Abstract
Background:
The manifestations of human immunodeficiency virus (HIV) infection and vitamin B12 deficiency overlap each other, so early diagnosis and intervention is important. The study aims to find out serum vitamin B12 level and its association with CD4 and CD8 count, clinical-staging, and hemato-biochemical status in newly diagnosed HIV positive cases.
Methodology:
Fifty-five confirmed HIV cases above 18 years of age and equal number of age and sex matched controls were recruited for the study. CD4 and CD8 counts were analyzed by Flow cytometer. Complete Blood Count, Serum vitamin B12, Folic acid, ferritin, and C-Reactive Protein (CRP) concentration were done.
Results:
Serum vitamin B12 was observed to be significantly low in HIV positive cases than healthy controls with a mean value of 240.62 ± 56.75 pg/ml and 317.57 ± 52.56 pg/ml, respectively. Decreased CD4 counts with elevated levels of ferritin and CRP was seen in HIV positive individuals. The subgroup analysis based on the levels of vitamin B12 was directly proportional to CD4 counts. CD8 counts also registered a significant association with serum B12 level, yet the response is not proportionate with the level of vitamin B12 deficiency. Nearly one-third of HIV positive cases revealed vitamin B12 deficiency.
Conclusion:
During the early stage, fast dividing immune cells cause increased consumption of micronutrients contributing toward vitamin B12 deficiency. It contributes to disorders in methylation affecting the immune function and NK Cell activity which increases the number of CD8 cells. Hence, vitamin B12 is a beneficial immunological modulator of HIV infection and can be a potent game changer in resource constrained set up.
Keywords: AIDS, CD4 count, CD8 count, HIV, immunomodulator, Vitamin B12
Introduction
Human immunodeficiency virus (HIV) infection continues to be a serious health issue in most parts of the world, with a global prevalence of 0.7% among adults, the majority being in developing countries with limited healthcare resources. Approximately 38 million people are currently living with HIV.[1] Because of its nature of progression in silence, the disease is usually detected in late stage.[2] HIV infection affects the immune system of the host affecting components of cellular immunity.[3] Being a chronic disease, its progression to acquired immunodeficiency syndrome (AIDS) is associated with anemia, micronutrient deficiency like folate and Vitamin B12 deficiency[4] and every possibility is there that the first point of contact for people living with HIV AIDS (PLHA) will be a primary care physician.
Nowadays, micronutrients have become a part of the immunotherapeutic approach against cancer, inflammatory diseases, autoimmune diseases, etc.[5] Recently, vitamin B12 attracted a great proportion of scientific and public interest because of its complex biological function and its role in modulating cellular immunity by increasing CD8 and NK cell activity.[6] Patients diagnosed with vitamin B12 deficiency have been reported to have suppressed natural killer cell activity with a decreased number of circulating lymphocytes.[7] Vitamin B12 supplementation has modified these immunological manifestations with augmentation of CD8 T cell & NK cell activity. Also, it improves the level of white blood cells and lymphocytes in rats fed with a low protein diet.[8] Recently, it has been suggested by Todorova et al.[6] that vitamin B12 could be a promising immunotherapeutic agent, though immunoglobulin levels were not found to be affected. As part of the cancer immunotherapy role of Cobalamin has also been strengthened as having a positive effect on anticancer defense.[9] However, the overall increase in cancer risk with elevated vitamin B12 makes the immunological role of vitamin B12 more confusing.[10]
Decreased serum vitamin B12 level has been noted in up to 20% of patients with AIDS, contributing toward hematologic and neurologic dysfunction in AIDS.[11,12] Antiretroviral therapy (ART) initiation has been shown to affect the vitamin B12 status of the patients.[13,14] Most of these studies have analysed Vitamin B12 status in late-stage being complicated with tuberculosis, neuropsychiatric manifestations, etc.[15] As HIV infection manifestations and vitamin B12 deficiency overlap each other, early diagnosis of this vitamin deficiency, and intervention are important to avoid irreversible damage.[16] Primary care givers and family physicians have a very important role to play here for early diagnosis and management of the condition to prevent further complications. Minimal data are available regarding Vitamin B12 status in HIV cases at an early stage and no study is undertaken till now in ART naïve subjects in this part of the Indian subcontinent.
The objective of our study was to find out serum Vitamin B12 concentration and its association with CD4 and CD8 count, clinical staging, and haemato-biochemical status in newly diagnosed HIV positive cases before initiation of ART.
Methodology
Study design and setting
This study was a case-control study conducted in the Department of Biochemistry from July 2018 to March 2020 at a tertiary care institute of eastern India. Ethical clearance was obtained from the Institutional Ethics Committee with reference no. IEC/AIIMS BBSR/PG Thesis/2018-19/14.
Selection of participants
Fifty-five patients above 18 years of age attending Integrated Counselling and Testing Centre (ICTC) clinic which included pre-operative cases, clinically symptomatic cases referred by the clinician and normal or high-risk cases directly walking to the clinic for HIV testing with a positive screening test (Meriscreen) and confirmatory test for HIV (HIV-TRIDOT test) and have not started with anti-retroviral therapy were included in the study after getting informed written consent from the participants. Seriously ill, pregnant women, patients taking vitamin supplements, and patients not able to answer the questions were excluded from the study. Fifty-five age and sex-matched, relatives, and attendants of patients found negative in screening test were enrolled as control after taking informed written consent from them.
Procedure
A detailed history of the diet with the frequency of intake of non-vegetarian diet and drug intake was recorded on each individual’s datasheet. Anthropometric measurements like height, weight, body mass index (BMI) measurements were done in both cases and controls. Clinical examination of the patient was also done before blood collection.
From both cases and controls 5 ml of blood were collected, 2ml in ethylenediaminetetraacetic acid (EDTA) vial, and 3 ml in a plain vial in the ICTC clinic itself. CD4 and CD8 counts were analyzed by Beckman Coulter Navios Flow cytometer, Complete Blood Count was done using Sysmex XT-4000 hematology analyzer, Serum Vitamin B12, Folic acid, ferritin was estimated with Siemens Advia XP chemiluminescence immunoassay (CLIA) analyzer and C-Reactive Protein (CRP) concentration in serum was estimated with Beckman Coulter AU480 Autoanalyzer. Quality control was maintained by using Bio-Rad internal quality control samples daily.
Statistical analysis
Statistical analysis was done with the SPSS-21 version. Parametric and non-parametric data were expressed as mean ± standard deviation. Student’s t-test and analysis of variance (ANOVA) test were used to analyze variables between cases and controls. Pearson correlation study was implemented for correlation study, a P value of < 0.05 was taken as significant.
Results
Participant characteristics
In the present study, mean age of the study participants was found to be 34.4 ± 4.4 years. 92.5% patients were married, 95% were educated, and employed. Out of all the 55 cases, 87.5% were chronic alcoholics. 85% of cases were in World Health Organisation (WHO) staging I and II, that is, early-stage, whereas only 15% were in late-stage (WHO III and IV). Most of them take a non-vegetarian diet frequently. BMI was significantly low in HIV positive cases in comparison to normal control.
Hematological parameters revealed features of anemia in HIV positive cases, with a significant fall in Hb%, PCV, MCHC, total RBC count with a marked rise in RDW CV(%). Absolute lymphocyte count and absolute basophil count registered marked fall in comparison to control group. A significant decline in CD4 count with a marked rise of CD8 count resulted in a significant fall (p < 0.05) in CD4/CD8 ratio in cases than controls [Table 1].
Table 1.
Hematological and immunological parameters in cases and controls
| Parameters | HIV positive cases (n=55) | HIV negative controls (n=55) | P a |
|---|---|---|---|
| Hemoglobin (gm/dl) | 10.07±2.90 | 13.63±1.28 | <0.001 |
| RBC (million cells/cu.mm) | 4.04±1.03 | 4.98±0.66 | <0.001 |
| PCV (%) | 36.09±9.74 | 42.70±3.76 | 0.001 |
| MCV (fl) | 89.47±11.98 | 86.68±9.43 | 0.321 |
| MCH (pg) | 26.57±3.65 | 26.95±3.46 | 0.677 |
| MCHC (gm/dl) | 29.746±2.04 | 31.046±1.27 | 0.004 |
| RDW CV (%) | 16.130±2.17 | 14.313±1.35 | 0.000 |
| WBC X1000 cells/cu.mm | 6.298±2.60 | 7.34±1.80 | 0.076 |
| Absolute neutrophils/cu.mm | 3971.55±32.43 | 4396.33±54.02 | 0.398 |
| Absolute lymphocytes/cu.mm | 1783.50±50.08 | 2340.33±56.61 | 0.008 |
| Absolute eosinophils/cu.mm | 231.10±28.56 | 383.00±48.57 | 0.106 |
| Absolute monocytes/cu.mm | 200.33±34.114 | 194.67±88.15 | 0.847 |
| Absolute Basophils/cu.mm | 16.40±3.22 | 31.33±4.87 | 0.005 |
| PlateletCountX1000cells/cu.mm | 228.40±75.92 | 254.37±85.02 | 0.217 |
| CD4 Count (cells/μL) | 295.80±189.93 | 1005.14±304.87 | <0.001 |
| CD8 Count (cells/μL) | 1034.71±760.85 | 585.58±229.25 | 0.003 |
| CD4/CD8 ratio | 0.31±0.19 | 1.83±0.57 | <0.001 |
a P<0.05 is significant
Significantly low serum vitamin B12 in HIV positive cases (p < 0.05) is seen with a mean value of 240.62 ± 56.75 pg/ml [Figure 1]. Prominent fall in patients of late-stage reflects fall in Vitamin B12 with disease severity. However, serum folic acid did not register any significant difference. Both ferritin and CRP registered a prominent rise in HIV positive cases than healthy controls (p < 0.001), showing an inflammatory response in these cases [Table 2].
Figure 1.
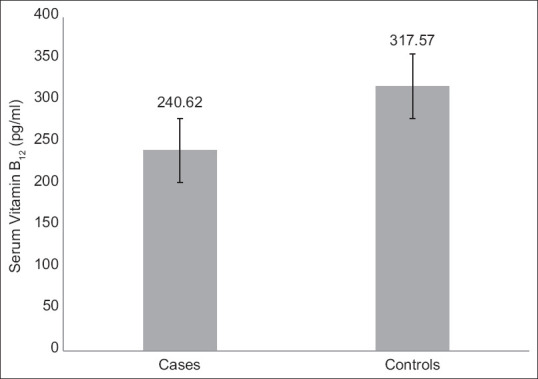
Serum vitamin B12 concentrations in cases and controls
Table 2.
Biochemical parameters in the study group
| Parameter | HIV positive cases (n=55) | HIV negative controls (n=55) | P a |
|---|---|---|---|
| Vitamin B12 (pg/ml) | 240.62±56.75 | 317.57±52.56 | 0.031 |
| Folic Acid (ng/ml) | 3.39±2.57 | 4.596±2.44 | 0.380 |
| Ferritin (ng/ml) | 692.00±109.19 | 76.45±18.88 | 0.001 |
| CRP (mg/L) | 18.5±2.5 | 1.95±0.19 | 0.001 |
a P<0.05 is significant
In our study, 35.45% of HIV infected patients had deficient levels of serum vitamin B12 (<200 pg/ml), 26.09% with a sub-normal level of serum vitamin B12 (200-300 pg/ml), and remaining 38.46% with a normal level of serum vitamin B12 (>300 pg/ml). Subgroup analysis with different vitamin B12 levels documented a significant parallel graded response with parameters of anemia like Hb%, PCV, and total RBC. A significant graded parallel trend was also noticed with absolute lymphocyte count and CD4 count, but not with CD8 count. However, ferritin and CRP revealed a proportionate response in inverse direction to vitamin B12 value showing the inflammatory response contributing to the disease process [Table 3].
Table 3.
Sub-group analysis of altered parameters in HIV-infected cases with relation to serum vitamin B12 concentration
| Parameters | Deficient Vitamin B12 levels (<200 pg/ml) (n=24) | Sub-normal VitaminB12 levels (200-300 pg/ml) (n=16) | Normal Vitamin B12 levels (>300 pg/ml) (n=15) | P a |
|---|---|---|---|---|
| Hb (gm/dl) | 9.65±2.62 | 10.97±2.64 | 13.59±2.18 | 0.008 |
| RBC (million cells/cu. mm) | 3.78±0.96 | 4.28±1.13 | 4.6±0.78 | 0.239 |
| PCV% | 33.41±9.83 | 37.83±9.96 | 45.02±6.19 | 0.041 |
| MCV (fl) | 99.58±15.08 | 90.72±9.01 | 88.15±8.81 | 0.037 |
| MCH (pg) | 25.63±2.54 | 25.9±2.94 | 29.91±4.00 | 0.007 |
| MCHC (gm/dl) | 29.16±2.38 | 29.22±2.06 | 30.16±1.98 | 0.530 |
| RDW CV(%) | 16.74±1.89 | 16.08±2.16 | 14.62±1.26 | 0.042 |
| Absolute lymphocytes/cu.mm | 1070±600.47 | 1866.6±890.15 | 2178.75±647.03 | 0.010 |
| CD4 count (cells/μL) | 121.2±15.06 | 273.2±141.83 | 483.2±140.69 | <0.001 |
| CD8 count (cells/μL) | 392.34±25.36 | 1147.6±804.75 | 1056.48±375.39 | 0.012 |
| Ferritin (ng/ml) | 1191.14±175.38 | 437.9±55.87 | 150.88±16.42 | 0.006 |
| CRP (mg/L) | 27.26±3.11 | 8.23±1.27 | 3.99±0.18 | 0.005 |
aP<0.05 is significant
Pearson correlation study documented a significant positive association between Vitamin B12 with Hb%, CD4 count and absolute lymphocyte count reflecting the contributory role of vitamin B12 on erythropoiesis and lymphocyte turnover in HIV positive cases.
Discussion
In the present study, majority of cases were within 30–50 years of age group having a male predominance (80%), which belongs to the sexually active and economically productive age group, similar to findings by Kumawat et al.,[17] stating that sexual mode to be the most common mode of HIV transmission, in India. Our study also revealed the prevalence of males, with male to female ratio of 4:1, which may be due to migration of male people in search of work from village/town areas to other metropolitan cities and staying away from the spouse for more extended periods.[18] Higher spouse positivity among females was also supported by the study conducted by Bhandarkar et al.[19]
We have got 70% of the males and 69.2% of females belonging to the low socioeconomic group getting their income from unskilled occupations, as shown by Gupta et al.,[20] stating that a big window of opportunity still exists to decrease the transmission of HIV by improving their socioeconomic class. At the time of diagnosis, 85% of cases were as per WHO clinical staging I & II of illness, that is, in the early stage of the disease as seen by Sonani et al.,[18] whereas at the time of registration, greater than two-third patients were in early-stage.
BMI is a critical indicator of nutritional status and one of the strong independent predictors of mortality in HIV-infected patients. The patients having BMI <18.5 kg/m2 were found to have 3-fold higher mortality rates than those with higher BMI, regardless of their tuberculosis status.[21] The significantly lower BMI (p value < 0.05) in HIV positive patients in the present study with a mean value of 18.22 ± 2.98 kg/m2 is being attributed to their poor nutritional status and viral infection.
Anemia in different grades is one of the commonest abnormalities seen in more than 50% of HIV patients. It is a predictor of the death being correlated with the progression of HIV disease. In the present study, anemia’s prevalence is 52.72% similar to observations of other researchers.[22]
The present study documented the mean level of hemoglobin in cases as 10.07 ± 2.90 gm/dl, which is significantly low compared to the control group indicating the role of HIV directly affecting the hematopoietic precursor cells. Mean levels of RBC, PCV, and MCHC were observed to be significantly low (p values < 0.05) compared to controls, supporting the presence of anemia. Multifactorial reasons like inflammatory cytokines released by lymphocytes, underlying chronic disease, mixed nutritional deficiencies, opportunistic infections, malnutrition, malabsorption, vitamin B12 deficiency, etc., might have contributed towards anemia.[12,23]
Our study also documented increased red cell distribution width with a mean value of 16.13 ± 2.17%, indicating a state of ineffective erythropoiesis, a marker of immunologic outcomes in HIV positive cases with the underlying inflammatory state by HIV viral load.[24] A significant fall in absolute lymphocyte count and absolute basophil count in HIV positive cases may be due to a decrease in the number of CD4+ T lymphocyte as well as the capture of basophils by HIV for mediating viral trans-infection of CD4+ T cells as suggested by Jiang et al.[25]
The direct cytopathic effect of HIV on CD4+ T cells with the destruction of the cell membranes leads to immune failure with accelerated destruction of matured CD4+ T cells. The present study also documented a significant fall in CD4 count in cases compared to controls. In the acute stage of HIV infected patients, there is an incomplete, ineffective expansion of HIV-specific CD8+ T cells to clear initial HIV infection and set a predictive viral load set-point with evidence of CD8+ T cell dysfunction.[26] Being unable to clear the virus in the initial stages of HIV infection, there occurs a persistent fight between the virus and the T cells during the course of HIV disease, leading to deterioration and exhaustion of the CD8+ T cell responses as seen by Boulassel et al.[27] The present study with a mean CD8+ T cell count of 1034.71 ± 760.85 cells/mL in HIV positive cases is in agreement with Boulassel et al.[27] and others with a potential explanation for the emergence of a “dysfunctional” CD8+ T cell population due to the lack of CD4+ T cells during the acute phase of HIV infection. The CD4/CD8 ratio of 0.31 ± 0.19 in these cases is similar to observations of other studies pointing toward the targeted cell death of circulating CD4 cells with expansion of CD8 cells.[28,29]
This study registered a mean value of 220.50pg/ml and 180.23pg/ml of vitamin B12 in the early stage and late stage of HIV infection, respectively, stating that deficiency of serum vitamin B12 leads to faster HIV progression. Mean serum vitamin B12 levels were found to be in the subnormal range as with Semeere et al.[30] having a suboptimal value of 221pg/ml. However, nearly one-third of HIV positive cases revealed vitamin B12 deficiency, 26.09% in the sub-normal range, and 38.46% with a normal range of vitamin B12. This is in contrast to the study done by Bruno et al.[31] in 2017, where the majority of HIV positive individuals had a normal range of serum vitamin B12 with mean levels of 315pg/ml. Multiple factors are responsible for the low levels of serum vitamin B12 in HIV positive patients, like chronic diarrhea leading to malabsorption, gastric mucosal damage by HIV and opportunistic infections, and intrinsic factor due to injury of the gastric mucosa.
During the early stages of HIV infection, due to increased lymphocyte turnover, fast dividing immune cells cause increased consumption of micronutrients contributing toward as one of the etiologies of vitamin B12 deficiency in HIV patients. Deficiency of vitamin B12 contributes to disorders in methylation affecting the immune function affecting NK Cell activity.[32] It increases the number of cytotoxic T cells and also participates in antibody synthesis. A recent study has also revealed that vitamin B12 is a cofactor of transcriptional regulators and anti-repressors.[33,34]
No significant difference was noted in serum folic acid in HIV-infected individuals when compared to controls. However, low serum vitamin B12 and folate levels affect the composition of intestinal microbiota in the gastrointestinal tract altering the gut barrier defense mechanism. With folate, vitamin B12 modulate the immune function by facilitating the production of cytotoxic T cells, natural killer cells by acting through folate receptor 4 (FR4) present in T regulatory cells.[35]
Our study also registered a significantly high serum ferritin (p value < 0.05) in HIV positive cases and supported the observation of other authors that this high serum ferritin level is associated with immune suppression and low CD4+ T cell counts in these cases.[36] A significant rise in CRP also suggests that acute inflammation and persistent unresolved infection might be the reasons for increased CRP levels like ferritin in HIV positive cases correlating with HIV disease progression like other studies.[37]
With sub-groups having deficient, sub-normal, and normal levels of vitamin B12 in HIV positive patients, the trend of association of vitamin B12 with Hb%, PCV%, MCH registered a graded response in a positive direction and with MCV, RDW in an inverse way showing the necessity of vitamin B12 for erythropoiesis. Its inverse association with MCV & RDW suggests the role of vitamin B12 in the maturation and effective RBC production. The absolute lymphocyte counts also revealed a similar type of graded response with vitamin B12 levels stating that vitamin B12 in HIV cases also supplements the immunomodulatory function necessary for the replication of immune cells. The proportionate trend in inverse direction with ferritin and CRP highlights the association of vitamin B12 deficiency in inflammation and immune response in these cases.[38]
Direct positive association and proportionate trend of CD4+ T cell with vitamin B12 reveal a supportive role of vitamin B12 on T helper lymphocyte production and its immunomodulatory role in HIV positive patients. Though the CD8+ T cell counts registered a significant association with serum B12 level, the response is not proportionate to the level of vitamin B 12 deficiency.
Conclusion
From this study, we can conclude that vitamin B12 is a beneficial modulator of the immunological and inflammatory status of HIV-infected patients. It should be included as part of rational and healthy nutrition in HIV-infected patients to ensure the proper functioning of the immune system and to avoid future adverse effects. It may prove to be beneficial in resource constrained set up to manage a large population of PLHA as seen in low- and middle-income countries. So, primary care givers and family physicians must be aware of this beneficial effects of vitamin B12 in the early stage so that immediate intervention cane be done after diagnosis rather than waiting for the deficiency manifestations to occur after initiation of ART. This will also reduce the risk of treatment failure. More prospective studies are needed to have a better understanding of its causal relationship, the effect of its supplementation on immune response highlighting immunomodulation triggering signal pathways in these HIV positive cases.
Authors’ contributions
KK: designed the study, collected, analyzed, and interpreted the data, wrote and edited the manuscript. GKS: designed the study, analyzed, and interpreted the data, wrote and edited the manuscript. AKS: designed the study, analysed and interpreted the data, edited the manuscript. MM: designed the study, interpreted the data, edited manuscript. All authors read and approved the final manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank all the staff of the ICTC and Clinical Biochemistry Laboratory of AIIMS Bhubaneswar. We especially thank all the participants who contributed to this study.
References
- 1.The Global HIV/AIDS Epidemic. KFF. 2020. [Last accessed on 2021 Dec 28]. Available from: https://www.kff.org/global-health-policy/fact-sheet/the-global-hivaids-epidemic .
- 2.The World health organisation-fact sheet1 HIV. 2020. [Last accessed on 2021 Dec 28]. Available from: https://www.who.int/hiv/abouthiv/fact_sheet_hiv.htm .
- 3.Walker NF, Meintjes G, Wilkinson RJ. HIV-1 and the immune response to TB. Future Virol. 2013;8:57–80. doi: 10.2217/fvl.12.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adhikari PM, Chowta MN, Ramapuram JT, Rao S, Udupa K, Acharya SD. Prevalence of Vitamin B12 and folic acid deficiency in HIV-positive patients and its association with neuropsychiatric symptoms and immunological response. Indian J Sex Transm Dis AIDS. 2016;37:178–84. doi: 10.4103/2589-0557.192117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soldati L, Di Renzo L, Jirillo E, Ascierto PA, Marincola FM, De Lorenzo A. The influence of diet on anti-cancer immune responsiveness. J Transl Med. 2018;16:75. doi: 10.1186/s12967-018-1448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todorova TT, Ermenlieva N, Tsankova G. Vitamin B12:Could it be a promising immunotherapy? Metodiev K, editor. Immunotherapy- Myths, Reality, Ideas, Future. InTechOpen. 2017:85–101. [Google Scholar]
- 7.Tamura J, Kubota K, Murakami H, Sawamura M, Matsushima T, Tamura T, et al. Immunomodulation by Vitamin B12:Augmentation of CD8+T lymphocytes and natural killer (NK) cell activity in Vitamin B12-deficient patients by methyl-B12 treatment. Clin Exp Immunol. 1999;116:28–32. doi: 10.1046/j.1365-2249.1999.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewicki S, Lewicka A, Kalicki B, Kłos A, Bertrandt J, Zdanowski R. The influence of Vitamin B12 supplementation on the level of white blood cells and lymphocytes phenotype in rats fed a low-protein diet. Cent Eur J Immunol. 2014;39:419–25. doi: 10.5114/ceji.2014.47723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang SC, Goldstein BY, Mu L, Cai L, You NC, He N, et al. Plasma folate, Vitamin B12, and homocysteine and cancers of the oesophagus, stomach, and liver in a Chinese population. Nutr Cancer. 2015;67:212–23. doi: 10.1080/01635581.2015.989375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arendt JHF, Farkas DK, Pederson L, Nexo E, Sorenson HT. Elevated plasma Vitamin B12 levels and cancer prognosis:A population-based cohort study. Cancer Epidemiol. 2016;40:158–65. doi: 10.1016/j.canep.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Hepburn MJ, Dyal K, Runser LA, Barfield RL, Hepburn LM, Fraser SL. Low serum Vitamin B12 levels in an outpatient HIV-infected population. Int J STD AIDS. 2004;15:127–33. doi: 10.1258/095646204322764334. [DOI] [PubMed] [Google Scholar]
- 12.Mutengo K, Mupeta F, Ngalamika O. Pernicious anemia and Vitiligo in an HIV patient:An unfamiliar case presentation. Case Rep Gastrointest Med. 2020;2020:7942453. doi: 10.1155/2020/7942453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goosen C, Baumgartner J, Mikulic N, Barnabas SL, Cotton MF, Zimmermann MB, et al. Examining associations of HIV and iron status with nutritional and inflammatory status, anemia, and dietary intake in South African School children. Nutrients. 2021;13:962. doi: 10.3390/nu13030962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezaei E, Sedigh Ebrahim-Saraie H, Heidari H, Ghane P, Rezaei K, Manochehri J, et al. Impact of vitamin supplements on HAART related haematological abnormalities in HIV-infected patients. Med J Islam Repub Iran. 2016;30:350. [PMC free article] [PubMed] [Google Scholar]
- 15.Ralapanawa DM, Jayawickreme KP, Ekanayake EM, Jayalath WA. Vitamin B12 deficiency with neurological manifestations in the absence of anaemia. BMC Res Notes. 2015;8:458. doi: 10.1186/s13104-015-1437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balt CA. An investigation of the relationship between Vitamin B12 deficiency and HIV infection. J Assoc Nurses AIDS Care. 2000;11:24–35. doi: 10.1016/S1055-3290(06)60419-6. [DOI] [PubMed] [Google Scholar]
- 17.Kumawat S, Kochar A, Sirohi P, Garhwal J. Socio-demographic and clinical profile of HIV/AIDS patients in HAART era at a tertiary care hospital in North-West Rajasthan, India. Int J Community Med Public Health. 2016;3:2088–93. [Google Scholar]
- 18.Sonani HP, Undhad AM, Savani GT. Clinical and socio-demographic profile of patients registered at ART centre, SMIMER, Surat. Natl J Community Med. 2011;2:130–2. [Google Scholar]
- 19.Bhandarkar PN, Mohd S, Kannan K, Jogdand GS. Socio-demographic Profile of HIV patients at ICTC, CAIMS, Karimnagar. Int J Biol Med Res. 2011;2:1023–5. [Google Scholar]
- 20.Gupta M. Profile of clients tested HIV positive in a voluntary counselling and testing centre of a District Hospital, Udupi. Indian J Community Med. 2009;34:223–6. doi: 10.4103/0970-0218.55288. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Naidoo K, Yende-Zuma N, Augustine S. A retrospective cohort study of body mass index and survival in HIV infected patients with and without TB co-infection. Infect Dis Poverty. 2018;7:35. doi: 10.1186/s40249-018-0418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meidani M, Rezaei F, Maracy MR, Avijgan M, Tayeri K. Prevalence, severity, and related factors of anemia in HIV/AIDS patients. J Res Med Sci. 2012;17:138–42. [PMC free article] [PubMed] [Google Scholar]
- 23.Gibellini D, Clò A, Morini S, Miserocchi A, Ponti C, Re MC. Effects of human immunodeficiency virus on the erythrocyte and megakaryocyte lineages. World J Virol. 2013;2:91–101. doi: 10.5501/wjv.v2.i2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campa A, Baum MK. Micronutrients and HIV infection. HIV Ther. 2010;4:437–69. [Google Scholar]
- 25.Jiang AP, Jiang JF, Guo MG, Jin YM, Li YY, Wang JH. Human blood-circulating basophils capture HIV-1 and mediate viral trans-infection of CD4+T cells. J Virol. 2015;89:8050–62. doi: 10.1128/JVI.01021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eller MA, Goonetilleke N, Tassaneetrithep B, Eller LA, Costanzo MC, Johnson S, et al. Expansion of inefficient HIV-Specific CD8 T cells during acute infection. J Virol. 2016;90:4005–16. doi: 10.1128/JVI.02785-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boulassel MR, Chomont N, Pai NP, Gilmore N, Sékaly RP, Routy JP. CD4 T cell nadir independently predicts the magnitude of the HIV reservoir after prolonged suppressive antiretroviral therapy. J Clin Virol. 2012;53:29–32. doi: 10.1016/j.jcv.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Petoumenos K, Choi JY, Hoy J, Kiertiburanakul S, Ng OT, Boyd M, et al. CD4:CD8 ratio comparison between cohorts of HIV-positive Asians and Caucasians upon commencement of antiretroviral therapy. Antivir Ther. 2017;22:659–68. doi: 10.3851/IMP3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han WM, Apornpong T, Kerr SJ, Hiransuthikul A, Gatechompol S, Do T, et al. CD4/CD8 ratio normalization rates and low ratio as prognostic marker for non-AIDS defining events among long-term virologically suppressed people living with HIV. AIDS Res Ther. 2018;15:13. doi: 10.1186/s12981-018-0200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semeere AS, Nakanjako D, Ddungu H, Kambugu A, Manabe YC, Colebunders R. Sub-optimal vitamin B-12 Levels among ART-naive HIV-positive individuals in an urban cohort in Uganda. PLoS One. 2012;7:e40072. doi: 10.1371/journal.pone.0040072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruno R, Scuderi D, Locatelli ME, Pampaloni A, Pinzone MR. Prevalence of micronutrients deficiencies in a cohort of HIV-positive individuals on ART. Infect Dis Trop Med. 2017;3:e431. [Google Scholar]
- 32.Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients. 2020;12:236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grudzien M, Rapak A. Effect of natural compounds on NK cell activation. J Immunol Res. 2018;2018:4868417. doi: 10.1155/2018/4868417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshii K, Hosomi K, Sawane K, Kunisawa J. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front Nutr. 2019;6:48. doi: 10.3389/fnut.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunisawa J, Hashimoto E, Ishikawa I, Kiyono H. A pivotal role of vitamin B9 in the maintenance of regulatory T cells in vitro and in vivo. PLoS One. 2012;7:e32094. doi: 10.1371/journal.pone.0032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kharb S, Lallar M, Ghalaut PS, Bala J, Kumavat M, Nanda S. Haemoglobin and serum ferritin concentration in anaemic and non-anaemic human HIV females in India. J Health Res Rev. 2018;5:22–5. [Google Scholar]
- 37.Shivakoti R, Yang WT, Berendes S, Mwelase N, Kanyama C, Pillay S, et al. Persistently elevated CRP in first year of ART despite virologic suppression is associated with HIV disease progression in resource constrained settings. J Infect Dis. 2016;213:1074–8. doi: 10.1093/infdis/jiv573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Daghri NM, Rahman S, Sabico S, Yakout S, Wani K, Al-Attas OS, et al. Association of Vitamin B12 with pro-inflammatory cytokines and biochemical markers related to cardiometabolic risk in Saudi subjects. Nutrients. 2016;8:460. doi: 10.3390/nu8090460. [DOI] [PMC free article] [PubMed] [Google Scholar]


