effect. Secondly, of the three included studies, one study by Ananthapanyasut et al.[11] had a small sample size, and the results were insignificant. Therefore, the pooled estimate of the present study was pulled towards the value of 1, which supports the null hypothesis. This may be considered a limitation of this meta-analysis. If we consider excluding the study by Ananthapanyasut et al.[11] from this meta-analysis, then the pooled OR was calculated to be 1.66 (1.03–2.66), indicating that the risk of AF among patients with diabetic nephropathy was 1.66 times higher as compared to diabetic patients without nephropathy, thereby indicating the effect of sample size on the summary estimate.
This study has some potential[15] limitations. A significant limitation of this meta-analysis pertains to the limited number of studies available in this research area. Thus, the overall sample size remained small for a summary measure estimation. Secondly, the study design of included studies differed. Of the four studies, two were retrospective cross-sectional studies, which weakened the power of the estimate as cross-sectional studies are considered epidemiologically weak studies in providing evidence for associations. Ideally, we would have included either cohort or case-control studies, but due to lack of relevant studies, we decided to include cross-sectional studies also. The third limitation pertains to the large heterogeneity among the studies, which was attributed to the small sample sizes, varied study designs, and different populations.
Despite the various limitations, the current meta-analysis was able to highlight many research issues that are important for controlling the rising number of AF cases worldwide. Large sample prospective studies like the Framingham study, the Malmo study, and the Atherosclerosis Risk in Communities (ARIC) study have explored multiple risk factors like advancing age, hypertension, obesity, smoking, previous chronic heart failure, myocardial infarction, and valve disease.[3,9,17,18] Similar findings were reported by Zethelius et al.[15]. In addition, a few studies have pointed towards the positive association of HbA1c with AF, although this is not a well-established risk factor.[15] An association between HbA1c and albuminuria as a result of diabetic microangiopathy has long been established, as has the link between the presence of both diabetes and albuminuria and the risk of CVD.[7] Therefore, there is some evidence for a relationship between HbA1c and AF, which should be further explored in more prospective cohort studies. Another important thing to observe is that like other non-communicable diseases, AF shares many risk factors, which can be addressed simultaneously.[9,10,18] Therefore, it is better to identify more of the risk factors, such as diabetic nephropathy or the role of HbA1c in diabetic nephropathy, for AF so that a multipronged strategy can be used to contain the rising burden of this disease.
Aside from the study by Zethelius et al.,[15] we could not find any other prospective study that reported an association between AF and diabetic nephropathy. In conclusion, the results of this meta-analysis do not show an increased risk of AF among patients with diabetic nephropathy. However, taking into account the study limitations, future research should be directed at further exploring a potential association between AF and diabetic nephropathy using large sample-sized prospective cohort studies.[9,10,18]
Author contributions
Arnous MM and Al Sayed KA conceived the study aims and design, made decisions on inclusion and exclusion of the articles contributed to the data extraction; Al Dalbhi SK and Al Saidan AA has contributed to study design, planned the analysis, interpreted the results, and drafted the final version of the paper manuscript development and critical review, final approval of the version to be published; Balghaith MA and Al Tahan TM have reviewed the manuscript critically, contributed to the article critically for important intellectual content and epidemiological aspects.
PRISMA 2009 checklist statement
The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation:A Global Burden of Disease 2010 Study. Circulation. 2013;129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death:Systematic review and meta-analysis. BMJ. doi:10.1136/bmj.i4482. 2016;354:i4482. doi: 10.1136/bmj.i4482. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death:The Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case–control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108:56–62. doi: 10.1016/j.amjcard.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tadic M, Cuspidi C. Type 2 diabetes mellitus and atrial fibrillation:From mechanisms to clinical practice. Arch Cardiovasc Dis. 2015;108:269–76. doi: 10.1016/j.acvd.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Gorst C, Kwok CS, Aslam S, Buchan I, Kontopantelis E, Myint PK, et al. Long-term glycemic variability and risk of adverse outcomes:A systematic review and meta-analysis. Diabetes Car. 2015;38:2354–69. doi: 10.2337/dc15-1188. [DOI] [PubMed] [Google Scholar]
- 7.Wadén J, Forsblom C, Thorn LM, Gordin D, Saraheimo M, Groop PH, et al. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes. 2009;58:2649–55. doi: 10.2337/db09-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adeniyi A, Folsom AR, Brancati FL, Desvorieux M, Pankow JS, Taylor H. Incidence and risk factors for cardiovascular disease in African Americans with diabetes:The Atherosclerosis Risk in Communities (ARIC) study. J Natl Med Assoc. 2002;94:1025–35. [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation:The Framingham Heart Study. Circulation. 2004;110:1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 10.Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation. 2017;136:583–96. doi: 10.1161/CIRCULATIONAHA.116.023163. [DOI] [PubMed] [Google Scholar]
- 11.Ananthapanyasut W, Napan S, Rudolph EH, Harindhanavudhi T, Ayash H, Guglielmi KE, et al. Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:173–81. doi: 10.2215/CJN.03170509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahlqvist S, Rosengren A, Gudbjörnsdottir S, Pivodic A, Wedel H, Kosiborod M, et al. Risk of atrial fibrillation in people with type 1 diabetes compared with matched controls from the general population:A prospective case-control study. Lancet Diabetes Endocrinol. 2017;5:799–807. doi: 10.1016/S2213-8587(17)30262-0. [DOI] [PubMed] [Google Scholar]
- 13.Mochizuki Y, Tanaka H, Matsumoto K, Sano H, Shimoura H, Ooka J, et al. Impaired mechanics of left ventriculo-atrial coupling in patients with diabetic nephropathy. Circ J. 2016;80:1957–64. doi: 10.1253/circj.CJ-16-0488. [DOI] [PubMed] [Google Scholar]
- 14.Papa G, Iurato M, Licciardello C, Maiorana R, Finocchiaro C, Pezzino V. Aging, diabetic nephropathy and multiple macrovascular involvement are associated with atrial fibrillation in type 2 diabetes mellitus. Br J Med Med Res. 2014;4:5154–66. [Google Scholar]
- 15.Zethelius B, Gudbjörnsdottir S, Eliasson B, Eeg-Olofsson K, Svensson A-M, Cederholm J. Risk factors for atrial fibrillation in type 2 diabetes:Report from the Swedish National Diabetes Register (NDR) Diabetologia. 2015;58:2259–68. doi: 10.1007/s00125-015-3666-9. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors:The Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–8. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malmo V, Nes BM, Amundsen BH, Tjonna A-E, Stoylen A, Rossvoll O, et al. Aerobic interval training reduces the burden of atrial fibrillation in the short term:A randomized trial. Circulation. 2016;133:466–73. doi: 10.1161/CIRCULATIONAHA.115.018220. [DOI] [PubMed] [Google Scholar]