Mast cell tumor (MCT) is the most common skin cancer in dogs and represents 16 to 21% of canine skin tumors (1,2). Clinical presentation of canine cutaneous MCT can vary from an asymptomatic, solitary dermal nodule to a rapidly growing, infiltrative mass (Figures 1, 2), and multiple skin nodules may be present in 11 to 14% of cases (3,4). Superficial tumors may be accompanied by cutaneous changes such as erythema, ulcer, and/or edema. Regardless of the clinical appearance, a diagnosis of MCT is readily achievable cytologically in most cases, but the first step after diagnostic confirmation may not always be surgical removal of the tumor(s). This is because although 70 to 85% of canine cutaneous MCTs can be cured by surgery alone, the remainder recur locally or metastasize systemically, requiring multimodal therapy including surgery, radiation therapy, and/or chemotherapy (5–8). Being aware of prognostic indicators that help identify these aggressively behaving mast cell tumors before surgical removal is important in guiding staging tests, and in helping owners with the decision to perform surgery.
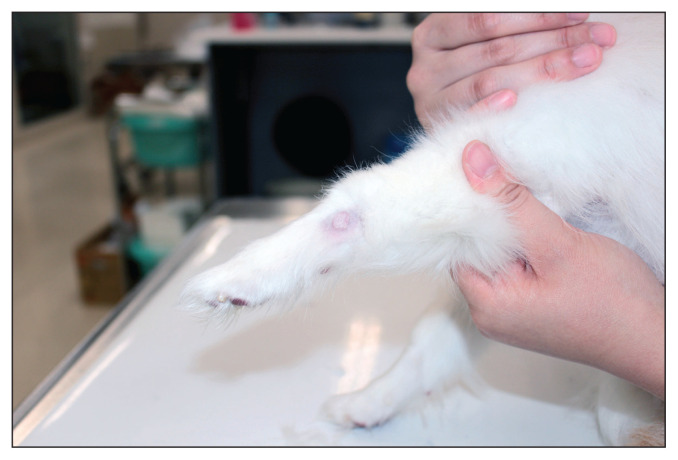
Figure 1.
A small, alopecic, dome-shaped cutaneous nodule on the left thoracic limb in a 12-year-old Pomeranian. The nodule had been present for 6 mo without progression before surgery. Histopathology confirmed the diagnosis of a Grade I/Low mast cell tumor.
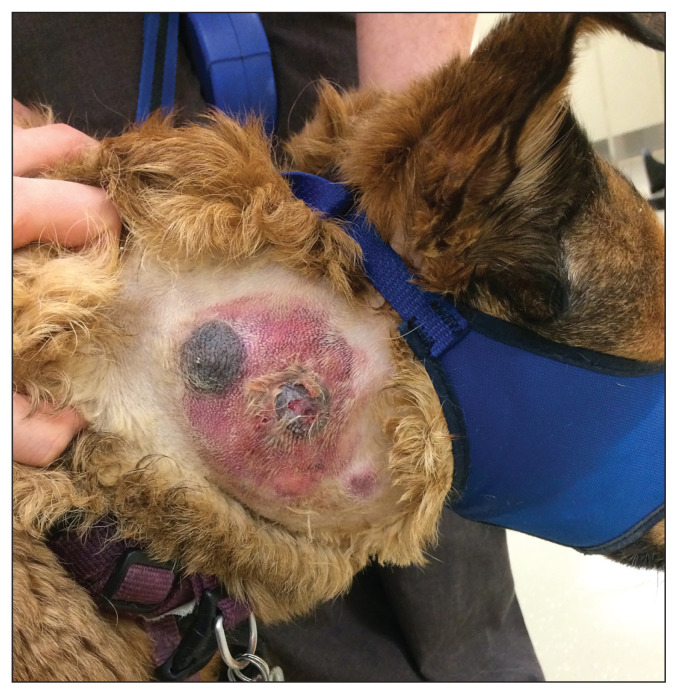
Figure 2.
A large, ulcerated, cutaneous mass on the right neck in an 8-year-old shepherd cross dog. Acute swelling, discharge, and pain had developed 2 wk before presentation. Metastatic mast cell tumors were present in the superficial cervical lymph nodes and spleen at the time of diagnosis. Only palliative systemic chemotherapy was applied to this patient.
Undoubtedly one of the most reliable methods to identify those 15 to 30% aggressive MCT cases is histological grading. Traditionally, cutaneous MCTs have been graded by the 3-tier Patnaik system (Grades I, II, III), based on 5 different histological findings such as mitotic activity and cellular morphology (9). However, variation in the clinical behavior of Patnaik intermediate grade MCTs and inconsistency in tumor grading among pathologists have led to development of the 2-tier Kiupel system (Low and High Grade) (10). These 2 grading systems are not mutually exclusive but rather complementary. Indeed, the current recommendation in prognostication of canine MCT is to use the combined grading scheme, histologically categorizing a MCT into Grade I/Low, II/Low, II/High, or III/High (11). Nearly 95% of Grade I/Low MCT can be successfully treated by surgery alone, whereas a median survival time of 108 d and 1-year survival rate of 16% have been reported for dogs with Grade III/High MCT (6). Mortality rates of Grade II/Low and II/High MCT are 17% and 56%, respectively, emphasizing the importance of subcategorizing the Patnaik Grade II MCT (5). Mitotic count (MC), which is a histologic grading criterion in both systems, has additional independent prognostic significance with a reported median survival time of 70 mo for dogs with low MC (≤ 5/10 high power field) versus 2 mo for dogs with high MC (> 5/10 high power field) following surgery (12).
Although histological prognostic clues are critical in predicting a patient’s outcome, one may wonder if we can foresee the prognosis of a dog with MCT before surgery provides histological samples. For instance, what if a senior dog presents with a cutaneous MCT and concurrent comorbidities that make general anesthesia or surgery challenging? Indeed, there are multiple pre-surgical factors that reportedly correlate with tumor grade, metastatic rate, or even survival of the affected dogs (Table 1).
Table 1.
Prognostic factors for canine mast cell tumors that can be assessed before surgery.
| Risk factors | Favorable | Unfavorable | Clinical significance | Reference(s) |
|---|---|---|---|---|
| Breed | Pug, boxer, golden retriever | Shar Pei, Rottweiler, Shih Tzu, French bulldog, pit bull | Associated with histological tumor grade | (13–15) |
| Number of skin tumors | — | — | No known association with prognosis | (3,16) |
| Progression | Slow | Fast | Associated with survival | (17) |
| Systemic symptoms | Asymptomatic | Clinically ill, tumor degranulated | Indicative for systemic metastasis Associated with survival |
(3,18) |
| Recurrence | — | Recurrent tumor | Associated with survival | (5,19) |
| Anatomic location | — | Perigenital, perioral/oral, mucocutaneous junction | Associated with histological tumor grade, metastatic potential and/or survival | (14,15,20,21) |
| Micromorphological location | Subcutaneous | — | Associated with metastatic potential and survival | (6,23) |
| Cytological grade | Low grade | High grade | Associated with histological tumor grade and/or survival | (24–26) |
The first of these factors is the breed of dog. Shar Pei is historically well-recognized as a breed predisposed to developing aggressive MCT (1,13). Recent epidemiological studies have also shown genetic predisposition to high grade MCT in other breeds such as Rottweiler, Shih Tzu, French bulldog, and pit bull (13–15). On the other hand, pug, boxer, and golden retriever breeds were more likely to develop low to intermediate grade MCT (13–15). The latter breeds are also well-recognized for developing multiple MCTs, but the presence of multiple tumors itself is not a poor prognostic indicator; the histological grade of individual tumors is more important for prognosis (3,16).
Clinical history and physical examination findings of an MCT are other important factors to evaluate. A localized, static tumor present for months to sometimes years tends to be clinically benign. In a study of 97 canine MCT cases, dogs with a MCT present over 7 mo had significantly longer survival (mean: 58 wk) than dogs in which tumor was discovered within 7 mo (mean: 19 to 22 wk) (17). Aggressive MCTs may present with systemic visceral disease or degranulation, with affected dogs showing lethargy, inappetence, vomiting, and/or suffering from diarrhea/melena (3,18). Tumor degranulation may locally result in ulcer, edema, or erythema of an MCT; these are also associated with poor prognosis (3,18). With or without degranulation, larger tumors may not be completely resectable, resulting in a higher risk of local recurrence if untreated with radiation therapy; this is strongly associated with poor prognosis (5,19).
Although canine MCTs can arise in any superficial locations on the body, cutaneous MCTs occurring in perigenital, peri/oral, or mucocutaneous junction are generally metastatic and histologically high grade (Figure 3) (13,15,20,21). Historically, MCTs on the head were also thought to be clinically aggressive, but this was likely because large surgical margins are difficult to obtain for a head MCT and therefore tumor control fails locally. Indeed, a study of 28 dogs with ear pinna MCT reported a favorable outcome overall, except for histologically high grade or high MC MCT, likely because wide surgical margins can be achievable with ear pinna amputation (22). In addition to the anatomic location, micromorphological location of MCT (skin versus subcutis) also affects the clinical behavior of MCTs. Subcutaneous MCTs are especially benign with a 1-year survival rate of 91 to 95% following surgery alone (6,23). Because of this exception, both Patnaik and Kiupel grading systems do not apply to subcutaneous MCTs, although historically subcutaneous MCTs were graded as Patnaik Grades II or III.
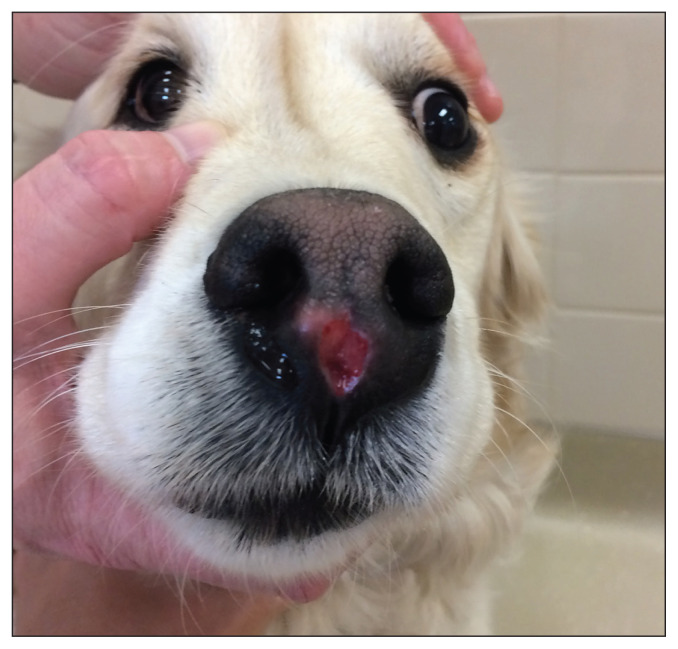
Figure 3.
An ulcerated, rapidly growing mass within the nasal planum in a 5-year-old golden retriever. Pre-surgical staging tests confirmed the presence of a regional lymph node metastasis. The dog was treated with a combination of nasal planectomy, adjuvant radiation therapy, and systemic chemotherapy due to the tumor location and presence of a metastatic lymph node.
Lastly, cytology can also be useful in predicting tumor behavior pre-surgically. Multiple studies have shown associations between cytological grade with histological grade and patient outcome (24–26). Cytology grading schemes use some of the histological grading parameters such as cellular granularity, nuclear morphology, and mitotic figures, to establish the tumor’s biological behavior. The published evidence supports the use of cytological grading in predicting histological grade of cutaneous MCTs; however, fine-needle aspirates capture characteristics of only a small proportion of cells in an entire tumor mass. A cytological grade, therefore, needs to be carefully interpreted, as both false-positive and false-negative results can occur.
Although none of these factors can be definitive as a sole prognostic factor and patient outcome needs to be assessed comprehensively, these clinicopathological factors can be informative and ideally are considered at the pre-surgical evaluation. For cases with one or more negative prognostic indicators, the authors encourage pet owners to consider thorough diagnostics such as the cytological assessment of draining lymph node(s), assessment, and abdominal ultrasound before surgery to identify regional and distant metastases.
Questions.
Which of the following histological tumor grades carry the highest metastatic potential in dogs with cutaneous mast cell tumor?
Patnaik grade I/Kiupel grade Low
Patnaik grade II/Kiupel grade Low
Patnaik grade II/Kiupel grade High
Patnaik grade III/Kiupel grade High
Answer: 4.
Which of the following clinical features is associated with shorter survival in dogs with mast cell tumor?
Subcutaneous location
Recurrent tumor
Thoracic location
Three concurrent cutaneous tumors present
Answer: 2.
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.London CA, Thamm DH. Mast cell tumors. In: Vail D, Thamm D, Liptak J, editors. Small Animal Clinical Oncology. 6th ed. Philadelphia, Pennsylvania: Elsevier; 2020. pp. 382–403. [Google Scholar]
- 2.Kiupel M. Tumor in Domestic Animals. 5th ed. Ames, Iowa: John Wiley & Sons; 2017. Mast cell tumor; pp. 176–195. [Google Scholar]
- 3.Mullins MN, Dernell WS, Withrow SJ, Ehrhart EJ, Thamm DH, Lana SE. Evaluation of prognostic factors associated with outcome in dogs with multiple cutaneous mast cell tumors treated with surgery with and without adjuvant treatment: 54 cases (1998–2004) J Am Vet Med Assoc. 2006;228:91–95. doi: 10.2460/javma.228.1.91. [DOI] [PubMed] [Google Scholar]
- 4.Pelt DR, Fowler JD, Leighton FA. Multiple cutaneous mast cell tumors in a dog: A case report and brief review. Can Vet J. 1986;27:259–263. [PMC free article] [PubMed] [Google Scholar]
- 5.Horta RS, Lavalle GE, Monteiro LN, Souza MCC, Cassali GD, Araújo RB. Assessment of canine mast cell tumor mortality risk based on clinical, histologic, immunohistochemical, and molecular features. Vet Pathol. 2018;55:212–223. doi: 10.1177/0300985817747325. [DOI] [PubMed] [Google Scholar]
- 6.Sabattini S, Scarpa F, Berlato D, Bettini G. Histologic grading of canine mast cell tumor: Is 2 better than 3? Vet Pathol. 2015;52:70–73. doi: 10.1177/0300985814521638. [DOI] [PubMed] [Google Scholar]
- 7.Stefanello D, Buracco P, Sabattini S, et al. Comparison of 2- and 3-category histologic grading systems for predicting the presence of metastasis at the time of initial evaluation in dogs with cutaneous mast cell tumors: 386 cases (2009–2014) J Am Vet Med Assoc. 2015;246:765–769. doi: 10.2460/javma.246.7.765. [DOI] [PubMed] [Google Scholar]
- 8.Vascellari M, Giantin M, Capello K, et al. Expression of Ki67, BCL-2, and COX-2 in canine cutaneous mast cell tumors: Association with grading and prognosis. Vet Pathol. 2013;50:110–121. doi: 10.1177/0300985812447829. [DOI] [PubMed] [Google Scholar]
- 9.Patnaik AK, Ehler WJ, MacEwen EG. Canine cutaneous mast cell tumor: Morphologic grading and survival time in 83 dogs. Vet Pathol. 1984;21:469–474. doi: 10.1177/030098588402100503. [DOI] [PubMed] [Google Scholar]
- 10.Kiupel M, Webster JD, Bailey KL, et al. Proposal of a 2-tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Vet Pathol. 2011;48:147–155. doi: 10.1177/0300985810386469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berlato D, Bulman-Fleming J, Clifford CA, et al. Value, limitations, and recommendations for grading of canine cutaneous mast cell tumors: A consensus of the Oncology-Pathology Working Group. Vet Pathol. 2021;58:858–863. doi: 10.1177/03009858211009785. [DOI] [PubMed] [Google Scholar]
- 12.Romansik EM, Reilly CM, Kass PH, Moore PF, London CA. Mitotic index is predictive for survival for canine cutaneous mast cell tumors. Vet Pathol. 2007;44:335–341. doi: 10.1354/vp.44-3-335. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds BD, Thomson MJ, O’Connell K, Morgan EJ, Gummow B. Patient and tumour factors influencing canine mast cell tumour histological grade and mitotic index. Vet Comp Oncol. 2019;17:338–344. doi: 10.1111/vco.12477. [DOI] [PubMed] [Google Scholar]
- 14.Mochizuki H, Motsinger-Reif A, Bettini C, Moroff S, Breen M. Association of breed and histopathological grade in canine mast cell tumours. Vet Comp Oncol. 2017;15:829–839. doi: 10.1111/vco.12225. [DOI] [PubMed] [Google Scholar]
- 15.Martins AL, Carvalho FF, Mesquita JR, Gärtner F, Amorim I. Analysis of risk factors for canine mast cell tumors based on the Kiupel and Patnaik grading system among dogs with skin tumors. Open Vet J. 2021;11:619–634. doi: 10.5455/OVJ.2021.v11.i4.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connell K, Thomson M. Evaluation of prognostic indicators in dogs with multiple, simultaneously occurring cutaneous mast cell tumours: 63 cases. Vet Comp Oncol. 2013;11:51–62. doi: 10.1111/j.1476-5829.2011.00301.x. [DOI] [PubMed] [Google Scholar]
- 17.Bostock DE. The prognosis following surgical removal of mastocytomas in dogs. J Small Anim Pract. 1973;14:27–41. doi: 10.1111/j.1748-5827.1973.tb06891.x. [DOI] [PubMed] [Google Scholar]
- 18.O’Keefe DA, Couto CG, Burke-Schwartz C, Jacobs RM. Systemic mastocytosis in 16 dogs. J Vet Intern Med. 1987;1:75–80. doi: 10.1111/j.1939-1676.1987.tb01990.x. [DOI] [PubMed] [Google Scholar]
- 19.Thamm DH, Mauldin EA, Vail DM. Prednisone and vinblastine chemotherapy for canine mast cell tumor — 41 cases (1992–1997) J Vet Intern Med. 1999;13:491–497. doi: 10.1892/0891-6640(1999)013<0491:pavcfc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Sfiligoi G, Rassnick KM, Scarlett JM, Northrup NC, Gieger TL. Outcome of dogs with mast cell tumors in the inguinal or perineal region versus other cutaneous locations: 124 cases (1990–2001) J Am Vet Med Assoc. 2005;226:1368–1374. doi: 10.2460/javma.2005.226.1368. [DOI] [PubMed] [Google Scholar]
- 21.Hillman LA, Garrett LD, de Lorimier LP, Charney SC, Borst LB, Fan TM. Biological behavior of oral and perioral mast cell tumors in dogs: 44 cases (1996–2006) J Am Vet Med Assoc. 2010;237:936–942. doi: 10.2460/javma.237.8.936. [DOI] [PubMed] [Google Scholar]
- 22.Schwab TM, Popovitch C, DeBiasio J, Goldschmidt M. Clinical outcome for MCTs of canine pinnae treated with surgical excision (2004–2008) J Am Anim Hosp Assoc. 2014;50:187–191. doi: 10.5326/JAAHA-MS-6039. [DOI] [PubMed] [Google Scholar]
- 23.Thompson JJ, Pearl DL, Yager JA, Best SJ, Coomber BL, Foster RA. Canine subcutaneous mast cell tumor: Characterization and prognostic indices. Vet Pathol. 2011;48:156–168. doi: 10.1177/0300985810387446. [DOI] [PubMed] [Google Scholar]
- 24.Camus MS, Priest HL, Koehler JW, et al. Cytologic criteria for mast cell tumor grading in dogs with evaluation of clinical outcome. Vet Pathol. 2016;53:1117–1123. doi: 10.1177/0300985816638721. [DOI] [PubMed] [Google Scholar]
- 25.Scarpa F, Sabattini S, Bettini G. Cytological grading of canine cutaneous mast cell tumours. Vet Comp Oncol. 2016;14:245–251. doi: 10.1111/vco.12090. [DOI] [PubMed] [Google Scholar]
- 26.Hergt F, von Bomhard W, Kent MS, Hirschberger J. Use of a 2-tier histologic grading system for canine cutaneous mast cell tumors on cytology specimens. Vet Clin Pathol. 2016;45:477–483. doi: 10.1111/vcp.12387. [DOI] [PubMed] [Google Scholar]