Abstract
Filarial parasite infestation not only affects the structure and function of lymphatic vessels but is also associated with extralymphatic pathology and disease. Incidence of renal involvement in microfilaria carriers has led to increased cognizance of extralymphatic presentation. Literature set forth clinical syndromes having extralymphatic manifestation of filaria. The diagnosis of filariasis is done by visualisation of microfilaria in peripheral blood smear, lymphatic tissue. Other modalities of diagnosis are Enzyme linked immunosorbent assay (ELISA), Immunochromatographic test. Diethyl carbamazine (DEC) provocation test usually is done to detect microfilaria in night blood smear due to the nocturnal periodicity of microfilaria. The drug DEC flushes the microfilaria into the peripheral circulation leading to high probability of detection. We present a case of a 59-year-old male who was diagnosed as nephrotic syndrome and after a DEC challenge we detected microfilaria in the peripheral smear confirming microfilaria-induced Nephrotic Syndrome after all other secondary conditions were excluded.
Keywords: Filaria, microfilaria, nephrotic syndrome
Introduction
Lymphatic filariasis affects globally over 120 million people in 73 countries throughout Asia, Africa, Western Pacific and South America.[1] Wuchereria bancrofti and Brugia malayi cause lymphatic filariasis by blockage of lymphatics and land up in elephantiasis. Filariasis is renowned to be present with lymphatic and extra-lymphatic symptoms. Extra lymphatic filarial manifestations require utmost importance which is lacking in the recent times leading to diagnostic misinterpretations.
Several hundreds of species have been identified; out of which eight species are known to affect humans. Among these, four species, namely Wuchereria bancrofti, Onchocerca volvulus, Loa loa, and Brugia malayi, lead to renal damage ranging from asymptomatic proteinuria, chyluria, microscopic hematuria, nephrotic syndrome, to acute glomerulonephritis.[1] The diagnosis of filariasis is established by eosinophilia along with microfilarial detection in peripheral blood smear.
In spite of various evidence-based studies since long describing glomerulonephritis, hematuria and proteinuria in filariasis their significance has been noted only in the recent times. Hematuria in filariasis was assumed to be due to mechanical glomerular damage by microfilaria in the circulation.[2] Later it was proved that circulating immune complexes get deposited in the basement membrane of the glomeruli which was believed to be causative factor for renal affection in filariasis identified in renal biopsies.[3]
Primary care physicians play an important role so as to rule out common infectious processes which are endemic in specific regions of this country. Primary care physicians are blessed with this specific opportunity because they are the first-line healthcare providers of the community. With their experience of dealing with large number of clinical manifestations of common problems, they should keep a strong index of suspicion of common disorders presenting in an uncommon way.
Case Report
A 59-year-old patient presented to us with complaints of swelling over bilateral lower limbs since two years which was insidious in onset, gradually progressive in nature and of pitting type which extends up to knee initially later it progressed to whole body and presents with generalised anasarca and facial puffiness. On asking leading questions he also gave history of episodic fever which was of high grade, intermittent associated with chills one to two attacks in two to three months.
Patient had no history of rash, burning micturition, vomiting, loose stools, cough, breathlessness, chest pain, yellowish discoloration of eyes, abdominal distension, blood in vomitings or stools, altered sensorium, drug intake. There was no history of tuberculosis, bronchial asthma or any allergies.
On admission; patient was febrile (temp-101° F) with tachycardia having heart rate of 110/minute with raised blood pressure of 150/100 mm Hg in right arm supine position, respiratory rate of 22/min of abdomino thoracic type, pedal edema of pitting type presents extending up to thigh. On systemic examination, cardiovascular system examination revealed normal heart sounds, respiratory examination revealed bilateral infrascapular minimal crackles, abdomen was normal. Central Nervous System (CNS) examination was normal.
Patients’ laboratory investigations revealed;
CBC-Hb was 13 gm%, TLC – 4500 cells/cumm, DLC – showed 9% eosinophils, Absolute eosinophil count was 410/mm3, Platelets 2.33 lakhs/cumm, Urine albumin +++, 24 hour urinary protein was 3500 mg, 4 RBCs present/HPF. Serum urea 56 mg/dl, creatinine 2.7 mg/dl. Total cholesterol 289 mg/dl, triglyceride 296 mg/dl, low density lipoprotein 176 mg/dl, high density lipoprotein 32 mg/dl, albumin 1.8 g/dl. Patients’ glomerular filtration rate has been reduced to 24.3 ml/min/1.73 m2, according to MDRD GFR Equation.
Viral markers such as Human Immunodeficiency Virus (HIV), hepatitis B, C were negative, malarial antigen was negative. P-ANCA-negative (1 u/ml), C-ANCA-negative (1.2 u/ml), ANA-0.3(Not detectable), ds DNA-0.5(Not detectable), C3-68 mg/dl, C4-9 mg/dl levels were low in range. Anti-streptolysin O (ASO) titre was negative. Patients urine output was decreased to 300–500 ml in 24 hours. In view of the patient being from an endemic area for filariasis and as per the history of recurrent fevers with chills over a period of two years, a possibility of lymphatic filariasis was kept and DEC challenge test was undertaken. A 100 mg tablet was given per orally at 12 am and a thick blood smear was examined for presence of microfilaria 45 minutes after the oral dose. The smear revealed microfilaria [Figure 1].
Figure 1.
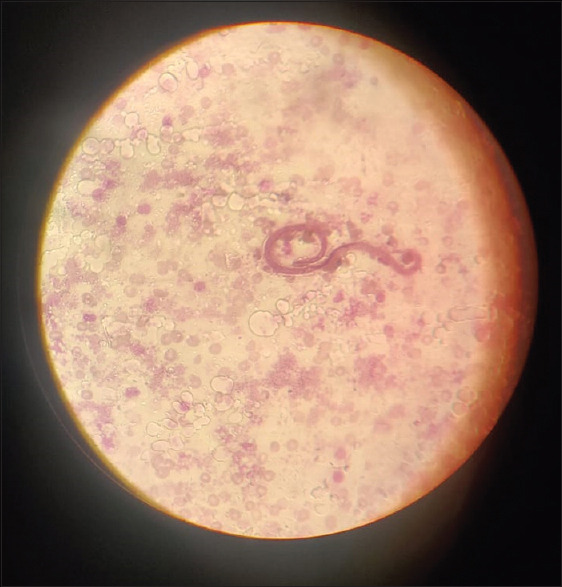
Thick peripheral blood smear with Giemsa stain showing microfilaria
Patient was tested positive for filarial antigenemia using BinaxNOW filariasis Immunochromatographic test (ICT).
USG abdomen and pelvis was done reveals bilateral raised cortical echotexture with altered corticomedullary differentiation suggestive of grade 3 renal parenchymal disease. A Kidney biopsy was planned but patient did not give consent for the same.
A probable diagnosis of Nephrotic Syndrome due to microfilaria was made.
Patient has been started on Diethyl carbamazine 100 mg thrice a day for 21 days, tablet doxycycline 100 mg bid for 14 days, Tab. Ramipril 5 mg once in night, rosuvastatin 40 mg once in night. Patient has been advised single doses of albendazole, diethylcarbamazine, ivermectin every year to sustain microfilaria clearance.
Patients condition was improved symptomatically, edema reduced, proteinuria reduced to 1.6 gm, blood pressure got normalised after three weeks follow up.
Discussion
Renal involvement in microfilaremic carriers manifests as microscopic hematuria and proteinuria which can be attributed to two mechanisms: 1) mechanical damage to glomeruli leading to hematuria and 2) deposition of immune complexes.[4]
Microfilarial antigens are increased in the circulation which can be correlated with heightened metabolic activity of adult worms. This leads to formation and deposition of immune complexes in glomerular basement membrane as well as direct mechanical damage to the tubulointerstitium.
Dreyer et al.[5] stated that therapy of filariasis with DEC can lead to proteinuria and hematuria in microfilaremic carriers, the reason being attributed to deposition of immune complexes due to disintegration of dead microfilaria. Rath et al. had observed histopathological changes in 9 out of 14 filariasis patients who underwent renal biopsies. Among those histopathological changes, mesangial cell hyperplasia was found to be common in 6 out of 9 patients. Granular IgG and C3 deposits were noted in the mesangium and walls of the renal capillaries in five of these nine patients.[6]
A study stated that filariasis patients had showed a spectrum of proliferative and non-proliferative glomerular involvement.[7] Traditional method for diagnosis of filariasis is by detection of microfilaria in the peripheral smear. However, this method was proved to be less sensitive.
Filariasis has a wide spectrum of presentation which makes the clinical diagnosis tricky. Hence a high index of clinical suspicion is required as kidney biopsy is indispensable to establish the diagnosis. ELISA and a rapid format immunochromatographic card test detects circulating filarial antigens with high sensitivity and specificity.[8]
DEC leads to microfilarial demise due to sheath disruption which induces and propagates apoptosis.[9] Poor microfilaricidal effect of DEC compared to ivermectin explains the presence of microfilaria in the renal biopsies even after treatment with DEC. DEC also has a role in destruction of adult worms in the lymphatics leading to immune complex formation and accentuation of renal pathology.[10] However this cannot be considered as an indication to stop DEC therapy because hematuria and/or proteinuria due to DEC is transient, and declining microfilarial load with continued treatment helps in resolution of renal pathology.[11] Filarial antigen load can be cut down by therapeutic apheresis before the start of DEC therapy.[12] Our patient showed dramatic response to DEC with improvement in renal function and resolution of hematuria and proteinuria within one week of initiation of DEC therapy.
Evidences suggested that there is no role of corcticosteroids in the management of microfilaria induced nephrotic syndrome.
In order to confirm the diagnosis and properly treat patients, primary care physicians will need to collaborate with nursing, administration, and public health officials. They may need extra surgical consultation depending on the degree of the deformity. To prevent subsequent infections and worse consequences, the patient may require wound care, hygiene awareness, and regular follow-up.
The key measures required to eliminate and further eradicate microfilaria as a public health hazard are chemotherapy prevention drugs, vector control measures, and programs to bring awareness among individuals, management of complications.
Because the treatment regimen is effective against many neglected tropical diseases (NTDs), microfilaria eradication efforts are combined with attempts to manage or remove other NTDs that are receptive to PC.
Community worker training, social mobilisation, and information, education, and communication (which is frequently localised) are ongoing expenses to programs that demand enough resources as a percentage of health expenditures. Given the investment in their training and the importance of their function in the community, it is also important to try to minimise community distributor attrition. Primary care physicians stand in the apex of such healthcare algorithm.
Oemijati S[13] in his study stated that DEC regimens came out with good results in Indonesia. Among those regimens, higher dose packs gave adverse effects and vice versa. Prevention and control programs were handled by trained leaders in their community primary care physicians who are the heads of primary healthcare units should identify the symptomatology, taking care for drug supply, and intervene for the adverse effects when occurred. Controlling communicable diseases through a healthcare-based strategy is currently being emphasized. The supportive activities to improve health at primary care level were carried out by volunteers through organisations supervised by healthcare units.
To the best of our knowledge, very few case reports were published highlighting the extralymphatic filarial manifestations with a special focus on renal involvement. Here, our patient had presented with recurrent fever, oliguria, and anasarca with a nephrotic range proteinuria on urine analysis. All the etiological factors of nephrotic syndrome were ruled out, but patient being from endemic area DEC provocation test was done and was found to be smear positive for microfilaria. Hence, a diagnosis of nephrotic syndrome secondary to filariasis was confirmed.
Conclusion
The main purpose of this study is to increase cognizance regarding extralymphatic filarial manifestations with a focus on renal involvement especially in endemic areas. The take home message of this case report is early diagnosis and appropriate timely intervention is crucial to minimise the progression of renal involvement in filariasis which needs further research in this aspect. Identifying endemic diseases at the primary care level can only be done by primary care physicians. Elimination at primary healthcare level helps in preventing potential morbidity in such cases of microfilaria involving renal system.
Patients’ consent form
Appropriate consent was taken from the relatives of the patient before making the case report. As this is a case report the university’s guideline does not require an ethical committee and/or Institutional Review Board (IRB) clearance for its scientific publication.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We thank microbiologist for providing pictures of Microfilaria.
Attributed to: Acharya Vinobha Bhave Rural Hospital, Sawangi, Wardha, 442001, Maharashtra, India.
References
- 1.Prakash GK, Vankalakunti M, Ballal HS. Microfilaria associated macroscopic hematuria and nephrotic range proteinuria. Indian J Nephrol. 2016;26:302–3. doi: 10.4103/0971-4065.161025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnakumar A, Valson AT, Duhli N, Mohapatra A, Tulsidas KS, Varughese S. Catching the worm early:An atypical case of bancroftian filarial nephropathy. Saudi J Kidney Dis Transpl. 2020;31:1101–5. doi: 10.4103/1319-2442.301177. [DOI] [PubMed] [Google Scholar]
- 3.Basu A, Wali M, Bhattacharya B, Rahman M, Ray Y, Goswami RP. Nephritic syndrome and anasarca in a case of lymphatic filariasis:A rare association. Asian Pacific Journal of Tropical Disease. 2016;6:486–8. [Google Scholar]
- 4.Shubham S, Ahuja A, Bhardwaj M. Microfilaria in kidney biopsy:A report of two cases. J Infect Public Health. 2018;11:732–4. doi: 10.1016/j.jiph.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Dreyer G, Dreyer P, Piessens WF. Extralymphatic disease due to bancroftian filariasis. Braz J Med Biol Res. 1999;32:1467–72. doi: 10.1590/s0100-879x1999001200003. [DOI] [PubMed] [Google Scholar]
- 6.Rath K, Nath N, Shaloumy M, Swain BK, Suchismita M, Babu BV. Knowledge and perceptions about lymphatic filariasis:a study during the programme to eliminate lymphatic filariasis in an urban community of Orissa, India. Trop Biomed. 2006;23:156–62. PMID:17322817. [PubMed] [Google Scholar]
- 7.Phulware RH, Singh SK, Singh G, Barwad A. Microfilaria presenting as nephrotic syndrome in a young female. IDCases. 2018;13:e00424. doi: 10.1016/j.idcr.2018.e00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Moamly AA, El-Sweify MA, Hafez MA. Using the AD12-ICT rapid-format test to detect Wuchereria bancrofti circulating antigens in comparison to Og4C3-ELISA and nucleopore membrane filtration and microscopy techniques. Parasitol Res. 2012;111:1379–83. doi: 10.1007/s00436-012-2870-5. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S, Vaibhao GS, Sanjay DK. Leptospirosis induced acute kidney injury in elderly:It's different?J Pharm Biomed Sci. 2014;12:1103–5. [Google Scholar]
- 10.Nana-Djeunga HC, Tchatchueng-Mbougua JB, Bopda J, Mbickmen-Tchana S, Elong-Kana N, Nnomzo'o E, et al. Mapping of bancroftian filariasis in Cameroon:Prospects for elimination. PLoS Negl Trop Dis. 2015;9:e0004001. doi: 10.1371/journal.pntd.0004001. doi:10.1371/journal.pntd.0004001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarangi J, Arava S, Kumar H. Microfilaria in urine cytology:Report of three cases with review of literature. Diagn Cytopathol. 2020;48:675–8. doi: 10.1002/dc.24423. [DOI] [PubMed] [Google Scholar]
- 12.Mandal T, Meena S, Singh R, Azad CS. Microfilaria in achylous hematuria:Can it imitate urolithiasis?Trop Parasitol. 2020;10:44–6. doi: 10.4103/tp.TP_27_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oemijati S. The role of primary health care in filariasis control in Indonesia. Southeast Asian J Trop Med Public Health. 1993;24((Suppl 2)):91–2. [PubMed] [Google Scholar]


