Abstract
Background
Sepsis still remains a major challenge in intensive care medicine with unacceptably high mortality among patients with septic shock. Due to current limitations of human CD19+CD24hiCD38hi Breg cells (Bregs) studies among sepsis, here, we tried to evaluate Bregs in severity and prognostic value in patients with sepsis.
Methods
Peripheral blood from 58 patients with sepsis and 22 healthy controls was analyzed using flow cytometry to evaluate the frequency and number of Bregs. All cases were divided into non-survived or survived group after 28 days followed up. Spearman's correlation analysis was performed on Bregs frequency and clinical indices. The area under the curve was acquired using the receiver operating characteristic analysis to assess the sensitivity and specificity of Bregs for outcome of sepsis. Survival curve analysis and binary logistic regression were applied to estimate the value of Bregs in prognosis among cases with sepsis.
Results
Sepsis patients had decreased proportions and number of Bregs. Sepsis patients with low frequency of Bregs were associated with an increased risk of septic shock. Bregs frequency is inversely associated with lactate, SOFA, and APACHE II and positively correlated with Tregs frequency. Low levels of Bregs closely correlated with septic outcomes. Numbers of Bregs were prediction factors for poor prognosis.
Conclusions
Frequency and number of Bregs decreased, and Bregs deficiency revealed poor prognosis in patients with sepsis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12865-022-00528-x.
Keywords: Sepsis, Flow cytometry, B-lymphocytes, Interleukin-10, Regulatory B cells, Prognosis
Introduction
Sepsis is defined clinically as the presence of acute syndrome of infection with the development of new organ dysfunction, an increase of 2 points in the Sequential (Sepsis Related) Organ Failure Assessment (SOFA) [1]. Sepsis remains one of the major challenges in intensive care medicine, its incidence increasing continuously over the past decades [2, 3]. Despite massive efforts in sepsis research, in-hospital mortality (20.3%) is broadly more common among patients with hospital-onset sepsis than those (18.3%) with community-onset sepsis [4, 5], mortality in patients with septic shock still remains unacceptably high [3]. Therefore, emphasis has been focused on identifying patients who are at a higher risk of developing septic shock, early treatment, and early prognosis assessment. Research in sepsis has shown that the pathology of sepsis results not only from the presence of an infection, but also from the dysregulated host response to an infection [6]. Recent accumulating evidence depicts that sepsis is characterized by an initial overwhelming production of proinflammatory cytokines followed by immunosuppression, with increase of the regulatory T cells (Tregs) population [7–9]. Interleukin-10–producing B cells are a newly described subset of B cells with regulatory function. Mizoguchi and collaborators identified regulatory B cells (Bregs) as an IL-10–producing B-cell subpopulation and introduced the term “regulatory B cells [10].” In humans, Blair and coworkers described Bregs as CD19+CD24hiCD38hi, a phenotype that generally defines human transitional B cells [11, 12]. Since these seminal observations, a considerable body of evidence of CD19+CD24hiCD38hi has been shown to prevent tissue injury, autoimmunity, chronic graft-versus-host disease (cGVHD), infection, and cancer [13–17]. So far, Bregs in sepsis, however, remain incompletely understood.
An increased frequency of circulatory Tregs in patients diagnosed with septic shock is significantly associated with immunosuppression, meanwhile percentage of Tregs increased as early as 3 days after shock, but the absolute number still was lower than in healthy donors [18]. Recent study supports Bregs, like Tregs, capable of negatively modulating T-cell–dependent autoimmune responses in an Ag-specific manner [19–21]. However, the function and clinical relevance of Bregs during sepsis remains unclear and little is presently known about the survival of patients following diagnosis. The prime aim of this exploratory study was to investigate Bregs in septic shock development and prognosis using a whole blood staining approach by flow cytometry. Our study demonstrated that sepsis patients with high frequency of Bregs had a lower risk of septic shock development. Our results showed that low levels of Bregs were associated with an increased risk of non-survival in patients with sepsis. This association might be due to decreased IL-10-producing B cells, and elevated IL-10-producing T cells. Findings indicated that Bregs might be applied to identify sepsis patients who are at risk of septic shock development and evaluate outcome during sepsis state.
Materials and methods
Subjects
Sepsis inclusion criteria were based on the Surviving Sepsis Campaign definition 3.0 [1]. Overall, 58 septic patients, diagnosed with sepsis (n = 26) and septic shock (n = 32) at intensive care unit (ICU) of Shanghai East Hospital between May 2016 and May 2018 were enrolled into study (Fig. 1). Healthy controls inclusion criteria were as follows: Matched age to the sepsis group of patients; good health; no history of chronic or metabolic diseases (e.g., hypertension, diabetes, coronary heart disease, hepatitis, and hyperthyroidism); and not using antibiotics within 3 days before enrollment. Healthy control group exclusion criteria were the same as those for sepsis.
Fig. 1.
Flow chart for sepsis patients
The study was approved by the Institutional Ethics Authorities (No. 2015-028, Tongji University, China). All enrolled patients and healthy controls signed informed consent forms.
Eight mL of peripheral venous blood samples collected from septic patients at day 1 after admission and from healthy controls were subjected to lymphocyte separation within 3 h and flow cytometry analysis. The following general clinical data were collected and recorded at day 1: demographic characteristics (age and sex), main diagnosis, vasopressor use, ICU days, mechanical ventilation, infection site, and pathogenic bacterial agent [based on qualitative culture or metagenomic next generation sequencing (mNGS)] (Table 1). The white blood cell (WBC), creatinine, bilirubin, C-reactive protein (CRP), procalcitonin (PCT), blood lactate (Lac), Acute Physiology and Chronic Health Evaluation II (APACHE II) score, and Sequential Organ Failure Assessment (SOFA) score of patients on the day of admission were recorded (Additional file 1: Table S1). Twenty eight-day follow-up was complete. All patients with sepsis were divided into survivors (Alive) and non-survivors (Dead).
Table 1.
Clinical data in elderly patients with sepsis and healthy donors
| Healthy | Sepsis | t/F | p | |
|---|---|---|---|---|
| n | 22 | 58 | ||
| Age (year) | 75.2 ± 7.0 | 78.6 ± 8.1 | 1.832 | 0.0707 |
| Male gender, n (%) | 10 (45.5) | 38 (65.5) | 0.167 | 0.1281 |
| Septic shock, n (%) | 26 (44.8%) | |||
| Mechanical ventilation, n (%) | 6 (10.3%) | |||
| Infection site, n(%) | Lung, 38 (65.5) | |||
| Biliary tract 9 (15.5) | ||||
| Digestive tract 5 (8.6) | ||||
| Urinary tract 3 (5.2) | ||||
| Skin/ soft tissue 3 (5.2) | ||||
| Etiology, n (%) | Bacterial, 38 (65.5) | |||
| Fungal, 9 (15.5) | ||||
| Viral, 11 (19.0) |
CD19+CD24hiCD38hi Bregs analysis by flow cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated from blood samples using standard Ficoll gradient centrifugation. The isolated PBMCs were stained at room temperature for 15 min with antibodies against CD45 (OKT3), CD3 (OKT3), CD19 (SJ25C1), CD20 (2H7), CD24 (ML5) and CD38 (HIT2). Cells were gated on CD19+ B cells and CD19− fraction after gating out CD3+ T cells. B cells were selected on a biparametric CD3−CD19+ dot-plot and Bregs were selected on CD24hi CD38hi circled from the CD19+ gate.
300 μL fresh whole blood was stained with antibodies against CD45 (OKT3) and CD33 (WM53) after lysing red blood cells. Then we selected WBC as CD45+ cells based on a biparametric CD45/SSC dot plot. By gating out CD33loSSChi polymorphonuclear (PMN), PBMCs were selected and the percentage of PBMCs in CD45+ cells was calculated. Absolute numbers of CD45+ lymphocytes per microliter were then calculated. Next, B cells were selected based on a biparametric CD19/SSC dot plot and the percentage of CD19+ B cells in PBMCs was calculated. Then, Bregs were gated based on CD3−CD19+CD24hiCD38hi and the percentage of Bregs in CD19+ cells was calculated. Meanwhile, the appropriate irrelevant anti-mouse isotype controls IgG1-FITC, APC and PE were used.
The absolute numbers of Bregs were calculated according to standard flow cytometry criteria for lymphocyte subset identification. The absolute number of CD19+ B lymphocytes was calculated by determining the percentage of CD19+ B cells in peripheral blood lymphocytes multiplied by the total number of lymphocytes per microliter measured using URIT-2900. The absolute number of CD19+CD24hiCD38hi was calculated by multiplying the total number of B lymphocytes calculated by the percentage of positive cells in CD19+ B cells. Absolute numbers were expressed as cells per milliliter.
Type 1 T helper (Th1) and Tregs analysis by flow cytometry
PBMCs were obtained from blood sample cells at day 1 after patients admission. The isolated PBMCs were stained for 15 min at room temperature with anti-CD3-BUV395, CD4-BV510, CD25-PE-Dazzle594, CD127-BV650, CXCR3-PECy7 and CCR6-BV785. PBMCs were suspended in staining buffer and detected by FACS Fortessa flow cytometry. T cells were selected on a biparametric CD3/SSC dot plot. Tregs were selected on CD4+CD25+CD127+ and Th1 were selected on CD4+CXCR3+CCR6−.
IL-10+ Breg cells analysis by flow cytometry
PBMCs were first stained with combinations of cell surface markers, including anti-human CD3, CD19, CD20 monoclonal antibody (BD) for 30 min at 4 ˚C, and then stimulated with 50 ng/mL phorbol 12-myristate 13-acetate (PMA) (Sigma), 1 μg/mL ionomycin (Sigma), and 5 μg/mL brefeldin A (Biolegend) for 5 h at 37˚C. Finally, cells were washed twice and stained with zombie yellow™ dye (Biolegend) to eliminate dead cells. Unstimulated cells were used as controls for the following analyses. Intracellular cytokine staining was then carried out using anti-human interleukin-10 (IL-10) monoclonal antibodies (BD) after permeabilization and fixation. Labeled cells were acquired on LSRFortessa flow cytometer (BD Biosciences), and data were processed using Flowjo software (Tree Star).
ELISA for plasma cytokine
A total of 150 μL plasma was collected after centrifugation on day 1 after diagnosis and then stored at −80 ℃ for further detection. Human plasma IL-10 was detected by ELISA kit (R&D Systems) per manufacturers instructions.
Statistical analysis
The clinical data were first tested for normality and homogeneity of variance. Data conforming to a normal distribution and homogeneity of variance were expressed as the mean ± SD, and the t-test or analysis of variance was used for statistical analysis. Data were shown as the mean ± SD. The χ2 test was used to compare the enumeration data. Mann–Whitney U-test was used for the comparison of the two groups. One-way analysis of variance (ANOVA) was for comparison among multiple groups, followed by Kruskal–Wallis test with Dunns multiple comparison test when the values were not normally distributed. Correlation analysis was analyzed using Pearson’s chi-square test. Statistical tests were performed using SPSS 20.0 (SPSS, Chicago, IL, USA) and GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA). p < 0.05 was considered statistically significant.
Results
Frequency of Bregs decreased in patients with sepsis
Blood samples were collected at day 1 after patients were admitted in ICU and prepared within 3 h for flow cytometry after collection. The frequency of classical human Bregs was distinguished by CD3−CD19+CD24hiCD38hi (Fig. 2A). 58 septic patients (38 males, 20 females) and 22 healthy controls were recruited for the study (Table 1, Additional file 1: Table S1). There was no significant age or sex differences between healthy controls and septic ones (Table 1). Infection sites were defined as lung, biliary tract, urinary tract, digestive tract, and skin/soft tissue. Among the patients with sepsis, pneumonia represented the initiation of infection (38/58); bacterial infection was the main cause of sepsis (38/58) (Table 1). We found that the frequency and absolute number of Bregs were both significantly decreased in septic patients compared with healthy controls (Fig. 2B–C). Frequency and absolute number of Bregs did not differ significantly among infection sites (Fig. 2D–E). No significant differences were observed for the etiology of sepsis (Fig. 2F–G).
Fig. 2.
Bregs were decreased in sepsis patients on day 1. A Gating strategy of Bregs in representative healthy and sepsis patients. > 1 million events in the lymphocyte gate were collected. B Bregs requency in total B cells and C absolute number per microlitre of blood of Bregs in all healthy and sepsis subjects. Absolute number of Bregs per microlitre of blood was calculated by multiplying the total number of B lymphocytes calculated by the percentage of positive cells in Bregs in lymphocytes. D Frequency and E absolute number of Bregs in sepsis patients caused by digestive tract, lung, biliary tract, urinary tract, and skin/soft tissue infection. F Frequency and G absolute number of Bregs in sepsis patients with bacterial, viral, and fungal causes. Two-tailed t-test with unequal variance or Kruskal–Wallis test. ****p < 0.0001
Low frequencies of Bregs were associated with development of septic shock in patients with sepsis
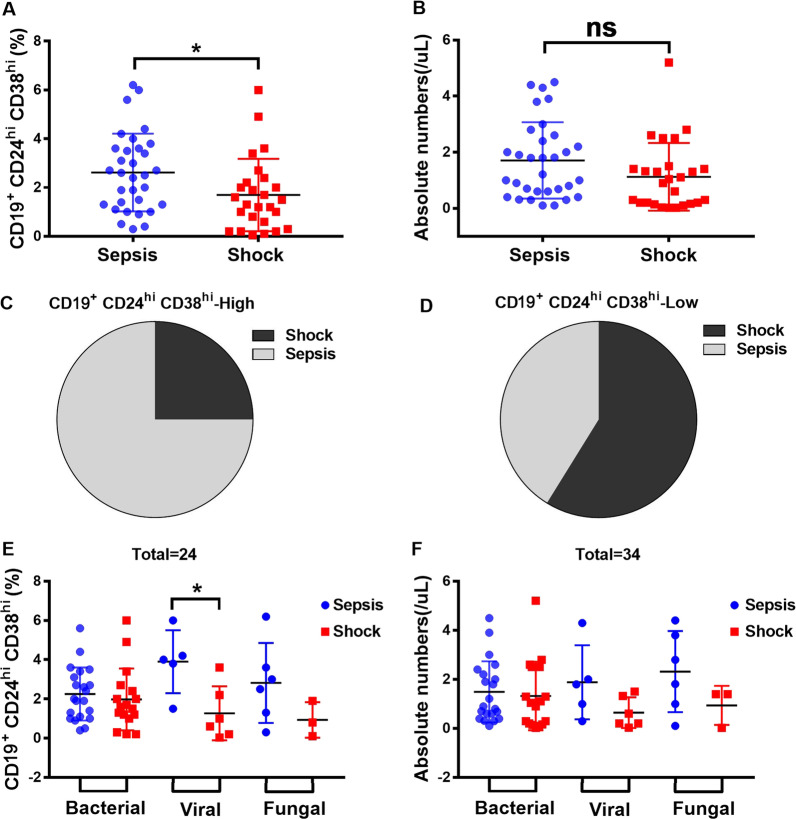
To investigate the potential role of Bregs in septic shock development in patients with sepsis, we assessed our study cohort for septic shock development (Additional file 2: Table S2). We found that the frequency of Bregs was markedly lower in septic shock group than that in sepsis group, but there was no difference in absolute number of Bregs (Fig. 3A–B). Sepsis patients with high Bregs had reduced risk of developing septic shock. Our study revealed that there was a significantly lower frequency of Bregs in patients who later developed septic shock compared to patients who did not (Fig. 3C, D). When sepsis-related shock was stratified by etiology type, our study revealed that viral sepsis patients who later developed shock had significantly less Bregs frequency, while Bregs counts were not different (Fig. 3E, F).
Fig. 3.
Low frequencies of Bregs were associated with an increased risk of septic shock development in patients with sepsis. A The Bregs in sepsis patients that did not develop septic shock versus those that developed septic shock. B The absolute count of Bregs in sepsis patients that did not develop septic shock versus those that developed septic shock. C Fraction of septic shock patients in Bregs high (%Bregs > 2.204%) patients versus that in Bregs low (%Bregs < 2.204%) patients. D Frequency and F absolute number of Bregs in sepsis patients versus septic shock patients, subdivided into bacterial, viral and fungal types. Two-tailed t test with unequal variance or Kruskal–Wallis test. *p < 0.05
IL-10+B cells did not match plasma IL-10 in septic patients
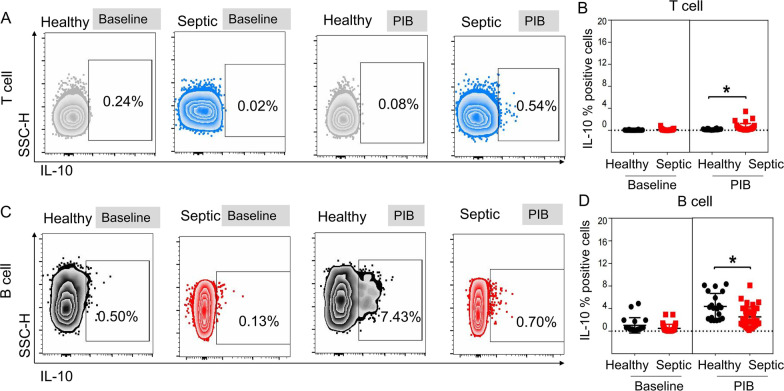
To identify circulating lymphocyte subsets producing IL-10, we detected IL-10 expressed on CD3 and CD19 between septic group and healthy one. Accordingly, we found the proportion of CD4+ T cells expressing IL-10 was significantly higher in septic patients than that in healthy group (Fig. 4A, B), while the CD19+ B cells expressing IL-10 were lower (Fig. 4C, D). Concentration of plasma IL-10 was significantly higher among septic group when compared to healthy groups (median [IQR] 7.2 [3.4–17.7] vs 1.2 [0.7–2.4], p = 0.0211) (Additional file 1: Table S1).
Fig. 4.
Analysis of IL-10 producing CD4+ T cells and CD19+ B cells. A, B Comparison of IL-10+ %CD4+ T cells between healthy group and sepsis group. C, D Comparison of IL-10+ %CD19+ B cells between healthy group and sepsis group. Results are presented as individual values in healthy donors (n = 12; black circles) and septic patients (n = 23; red squares) and medians ± IQR. Two-tailed t-test with unequal variance or Mann–Whitney U test. *p < 0.05
Bregs frequency was inversely associated with lactate, SOFA and APACHE II and positively correlated with Tregs
Clinical severity scores such as SOFA, APACHE II and available assays such as blood lactate reflect the severity of septic patients. Here, we found that there were no correlation between Bregs frequency at day 1 and PCT, CRP and plasma IL-10 (Fig. 5A, B, D). Bregs frequency at day 1 was inversely associated with lactate, SOFA, and APACHE II (Fig. 5C, E, F). We examined the correlation between Bregs and T cell subsets. There was no correlation between the frequency of Bregs and Th1 (Fig. 5G), while Bregs were positively correlated with Tregs (Fig. 5H) (R2 = 0.0897, p = 0.0224).
Fig. 5.
The frequency of Bregs was inversely associated with lactate, SOFA, and APACHE II and positively correlated with Tregs frequency. A–H The correlations between the frequencies of Bregs and PCT, CRP, lactate, plasma IL-10, SOFA, APACHE II, CD4+CXCR3+CCR6− T cells, and CD4+CD25+CD127+ T cells in all sepsis patients at day 1 were shown. p values represented Pearson’s correlation coefficient
Low levels of Bregs were associated with an increased risk of non-survival in septic patients
Of the 35 patients, who died during hospitalization, 20 (57.1%) patients had septic shock during their course, meanwhile, 6 out of 23 patients who survived had septic shock (Additional file 3: Table S3, p = 0.036). We observed that patients non-survivor had decreased frequency and numbers of Bregs than survivor patients (Fig. 6A–B). Furthermore, lactate, SOFA, and APACHE II, established sepsis severity markers, were elevated in non-survivor group (Fig. 6E, H, I), consistent with a causal association of Bregs changes with non-survivor in septic patients. There were no significant differences in PCT, CRP, creatinine, and bilirubin between non-survival group and survival group (Fig. 6 C, D, F, G).
Fig. 6.
Low levels of Bregs were associated with an increased risk of death in patients with sepsis. A Frequency and B absolute number of Bregs in sepsis patients were decreased in dead patients. C PCT, D CRP, E lactate, F creatinine, G bilirubin, H APACHE II, and I SOFA were compared between alive and dead groups. Two-tailed t-test with unequal variance or Kruskal–Wallis test. *p < 0.05; **p < 0.01; ***p < 0.001
Bregs exerted a good predictive value for 28-day mortality
Mortality predictions were explored for septic patients who died within 28 days. We used the factors mentioned above (Fig. 6) to develop a ROC (receiver operator characteristic curve) for predictive assessment for mortality in septic patients. The area under the ROC curve of PCT, CRP, and lactate were 0.53 (95% CI 0.37–0.69), 0.51 (95% CI 0.36–0.67), 0.67 (95% CI 0.52–0.81) (Fig. 7A–C). The area under the ROC curve of Bregs frequency, SOFA and APACHE II were 0.78 (95% CI 0.66–0.90), 0.67 (95% CI 0.53–0.81), 0.80 (95% CI 0.69–0.92) (Fig. 7D). Moreover, patients with higher frequency and number of Bregs levels (> 2.204%, > 1.444 /µL, respectively) had a significantly better outcome than those with lower levels (< 2.204%, < 1.444 /µL, respectively) (p = 0.0293, p = 0.0052, respectively) especially in 28-day mortality (Fig. 7E, F).
Fig. 7.
Bregs exerted a good predictive value for 28-day mortality. A Survival ROC curve of PCT; B Survival ROC curve of CRP; C Survival ROC curve of lactate; D Survival ROC curve of frequency of Bregs, SOFA score, and APACHE II score; E Kaplan–Meier survival curves of frequency of Bregs in Kaplan–Meier plotter cohort; F Kaplan–Meier survival curves of absolute number of Bregs in Kaplan–Meier plotter cohort
Frequency and numbers of Bregs were prediction factors for poor prognosis.
Binary logistic regression analysis (entry method) was performed on the frequency and numbers of Bregs, APACHE II score, and SOFA score, which had statistically significant differences between the survival and the non-survival group, to determine the independent outcome risk factors. The method of screening variables is forward: LR method and the regression coefficient of absolute Bregs value is −0.642, showing a significance level of 0.023 (Table 2, p < 0.05), which means that the absolute Bregs number has a significant negative impact on the mortality in septic patients. And the odds ratio (OR value) was 0.526, meaning that when the absolute number of Bregs increased by one unit, the mortality of septic patients decreased by 0.526 times. In a word, the results showed that the numbers of Bregs were independent risk factors affecting the prognosis of elderly septic patients.
Table 2.
The influence of independent variables on prognosis of patients with sepsis at 28 days
| β | S.E. | Wals | p | OR | OR 95% CI | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| SOFA score | 0.080 | 0.103 | 0.608 | 0.436 | 1.083 | 0.886 | 1.325 |
| Bregs frequency | −0.261 | 0.251 | 1.082 | 0.298 | 0.770 | 0.471 | 1.260 |
| Bregs count | −0.642 | 0.282 | 5.169 | 0.023 | 0.526 | 0.303 | 0.915 |
| APACHE II score | 0.118 | 0.094 | 1.561 | 0.211 | 1.125 | 0.935 | 1.354 |
| Stable | −0.553 | 1.664 | 0.110 | 0.740 | 0.575 | ||
Discussion
Sepsis is defined as a life-threatening organ dysfunction due to a dysregulated host response following an infection [1]. Sepsis is treatable, and timely implementation of targeted interventions can improve prognosis [22–24]. Because the mechanism of sepsis pathogenesis remains unclear, accurately identifying sepsis patients who are at higher risk of septic shock, while important for public health leaders, healthcare providers, medical personnel, and researchers, remains a great challenge. Recent studies suggest that Bregs have had an essential role in regulating inflammation and autoimmune diseases, very little is known about their involvement in sepsis. Here, we examined the frequency and absolute number of the Bregs population in septic patients at day 1 after admission. We found that septic patients had decreased Bregs frequency and absolute number compared to healthy controls. Moreover, we found a reduced levels of Bregs in sepsis patients who developed septic shock and in non-survival group compared to the survival one. Binary logistic regression analysis identified that Bregs absolute number represented an independent prognostic factor for poor outcome in septic patients.
Bregs can impair immune responses and be linked to increased disease severity during infections. Data on CD19+CD24hiCD38hi regulatory B cells are conflicting in cohort studies of patients with sepsis. Contrary to our results, Pan X et al. showed that the percentage of CD19+CD24hiCD38hi regulatory B cells was higher in neonatal patients with sepsis than healthy controls by using flow cytometry [25]. Whereas, the fraction of CD19+CD24hiCD38hi regulatory B cells on adult patients with sepsis was not altered [26]. Such differences may be attributed to the complexity of sepsis and physiological environments within individuals. In addition to the role in sepsis, a recent study showed Bregs revealed a deficiency proportion of IgM memory and transitional subsets in chronic GVHD [27]. Two other studies confirmed the protective effect of Bregs on mouse cGVHD models [14, 28]. Meanwhile, CD19+CD24hiCD38hi regulatory B cells are also known to play an important role in the pathogenesis of systemic lupus erythematosus (SLE), because the percentage of CD19+CD24hiCD38hi regulatory B cells was significantly increased in peripheral blood from patients with SLE [29]. Cui et al. [30] have shown that Bregs significantly decreased in patients with RA.
Moreover, we found a reduced level of Bregs in sepsis patients who developed septic shock. Interestingly, when septic patients were divided into two layers, sepsis and septic shock patients, percent of Bregs was lower in viral septic shock compared to septic patients, indicating the lower the Bregs, the more likely they are to predict viral septic. Peripheral blood CD19+CD24hiCD38hi regulatory B cells were significantly reduced in patients with severe COVID-19 compared with those with mild disease, which corresponds to the expansion of extrafollicular B cells [31]. We also found Bregs were closely correlated with the progression of sepsis. There was a close correlation between Bregs and progression of sepsis. We observed that there was a positive correlation between the frequency of Bregs and frequency of Tregs in peripheral blood. In particular, we also observed that the frequency of Bregs was inversely associated with artery Lac, SOFA, and APACHE II. It was suggested that the frequency of Bregs had a significant negative correlation with the disease process. Binary logistic regression analysis identified that Bregs absolute number represented an independent prognostic factor for poor outcome in septic patients. Altogether, our results demonstrated that Bregs have been proposed to mediate the anti-inflammatory function and may exert protective effects during sepsis. Bregs have been shown to maintain immune tolerance in humans and mice including secretion of IL-35, TGF-β, IL-10, and through the expression of FasL, GITRL, PD-1, and CD73 [32, 33]. IL-10, a major anti-inflammatory cytokine, suppresses both Th1 and type 2 T helper (Th2) polarization and inhibits antigen presentation and proinflammatory cytokine production by macrophages, lymphocytes, monocytes, dendritic cell and some epithelial cells [34, 35]. Anti-inflammatory response, including the anti-inflammatory cytokine IL-10, IL-LRA, SIL-LR, STNFR-I and STNFR-II, parallel the overproduction of proinflammatory cytokines, may induce an immunosuppressive state in patients with sepsis [36]. We revealed that plasma IL-10 was higher in septic patients than healthy donors, which indicated very early immunosuppression or immunoparalysis in elderly septic patients. Contrary to data from the literature [37], we observed that the ability of B cells secreting IL-10 decreased, which may be due to different stimulation conditions, B cell exhaustion, decreasing numbers and impaired function of B cells. Finally, we observed an increase in IL-10 secreting by CD4+ T cells in septic patients, which is consistent with prvious published literature on Tregs in sepsis and septic shock [38, 39]. Previous findings by Roth et al. showed that increased IL-10 concentrations in sepsis may be associated with susceptibility of Th1 to apoptosis, leading to a prevalence of Th2 cells, which is known for their IL-10 production. CD14+ monocytes, not CD4+ T cells nor multipotent adult progenitor cells, are resposible for IL-10 production [40]. In addition, B cells from acute COVID-19 patients showed increased IL-6 and decreased IL-10 production compared with healthy B cells. Age-related declines in the number and function of Bregs may contribute to "inflammation" or the “chronic inflammation” observed with ageing [41]. Age‐associated reductions in Bregs frequency, as well as impaired IL‐10 production, were observed in healthy older donors (> 60 years) compared to healthy younger donors (20‐40 years old) [41]. Patients that succumbed to shock might be due to B cells exhibited a decrease in IL-10 secretion, indicating that both anti-inflammatory cytokines, inhibition of B cells in the initial stage of sepsis in elderly patients, and exogenous IL-10 had protective effects in sepsis.
APACHE II score is a classical standard to evaluate the severity of the disease in critically ill patients, and patients can be classified according to the severity of the disease. Studies have shown that APACHE II score is negatively correlated with ICU length of stay, and positively correlated with mortality [42]. SOFA score is also a simple and effective method to evaluate organ dysfunction/failure in critically ill patients. High SOFA score is associated with high mortality [42]. Our study demonstrated that APACHE II score, SOFA score, lactate, and Bregs differed significantly between dead and alive patients. Moreover, percentage of Bregs were negatively correlated with severity scores, and significantly correlated with non-survival of septic patients. All this data indicates that changes in Bregs may predict the prognosis in septic patients.
Conclusions
Overall, our results indicate that patients with sepsis had a lower frequency and number of Bregs, which seemed to be closely associated with disease progression, predicting a shorter overall survival and poor prognosis. Circulating Bregs numbers may be used as a reliable supplementary resource in the determination of sepsis severity. A longitudinal study of Bregs activity in sepsis patients should be explored as a test for disease progression/resolution. It is noteworthy that Bregs provides a novel insight into the dynamics underlying sepsis progression. All these data suggest Bregs have a potential immunosuppressant function in sepsis.
Supplementary Information
Additional file 1. Table S1. Clinical and laboratory data in septic patients and healthy donors.
Additional file 2. Table S2. Clinical and laboratory data between septic and septic shock patients.
Additional file 3. Table S3. Clinical and laboratory data between survivors and non-survivors of sepsis.
Additional file 4. All original data generated or analyzed in the study.
Acknowledgements
The authors thank Xiaoming Zhang for flow cytometry support (Key laboratory of Molecular Virology & Immunology, Institut Pasteur of Shanghai, Shanghai, China), Lunxian Tang, Hong Sun, Qian Yang, Xiandong Liu, Huijuan Ren, and Xiaowei Bao for clinical support (Shanghai East Hospital, Shanghai, China).
Abbreviations
- ANOVA
One-way analysis of variance
- APACHE II
Acute physiology and chronic health evaluation II
- Bregs
Regulatory B cells
- cGVHD
Chronic graft-versus-host disease
- CRP
C-reactive protein
- HBV
Hepatitis B virus
- ICU
Intensive care unit
- IL-10
Interleukin-10
- Lac
Lactate
- mNGS
Metagenomic next generation sequencing
- PBMCs
Peripheral blood mononuclear cells
- PCT
Procalcitonin
- ROC
Receiver operator characteristic curve
- Th1
Type 1 T helper
- Th2
Type 2 T helper
- Tregs
Regulatory T cells
- SOFA
Sequential organ failure assessment
- WBC
White blood cell
Author contributions
Jianwen Bai designed the research. Siting Liu, Mingzheng Xu and Chunmei Wang included and collected data. Mingzheng Xu and Jianwen Bai provided reagents, and quality control. Fengying Leng searched literature. Chunmei Wang, Rui Gao, and Huihui Xu wrote the manuscript. Chunmei Wang, Fangjie Huo and Huihui Xu performed the experiments, and analyzed data. All of the authors contributed to the article and approved the final version. All authors read and approved the final manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (Grant Nos. 82003182, 81670067, and 82070073) and Shanghai Pudong New Area summit (emergency medicine and critical care) construction project (Grant No. PWYgf2021-03).
Availability of data and materials
All original data generated or analyzed in the study are included in this article/supplementary materials (Additional file 4). Further inquiries can be directed to the corresponding author.
Declarations
Ethics approval and consent to participate
All experiments were performed according to relevant guidelines and regulations. The written informed consent was provided by all the recruited patients. This study was approved by the Research Ethics Board of East Hospital, Tongji University (Grant No. 2015–028).
Consent for publication
All authors have contributed to, read and approved the final version of this manuscript.
Competing interests
All authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chunmei Wang and Huihui Xu have contributed equally to this work and share first authorship
Contributor Information
Mingzheng Xu, Email: xmz878@126.com.
Jianwen Bai, Email: baijianwen1019@163.com.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. VIRULENCE. 2014;5:4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walkey AJ, Wiener RS, Lindenauer PK. Utilization patterns and outcomes associated with central venous catheter in septic shock: a population-based study. CRIT CARE MED. 2013;41:1450–1457. doi: 10.1097/CCM.0b013e31827caa89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, Jernigan JA, Martin GS, Septimus E, Warren DK, Karcz A, Chan C, Menchaca JT, Wang R, Gruber S, Klompas M. Incidence and trends of sepsis in US Hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318:1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baghdadi JD, Brook RH, Uslan DZ, Needleman J, Bell DS, Cunningham WE, Wong MD. Association of a care bundle for early sepsis management with mortality among patients with hospital-onset or community-onset sepsis. JAMA Intern Med. 2020;180:707–716. doi: 10.1001/jamainternmed.2020.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones SL, Ashton CM, Kiehne LB, Nicolas JC, Rose AL, Shirkey BA, Masud F, Wray NP. Outcomes and resource use of sepsis-associated stays by presence on admission, severity, and hospital type. Med Care. 2016;54:303–310. doi: 10.1097/MLR.0000000000000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daviaud F, Grimaldi D, Dechartres A, Charpentier J, Geri G, Marin N, Chiche JD, Cariou A, Mira JP, Pene F. Timing and causes of death in septic shock. Ann Intensive Care. 2015;5:16. doi: 10.1186/s13613-015-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiraki S, Ono S, Tsujimoto H, Kinoshita M, Takahata R, Miyazaki H, Saitoh D, Hase K. Neutralization of interleukin-10 or transforming growth factor-beta decreases the percentages of CD4+ CD25+ Foxp3+ regulatory T cells in septic mice, thereby leading to an improved survival. Surgery. 2012;151:313–322. doi: 10.1016/j.surg.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 11.Mauri C, Blair PA. The incognito journey of a regulatory B cell. Immunity. 2014;41:878–880. doi: 10.1016/j.immuni.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 13.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 14.Le Huu D, Matsushita T, Jin G, Hamaguchi Y, Hasegawa M, Takehara K, Tedder TF, Fujimoto M. Donor-derived regulatory B cells are important for suppression of murine sclerodermatous chronic graft-versus-host disease. Blood. 2013;121:3274–3283. doi: 10.1182/blood-2012-11-465658. [DOI] [PubMed] [Google Scholar]
- 15.Sarvaria A, Basar R, Mehta RS, Shaim H, Muftuoglu M, Khoder A, Sekine T, Gokdemir E, Kondo K, Marin D, Daher M, Alousi AM, Alsuliman A, Liu E, Oran B, Olson A, Jones RB, Popat U, Hosing C, Champlin R, Shpall EJ, Rezvani K. IL-10+ regulatory B cells are enriched in cord blood and may protect against cGVHD after cord blood transplantation. Blood. 2016;128:1346–1361. doi: 10.1182/blood-2016-01-695122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang M, Rui K, Wang S, Lu L. Regulatory B cells in autoimmune diseases. Cell Mol Immunol. 2013;10:122–132. doi: 10.1038/cmi.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He Y, Qian H, Liu Y, Duan L, Li Y, Shi G. The roles of regulatory B cells in cancer. J Immunol Res. 2014;2014:215471. doi: 10.1155/2014/215471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hein F, Massin F, Cravoisy-Popovic A, Barraud D, Levy B, Bollaert PE, Gibot S. The relationship between CD4+CD25+CD127- regulatory T cells and inflammatory response and outcome during shock states. Crit Care. 2010;14:R19. doi: 10.1186/cc8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 20.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, Hall RP, St CE, Tedder TF. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin W, Cerny D, Chua E, Duan K, Yi JT, Shadan NB, Lum J, Maho-Vaillant M, Zolezzi F, Wong SC, Larbi A, Fink K, Musette P, Poidinger M, Calbo S. Human regulatory B cells combine phenotypic and genetic hallmarks with a distinct differentiation fate. J Immunol. 2014;193:2258–2266. doi: 10.4049/jimmunol.1303214. [DOI] [PubMed] [Google Scholar]
- 22.Evans I, Phillips GS, Alpern ER, Angus DC, Friedrich ME, Kissoon N, Lemeshow S, Levy MM, Parker MM, Terry KM, Watson RS, Weiss SL, Zimmerman J, Seymour CW. Association between the New York Sepsis Care Mandate and in-hospital mortality for pediatric sepsis. JAMA. 2018;320:358–367. doi: 10.1001/jama.2018.9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving sepsis Campaign: International guidelines for management of sepsis and septic shock: 2016. CRIT Care Med. 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 24.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, Lemeshow S, Osborn T, Terry KM, Levy MM. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376:2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan X, Ji Z, Xue J. Percentage of peripheral CD19+CD24hiCD38hi regulatory B cells in neonatal sepsis patients and its functional implication. Med Sci Monit. 2016;22:2374–2378. doi: 10.12659/MSM.895421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shankar-Hari M, Fear D, Lavender P, Mare T, Beale R, Swanson C, Singer M, Spencer J. Activation-associated accelerated apoptosis of memory B cells in critically Ill patients with sepsis. Crit Care Med. 2017;45:875–882. doi: 10.1097/CCM.0000000000002380. [DOI] [PubMed] [Google Scholar]
- 27.Khoder A, Sarvaria A, Alsuliman A, Chew C, Sekine T, Cooper N, Mielke S, de Lavallade H, Muftuoglu M, Fernandez CI, Liu E, Muraro PA, Alousi A, Stringaris K, Parmar S, Shah N, Shaim H, Yvon E, Molldrem J, Rouce R, Champlin R, McNiece I, Mauri C, Shpall EJ, Rezvani K. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood. 2014;124:2034–2045. doi: 10.1182/blood-2014-04-571125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber M, Stein P, Prufer S, Rudolph B, Kreft A, Schmitt E, Bopp T, Roers A, Schild H, Fillatreau S, Radsak MP. Donor and host B cell-derived IL-10 contributes to suppression of graft-versus-host disease. Eur J Immunol. 2014;44:1857–1865. doi: 10.1002/eji.201344081. [DOI] [PubMed] [Google Scholar]
- 29.Wang T, Li Z, Li X, Chen L, Zhao H, Jiang C, Song L. Expression of CD19+CD24highCD38high B cells, IL10 and IL10R in peripheral blood from patients with systemic lupus erythematosus. Mol Med Rep. 2017;16:6326–6333. doi: 10.3892/mmr.2017.7381. [DOI] [PubMed] [Google Scholar]
- 30.Cui D, Zhang L, Chen J, Zhu M, Hou L, Chen B, Shen B. Changes in regulatory B cells and their relationship with rheumatoid arthritis disease activity. Clin Exp Med. 2015;15:285–292. doi: 10.1007/s10238-014-0310-9. [DOI] [PubMed] [Google Scholar]
- 31.Woodruff M, Ramonell R, Cashman K, Nguyen D, Saini A, Haddad N, Ley A, Kyu S, Howell JC, Ozturk T, Lee S, Chen W, Estrada J, Morrison-Porter A, Derrico A, Anam F, Sharma M, Wu H, Le S, Jenks S, Tipton CM, Hu W, Lee FE, Sanz I. Dominant extrafollicular B cell responses in severe COVID-19 disease correlate with robust viral-specific antibody production but poor clinical outcomes, medRxiv. (2020).
- 32.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Floudas A, Amu S, Fallon PG. New Insights into IL-10 dependent and IL-10 independent mechanisms of regulatory B Cell immune suppression. J Clin Immunol. 2016;36(Suppl 1):25–33. doi: 10.1007/s10875-016-0263-8. [DOI] [PubMed] [Google Scholar]
- 34.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 35.Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 36.Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235–243. doi: 10.1378/chest.112.1.235. [DOI] [PubMed] [Google Scholar]
- 37.Gustave CA, Gossez M, Demaret J, Rimmele T, Lepape A, Malcus C, Poitevin-Later F, Jallades L, Textoris J, Monneret G, Venet F. Septic shock shapes B cell response toward an exhausted-like/immunoregulatory profile in patients. J Immunol. 2018;200:2418–2425. doi: 10.4049/jimmunol.1700929. [DOI] [PubMed] [Google Scholar]
- 38.Jiang LN, Yao YM, Sheng ZY. The role of regulatory T cells in the pathogenesis of sepsis and its clinical implication. J Interferon Cytokine Res. 2012;32:341–349. doi: 10.1089/jir.2011.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fabri A, Kandara K, Coudereau R, Gossez M, Abraham P, Monard C, Cour M, Rimmele T, Argaud L, Monneret G, Venet F. Characterization of circulating IL-10-producing cells in septic shock patients: a proof of concept study. FRONT IMMUNOL. 2020;11:615009. doi: 10.3389/fimmu.2020.615009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valentin-Torres A, Day C, Taggart JM, Williams N, Stubblefield SR, Roobrouck VD, Beyens J, Ting AE. Multipotent adult progenitor cells induce regulatory T cells and promote their suppressive phenotype via TGFbeta and monocyte-dependent mechanisms. Sci Rep. 2021;11:13549. doi: 10.1038/s41598-021-93025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duggal NA, Upton J, Phillips AC, Sapey E, Lord JM. An age-related numerical and functional deficit in CD19(+) CD24(hi) CD38(hi) B cells is associated with an increase in systemic autoimmunity. Aging Cell. 2013;12:873–881. doi: 10.1111/acel.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morkar DN, Dwivedi M, Patil P. Comparative study of sofa, Apache Ii, Saps Ii, as a predictor of mortality in patients of sepsis admitted in medical ICU. J Assoc Physicians India. 2022;70:11–12. [Google Scholar]
- 43.Bauer M, Gerlach H, Vogelmann T, Preissing F, Stiefel J, Adam D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit Care. 2020;24:239. doi: 10.1186/s13054-020-02950-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. Clinical and laboratory data in septic patients and healthy donors.
Additional file 2. Table S2. Clinical and laboratory data between septic and septic shock patients.
Additional file 3. Table S3. Clinical and laboratory data between survivors and non-survivors of sepsis.
Additional file 4. All original data generated or analyzed in the study.
Data Availability Statement
All original data generated or analyzed in the study are included in this article/supplementary materials (Additional file 4). Further inquiries can be directed to the corresponding author.