Abstract
In shigellosis, the network of cellular interactions mediated by a balance of pro- and anti-inflammatory cytokines or chemokines is clearly tipped toward acute destructive inflammation of intestinal tissues by the bacterial invader. This work has addressed the role played by interleukin-8 (IL-8) in a rabbit model of intestinal invasion by Shigella flexneri. IL-8, which is largely produced by the epithelial cells themselves, appears to be a major mediator of the recruitment of polymorphonuclear leukocytes (PMNs) to the subepithelial area and transmigration of these cells through the epithelial lining. Neutralization of IL-8 function by monoclonal antibody WS-4 caused a decrease in the amount of PMNs streaming through the lamina propria and the epithelium, thus significantly attenuating the severity of epithelial lesions in areas of bacterial invasion. These findings are in agreement with our previous work (31). In contrast to the PMNs, the bacteria displayed increased transepithelial translocation, as well as overgrowth in the lamina propria and increased passage into the mesenteric blood. By mediating eradication of bacteria at their epithelial entry site, although at the cost of severe epithelial destruction, IL-8 therefore appears to be a key chemokine in the control of bacterial translocation.
Inflammation is a nonspecific response to various tissue injuries, including infection. In its acute form, massive changes in microcirculation cause leakage of fluid and accumulation of leukocytes, particularly polymorphonuclear leukocytes (PMNs), at the site of injury (14). In humans, the colonic mucosa undergoes inflammation in the case of inflammatory bowel diseases (IBDs) of unknown origin (i.e., ulcerative colitis) or in the course of acute infections such as bacillary dysentery in which shigellae, the etiological pathogens, invade mucosal tissues. In both cases, inflammation is characterized by diffuse erythema and swelling of the mucosa, focal hemorrhages, and a purulent exudate. Small aphthoid ulcerations may also be observed, possibly corresponding to destruction of the lymphoid structures associated with mucosal tissues (17). In the case of bacillary dysentery, these aphthoid ulcerations are often seen at the early stage of the disease and colocalize with lymphoid follicles (26). At a later stage of shigellosis, the histopathological lesions cannot be differentiated from those observed in patients presenting with acute ulcerative colitis. Vascular degeneration (i.e., swelling of endothelial cells) and massive margination of PMNs are observed, leading to streaming of these PMNs through the lamina propria and the epithelial lining toward the intestinal lumen. This results in acute cryptitis and the appearance of large ulcerations due to extensive epithelial detachment. These inflammatory lesions extend far beyond the localized areas of bacterial invasion, suggesting that a cascade of proinflammatory mediators causes dramatic amplification of the process, which escapes its initiating factor. It is therefore essential to identify the components of this cascade.
Cytokines are well recognized as key mediators of the inflammatory cascade causing IBD. Proinflammatory cytokines such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-α) and chemokines such as IL-8 and related molecules are detected in IBD-affected tissues (22, 40). In several experimental animal models of IBD, an example being the study of IL-1 and its receptor antagonist IL-1ra in a rabbit model of colitis (8), the role of these cytokines and chemokines has been addressed. The failure of anti-inflammatory cytokines to downregulate inflammation is also considered. IL-10, for instance, suppresses macrophage and T-cell activation, production of cytokines (i.e., IL-1, IL-6, and TNF-α), and cellular differentiation (29). Moreover, IL-10 knockout mice develop severe enterocolitis unless the animals are maintained under germfree conditions, thereby demonstrating both that IL-10 is a regulator of intestinal inflammation and that the intestinal bacterial flora is a driving force for this inflammation (24). Correlatively, IBD patients have been shown to express decreased levels of IL-10 in serum (20). Noncytokine proinflammatory factors are involved as well, such as nitric oxide, eicosanoids, and histamine (45). However, the actual role and importance of these various factors are difficult to assess.
In shigellosis, several lines of evidence also indicate that cytokines and chemokines are mediators of tissue damage. Immunohistochemical studies of patients in the acute and convalescent stages of infection by Shigella flexneri and S. dysenteriae 1 have shown a broad pattern of local hyperproduction of IL-1, IL-4, IL-6, IL-8, TNF-α, and gamma interferon (IFN-γ) (34, 35). The latter has recently been shown to be essential for early Shigella killing in a mouse pulmonary model of shigellosis (47). Severe disease was observed in patients with an increase in the number of IL-1β-, IL-6-, TNF-α-, and IFN-γ-producing cells. Monocytes expressed IL-1, IL-4, TNF-α, and IFN-γ, whereas epithelial cells accounted for the production of IL-6 and IL-8 (34). In the rabbit ligated-loop model of shigellosis, intravenous (i.v.) infusion of IL-1ra controls the inflammatory symptoms (38), thus emphasizing the role of IL-1 in the generation of lesions. In addition, control of PMN migration into infected tissues by injection of an anti-CD18 monoclonal antibody (MAb) abrogates inflammatory symptoms and consequent tissue destruction, thereby indicating that PMNs are major effectors of the disease process (31).
The pathways leading to intestinal inflammation in shigellosis are currently being analyzed. Evidence from human patients (26), macaque monkeys (37), and the rabbit ligated-loop model (31, 39, 46) indicates that the follicle-associated epithelium, particularly M cells, represents the major early portal of Shigella entry. As shigellae reach the dome of the lymphoid follicle, which contains high numbers of resident macrophages, the bacteria are phagocytosed and cause rapid apoptotic death of these macrophages, as observed both in vitro (51) and in vivo (53). When pretreated with lipopolysaccharide (LPS) and subsequently challenged with invasive shigellae, apoptotic macrophages release large amounts of IL-1β (52). This dual activity of Shigella, which is able to cause both macrophage apoptotic death and the release of IL-1β, is currently attributed to the ability of IpaB, a 62-kDa bacterial invasin, to activate caspase 1 (6). After phagocytosis by macrophages in the dome region of lymphoid follicles, shigellae may initiate intestinal inflammation by triggering the IL-1 cascade (54, 55). However, a link needs to be established between initiation of inflammation in the follicular zones, which represent a limited surface of the intestine, and the extensive inflammatory destruction observed at distant sites, both in the crypts and at the epithelial surface.
The present work is part of an attempt to characterize these proinflammatory factors. It was specifically aimed at exploring the role of IL-8 as a mediator of mucosal inflammation in the rabbit ligated-intestinal-loop model of shigellosis, particularly at sites distant from the follicular zones. Inhibition of IL-8 function was obtained by injecting the animals, prior to infection, with WS-4, a murine MAb which has been elicited against human IL-8 and also neutralizes rabbit IL-8 (23, 50). Our results show that IL-8 causes PMN-mediated arrest of Shigella translocation through the intestinal epithelium into the lamina propria at the cost of massive epithelial destruction.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. flexneri M90T (an invasive isolate belonging to serotype 5) was used throughout the experiments (36). Alternatively, strain BS176, a plasmidless, noninvasive derivative of M90T was used. Bacteria were routinely grown in tryptic soy broth (TSB; Difco Laboratories, Detroit, Mich.). For animal infections, a confluent culture was obtained on TSB agar after overnight growth at 37°C. From these cultures, a bacterial suspension was established in saline at a concentration of 1010 bacteria/ml.
Rabbit intestinal-loop infection assay.
Sixteen New Zealand White rabbits (SSC-Cegav, Les Hautes Noës, Saint-Mars-d’Egrenne, France) weighing 2.5 to 3.0 kg were used in this study. Animals were fasted for 24 h before infection. Experiments were always performed on a pair of rabbits which received either neutralizing anti-IL-8 MAb WS-4 or a control MAb 30 min prior to the surgical procedure. Anti-human IL-8 MAb WS-4 immunoglobulin G1-κ ([IgG1-κ] type) (23), which is known to also neutralize rabbit IL-8 (50), was injected i.v. at a dose of 5 mg/kg in a 5-ml volume of saline. Control animals received the same dose of an irrelevant MAb, a mouse anti-yeast glutathione reductase MAb (Oriental Yeast Co., Ltd., Azusawa, Japan). General anesthesia was then obtained by injection of acepromazine (250 μg/kg) and ketamine (20 mg/kg), animals were laparotomized, the intestine was exposed, and eight intestinal segments 10 cm long were ligated. The blood supply was carefully preserved while ligation was performed. Into each loop, 0.5 ml of the bacterial suspension was injected. The abdominal cavity was then closed, and infection was allowed to proceed for 2, 4, or 8 h before titration of IL-8 in tissues and for 8 h for the IL-8 neutralization experiments and their controls.
At the end of the infection period, animals were again anesthetized and laparotomized. In order to count bacteria reaching the portal venous system, 50 to 100 μl of blood was drawn from the mesenteric vein draining the relevant loop, and dilutions were plated on TSB agar. Animals were then sacrificed by i.v. injection of a 10-ml air bolus. The ligated loops were emptied, the volume of fluid was measured, and depending on the subsequent step, loops were either filled with 5 ml of a gentamicin solution (50 μg/ml in phosphate-buffered saline [PBS]) for counting of invasive bacteria or opened and dissected for histopathological analysis. Therefore, a total of 32 blocks were subjected to histopathological analysis in control animals, and a similar number of blocks were analyzed in MAb WS-4-treated animals. In addition, similar numbers of tissue samples were processed for counting of tissue-associated bacteria and for dosing IL-8 concentrations by enzyme-linked immunosorbent assay (ELISA).
Bacterial counts in tissue samples.
Experiments were carried out as already described (31). Loops used for determination of bacterial counts were treated with gentamicin. Intestinal-tissue samples similar in size were obtained by punching out disks 8-mm in diameter with a skin biopsy apparatus (Biopsy Punch; Stiefel, Nanterre, France). Extensive washing of the samples was performed with 0.1 M PBS to eliminate gentamicin, and cold PBS was added to prevent bacterial growth. Tissue samples were then ground with an Ultra-Turrax apparatus (Janke & Kunkel GmbH, Staufen, Germany) in cold PBS. A 1/10 solution was then obtained, briefly incubated at 37°C, and serially diluted before plating on TSB agar plates. CFUs were counted after overnight incubation at 37°C, and the number of bacteria was standardized for an area of 1 cm2 of bacterial mucosa.
Tissue sampling for histopathological analysis, staining, immunostaining, and recording of results.
All tissue samples were immediately fixed in 4% formalin, dehydrated, and embedded in paraffin. Sequential sections were taken at various levels of the samples. Thin cuts of 5 μm were made. Histopathological analysis of infected tissues by classical microscopic observation followed hematoxylin-eosin staining or immunostaining for bactericidal permeability-increasing (BPI) proteins of PMNs, bacterial LPS, and IL-8.
These staining procedures were performed and used as follows. On hematoxylin-eosin-stained sections, the length-to-width (L/W) ratio of the villi was calculated. In each of the 64 sections examined, 100 villi were measured, their lengths and widths were recorded, and the L/W ratio was calculated. The mean ratios were therefore computed for 3,200 villi in control rabbits, as well as in rabbits in which IL-8 had been neutralized. The presence of PMNs in villus tissues was recorded. Rabbit PMNs contain eosinophilic granules; they can therefore be detected on hematoxylin-eosin-stained sections. These data were confirmed by staining with a polyclonal serum directed against BPI proteins from human PMNs which also recognizes rabbit BPI proteins, a kind gift of Jerald Weiss, The Skirball Institute for Molecular Medicine, New York University (48). Immunostaining was carried out as follows. Histosections were deparaffinized and rehydrated. Endogenous peroxidases were blocked by 0.3% hydrogen peroxide in methanol. Saturation was achieved by incubation in 5% nonimmune goat serum. Incubation was then carried out overnight at 4°C with goat anti-rabbit BPI polyclonal serum used at a dilution of 1/2,000 in PBS containing 1% bovine serum albumin (BSA). After washing, a rabbit biotinylated anti-goat antibody used at a 1/600 dilution was added, and incubation was carried out for 1 h at room temperature. The reaction was then amplified by using the Vectastain ABC kit (Vector Laboratories, Inc., Burlingame, Calif.). The chromogenic substrate used was diaminobenzidine (Vector Laboratories, Inc.).
For generation of data concerning PMN invasion of intestinal tissues, counts were determined in histosections stained with hematoxylin-eosin. Briefly, PMNs were counted in the epithelial lining, the lamina propria, and the area located between crypts. The mean value was calculated for each of the 3,200 villi observed in control and MAb WS-4-treated rabbits. The BPI staining technique provided similar counting.
Immunohistochemical staining for bacterial LPS was carried out on thin cuts after deparaffinization, rehydration, and neutralization of endogenous tissue peroxidases. A biotinylated IgG MAb against S. flexneri 5a LPS was used, and a MAb directed against S. flexneri 2a LPS was used as a control. Incubation was carried out overnight at 4°C with the MAb used at a concentration of 5 μg/ml in PBS containing 1% BSA. The chemical reaction was carried out by using the Vectastain ABC kit (Vector Laboratories, Inc.) as described above. This staining procedure allowed vizualization of bacteria in villus tissues, both in the epithelial lining and in the lamina propria.
Immunohistochemical staining for IL-8 was conducted as follows. After deparaffinization, rehydration, and neutralization of endogenous tissue peroxidases, thin sections were incubated overnight at 4°C with MAb WS-4 at a concentration of 5 μg/ml in PBS containing 1% BSA. After washing, biotinylated anti-mouse IgG polyclonal rabbit serum was added at a dilution of 1/400. After 1 h of incubation at room temperature, the preparation was washed and the chemical reaction was performed as described above.
Rabbit IL-8 ELISA protocol.
Dosage of IL-8 was carried out on tissue samples which were processed as follows. Punch biopsy samples 8 mm in diameter (Stiefel) were put in 2 ml of cold PBS containing a cocktail of protease inhibitors (0.05% [wt/vol] sodium azide, 1-μg/ml aprotinin, 1-μg/ml leupeptin, 1-μg/ml pepstatin A, 1 mM AEBSF [all from Sigma]) and homogenized in ice for 10 s by using an Ultra-Turrax homogenizer (Janke & Kunkel). Centrifugation was then carried out at 100,000 × g for 1 h at 15°C. Supernatants were immediately stored at −80°C until further ELISA dosage. This ELISA procedure was carried out as previously described (50). Briefly, wells of microtiter plates were coated with WS-4, the neutralizing anti-IL-8 MAb. After three washings with 0.05% Tween–PBS, saturation with 150 μl of 1% BSA in PBS for 1 h at 37°C, and three additional washings with 0.05% Tween–PBS, samples were added in a final volume of 100 μl after dilution with 0.5% BSA in Tween-PBS. Incubation was carried out overnight at 4°C. After five washings in Tween-PBS, guinea pig anti-rabbit IL-8 polyclonal serum diluted to 1 μg/ml was added and the samples were incubated for 2 h at 37°C. After five washings with Tween-PBS, 100 μl of a 1/10,000 dilution of peroxidase-labeled, affinity-purified anti-guinea pig IgG was added in 0.5% BSA–Tween–PBS. Incubation was carried out for 2 h at 37°C. After five washings in Tween-PBS, 100 μl of the chromogen o-phenylenediamine dihydrochloride was added and incubation was carried out for 30 min at room temperature. The reaction was stopped by adding 4 N H2SO4, and the optical density was read at 490 nm.
Statistical analysis.
The nonparametric Mann-Whitney test (i.e., rank-sum test) was used for determination of the statistical significance of differences between mean values. A probability of P < 0.05 was used to define this significance.
RESULTS
Concentration of IL-8 in infected intestinal tissues.
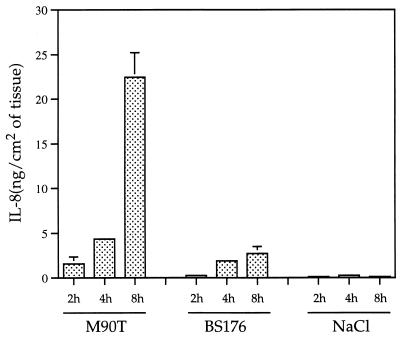
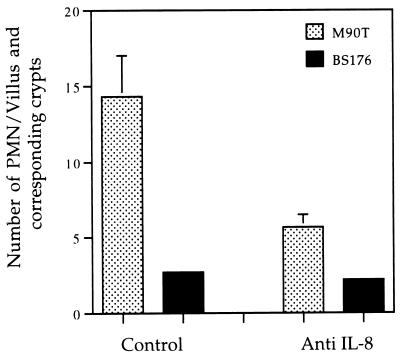
The kinetics of IL-8 production by infected rabbit intestinal tissues are shown in Fig. 1. Animals were infected with either wild-type invasive strain M90T or noninvasive strain BS176 and sacrificed after 2, 4, or 8 h of infection. Tissue samples from loops injected with saline were used as noninfected controls. Tissue samples from loops infected with strain M90T showed a striking increase in the concentration of IL-8 which, after 8 h of infection, appeared to be eightfold greater than the concentration measured in tissue samples from loops infected with strain BS176. This difference was highly significant (P < 0.01). These results demonstrated the strong correlation between IL-8 production and expression of the invasive phenotype of Shigella.
FIG. 1.
Concentration of IL-8 in intestinal tissue samples depending on time of infection and expression (M90T), or lack of expression (BS176), of the plasmid-encoded invasive phenotype of Shigella.
Immunolocalization of IL-8 in mucosal tissues.
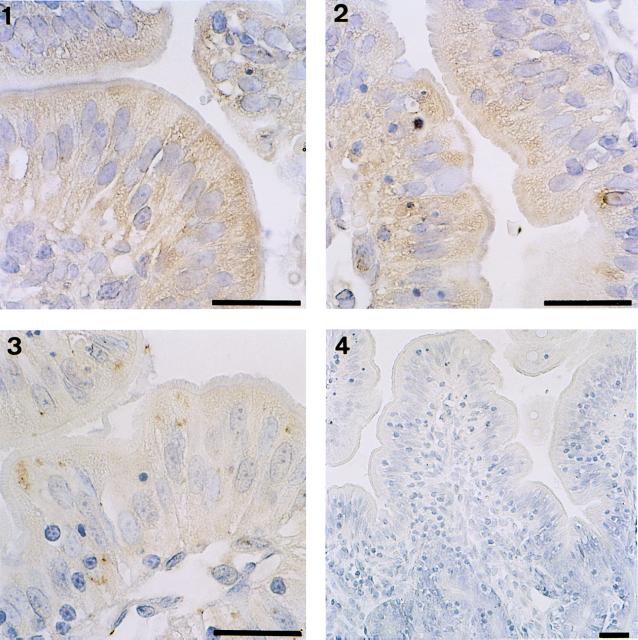
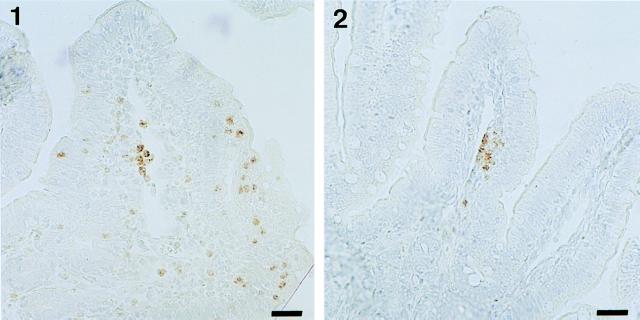
As shown in Fig. 2, immunohistochemical staining of IL-8 in tissue sections corresponding to samples taken from intestinal loops infected with either M90T (Fig. 2, panels 1 and 2) or BS176 (Fig. 2, panel 3) for 8 h revealed that the epithelial cells themselves accounted for the major part of IL-8 production, a limited amount of this chemokine being observed associated with cells in the lamina propria. In addition, M90T clearly elicited higher production of IL-8 than did BS176, the latter eliciting limited patchy zones associated with the intracellular compartment, thus confirming the data shown in Fig. 1. Figure 2, panel 4, is shown as a control for the specificity of the staining technique.
FIG. 2.
Immunostaining for IL-8 in tissue sections corresponding to samples obtained from loops infected for 8 h with M90T (panels 1 and 2) or BS176 (panel 3). In panel 4, a negative control is shown in which MAb WS-4 was omitted. Bars, 10 μm.
Effect of IL-8 neutralization on tissue lesions in the course of intestinal infection with M90T.
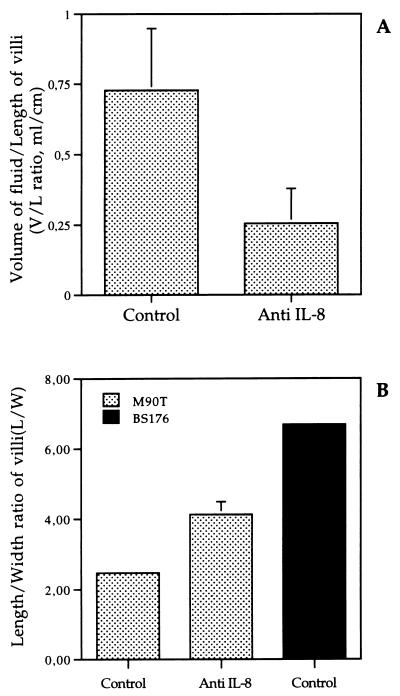
Severity of tissue inflammation in the course of experimental shigellosis can be approximated by measuring the average amount of exudative fluid produced per unit of intestinal length (V/L ratio) and by calculating the index of intestinal atrophy, which corresponds to the average villus L/W ratio. As shown in Fig. 3A, after 8 h of infection, the average V/L ratio was 0.73 ± 0.22 ml/cm in M90T-infected loops of animals that had received the control MAb, roughly threefold higher than the average V/L ratio of 0.25 ± 0.12 ml/cm observed in rabbits that had received IL-8-neutralizing MAb WS-4 (P < 0.05). Loops infected with BS176 were always devoid of fluid.
FIG. 3.
Evaluations of intestinal alterations based on the V/L ratio of intestinal loops (A) and of the villus atrophy index reflected by the L/W ratio of villi (B). Control refers to animals treated with the control MAb.
Accordingly, acute intestinal atrophy was much more pronounced in the absence of IL-8 neutralization. As shown in Fig. 3B, the average L/W ratio was twofold lower for villi in M90T-infected loops of rabbits receiving the control MAb (i.e., 2.47 ± 0.04) than for villin M90T-infected loops of rabbits in which IL-8 was neutralized (i.e., 4.12 ± 0.37). This difference reached statistical significance (P < 0.05). Loops infected with noninvasive strain BS176 showed a mean L/W ratio of 6.7, very close to the usual L/W ratio observed in noninfected intestinal loops (data not shown).
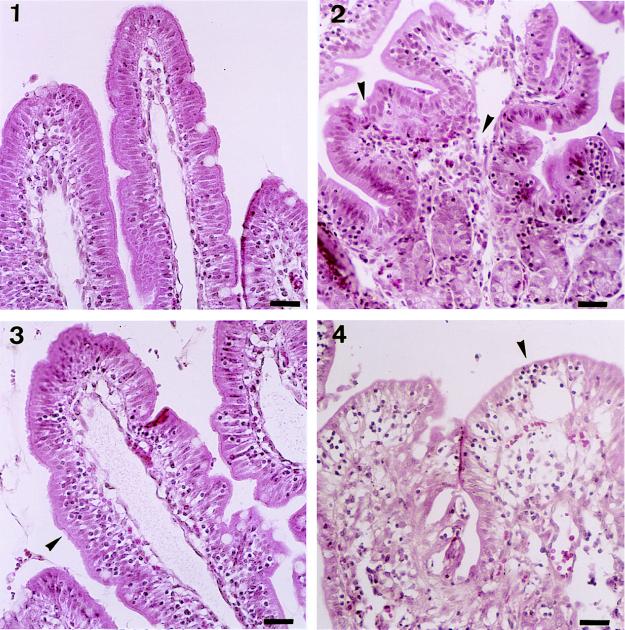
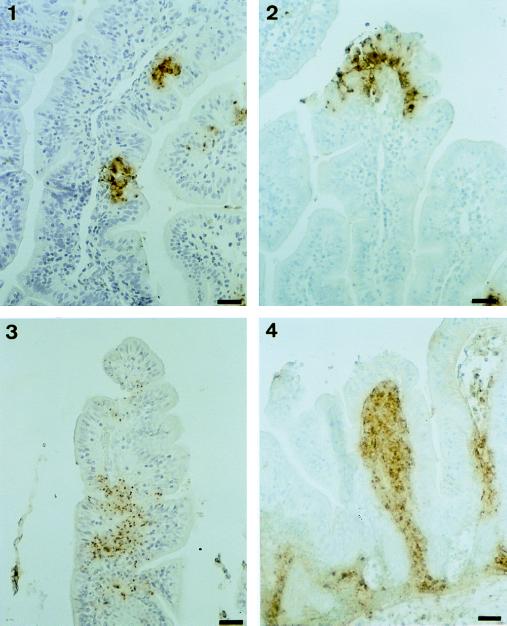
Further histopathological analysis was performed on tissue sections taken from intestinal loops of control and anti-IL-8 MAb-treated animals. Characteristic observations are summarized in Fig. 4. As a reference, typical villi observed after infection with noninvasive strain BS176 in control rabbits are shown in Fig. 4, panel 1. These villi are long and thin, showing a high L/W ratio; their epithelial lining is not altered and shows no sign of significant inflammatory infiltrate, except at the very tips of a limited percentage of villi. In general, neither the epithelium nor the lamina propria was affected. Conversely, a typical villus observed after 8 h of infection of a rabbit treated with the control MAb and infected with M90T is shown in Fig. 4, panel 2. PMNs in rabbits harbor eosinophilic granules that stain red with acidophilic dyes. They are shown, between two arrowheads, streaming through the lamina propria before invading and crossing the epithelial lining. On their way to the lumen, these PMNs disrupt the epithelial structure and cause the formation of an abscess which develops in the epithelium itself. In other areas of the epithelium, mononuclear cells are seen infiltrating the intercellular space; this will be considered later. In Fig. 4, panel 3, typical villi observed after 8 h of infection in rabbits treated with anti-IL-8 MAb WS-4 are shown. PMNs are barely present in association with the epithelium, and disruption of the epithelial lining is rarely observed. It is clear from these data that neutralization of IL-8 prevents the formation of PMN-mediated epithelial abscesses. Figure 4, panel 3, also shows that prevention of PMN migration, particularly into the epithelial lining, prevents progressive accumulation of mononuclear cells in the epithelial paracellular space at a location similar to that of intraepithelial lymphocytes. Pockets containing several mononuclear cells may form (Fig. 4, panel 4). From early to later stages of the process, these cells are surrounded by a clear halo of increasing diameter, corresponding to a process that tends to alter the epithelial structure and to give it a vacuolar aspect, suggesting a cytotoxic effect. A similar process occurs in the crypts (data not shown). Our extensive examination of tissue sections at different periods of infection indicates that the wave of migration of these mononuclear cells into the epithelium occurs between 2 and 4 h of infection, thereby preceding the wave of migration of PMNs. It is not affected by IL-8 neutralization (data not shown) and may cause early cytotoxic destabilization of the barrier. Between 4 and 8 h of infection, it is overwhelmed by PMN invasion of the lamina propria and epithelium, and the lesions caused by PMNs tend to mask the cytotoxic effect of these yet-to-be-defined mononuclear cells.
FIG. 4.
Haematoxylin-eosin staining of tissue sections corresponding to samples obtained from loops infected for 8 h with BS176 (panel 1) and M90T (panels 2, 3, and 4). Panel 2 corresponds to a sample from a rabbit treated with the control MAb, whereas panels 3 and 4 correspond to samples from rabbits treated with anti-IL-8 MAb WS-4. In panel 2, arrowheads define areas in which PMNs are streaming through the lamina propria and the epithelium. In panel 3, the arrowhead points to mononuclear cells infiltrating the epithelium in animals in which IL-8 has been neutralized. Bars, 10 μm.
Data shown in Fig. 5 confirm that the cells invading the epithelium in animals treated with the control antibody and infected with M90T were PMNs. Immunostaining was performed on the same tissue sections as those shown in Fig. 4 with an anti-BPI polyclonal serum that specifically stains PMN granules (48). In Fig. 5, panel 1, tissue sections corresponding to rabbits treated with the control antibody and infected for 8 h with M90T show BPI-positive cells (i.e., PMNs) strongly invading the epithelial lining. Conversely, Fig. 5, panel 2, shows that in rabbits treated with MAb WS-4 and infected for 8 h with M90T, some PMNs can migrate to the lamina propria and eventually accumulate but do not significantly invade the epithelial lining.
FIG. 5.
Immunostaining of the BPI protein characteristic of PMN granules. Panel 1 is a tissue section corresponding to a sample obtained from a loop infected with M90T for 8 h in a rabbit treated with the control antibody. PMNs streaming through the lamina propria and invading the epithelial lining are shown. Panel 2 is a tissue section corresponding to a sample obtained from a loop infected with M90T for 8 h in a rabbit in which IL-8 has been neutralized by MAb WS-4. A limited number of PMNs gain access to the lamina propria. These PMNs do not significantly invade the epithelial lining. Bars, 10 μm.
Data exemplified in Fig. 4 and 5 were quantified. For this purpose, the PMNs present in 100 villi and in their respective crypts were counted, and mean numbers were computed from the 32 sections observed from rabbits treated with the control MAb and an equivalent number of sections from rabbits in which IL-8 was neutralized. Figure 6 shows that the mean number of PMNs infiltrating the villi and corresponding crypts after 8 h of infection with M90T was about threefold lower in animals in which IL-8 was neutralized (P < 0.05). This number, however, did not reach the background level of animals infected with noninvasive strain BS176. It should be noted that this global evaluation does not reflect the sublocalization of PMNs. In animals treated with the control antibody, PMNs showed extensive invasion that encompassed the crypts, the lamina propria, and the epithelium. In animals in which IL-8 was neutralized, a global decrease of PMN influx was observed. These PMNs could accumulate in the vicinity of the crypts and in the lamina propria but did not significantly invade the epithelial lining, as already illustrated in Fig. 4 and 5.
FIG. 6.
Enumeration of PMNs in villi and crypts, depending on the invasive phenotype of Shigella and neutralization of IL-8. Control refers to animals treated with the control MAb.
Effect of IL-8 neutralization on mucosal invasion by M90T.
Immunohistochemical staining was performed with an anti-LPS MAb in order to localize M90T in infected intestinal samples taken from control rabbits or rabbits undergoing IL-8 neutralization. Figure 7 shows a striking difference in bacterial localization depending on whether IL-8 is functional or neutralized. Figure 7, panels 1 and 2, shows different stages of progression of M90T after 8 h of infection in control rabbits. Strikingly, bacteria tend to remain associated with the epithelial lining, confirming their ability to invade, grow inside, and disseminate in epithelial cells. They barely invade the lamina propria after crossing the epithelial barrier, even after 8 h of infection. This may be due to efficient IL-8-mediated recruitment of PMNs in the immediate vicinity of the infected epithelium, causing localization of the infectious focus at the initial site of bacterial invasion but also resulting in destructive abscess formation. Figure 7, panels 3 and 4, shows a dramatic difference in the location of bacteria after 8 h of infection with M90T in rabbits in which IL-8 was neutralized. Bacteria are barely seen associated with epithelial cells. On the other hand, they are essentially seen in a posttranslocation position, at various stages of invasion of the lamina propria. In some cases (Fig. 7, panel 4), the lamina propria can be massively infected. To some extent, we could observe mirror images of bacterial colonization with regard to the presence of the bacteria in the epithelium or in the lamina propria in control or IL-8-neutralized rabbits, respectively.
FIG. 7.
Immunostaining of bacterial LPS. Panels 1 and 2 are tissue sections corresponding to samples obtained from loops infected with M90T for 8 h in rabbits treated with the control MAb. Abscess formation localized to the epithelium is shown at different stages of severity. Panels 3 and 4 are tissue sections corresponding to samples obtained from loops infected with M90T for 8 h in rabbits in which IL-8 has been neutralized by MAb WS-4. Bacterial diffusion in the lamina propria is a characteristic of this situation with relatively limited presence in the epithelium. Bars, 10 μm.
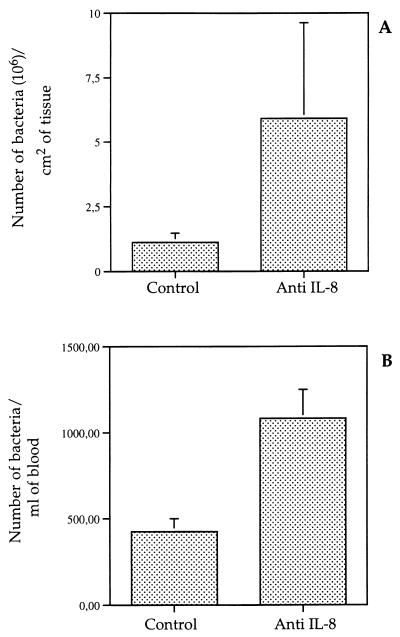
In an attempt to quantify the effect of neutralizing IL-8 on the number of bacteria invading the intestinal mucosa, bacteria associated with tissues were enumerated, as well as bacteria appearing in the mesenteric blood after crossing the intestinal barrier. As shown in Fig. 8A, the mean number of invasive bacteria was about threefold higher when IL-8 was neutralized than in controls (P < 0.05). Figure 8B shows that the mean number of bacteria crossing the intestinal barrier was twice as high when IL-8 was neutralized as in controls (P < 0.05).
FIG. 8.
Enumeration of M90T bacteria in villus tissues (A) and in efferent mesenteric blood (B), depending on neutralization of IL-8. Control refers to rabbits treated with the control MAb.
DISCUSSION
Cytokines and chemokines are key mediators that orchestrate the host immunoinflammatory response to bacterial infections (49). Those that activate leukocytes (particularly PMNs) to produce inflammation are essential for the early, nonspecific eradication of invading microbes, often at the price of tissue destruction, which may be quite deleterious and possibly lethal to the host. Shigellosis is a clear example of this situation, in which a limited number of microbes causes acute destructive inflammation of the colonic mucosa. Control of the proper balance between bacterial eradication and tissue destruction requires an understanding of the complex network of interactions mediated by pro- and anti-inflammatory cytokines at the initial stage of tissue invasion by Shigella.
IL-8 is a CXC chemokine which is chemotactic for PMNs. In addition to being produced by macrophages, along with IL-1, IL-6, and TNF-α, in situations of infection or tissue injury, it is also produced by other cells such as PMNs themselves, T cells, endothelial cells, and epithelial cells (2, 9). LPS, viruses, cytokines (IL-1, TNF-α), yeasts, and bacterial invasion of tissues are among the infection-related factors that cause IL-8 expression. In addition to chemotaxis, it increases adherence of PMNs to endothelial cells, thereby promoting their transendothelial migration, IL-8 also induces degranulation, respiratory burst, and LTB4 release by PMNs.
We have addressed the role of IL-8 in experimental shigellosis, a disease characterized by a massive influx of PMNs in the intestinal mucosa. Several observations pointed to IL-8 as a major candidate. (i) In human diseases characterized by massive PMN infiltration, IL-8 is detected at high concentrations in the corresponding bodily fluids in infections such as acute peritonitis, bacterial meningitis, endotoxemia, acute respiratory distress syndrome, Helicobacter pylori gastritis, and urinary tract infection (19). (ii) The use of blocking antibodies against IL-8 in animal models has shown attenuation of both clinical and histopathological symptoms in several situations characterized by acute inflammation (44); this includes rabbit models of LPS-induced dermatitis, arthritis, and immune complex glomerulonephritis (18), lung reperfusion injury (41), and endotoxin-induced pleurisy (5). These experiments have confirmed that IL-8 is a key mediator of tissue damage caused by acute inflammation. (iii) Finally, IL-8 is likely to be a key mediator in the pathogenesis of IBDs, particularly ulcerative colitis (10, 16, 33). The role of intestinal epithelial cells as a source of IL-8 in IBDs remains controversial. In Crohn’s disease and ulcerative colitis, colonic crypt cells produce elevated levels of IL-8, and cells isolated from inflamed areas express more IL-8 than do cells from normal areas (27). Also, short-chain fatty acid butyrate reduces in vitro secretion of IL-8 from isolated crypt cells (15). Other studies have shown, however, that infiltrating macrophages and PMNs are the major source of IL-8 (10, 16).
In situations of intestinal infection, intestinal epithelial cells, which are the first cellular barrier against the pathogen, act as sentinels (43) by orchestrating early nonspecific immune responses by the secretion of cytokines and chemokines. This is particularly obvious for colonic epithelial cells (i.e., the T84, HT-29, and Caco-2 cell lines and freshly isolated colonic cells). Gram-negative invasive bacteria appear to stimulate the highest levels of chemokines such as IL-8, MCP-1, and granulocyte-macrophage colony-stimulating factor and of TNF-α (21).
In patients, in the course of shigellosis, secretion of cytokines, particularly IL-8, in stool extracts is correlated with disease severity (35). As expected from the previous data, experimental shigellosis in the rabbit ligated-loop model of infection was associated with increasing levels of tissue IL-8, which reached a concentration eightfold higher after 8 h of infection when the infection was carried out with a wild-type invasive microorganism than when it was done with a noninvasive control. Although as already discussed for IBD, recruited monocytes and the PMN themselves may account for the production of a significant part of IL-8, immunostaining experiments indicated that IL-8 expression was essentially associated with epithelial cells, regardless of their invasion by bacteria, with areas of expression extending far beyond these zones of bacterial invasion. Similarly, in a SCID mouse model of human embryonic xenotransplantation followed by infection with Entamoeba histolytica trophozoites, IL-8 was essentially produced by epithelial cells, even at locations far from areas of mucosal damage (42), thereby confirming previous in vitro data (13). However, in in vitro assays, bacterial invasion of the cells is required to elicit significant basal secretion of IL-8 (11, 12). In addition, as shown experimentally with Salmonella typhimurium, factors other than IL-8 are required in order to allow PMN transmigration across an in vitro-reconstituted epithelial monolayer. IL-8 may attract PMNs from a distance but may not be directly responsible for their transmigration (28).
In the case of Shigella infection in similar in vitro systems, PMN transmigration also occurs and facilitates bacterial invasion via the basolateral pole of epithelial cells (32), and LPS accounts for about 50% of PMN transmigration (3). LPS acts both by induction of IL-8 production by T84 cells but also directly via its ability to transcytose through the epithelial lining from its apical to its basolateral pole (4). It was therefore essential to examine where, exactly, IL-8 is involved during the infectious process in an experimental model of infection that closely reflects tissue invasion.
Neutralization of IL-8 in rabbits during infection with invasive Shigella had a significant effect, both in attenuating the severity of the lesions and in loosening the barrier effect of the intestinal epithelium against Shigella translocation. This indicates that in spite of the redundancy of the chemokine system (9), as observed in other models reported above, IL-8 is a major chemoattractant for PMNs in infected tissues. IL-8 neutralization has a pleiotropic effect on intestinal tissues infected with Shigella which can be summarized in three parts.
(i) The global decrease in the severity of intestinal lesions can be directly assigned to poor recruitment of PMNs in the lamina propria and epithelial lining, which prevents abscess formation in the presence of invading bacteria but also prevents global attraction of PMNs, even to zones of the epithelium which are not affected by bacterial invasion. In addition, it is likely that PMNs that are still recruited when IL-8 is neutralized do not reach the activation level (i.e., in terms of adherence properties, induction of an oxidative burst, and release of bactericidal or cytotoxic granules) of the PMNs recruited in response to invasive Shigella in control rabbits.
(ii) A decrease in PMN influx caused by the neutralization of IL-8 limits epithelial destruction by lowering the number and size of abscesses, as well as extensive epithelial detachment; this decrease has revealed a subjacent wave of mononuclear cells, mostly lymphocytes, infiltrating the paracellular space of the epithelial lining. This wave precedes the PMN influx in response to the presence of luminal invasive bacteria, but its presence and effect on the epithelial barrier are normally quickly overwhelmed by the influx and cytotoxicity of PMNs. We are currently characterizing these cells which seem to exert a strong cytotoxic effect on the epithelial lining. They may also regulate the transmigration of PMNs through the epithelial lining by producing IFN-γ (1, 7, 25). If this is the case, early infiltration of the epithelium by these mononuclear cells may promote extension of inflammation at a distance from the infection foci and facilitate bacterial crossing of the epithelial barrier, which is necessary for Shigella to penetrate epithelial cells via the basolateral pole (30–32). Extensive loosening of junctional structures may facilitate access of bacteria or bacterial products of the luminal flora to subepithelial tissues and trigger extensive and diffuse PMN influx beyond foci of Shigella infection.
(iii) Neutralization of IL-8 also had a dramatic effect on the characteristics of mucosal invasion by Shigella, both qualitatively and quantitatively. Qualitatively, the domain of bacterial infection expanded deeply and diffusely into the lamina propria, instead of remaining restricted to the epithelium. IL-8 produced by epithelial cells may therefore play a major role in limiting Shigella infection to the villus surface at the initial site of invasion, the epithelium. This is achieved by recruitment of PMNs which have the ability to control and restrict the infectious focus at the cost of severe epithelial destruction. One can conclude from these experiments that IL-8 is a chemokine essential for maintenance of the antitranslocating potential of the intestinal epithelial barrier. Whether this notion can be generalized to the translocation of noninvasive bacteria which do not seem to elicit significant IL-8 expression by epithelial cells (21) remains to be demonstrated. Quantitatively, the number of bacteria associated with tissues appeared to be threefold higher when IL-8 was neutralized. Three major factors may explain this difference: a higher rate of translocation of bacteria across the epithelial barrier, a diminished number of PMNs recruited to the lamina propria, and a decreased bactericidal function of these PMNs. As a consequence, the number of bacteria achieving complete translocation and appearing in the mesenteric venous blood doubled when IL-8 was neutralized, indicating relative but significant alteration of the intestinal barrier.
Not only do these results indicate the important function that IL-8 has in the pathogenesis of shigellosis, but they also suggest caution when considering the use of an anti-IL-8 strategy against acute inflammatory diseases, particularly IBD and severe cases of infectious enterocolitis.
ACKNOWLEDGMENTS
We thank Nicole Wuscher for excellent technical expertise in histopathology, Michelle Rathman for careful reading of the manuscript, and Colette Jacquemin for its editing.
REFERENCES
- 1.Adams R B, Planchon S M, Roche J K. Interferon-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol. 1993;150:2356–2363. [PubMed] [Google Scholar]
- 2.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotatic cytokines CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 3.Beatty W L, Sansonetti P J. Role of lipopolysaccharide in signaling to subepithelial polymorphonuclear leukocytes. Infect Immun. 1997;65:4395–4404. doi: 10.1128/iai.65.11.4395-4404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beatty, W. L., S. Méresse, P. Gounon, J. Davoust, J. Mounier, P. J. Sansonetti, and J.-P. Gorvel. Trafficking of Shigella lipopolysaccharide in polarized intestinal epithelial cells. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 5.Broaddus V C, Boylan A M, Hoeffel J M, Kim K J, Sadick M, Chuntharapai A, Hebert C A. Neutralization of IL-8 inhibits neutrophil influx in a rabbit model of endotoxin mediated pleurisy. J Immunol. 1994;152:2960–2967. [PubMed] [Google Scholar]
- 6.Chen Y, Smith M R, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15:3853–3860. [PMC free article] [PubMed] [Google Scholar]
- 7.Colgan S P, Parkos C A, Matthews J B, D’Andrea L, Awtrey C S, Lichtman A, Delp-Archer C, Madara J L. Interferon-gamma induces a cell surface phenotype switch on T84 intestinal epithelial cells. Am J Physiol. 1994;267:C402–C410. doi: 10.1152/ajpcell.1994.267.2.C402. [DOI] [PubMed] [Google Scholar]
- 8.Cominelli F, Bortolami M, Pizzaro T T, Monsacchi L, Ferretti M, Brewer M T, Eisenberg S, Ng R K. Rabbit interleukin-1 receptor antagonist: cloning, expression, functional characterization and regulation during inflammation. J Biol Chem. 1994;269:6962–6970. [PubMed] [Google Scholar]
- 9.Curf S H A, Meis J F G M, Hoogkamp-Korstanje J A A. A primer on cytokines: sources, receptors, effects, and inducers. Clin Microbiol Rev. 1997;10:742–780. doi: 10.1128/cmr.10.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daig R, Andus T, Aschenbrenner E, Falk W, Schölmerich J, Gross V. Increased interleukin-8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut. 1996;38:216–222. doi: 10.1136/gut.38.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckmann L, Jung H C, Schurer-Maly C, Panja A, Morzycka-Wroblewska E, Kagnoff M F. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin-8. Gastroenterology. 1993;105:1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 12.Eckmann L, Kagnoff M F, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckmann L, Reed S L, Smith J R, Kagnoff M F. Entamoeba histolytica trophozoites induce an inflammatory cytokine response by cultured human cell through the paracrine action of cytolytically released interleukin-1α. J Clin Invest. 1995;96:1269–1279. doi: 10.1172/JCI118161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fantone J C, Ward P A. Inflammation. In: Rubin E, Farber J L, editors. Pathology. Philadelphia, Pa: Lippincott; 1988. pp. 34–64. [Google Scholar]
- 15.Gibson P, Rosella P. Interleukin-8 section by colonic crypt in vitro: responses to injury suppressed by butyrate and enhanced in inflammatory bowel disease. Gut. 1995;37:536–543. doi: 10.1136/gut.37.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimm M C, Elsbury S K O, Pavli P, Doe W F. Interleukin-8: cells of origin in inflammatory bowel disease. Gut. 1996;38:90–98. doi: 10.1136/gut.38.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hale T L. Bacillary dysentery. In: Hansler W J, Sussman M, editors. Microbiology and microbial infections. 3 bacterial infections. London, England: Arnold; 1998. pp. 479–493. [Google Scholar]
- 18.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukocyte Biol. 1994;56:559–564. [PubMed] [Google Scholar]
- 19.Harada, A., N. Mukaida, and K. Matsushima. 1996. Interleukin-8 as a novel target for intervention therapy in inflammatory diseases. Mol. Med. Today 482–489. [DOI] [PubMed]
- 20.Ishida H. Clinical implications of IL-10 in patients with immune and inflammatory diseases. Jpn J Clin Pathol. 1994;42:843–852. [PubMed] [Google Scholar]
- 21.Jung H C, Eckmann L, Yang S K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Investig. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kam L, Pizzarro T T, Cominelli F. Cytokines and chemokines in inflammatory bowel diseases. Curr Opin Gastroenterol. 1995;11:305–309. [Google Scholar]
- 23.Ko Y, Mukaida N, Panyutich A, Voitenk N N, Matsushima K, Kawai T, Kasahara T. Establishment of a sensitive enzyme-linked immunosorbent assay for human interleukin-8. J Immunol Methods. 1993;149:227–235. doi: 10.1016/0022-1759(92)90254-q. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10 deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 25.Madara J L, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Investig. 1989;83:724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathan M M, Mathan V I. Morphology of rectal mucosa of patients with shigellosis. Rev Infect Dis. 1991;13(Suppl. 4):S314–S318. doi: 10.1093/clinids/13.supplement_4.s314. [DOI] [PubMed] [Google Scholar]
- 27.Mazzucchelli L, Hauser C, Zgraggen K, Wagner H, Hess M, Laissue J A, Mueller C. Expression of interleukin-8 in inflammatory bowel disease is related to the histological grade of inflammation. Am J Pathol. 1994;144:997–1007. [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick B A, Hofman P M, Kim J, Carnes D K, Miller S I, Madara J L. Surface attachment of Salmonella typhimurium to intestinal epithelial imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore K W, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann T R. Interleukin 10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 30.Mounier J, Vasselon T, Hellio R, Lesourd M, Sansonetti P J. Shigella flexneri enters human colonic Caco-2 epithelial cells through their basolateral pole. Infect Immun. 1992;60:237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perdomo J J, Cavaillon J M, Huerre M, Ohayon H, Gounon P, Sansonetti P J. Acute inflammation causes epithelial invasion and mucosal destruction in experimental shigellosis. J Exp Med. 1994;180:1307–1319. doi: 10.1084/jem.180.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perdomo J J, Gounon P, Sansonetti P J. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayer by Shigella flexneri. J Clin Investig. 1994;93:633–643. doi: 10.1172/JCI117015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raob Y, Gerdin B, Ahlstedt S, Hällgren R. Neutrophil mucosal involvement is accompanied by enhanced local production of interleukin-8 in ulcerative colitis. Gut. 1993;34:1203–1206. doi: 10.1136/gut.34.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raqib R, Lindberg A A, Wrething B, Bardhan P K, Andersson U, Andersson J. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect Immun. 1995;63:289–296. doi: 10.1128/iai.63.1.289-296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raqib R, Wretlind B, Andersson J, Lindberg A A. Cytokine secretion in acute shigellosis is correlated to disease activity and directed more to stools than plasma. J Infect Dis. 1995;171:376–384. doi: 10.1093/infdis/171.2.376. [DOI] [PubMed] [Google Scholar]
- 36.Sansonetti P J, Kopecko D J, Formal S B. Involvement of a large plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982;35:852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sansonetti P J, Arondel J, Fontaine A, d’Hauteville H, Bernardini M L. OmpB (osmo-regulation) and icsA (cell to cell spread) mutants of Shigella flexneri. Evaluation as vaccine candidates. Probes to study the pathogenesis of shigellosis. Vaccine. 1991;9:416–422. doi: 10.1016/0264-410x(91)90128-s. [DOI] [PubMed] [Google Scholar]
- 38.Sansonetti P J, Arondel J, Cavaillon J M, Huerre M. Role of IL-1 in the pathogenesis of experimental shigellosis. J Clin Investig. 1995;96:884–892. doi: 10.1172/JCI118135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sansonetti P J, Arondel J, Cantey R J, Prévost M-C, Huerre M. Infection of rabbit Peyer’s patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infect Immun. 1996;64:2752–2764. doi: 10.1128/iai.64.7.2752-2764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sartor R B. Pathogenic and clinical relevance of cytokines in inflammatory bowel diseases. Immunol Res. 1991;10:465–471. doi: 10.1007/BF02919743. [DOI] [PubMed] [Google Scholar]
- 41.Sekido N, Mukaida N, Harada A, Nakanishi I, Watanabe Y, Matsushima K. Prevention of lung reperfusion injury in rabbits by a monoclonal antibody against interleukin-8. Nature. 1993;365:654–657. doi: 10.1038/365654a0. [DOI] [PubMed] [Google Scholar]
- 42.Seydel K B, Li E, Swanson P E, Stanley S L. Human intestinal epithelial cells produce proinflammatory cytokines in response to infection in a SCID mouse-human intestinal xenograft model of amebiasis. Infect Immun. 1997;65:1631–1639. doi: 10.1128/iai.65.5.1631-1639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stadnyk A W, Waterhouse C C M. Epithelial cytokines in intestinal inflammation and mucosal immunity. Curr Opin Gastroenterol. 1997;13:510–517. [Google Scholar]
- 44.Vogels M T E, Lindery E J D, Curf J H A J, Ehing W M C, van den Meer J W M. Effect of interleukin-8 on nonspecific resistance to infection in neutropenic and normal mice. Antimicrob Agents Chemother. 1993;37:276–280. doi: 10.1128/aac.37.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallace J L, Beck P L. Inflammatory mediators in inflammatory bowel disease. Curr Opin Gastroenterol. 1996;12:334–339. [Google Scholar]
- 46.Wassef J S, Keren D F, Mailloux J L. Role of M cells in initial antigen uptake and in ulcer formation in the rabbit intestinal loop model of shigellosis. Infect Immun. 1989;57:858–863. doi: 10.1128/iai.57.3.858-863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Way S S, Borczuk A C, Domitz R, Goldberg M. An essential role for gamma interferon in innate resistance to Shigella flexneri infections. Infect Immun. 1998;66:1342–1348. doi: 10.1128/iai.66.4.1342-1348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinrauch Y, Foreman A, Shu C, Zarember K, Levy O, Elsbach P, Weiss J. Extracellular accumulation of potently microbicidal bactericidal/permeability-increasing protein and p15s in an evolving sterile rabbit peritoneal inflammatory exudate. J Clin Investig. 1995;95:1916–1924. doi: 10.1172/JCI117873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson M, Seymour R, Henderson B. Bacterial perturbation of cytokine networks. Infect Immun. 1998;66:2401–2409. doi: 10.1128/iai.66.6.2401-2409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yokoi K, Mukaida N, Harada A, Watanabe Y, Matsushima K. Prevention of endotoxemia-induced acute respiratory distress syndrome-like lung injury in rabbits by a monoclonal antibody to IL-8. Lab Investig. 1997;76:375–384. [PubMed] [Google Scholar]
- 51.Zychlinsky A, Prévost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 52.Zychlinsky A, Fitting C, Cavaillon J M, Sansonetti P J. Interleukin 1 is released by macrophages during apoptosis induced by Shigella flexneri. J Clin Investig. 1994;94:1328–1332. doi: 10.1172/JCI117452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zychlinsky A, Thirumalai K, Arondel J, Cantey J R, Aliprantis A O, Sansonetti P J. In vivo apoptosis in Shigella flexneri infections. Infect Immun. 1996;64:5357–5365. doi: 10.1128/iai.64.12.5357-5365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zychlinsky A, Sansonetti P J. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death ? Trends Microbiol. 1997;5:201–204. doi: 10.1016/S0966-842X(97)01044-5. [DOI] [PubMed] [Google Scholar]
- 55.Zychlinsky A, Sansonetti P J. Host/pathogen interactions. Apoptosis in bacterial pathogenesis. J Clin Investig. 1997;100:493–496. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]