Abstract
Previous findings indicate that the cystic fibrosis transmembrane conductance regulator (CFTR) is a ligand for Pseudomonas aeruginosa ingestion into respiratory epithelial cells. In experimental murine keratitis, P. aeruginosa enters corneal epithelial cells. We determined the importance of CFTR-mediated uptake of P. aeruginosa by corneal cells in experimental eye infections. Entry of noncytotoxic (exoU) P. aeruginosa into human and rabbit corneal cell cultures was inhibited with monoclonal antibodies and peptides specific to CFTR amino acids 108 to 117. Immunofluorescence microscopy and flow cytometry demonstrated CFTR in the intact murine corneal epithelium, and electron microscopy showed that CFTR binds to P. aeruginosa following corneal cell ingestion. In experimental murine eye infections, multiple additions of 5 nM CFTR peptide 103-117 to inocula of either cytotoxic (exoU+) or noncytotoxic P. aeruginosa resulted in large reductions in bacteria in the eye and markedly lessened eye pathology. Compared with wild-type C57BL/6 mice, heterozygous ΔF508 Cftr mice infected with P. aeruginosa had an approximately 10-fold reduction in bacterial levels in the eye and consequent reductions in eye pathology. Homozygous ΔF508 Cftr mice were nearly completely resistant to P. aeruginosa corneal infection. CFTR-mediated internalization of P. aeruginosa by buried corneal epithelial cells is critical to the pathogenesis of experimental eye infection, while in the lung, P. aeruginosa uptake by surface epithelial cells enhances P. aeruginosa clearance from this tissue.
In several recent studies, our laboratory has provided evidence that the cystic fibrosis (CF) transmembrane conductance regulator (CFTR) can serve as an epithelial cell receptor for internalization of both Pseudomonas aeruginosa (24, 26) and Salmonella typhi (23). The complete outer-core polysaccharide portion of the lipopolysaccharide (LPS) of P. aeruginosa was identified as a ligand for CFTR in these studies (24, 26). We have proposed that CFTR-mediated lung epithelial cell internalization of P. aeruginosa is part of the host defense mechanism for clearing P. aeruginosa from this tissue. Since many severe cases of CF are associated with CFTR mutations that greatly reduce or eliminate expression of CFTR protein, this deficiency may underlie the inability of many CF patients to clear P. aeruginosa from the lung.
P. aeruginosa is also the bacterial pathogen most commonly involved in keratitis associated with eye trauma and with the use of contact lenses (3, 27, 32, 34). Numerous P. aeruginosa and host factors have been reported to contribute to pathogenesis, including binding of bacterial pili and LPS to asialo-GM1 receptors (12), elaboration of bacterial and host proteases (6, 15, 29, 36), and intracellular uptake of P. aeruginosa by corneal epithelial cells (9, 11). However, where corneal epithelial cell ingestion of P. aeruginosa falls in the spectrum of critical to irrelevant to the pathologic process is not known. Although we have shown that the LPS outer-core polysaccharide is the bacterial ligand for corneal epithelial cell uptake of P. aeruginosa (39), it has not been established whether CFTR is expressed in corneal epithelium and can serve as a receptor for bacteria during eye infection. We have also shown that addition of complete core LPS inhibits the ingestion of P. aeruginosa by corneal epithelial cells in vitro, but addition of this polysaccharide to infectious inocula of P. aeruginosa applied to the injured eyes of mice resulted in only modest effects on corneal infection and eye pathology (40). These modest effects were felt to be due to the difficulty that a monovalent, exogenous LPS inhibitor added to the infectious inoculum of P. aeruginosa would have in competing with a highly repetitive endogenous LPS ligand on the bacterial surface for binding and internalization into corneal cells. This situation would render the bacterial cells fairly insensitive to competition by the soluble inhibitor.
Following the identification of the first extracellular domain of CFTR as a lung epithelial cell receptor for P. aeruginosa, we investigated whether CFTR could also serve as a receptor for uptake of P. aeruginosa during eye infections and the role that this uptake played in pathogenesis. Use of CFTR-specific peptides and the availability of transgenic mouse strains with Cftr mutations provided better experimental systems for further study of P. aeruginosa corneal cell internalization in the pathogenic process than the competition experimental protocols used previously (40). If CFTR peptides could effectively block internalization of P. aeruginosa by corneal cells, then delivery of the peptides along with the infectious inoculum followed by multiple, subsequent applications of CFTR peptides could help determine the role of corneal cell internalization in the pathologic process. Transgenic mice either heterozygous or homozygous for the ΔF508 Cftr allele provide a clear system for testing the hypothesis that CFTR-mediated uptake of P. aeruginosa is critical for the development of infection and disease. The ΔF508 Cftr allele encodes a temperature-sensitive variant of CFTR that fails to be exported to the plasma membrane of cells (4, 5). Mutant Cftr animals also provide a system for bacteriologic and histologic analysis of the progression of infection and development of eye pathology. In this work, we evaluated whether CFTR is expressed in corneal cells, whether CFTR serves as a receptor for uptake of P. aeruginosa during experimental eye infection, and the role of epithelial cell internalization of P. aeruginosa during infection on pathogenesis of experimental keratitis.
MATERIALS AND METHODS
Bacterial strains.
For in vitro studies and experimental eye infections, we used P. aeruginosa 6294, 6487, 6073, 6077, and 6206, isolated from human corneal infections, and PAO1, provided by Michael Vasil, Denver, Colo. Strains 6073, 6077, and 6206 contain the exoU gene (7, 8) and are cytotoxic for corneal epithelial cells in vitro (11). The other strains lack the exoU gene and readily invade corneal cells in vitro (8, 11). Strains were grown for inoculation into corneal cell cultures and mouse eyes as described previously (28).
Cell culture.
Rabbit corneal cells were obtained from Pel-Freeze Biologicals (Rogers, Ark.) and cultured in SHEM medium as described elsewhere (39). Primary cultures of human corneal cells were established from corneal rims obtained during corneal transplant surgery. The tissues were rinsed in Hanks’ balanced salt solution; the endothelium and stroma were peeled off, and the corneal epithelial layer was released from the remaining tissue by treatment with 2 mg of dispase II (Boehringer Mannheim, Indianapolis, Ind.) per ml for 1 h at 37°C. Sheets of corneal cells could then be placed into Keratinocyte-SF medium supplemented with 0.1 ng of epidermal growth factor/ml and with 50 ng bovine pituitary extract/ml (Life Technologies, Gibco, Burlington, Ontario, Canada) for culture at 37°C in 5% CO2. The medium was changed twice a week until the flasks were confluent.
Monolayer cultures of human corneal cells were established in 96-well tissue culture plates by treating the cells in the flasks with dispase II to release them and then seeding the cells at 105 per well, using the medium described above for culture. Cells were used within 24 h for bacterial ingestion assays. Polarized cultures of human corneal cells were established on 6.5-mm-diameter Transwell filters (Corning Costar Corp., Cambridge, Mass.) with 0.4-μm-diameter pores as described previously (23), using the supplemented Keratinocyte-SF medium. After 5 days at 37°C in 5% CO2, the individual wells were checked for formation of tight junctions by addition of [3H]mannitol to the apical side of the cell culture and collection of fluid from the basal side 2 h later for analysis of radioactivity. [3H]mannitol placed into an empty Transwell unit showed equal counts on both sides of the membrane, whereas all wells used for bacterial ingestion experiments allowed <8% of the radioactivity to translocate to the basal side of the cells.
Peptides and MAbs.
Peptides corresponding to CFTR amino acids 103 to 117, amino acids 103 to 112, and amino acids 108 to 117 or scrambled versions of peptide 108-117 or peptide 103-117 were obtained from Biosynthesis Corp, Lewisville, Tex. Immunoglobulin M (IgM) monoclonal antibodies (MAbs) used were as described previously (37) and included (i) CF3, specific to human CFTR peptide 103-117 (located in the first predicted extracellular domain of CFTR); (ii) CF4, specific to human CFTR peptide 881-910; (iii) CF8, specific to human CFTR residues 1035 to 1050 (representing a 16-amino-acid region located in the cytoplasmic domain connecting the fifth and sixth transmembrane domains (37); and (iv) an irrelevant IgM MAb (VF8) specific to Staphylococcus epidermidis capsular polysaccharide/adhesin.
Cellular ingestion assays.
Ingestion of P. aeruginosa by cultured cells, and inhibition of this ingestion with CFTR peptides and MAbs, was measured by gentamicin exclusion assays as described previously (39). In brief, about 106 CFU of P. aeruginosa, along with appropriate peptides or MAb inhibitors, was added to monolayers of 105 cultured corneal cells. For polarized human corneal cells, 2 × 107 CFU of P. aeruginosa was added to the cultures that contained about 2 × 106 cells. Infected cells were placed for 3 h at 37°C in 5% CO2. Nonadherent bacteria were washed away, and 200 μg of gentamicin/ml was added to kill extracellular bacteria. After 1 h of exposure to antibiotic, cells were washed and intracellular bacteria were released from cells by lysis with 0.5% Triton X-100, diluted, and plated for bacterial enumeration.
Mice.
Wild-type C57BL/6 mice and C57BL/6 mice either homozygous or heterozygous for the ΔF508 allele of Cftr were obtained from The Jackson Laboratory (Bar Harbor, Maine) for use in this study. Breeding pairs of heterozygous ΔF508 Cftr mice (C57BL/6-Cftrtm1Kth) (41) were maintained on normal lab chow and water; litters of about 2 weeks of age and their dams were placed on water containing golytely (Braintree Laboratories, Braintree, Mass.) (1) and an elemental liquid diet of Peptamin (Clintec Nutrition Co., Deerfield, Mich.). Pups were maintained on these liquids after weaning until genotyping for Cftr alleles was carried out as described elsewhere (41). Homozygous ΔF508 Cftr mice were kept on this diet, while wild-type and heterozygous mice were returned to normal lab chow and water.
Murine model of corneal infection.
Scratch-injured eyes of anesthetized mice were infected as described previously (28, 40). For some experiments, 5 nM either the cognate CFTR peptide 103-117 or the scrambled version of this peptide was added to the infectious inoculum of P. aeruginosa. Total and intracellular P. aeruginosa organisms in the corneal epithelium 24 or 48 h after infection were quantified as described elsewhere (40). Briefly, mice with infected eyes were killed by CO2 inhalation, and the corneas dissected from the eyes with a sterile scalpel blade and then removed with microdissecting scissors. Corneas were either homogenized in tryptic soy broth containing Triton X-100 (0.5%) for enumeration of total bacteria (both bound to cells and internalized by them) or exposed to 200 μg of gentamicin/ml for 1 h to kill extracellular bacteria, washed extensively to remove the antibiotic, and homogenized in 0.5% Triton X-100 to release intracellular bacteria for enumeration by dilution and plating. Corneal pathology was scored by an observer masked to any information about the animal or its infection status as described elsewhere (40). The following scale was used on eyes 24 or 48 h after infection: grade 0, eye macroscopically identical to the uninfected contralateral control eye; grade 1, faint opacity partially covering the pupil; grade 2, dense opacity covering the pupil; grade 3, dense opacity covering the entire anterior segment; and grade 4, perforation of the cornea. For some of the results reported, we collapsed the categorization of eye pathology into mild (mice with pathology scores of <2) or severe (mice with pathology scores of ≥2) to simplify presentation of data. Corneas infected in vivo and used for microscopy were prepared as described elsewhere (9, 10). All animals were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for Use of Animals in Ophthalmic and Vision Research.
In vitro infection of corneas.
Mice were killed by an overdose of carbon dioxide. Three 1-mm-long scratches were made in the corneal epithelium and superficial stroma with a 25-gauge needle, and the entire eye was removed and placed in a tissue culture plate on a piece of sterile gauze soaked with sterile Ham’s F-12 medium. Each cornea was immediately inoculated with 10 μl of P. aeruginosa suspended in Ham’s F-12 at a concentration of 2 × 1010 CFU/ml. The eyes were then incubated at 37°C in room air for 3 h and washed twice in sterile F-12 medium, and the corneas were dissected from the eyes. The corneas were then washed twice (for microscopy or flow cytometry) or five times (for reverse transcription [RT]-PCR samples) in sterile F-12 medium.
Microscopy.
Corneas were fixed in 10% neutral-buffered formamide (Sigma) and sectioned for immunofluorescence microscopy (24). The corneas were treated with the CFTR-specific MAb CF8 or control IgM MAb VF8 (described above), followed by fluorescein-conjugated goat anti-mouse IgM (Sigma). Extensive studies have documented the cross-reactivity of the human CFTR-specific MAbs with murine CFTR (23, 24). In addition, although the three CFTR-specific MAbs have been reported to cross-react with non-CFTR antigens expressed by human cells in culture (37), the methodology that we use to stain murine tissues and cells from humans with these MAbs (23, 24) does not result in any detectable antibody binding to tissues or cells from ΔF508 Cftr homozygote mice or humans.
For immunoelectron microscopy, corneas were fixed for 3 h at room temperature in 200 mM HEPES (pH 7.5) containing 4% paraformaldehyde. Tissue sections were embedded in Epon/Araldite (Electron Microscopy Sciences, Fort Washington, Pa.) and mounted on nickel grids. Before addition of antibodies, the tissue sections were etched by incubation in a saturated solution of sodium metaperiodate for 3 min. The grids were then blocked to prevent nonspecific staining by incubation in phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 0.1% Triton X-100 (blocking buffer) for 15 min. All of the following washes and incubations were performed at room temperature, and all washes were 5 min in duration. The grids were next incubated for 1 h with CFTR-specific MAb CF8 or control MAb VF8, washed three times in blocking buffer, incubated for 1 h with rabbit anti-mouse IgM (Zymed Laboratories, Inc., South San Francisco, Calif.), washed three times in blocking buffer, incubated for 30 min with 20-nm colloidal gold-labeled protein A (Sigma) diluted to 1 μg of protein A/ml in blocking buffer, and washed again three times in blocking buffer and then twice in PBS. The tissue was then fixed in 1% glutaraldehyde in PBS for 5 min and finally washed twice in distilled water.
RT-PCR analysis.
Total RNA was prepared with an RNEasy kit (Qiagen, Valencia, Calif.), and cDNA was then prepared with the Superscript II preamplification kit (Gibco, Grand Island, N.Y.) according to the manufacturer’s protocol for cDNA synthesis of transcripts with high GC content. For each reaction, a duplicate one, identical to the first, but lacking reverse transcriptase, was carried out.
Semiquantitative PCR employed the cDNA products as substrate. Samples were compared with use of several dilutions of known concentrations of cDNA all amplified with a constant number of PCR cycles. PCRs were done by using a touchdown PCR protocol as described previously (13), with the annealing temperature decreasing from 60 to 45.5°C over 30 cycles, followed by an additional 30 cycles with an annealing temperature of 45°C. The following murine Cftr-specific primers were obtained from Operon Technologies, Inc. (Alameda, Calif.): CFTRFOROUT (5′-ATTACTGGAGAAAGTACAACAAAG-3′), complementary to a portion of Cftr exon 9; and CFTRREVOUT (5′-AATACTTGTTCTTCAGTAAAAAC-3′), complementary to a portion of Cftr exon 12. These primers are predicted to amplify a product of 540 bp.
Electrophoresis was used to analyze 20 μl of each 50-μl reaction in 1.8% agarose along with a 1-kb DNA ladder size standard (Gibco). The gel was stained in 0.5 μg of ethidium bromide/ml for 2 to 3 h, photographed, and scanned at 150 dots/in. with VistaScan software, version 1.2.2 (UMAX Data Systems, Hsinchu, Taiwan). The intensity of each PCR product was determined with the NIH Image software package (version 1.61), and the density of each reaction product was plotted versus the dilution factor of the initial concentration of cDNA in the PCR. A linear regression analysis of the data was undertaken to determine the cDNA dilution factor that would yield a PCR product density of zero, which could then be used to compare the different samples for the relative amounts of mRNA initially present. The higher the endpoint dilution factor, the more mRNA that was initially present.
Flow cytometric analysis.
Corneas were dissected from control, uninfected eyes or from eyes infected in vitro with P. aeruginosa, washed once in PBS, and incubated overnight at 4°C in a 0.25% solution of trypsin (Gibco). The following day, trypsin was inhibited by addition of medium containing serum. The corneas were washed once in sterile, cold PBS and transferred to a 24-well tissue culture plate (Costar, Cambridge, Mass.) containing 2.0 ml of sterile, cold PBS per well. For each set of experimental conditions, 4 to 10 identically treated corneas were pooled. The corneas were minced with sterile dissection scissors and then stirred vigorously in the 24-well plate at 4°C for 2 h to dissociate the epithelial cells from the corneal stroma. The epithelial cell suspensions were transferred to microcentrifuge tubes and centrifuged for 1 min at 225 × g to pellet debris. Each supernatant (containing the epithelial cells) was split into two equal (0.8-ml) portions (for staining with CFTR-specific or control antibodies), and transferred to separate microcentrifuge tubes; the epithelial cells were pelleted at 225 × g for 5 min, washed once in 0.5 ml of PBS, and resuspended in 0.5 ml of staining buffer (PBS containing 1% bovine serum albumin [Amresco, Solon, Ohio], 2% normal goat serum [Sigma], and 0.04% NaN3]. The samples were incubated at 4°C for 30 min to block nonspecific binding, pelleted at 225 × g for 5 min, and incubated for 60 min with 0.5 ml of either CF3 or VF8 mouse IgM MAb (10 μg/ml in staining buffer). Cells were washed twice in 0.5-ml portions of staining buffer, and then incubated for 30 min with fluorescein isothiocyanate-conjugated goat anti-mouse IgM (Sigma) diluted 1:100 in staining buffer. Cells were washed twice in 0.5-ml portions of staining buffer and then fixed in 0.2 ml of PBS containing 1% paraformaldehyde.
Samples were analyzed on a Facscalibur flow cytometer (Becton Dickinson, Sparks, Md.), and the CellQuest software package (Becton Dickinson) was used for data acquisition and analysis. Collected events were gated by using forward scatter × side scatter to avoid cell debris. For each sample, at least 5,000 gated events were collected. For each set of like samples, the mean fluorescent signal obtained with the irrelevant VF8 primary antibody was subtracted from that obtained with the CFTR-specific CF3 primary antibody.
Statistical analysis.
Differences between mouse groups in the proportions of animals with eye pathology scores of ≥2 were calculated by Fisher’s exact test (31). Differences in eye pathology scores were determined by Kruskal-Wallis nonparametric analysis of variance (ANOVA). For continuous variables, unpaired t tests or ANOVA analysis was carried out on either normally distributed or log-transformed results; the Fisher probable least squares difference (PLSD) statistic was used for pairwise comparisons when three or more groups were compared.
RESULTS
CFTR-mediated uptake of P. aeruginosa by cultured corneal cells.
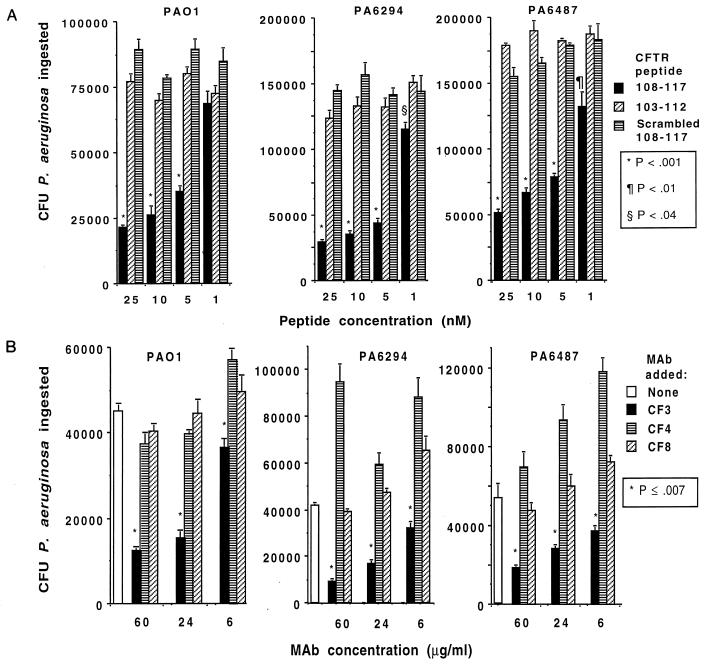
Previous work has established that strains of P. aeruginosa can be classified as either invasive (noncytotoxic) or cytotoxic to cultured cells, including corneal epithelial cells (11). Cytotoxic strains contain the exoU gene (7, 8), and cellular ingestion of these strains is difficult to measure due to the ability of the bacteria to rapidly lyse cells during in vitro ingestion assays. Entry of invasive strains lacking the exoU gene into cultured cells can be measured by gentamicin exclusion assays. Previous work has also shown that CFTR-mediated uptake of P. aeruginosa into cells (24) can be inhibited with antibodies and peptides corresponding to the first predicted extracellular domain of CFTR. To determine whether corneal cells also used CFTR to ingest P. aeruginosa primary cultures of rabbit or human corneal cells were incubated with three noncytotoxic P. aeruginosa strains along with CFTR-specific peptides and MAbs. CFTR peptide 108-117, predicted to be in the first extracellular domain of this protein, inhibited uptake of all three P. aeruginosa strains by rabbit corneal cells when added during the ingestion assays (Fig. 1A), whereas neither a peptide comprising CFTR amino acids 103 to 112 nor a scrambled version of peptide 108-117 had any inhibitory effect on bacterial internalization. A 30-mer peptide (CFTR 881-900) predicted to encompass the fourth extracellular domain of CFTR was without effect on P. aeruginosa internalization (data not shown).
FIG. 1.
Inhibition of uptake of three P. aeruginosa strains (indicated above each graph) by rabbit corneal cells grown in vitro by use of synthetic peptides corresponding to the first extracellular domain of CFTR (CFTR peptide 108-117; A) or a MAb to CFTR peptide 103-117 (MAb CF3; B) to inhibit cellular ingestion. Bars indicate means of six to nine replicates; error bars indicate the SE. P values were determined by ANOVA and Fisher PLSD for pairwise differences. For peptide inhibitions, peptide 103-112 and the scrambled 108-117 peptide gave results equivalent to those obtained when no peptide was added.
MAb CF3, specific to the first predicted extracellular domain of CFTR, inhibited P. aeruginosa uptake by corneal cells (Fig. 1B), whereas MAb CF8, specific to a cytoplasmic domain, was without effect on P. aeruginosa entry into corneal cells. MAB CF4, specific to the predicted fourth extracellular domain of CFTR, was usually without effect but in some experiments showed enhancement of bacterial uptake. Although we do not know why enhanced P. aeruginosa uptake was sometimes measured with MAb CF4, it was not consistent, did not always show a dose-response effect, and enhanced uptake may be observed at times due to unexpected biologic activities of MAb CF4 and either the corneal cells or the P. aeruginosa strain being tested. Also, the differences in uptake of P. aeruginosa cells in different experiments (e.g., Fig. 1) is due to variability in the passage level of the cell cultures and variations in the actual inocula of bacteria used, and so relative and not absolute levels of bacterial uptake within each experiment must be compared.
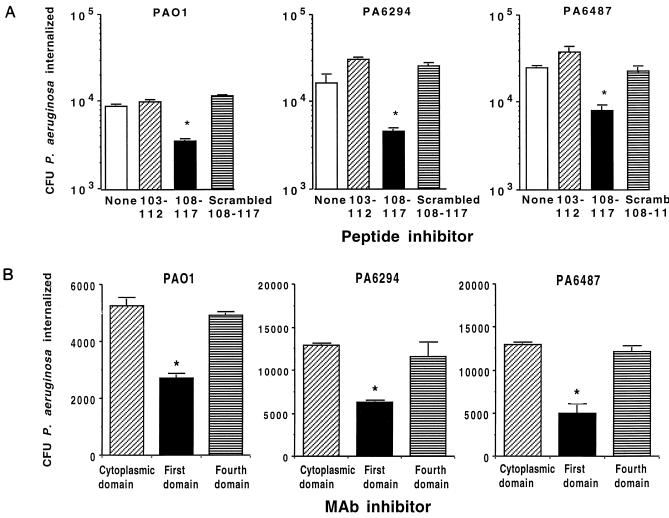
A comparable effect of CFTR peptide 108-117 and a MAb to this peptide was achieved with monolayer cultures of human corneal cells and the three P. aeruginosa strains (Fig. 2). When polarized cultures of human corneal epithelial cells grown on Transwells were evaluated for bacterial ingestion, CFTR peptide 108-117 inhibited uptake of P. aeruginosa 6294 by 59% ± 5% (mean ± standard error [SE]), while the scrambled version of this peptide was without effect (P <0.001, ANOVA and Fisher PLSD). In a comparable manner, 24 μg of MAb CF3/ml, raised to CFTR peptide 103-117, inhibited polarized corneal cell uptake of P. aeruginosa 6294 by 70% ± 5%, whereas MAb CF4, raised to CFTR peptide 881-900, had no inhibitory effect (P <0.001, ANOVA and Fisher PLSD). These results indicate that corneal cells, like respiratory cells (24), use CFTR to internalize a proportion of infecting P. aeruginosa cells. Binding of CFTR to P. aeruginosa during entry into cultured corneal cells was confirmed by immunoelectron microscopy (data not shown).
FIG. 2.
Inhibition of uptake of three P. aeruginosa strains (indicated above each graph) by human corneal cells grown in vitro by use of 20 nM synthetic peptides corresponding to the first extracellular domain of CFTR (upper graphs) or MAbs (60 μg/ml) to CFTR peptides (lower graphs). Bars indicate means of six to nine replicates; error bars represent the SE. Asterisks indicate P values of <0.01 determined by ANOVA and Fisher PLSD for pairwise differences.
Evaluation of CFTR expression in mouse corneas.
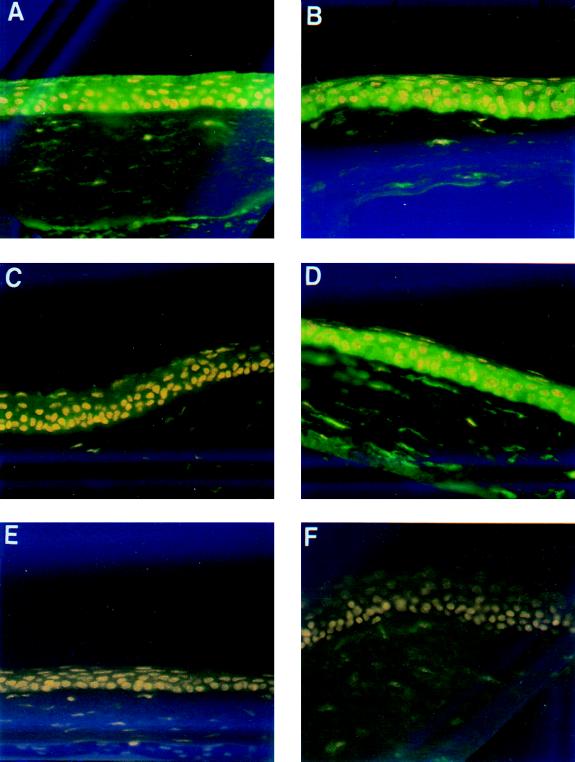
The expression of CFTR in the lung, intestine, sweat glands, pancreas, and other exocrine tissues is well documented (14); it is also expressed in kidney (19), hypothalamus (21), and lymphocytes (20). However, a Medline search (search terms CFTR or cystic fibrosis and cornea) failed to find any documents reporting analysis of CFTR expression in the cornea. We therefore determined the expression of CFTR in the corneal epithelium of C57BL/6 mice either with two wild-type Cftr alleles or with one or two ΔF508 Cftr alleles both before and 3 h after application of P. aeruginosa to injured, enucleated eyes maintained in organ culture. Staining for CFTR expression in the corneal epithelium of uninfected wild-type mice by immunofluorescence microscopy showed moderately heavy fluorescence throughout the epithelium, which indicates the endogenous production of CFTR by most cells in this tissue (Fig. 3A). RT-PCR analysis using mRNA isolated from wild-type corneas and primers specific for murine Cftr yielded the expected product of 540 bp (not shown), confirming corneal expression of CFTR in normal mice. In contrast, the corneal epithelium of uninfected heterozygous ΔF508 mice appeared much duller in staining reactions (Fig. 3C), suggesting decreased endogenous expression of CFTR in the corneas from ΔF508 Cftr heterozygotes. Three hours after infection with P. aeruginosa, there was comparable, uniform staining of the epithelial cells from both the wild-type and heterozygous corneas using the CFTR-specific MAb CF3 (Fig. 3B and D). Corneas that were scratched but not infected (i.e., mock infected) appeared identical to the uninfected corneas shown in Fig. 3A and C. Corneas from mice homozygous for the ΔF508 Cftr allele showed no detectable staining for CFTR protein even after P. aeruginosa infection (Fig. 3E), appearing the same as wild-type corneas treated with an irrelevant IgM MAb (Fig. 3F). Although MAb CF3 used to detect CFTR in the cornea has been reported to cross-react with unidentified proteins expressed by human cells in culture (37), we have consistently found no reactivity of this MAb with many tissues from mice homozygous for ΔF508 Cftr (23, 24), including the cornea as shown here.
FIG. 3.
Immunohistochemical stain of mouse corneas for CFTR expression. The epithelial layer lies at the top of each section in the micrographs, and the acellular stromal layer is on the bottom of each section. Corneas stained with MAb CF3, specific to CFTR peptide 103-117, were from an uninfected, wild-type mouse (A), a P. aeruginosa-infected wild-type mouse (B), an uninfected ΔF508 heterozygous Cftr mouse (C), a P. aeruginosa-infected heterozygous ΔF508 Cftr mouse (D), and a P. aeruginosa-infected homozygous ΔF508 Cftr mouse (E). (F) Uninfected cornea from wild-type mouse stained with irrelevant MAb. All micrographs were taken at a magnification of ×400.
Flow cytometric analysis of CFTR expression in corneas of wild-type and ΔF508 Cftr heterozygotes confirmed the observations made by immunofluorescence microscopy. Corneal epithelial cells from wild-type mice had a baseline mean fluorescence intensity of 17.1 ± 2.2 (standard deviation [SD]) U, whereas the epithelial cells from mice heterozygous for the ΔF508 Cftr allele had a value of 0 ± 2.2 (SD) U. Following P. aeruginosa infection for 3 h, the mean fluorescence in the wild-type corneas more than doubled, to a value of 36.8 ± 3.0 (SD) U, and the mean fluorescence intensity in corneas from ΔF508 Cftr heterozygous mice increased to 22.8 ± 3.5 (SD) U. Thus, the differences in the level of CFTR in the corneas of uninfected wild-type mice compared with those of ΔF508 Cftr heterozygotes observed by immunofluorescence were confirmed by quantitative flow cytometric analysis. This method also confirmed the increase in expression of CFTR on corneal cells of heterozygous ΔF508 Cftr mice following P. aeruginosa infection. The basis for low endogenous expression of CFTR on corneas from heterozygous ΔF508 Cftr mice is unclear at this time.
Effect of CFTR peptides on infection and pathology in scratch-injured murine eyes.
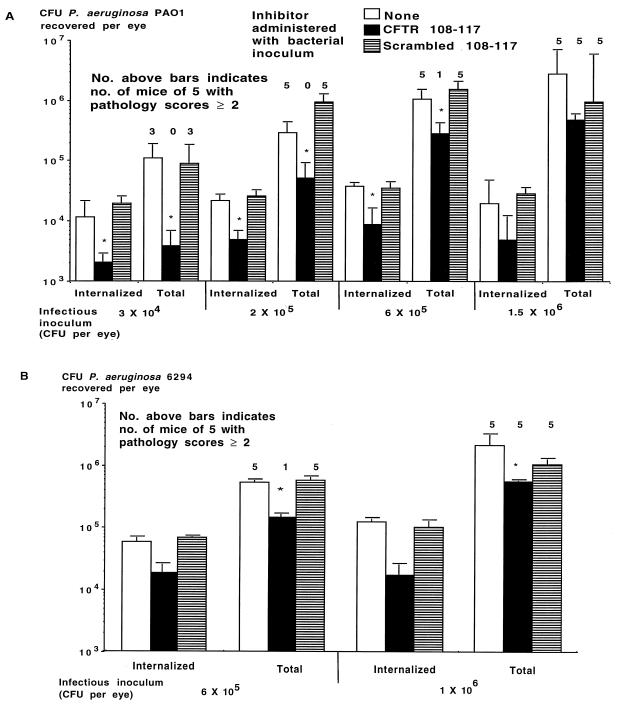
Using a previously described model of P. aeruginosa eye infection (28), we evaluated whether the addition of CFTR peptide 108-117 to the infectious inoculum could affect the course of P. aeruginosa corneal infection in vivo. When given once along with an infectious inoculum containing either of two noncytotoxic P. aeruginosa strains, PAO1 (Fig. 4A) or 6294 (Fig. 4B), the cognate CFTR peptide, but not a scrambled version of this peptide, reduced both the level of corneal cell ingestion and the total level of P. aeruginosa in the eye 24 h after infection when the challenge dose was <106 CFU per eye. Peptide treatment also reduced the consequent pathology in the eye by 48 h after infection (Fig. 4), which remained unchanged for up to 7 days after infection, when the experiment ended. When the infectious dose was ≥106 CFU of P. aeruginosa per eye, the CFTR peptide reduced both total and internalized bacterial levels in the corneal cells 24 h after infection for strain 6294 but not for strain PAO1, but at this higher challenge dose, CFTR peptide administration had no effect on eye pathology for either strain (all mice had pathology scores of ≥2). Under these conditions, a single dose of CFTR peptide 108-117 inhibited eye pathology due to a modest infectious challenge with noncytotoxic P. aeruginosa strains.
FIG. 4.
Effect of administration of a single dose of CFTR peptide along with the indicated infectious inoculum of noncytotoxic P. aeruginosa on levels of internalized and total CFU per eye and the development of eye pathology with a score of ≥2. (A) Challenge with P. aeruginosa PAO1; (B) challenge with P. aeruginosa 6294. Bars indicate means of six to nine replicates; error bars indicate the SE. Asterisks indicate P values of <0.001 determined by ANOVA and Fisher PLSD for pairwise differences.
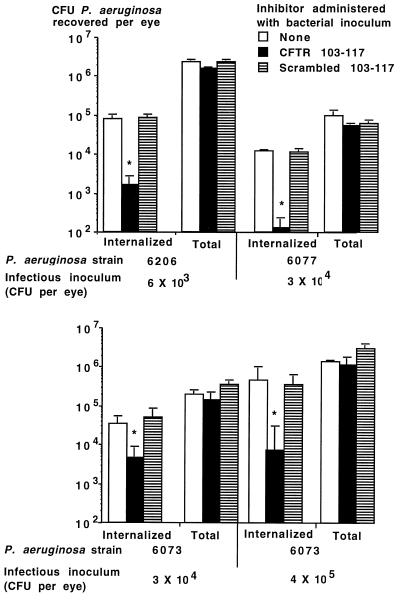
When low doses (6 × 103 to 4 × 105 per eye) of P. aeruginosa cytotoxic strains 6073, 6077, and 6206 were inoculated onto scratch-injured eyes of C57BL/6 mice along with CFTR peptide 103-117 (encompassing all 15 amino acids predicted to make up the first extracellular domain of CFTR), we achieved a significant reduction in the levels of bacteria internalized into corneal cells compared with levels achieved with either no peptide or the control, scrambled version of the peptide (Fig. 5). This result establishes that cytotoxic strains of P. aeruginosa measurably enter corneal cells via CFTR during in vivo infection. Despite this reduction in internalization levels, there was no significant effect on either total levels of P. aeruginosa in the eye or the eye pathology scores achieved with the cytotoxic P. aeruginosa strains. All mice had pathology scores of ≥2 48 h after infection.
FIG. 5.
Effect of administration of a single dose of CFTR peptide along with the indicated strain and infectious inoculum of cytotoxic P. aeruginosa on levels of internalized and total CFU per eye. Bars indicate means of six to nine replicates; error bars indicate the SE. Asterisks indicate P values of <0.001 determined by ANOVA and Fisher PLSD for pairwise differences.
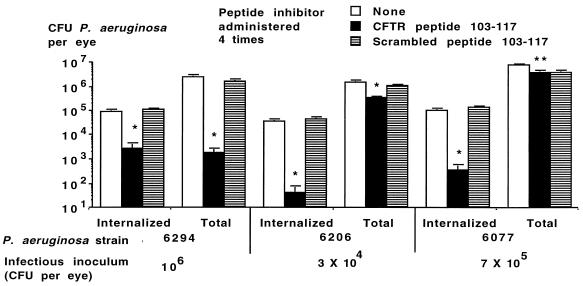
We next evaluated whether multiple doses of CFTR peptide 103-117 given at the time of infection and 1, 3, and 6 h after infection could be more effective than a single dose in modulating the course of P. aeruginosa corneal infection. When given along with a dose of 106 CFU per eye of noncytotoxic strain 6294, multiple doses of the cognate CFTR peptide significantly reduced both internalized and total levels of P. aeruginosa in the eye and prevented the development of severe eye pathology (Fig. 6 and Table 1). Similarly, four doses of CFTR peptide 103-117 provided significant reductions in total and internalized levels of P. aeruginosa in the eyes of mice challenged with highly virulent cytotoxic strains 6206 and 6077 (Fig. 6). A concomitant reduction in the overall eye pathology scores was also achieved (Table 1). These results show that when sufficient amounts of CFTR peptides are used to inhibit CFTR-mediated internalization of P. aeruginosa during corneal infection, a significant reduction in bacterial burdens and resultant pathology can be achieved.
FIG. 6.
Effect of administration of four doses of CFTR peptide along with the indicated strain and infectious inoculum of P. aeruginosa on levels of internalized and total CFU per eye. Bars indicate means of six to nine replicates; error bars indicate the SE. Single asterisks indicate P values of <0.001 determined by ANOVA and Fisher PLSD for both pairwise differences; double asterisks indicate P values of <0.001 in comparison to no inhibitor only by Fisher PLSD calculation.
TABLE 1.
Effect of four doses of CFTR peptides on pathology scores in eyes of mice infected with P. aeruginosa strains
| P. aeruginosa strain | Infecting dose (CFU/eye) | Treatment resulting in pathology scores of ≥2 (no. of mice/total)a
|
||
|---|---|---|---|---|
| No peptide | CFTR 103-117 | Scrambled peptide | ||
| 6294 | 1 × 106 | 6/6 | 0/5* | 5/5 |
| 6206 | 3 × 104 | 7/9 | 3/10† | 9/9 |
| 6077 | 7 × 105 | 5/5 | 0/5* | 5/5 |
*, P < 0.01 Fisher’s exact test; †, P < 0.05, Fisher’s exact test.
P. aeruginosa corneal infections in CF mice.
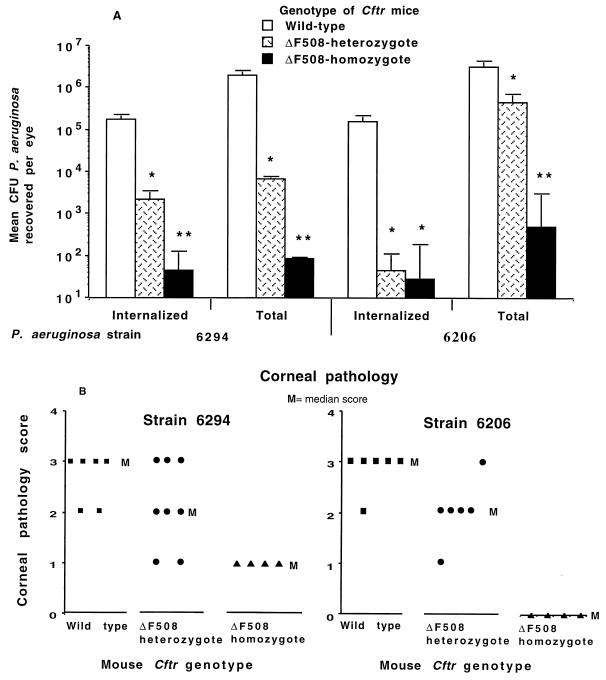
To confirm that CFTR-mediated corneal cell uptake of P. aeruginosa is critical to development of eye pathology, we infected either wild-type C57BL/6 mice or ΔF508 Cftr heterozygote or homozygote mice derived from the C57BL/6-Cftrtm1Kth transgenic line with ∼2 × 105 CFU of either P. aeruginosa 6294 (noncytotoxic) or 6206 (cytotoxic) per eye and 48 h later determined the eye pathology and the levels of total and internalized P. aeruginosa in the corneal epithelium. Compared with wild-type mice, heterozygous ΔF508 Cftr mice had an >85% decrease in the levels of total and internalized P. aeruginosa in the eye (Fig. 7A) and a significant reduction in the corneal pathology scores (Fig. 7B). Homozygous ΔF508 Cftr mice were nearly completely resistant to P. aeruginosa corneal infection, with only minimal levels of organisms in the eye and little to no significant eye pathology. We cannot readily explain the comparable levels of internalized P. aeruginosa 6206 bacteria in heterozygote and homozygote ΔF508 Cftr mice (Fig. 7A), but this result may reflect the cytotoxic character of this strain that yields a greater reduction in internalized bacteria than would be predicted solely from the proportional reduction in total bacterial levels in heterozygous mice. Nonetheless, these results confirm a critical role for CFTR-mediated uptake of P. aeruginosa in the pathogenesis of experimental murine corneal infection.
FIG. 7.
Infection of wild-type C57BL/6 mice or ΔF508 Cftr heterozygous or homozygous mice with 2 × 105 CFU per eye of either P. aeruginosa 6294 (noncytotoxic, exoU) or strain 6206 (cytotoxic, exoU+). (A) Total and internalized levels of P. aeruginosa in the corneas 48 h after infection. Bars indicate means of 4 to 12 eyes; error bars indicate the SE. Single asterisks indicate P values of ≤0.02 determined by ANOVA and Fisher PLSD for a pairwise difference from wild-type mice; double asterisks indicate P values of 0.001 by Fisher PLSD in comparison to both wild-type and ΔF508 Cftr heterozygotes. (B) Corneal pathology scores at 48 h for individual mice of the indicated Cftr genotype infected with the P. aeruginosa strain indicated at the top. P = 0.01 for strain 6294 and P = 0.003 for strain 6206 by Kruskal-Wallis nonparametric ANOVA.
Cftr mRNA expression and effect of endogenous CFTR expression on corneal infection.
To determine whether changes in the transcription of Cftr into mRNA occurred following P. aeruginosa infection, we used a semiquantitative RT-PCR method to analyze mRNA levels in corneas at various times postinfection. The expected band of 540 bp was readily detectable from mRNA isolated from corneas of wild-type C57BL6 mice but not with mRNA from corneas of Cftr knockout mice used as a control (data not shown). However, there was little to no change in Cftr mRNA levels over a 4-h infection period. Since previous results (9) indicate that P. aeruginosa enters corneal cells as early as 15 min after infection, it appeared that both apical and possibly subapical membrane stores (18, 30, 38) of CFTR are the major source of protein recruited for interaction with P. aeruginosa. Alternately, other translational or posttranslational consequences of P. aeruginosa interactions with epithelial cells could affect the rate of CFTR protein synthesis.
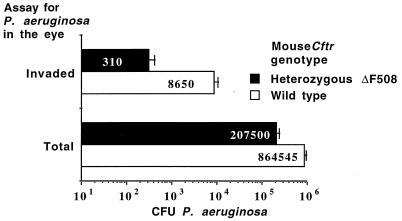
To test the idea that endogenous amounts of CFTR affected the course of P. aeruginosa survival on infected corneas, we compared the total and internalized CFU of P. aeruginosa 6294 on and in the corneas of wild-type and ΔF508 Cftr heterozygote C57BL/6 mice 15 min postinfection. A high challenge dose (108 CFU) of bacteria was used because previous work indicated that the vast majority of P. aeruginosa bacteria applied to the eye in this model (28) are gone after 15 min, and it was expected that heterozygous Cftr mice would internalize few P. aeruginosa cells. A high infectious dose was therefore needed for the analysis to have sufficient sensitivity to detect differences in ingestion and retention of bacteria on the corneal surface shortly after infection. Heterozygote ΔF508 Cftr mice had 76% fewer total (P < 0.001, t test) and 96% fewer internalized (P < 0.001, t test) CFU of P. aeruginosa in the corneal tissue 15 min postinfection compared with wild-type mice (Fig. 8). Thus, the initial level of CFTR in the cornea (Fig. 3) correlated with both internalization and retention of P. aeruginosa in this tissue (Fig. 8), which also correlated with bacterial levels and disease pathology that occurred later in the infectious process (Fig. 7).
FIG. 8.
Effect of Cftr genotype on total and internalized levels of P. aeruginosa in the murine eye 15 min after infection. Bars and white numbers indicate means of 10 to 12 eyes; error bars indicate the SE. Asterisks indicate P values of <0.001 determined by t tests.
Electron microscopic visualization of CFTR bound to P. aeruginosa in the corneas of infected mice.
To confirm that CFTR interacted directly with P. aeruginosa during corneal infection, murine eyes infected with P. aeruginosa 6294 were obtained for immunoelectron microscopic analysis of CFTR binding to microbial cells. In these specimens a CFTR-specific MAb (Fig. 9B and 9D), but not a control MAb (Fig. 9A and 9C), clearly showed CFTR attached to bacteria both inside corneal cells (Fig. 9B) and in the acellular stromal layer below the corneal epithelium (Fig. 9D). This latter result indicates that the bacteria in the stroma had previously entered epithelial cells and were then released, apparently with CFTR still bound to the bacterial surface. We were also able to visualize the bacterial LPS surrounding the invading P. aeruginosa cells by using an LPS-specific MAb (Fig. 9E) to authenticate that the observed bacteria were from the infectious inoculum and not an endogenous or contaminating organism infecting the eye after injury. Eyes from mice with ΔF508 Cftr alleles were not investigated due to the low numbers of P. aeruginosa present, a situation that we have previously shown leads to an inability to visualize the infecting bacteria by electron microscopy (9). Comparable binding of CFTR to P. aeruginosa strain PAO1 during murine eye infections was also seen by immunoelectron microscopy (data not shown).
FIG. 9.
Immunoelectron microscopy showing CFTR bound to P. aeruginosa in eyes of infected mice. The detection reagent was 20-nm gold-labeled protein A. (A) Irrelevant control MAb reacted with intracellular P. aeruginosa (none of the sections treated with the irrelevant MAb had gold beads associated with bacteria); (B) CFTR-specific MAb CF8 reacted with intracellular P. aeruginosa; (C) irrelevant control MAb reacted with intrastromal P. aeruginosa; (D) CFTR-specific MAb CF8 reacted with intrastromal P. aeruginosa; (E) LPS-specific MAb reacting with P. aeruginosa cells. Bars in all micrographs represent 200 nm.
DISCUSSION
The finding that CFTR is a cellular receptor for P. aeruginosa critical for efficient clearance of this organism from the lung (24) has indicated a potential mechanism that would account for the hypersusceptibility of CF patients to lung infection with this single bacterial species. Here we report that CFTR is expressed in the corneal epithelium and also serves as a receptor for cellular uptake of P. aeruginosa by rabbit, mouse, and human corneal epithelial cells. In an experimental murine P. aeruginosa keratitis model, inhibiting CFTR-mediated bacterial ingestion with peptides decreased the level of both total and internalized bacteria in the eye, with a consequent reduction in the severity of eye pathology due to infection. A significant reduction in infection and pathology was achieved in C57BL/6 mice with one ΔF508 Cftr allele, and almost complete resistance to infection occurred in ΔF508 Cftr homozygous CF mice, confirming a critical role of CFTR in the pathogenesis of P. aeruginosa corneal infection.
On the surface, the effects we report here for CFTR in P. aeruginosa eye infection seem to be the opposite of those that we have proposed operate in the lung (24–26). The differences in disease outcomes from the same bacterium-epithelial cell interaction are likely explained by the differences in location of CFTR-expressing epithelial cells in the two pathologic states. In the lung, P. aeruginosa interacts with CFTR-expressing epithelial cells on the surface of the airway. This interaction clearly leads to bacterial ingestion in vivo (8, 24), and we have proposed that desquamation of epithelial cells containing internalized bacteria contributes to bacterial clearance from the lung (24–26). Mulvey et al. have recently shown a comparable mechanism for clearance for uropathogenic Escherichia coli from infected mouse bladders (22). We have also hypothesized that CFTR-mediated epithelial cell ingestion of P. aeruginosa leads to a regulated inflammatory reaction that helps clear this organism from the lung, although specific data to support this idea have not yet been generated. In the eye, P. aeruginosa is internalized by epithelial cells below the tissue surface. In this situation, the cells are unable to desquamate from the eye due to their subsurface location. Instead, as previously shown (9), ingested P. aeruginosa leave endosomal vesicles in corneal cells and enter the cytoplasm. Results reported here indicate that P. aeruginosa is also released by an unknown mechanism from the cellular cytoplasm, where it can enter the acellular subepithelial stroma with CFTR still attached to the bacterial surface. Thus, the subsurface corneal cells containing ingested P. aeruginosa serve as a reservoir protecting P. aeruginosa from clearance mechanisms and potentiating their overall numbers in the eye. Increased levels of bacteria in the eye result in increased inflammation and tissue damage, events that underlie the development of significant corneal tissue pathology.
Further reconciliation of the role of CFTR-mediated P. aeruginosa ingestion in defense of the lung and susceptibility to pathologic damage in the eye stems from the plausible proposal that in the uninjured eye, CFTR-mediated P. aeruginosa ingestion by surface corneal cells protects the eye from infection. In support of this proposal is the hypersusceptibility of users of extended-wear contact lenses to P. aeruginosa corneal infection (16, 17, 27, 32). Overall, these individuals have a relative risk for microbial keratitis up to 36.8 times (95% confidence interval, 12.6 to 107.6) that of people who use daily-wear, rigid, gas-permeable contact lenses (33). Numerous factors have been proposed to account for the increased susceptibility to infection from use of extended-wear lenses, including accumulation of lens coatings in the eye, increased presence of bacteria adherent to lenses, irritation from bacteria or debris trapped beneath the lens, and lack of adequate lens care hygiene (35). Our results suggest that one potential additional mechanism of infection involves entrapment of surface corneal cells with ingested P. aeruginosa prolonging the residence time of the bacteria on the eye. This would allow P. aeruginosa to grow and damage the corneal surface, leading to frank infection and pathology. Thus, in the eye, compromise of the normal host defense of CFTR-mediated epithelial cell ingestion of P. aeruginosa may promote infection.
We documented a critical role for CFTR-mediated ingestion of P. aeruginosa in the pathogenesis of corneal infection by showing that CFTR peptides 103-117 and 108-117 inhibited bacterial ingestion, with a consequent reduction in both total bacterial counts in the eye and levels of corneal tissue damage. In addition, reduction or elimination of membrane CFTR protein in animals with one or two ΔF508 Cftr alleles led to marked diminutions in P. aeruginosa levels and corneal pathology following infection. The >50% reduction in P. aeruginosa levels in the eye in heterozygote animals with one wild-type and one ΔF508 Cftr allele indicates a greater impact of the ΔF508 allele on the overall CFTR levels than would be expected in an animal with one wild-type Cftr allele. A >50% reduction in basal CFTR levels in the corneas of heterozygote ΔF508 Cftr mice was documented both in immunofluorescence studies and by flow cytometric analysis. For another ΔF508 Cftr strain of mouse, we recently reported a potential selective advantage in resistance to typhoid fever in heterozygote ΔF508 CFTR humans, since ΔF508 Cftr heterozygote mice had an 86% reduction in translocation of S. typhi from their gastrointestinal (GI) epithelium. The 85 to 95% reduction in total levels of P. aeruginosa in the eyes of infected heterozygote CF mice used here, which were derived by Zeiher et al. by inserting a neomycin resistance gene into exon 10 of mouse ΔF508 Cftr as a selectable marker (41), is thus similar to the effect on S. typhi GI translocation in heterozygote mice derived by Colledge et al. (2), who inserted a hypoxanthine phosphoribosyltransferase minigene into exon 10 of mouse ΔF508 Cftr as a selectable marker. Finally, the effect of the insertion of the neomycin resistance cassette into exon 10 of mouse Cftr was reported not to have an effect on mRNA transcription or mouse phenotype (41), which indicates that in heterozygote ΔF508 Cftr mice it is likely the mutant allele, and not the selectable marker, that reduces by >50% the amount of wild-type CFTR present in GI and corneal tissues. Whether there is a comparable effect of the ΔF508 CFTR allele in heterozygote humans, particularly in response to bacterial pathogens, is not known at this time.
Before the findings in this report, it was not clear whether corneal epithelial uptake of P. aeruginosa was a critical factor for inducing keratitis or merely an associated phenomenon. Although a role for CFTR-mediated P. aeruginosa ingestion in human infection must be speculatively extrapolated from the results obtained here for mice, we were able to show that human corneal cells in culture ingested P. aeruginosa via CFTR. Overall our findings further document the importance of CFTR-P. aeruginosa interactions in the pathogenesis of infection, establish that in vitro cytotoxicity of P. aeruginosa for cultured corneal and other epithelial cells does not reflect the in vivo cellular ingestion of cytotoxic P. aeruginosa strains during corneal infection, and extend our understanding of the role of CFTR as a receptor for P. aeruginosa to another important clinical situation.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
This work was supported by NIH grants AI 22535, HL 58398, and EY 06805.
We thank Ken Kenyon, Massachusetts Eye and Ear Clinic, Boston, for provision of human corneal tissue for culture.
REFERENCES
- 1.Clarke L L, Gawenis L R, Franklin C L, Harline M C. Increased survival of CFTR knockout mice with an oral osmotic laxative. Lab Anim Sci. 1996;46:612–618. [PubMed] [Google Scholar]
- 2.Colledge W H, Abella B S, Southern K W, Ratcliff R, Jiang C W, Cheng S H, Macvinish L J, Anderson J R, Cuthbert A W, Evans M J. Generation and characterization of a ΔF508 cystic fibrosis mouse model. Nat Genet. 1995;10:445–452. doi: 10.1038/ng0895-445. [DOI] [PubMed] [Google Scholar]
- 3.Coster D J, Badenoch P R. Host, microbial, and pharmacological factors affecting the outcome of suppurative keratitis. Br J Ophthalmol. 1987;71:96–101. doi: 10.1136/bjo.71.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denning G M, Anderson M P, Amara J F, Marshall J, Smith A E, Welsh M J. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- 5.Denning G M, Ostedgaard L S, Cheng S H, Smith A E, Welsh M J. Localization of cystic fibrosis transmembrane conductance regulator in chloride secretory epithelia. J Clin Investig. 1992;89:339–349. doi: 10.1172/JCI115582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engel L S, Hobden J A, Moreau J M, Callegan M C, Hill J M, O’Callaghan R J. Pseudomonas deficient in protease IV has significantly reduced corneal virulence. Investig Ophthalmol Vis Sci. 1997;38:1535–1542. [PubMed] [Google Scholar]
- 7.Finck-Barbancon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish J P, Fleiszig S M, Wu C, Mende-Mueller L, Frank D W. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 8.Fleiszig S M J, Wiener-Kronish J P, Miyazaki H, Vallas V, Mostov K E, Kanada D, Sawa T, Yen T S B, Frank D W. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleiszig S M J, Zaidi T S, Fletcher E L, Preston M J, Pier G B. Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect Immun. 1994;62:3485–3493. doi: 10.1128/iai.62.8.3485-3493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleiszig S M J, Zaidi T S, Pier G B. Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect Immun. 1995;63:4072–4077. doi: 10.1128/iai.63.10.4072-4077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleiszig S M J, Zaidi T S, Preston M J, Grout M, Evans D J, Pier G B. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect Immun. 1996;64:2288–2294. doi: 10.1128/iai.64.6.2288-2294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S K, Berk R S, Masinick S, Hazlett L D. Pili and lipopolysaccharide of Pseudomonas aeruginosa bind to the glycolipid asialo GM1. Infect Immun. 1994;62:4572–4579. doi: 10.1128/iai.62.10.4572-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hecker K H, Roux K H. High and low annealing temperatures increase both specificity and yield in touchdown and stepdown PCR. BioTechniques. 1996;20:478–485. doi: 10.2144/19962003478. [DOI] [PubMed] [Google Scholar]
- 14.Jilling T, Kirk K L. The biogenesis, traffic, and function of the cystic fibrosis transmembrane conductance regulator. Int Rev Cytol. 1997;172:193–241. doi: 10.1016/s0074-7696(08)62361-x. [DOI] [PubMed] [Google Scholar]
- 15.Kernacki K A, Fridman R, Hazlett L D, Lande M A, Berk R S. In vivo characterization of host and bacterial protease expression during Pseudomonas aeruginosa corneal infections in naive and immunized mice. Curr Eye Res. 1997;16:289–297. doi: 10.1076/ceyr.16.4.289.10686. [DOI] [PubMed] [Google Scholar]
- 16.Liesegang T J. Contact lens-related microbial keratitis. Part I: epidemiology. Cornea. 1997;16:125–131. [PubMed] [Google Scholar]
- 17.Liesegang T J. Contact lens-related microbial keratitis. Part II: pathophysiology. Cornea. 1997;16:265–273. [PubMed] [Google Scholar]
- 18.Lukacs G L, Chang X B, Kartner N, Rotstein O D, Riordan J R, Grinstein S. The cystic fibrosis transmembrane regulator is present and functional in endosomes. Role as a determinant of endosomal pH. J Biol Chem. 1992;267:14568–14572. [PubMed] [Google Scholar]
- 19.Mohamed A, Ferguson D, Seibert F S, Cai H M, Kartner N, Grinstein S, Riordan J R, Lukacs G L. Functional expression and apical localization of the cystic fibrosis transmembrane conductance regulator in MDCK I cells. Biochem J. 1997;322:259–265. doi: 10.1042/bj3220259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moss R B, Bocian R C, Hsu Y P, Dong Y J, Kemna M, Wei T, Gardner P. Reduced IL-10 secretion by CD4+ T lymphocytes expressing mutant cystic fibrosis transmembrane conductance regulator (CFTR) Clin Exp Immunol. 1996;106:374–388. doi: 10.1046/j.1365-2249.1996.d01-826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulberg A E, Weyler R T, Altschuler S M, Hyde T M. Cystic fibrosis transmembrane conductance regulator expression in human hypothalamus. Neuroreport. 1998;9:141–144. doi: 10.1097/00001756-199801050-00028. [DOI] [PubMed] [Google Scholar]
- 22.Mulvey M A, Lopez-Boado Y S, Wilson C L, Roth R, Parks W C, Heuser J, Hultgren S J. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 23.Pier G B, Grout M, Zaidi T, Meluleni G, Mueschenborn S S, Banting G, Ratcliff R, Evans M J, Colledge W H. Salmonella typhi uses CFTR to enter intestinal epithelial cells. Nature. 1998;392:79–82. doi: 10.1038/30006. [DOI] [PubMed] [Google Scholar]
- 24.Pier G B, Grout M, Zaidi T S. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci USA. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pier G B, Grout M, Zaidi T S, Goldberg J B. How mutant CFTR may contribute to Pseudomonas aeruginosa infection in cystic fibrosis. Am J Respir Crit Care Med. 1996;154:S175–S182. doi: 10.1164/ajrccm/154.4_Pt_2.S175. [DOI] [PubMed] [Google Scholar]
- 26.Pier G B, Grout M, Zaidi T S, Olsen J C, Johnson L G, Yankaskas J R, Goldberg J B. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poggio E C, Glynn R J, Schein O D, Seddon J M, Shannon M J, Scardino V A, Kenyon K R. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N Engl J Med. 1989;321:779–783. doi: 10.1056/NEJM198909213211202. [DOI] [PubMed] [Google Scholar]
- 28.Preston M J, Fleiszig S M J, Zaidi T S, Goldberg J B, Shortridge V D, Vasil M L, Pier G B. Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect Immun. 1995;63:3497–3501. doi: 10.1128/iai.63.9.3497-3501.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Preston M J, Seed P C, Toder D S, Iglewski B H, Ohman D E, Gustin J K, Goldberg J B, Pier G B. Contribution of proteases and LasR to the virulence of Pseudomonas aeruginosa during corneal infections. Infect Immun. 1997;65:3086–3090. doi: 10.1128/iai.65.8.3086-3090.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prince L S, Workman R B, Jr, Marchase R B. Rapid endocytosis of the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci USA. 1994;91:5192–5196. doi: 10.1073/pnas.91.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosner B. Fundamentals of biostatistics. Boston, Mass: Duxbury Press; 1990. pp. 474–525. [Google Scholar]
- 32.Schein O D, Glynn R J, Poggio E C, Seddon J M, Kenyon K R. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. A case-control study. N Engl J Med. 1989;321:773–778. doi: 10.1056/NEJM198909213211201. [DOI] [PubMed] [Google Scholar]
- 33.Stapleton F, Dart J K, Minassian D. Risk factors with contact lens related suppurative keratitis. CLAO J. 1993;19:204–210. [PubMed] [Google Scholar]
- 34.Stapleton F, Dart J K, Seal D V, Matheson M. Epidemiology of Pseudomonas aeruginosa keratitis in contact lens wearers. Epidemiol Infect. 1995;114:395–402. doi: 10.1017/s0950268800052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stern G A. Pseudomonas keratitis and contact lens wear: the lens/eye is at fault. Cornea. 1990;9:S36–S38. doi: 10.1097/00003226-199010001-00015. [DOI] [PubMed] [Google Scholar]
- 36.Twining S S, Kirschner S E, Mahnke L A, Frank D W. Effect of Pseudomonas aeruginosa elastase, alkaline protease, and exotoxin A on corneal proteinases and proteins. Investig Ophthalmol Vis Sci. 1993;34:2699–2712. [PubMed] [Google Scholar]
- 37.Walker J, Watson J, Holmes C, Edelman A, Banting G. Production and characterisation of monoclonal and polyclonal antibodies to different regions of the cystic fibrosis transmembrane conductance regulator (CFTR): detection of immunologically related proteins. J Cell Sci. 1995;108:2433–2444. doi: 10.1242/jcs.108.6.2433. [DOI] [PubMed] [Google Scholar]
- 38.Webster P, Vanacore L, Nairn A C, Marino C R. Subcellular localization of CFTR to endosomes in a ductal epithelium. Am J Physiol. 1994;267:C340–C348. doi: 10.1152/ajpcell.1994.267.2.C340. [DOI] [PubMed] [Google Scholar]
- 39.Zaidi T S, Fleiszig S M J, Preston M J, Goldberg J B, Pier G B. Lipopolysaccharide outer core is a ligand for corneal cell binding and ingestion of Pseudomonas aeruginosa. Investig Ophthalmol Vis Sci. 1996;37:976–986. [PubMed] [Google Scholar]
- 40.Zaidi T S, Preston M J, Pier G B. Inhibition of bacterial adherence to host tissue does not markedly affect disease in the murine model of Pseudomonas aeruginosa corneal infection. Infect Immun. 1997;65:1370–1376. doi: 10.1128/iai.65.4.1370-1376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeiher B G, Eichwald E, Zabner J, Smith J J, Puga A P, Mccray P B, Capecchi M R, Welsh M J, Thomas K R. A mouse model for the ΔF508 allele of cystic fibrosis. J Clin Investig. 1995;96:2051–2064. doi: 10.1172/JCI118253. [DOI] [PMC free article] [PubMed] [Google Scholar]