Abstract
Pulmonary alveolar proteinosis (PAP) is a rare form of chronic interstitial lung disease, characterised by the intra-alveolar accumulation of lipoproteinaceous material. Numerous conditions can lead to its development. Whereas the autoimmune type is the main cause in adults, genetic defects account for a large part of cases in infants and children. Even if associated extra-respiratory signs may guide the clinician during diagnostic work-up, next-generation sequencing panels represent an efficient diagnostic tool. Exome sequencing also allowed the discovery of new variants and genes involved in PAP. The aim of this article is to summarise our current knowledge of genetic causes of PAP.
Short abstract
Genetic PAP occurs in young children and is often associated with a poor prognosis. Next-generation sequencing panels represent an efficient diagnostic tool. Promising treatments are currently being developed for some specific entities. https://bit.ly/2TRsJd1
Introduction
Pulmonary alveolar proteinosis (PAP) is a rare cause of chronic interstitial lung disease (ILD). It is characterised by alveolar accumulation of lipoproteinaceous material derived from surfactant [1] and results from an altered surfactant production, removal or both. Diagnosis is suggested on chest computed tomography (CT), showing a typical “crazy-paving” appearance and alveolar consolidations [2], and also on the macroscopic milky appearance of the bronchoalveolar lavage fluid (BALF). It is confirmed by microscopic examination of BALF, which shows foamy alveolar macrophages and extracellular globular hyaline material found homogeneously positive for periodic acid–Schiff (PAS) staining. Diagnosis rarely needs histological examination of lung biopsy specimens [3]. PAP can be autoimmune, secondary and genetic. Autoimmune PAP is mainly diagnosed in adults and is related to the presence of anti-granulocyte–macrophage colony-stimulating factor (GM-CSF) auto-antibodies. Secondary forms may occur during the course of congenital or acquired immune deficiencies and haematological disorders, and can be triggered by toxic inhalations, some infectious agents or some antiproliferative and immunosuppressive drugs. Genetic forms are usually diagnosed in children. The aim of this article is to summarise our current knowledge of genetic causes of PAP. Genetic mutations are involved in three different PAP forms, classified according to pathogenic mechanisms: hereditary, secondary and congenital. The so-called hereditary PAP is caused by mutations in CSF2RA or CSF2RB that impair the GM-CSF signalling pathway required for normal surfactant clearance by alveolar macrophages. Other genetic mutations that affect function and/or number of mononuclear phagocytes can also lead to PAP. Congenital PAP refers to mutations in the genes required for normal surfactant production. First, disorders where PAP is the hallmark of the genetic defect will be detailed. These include mutations in CSF2RA and CSF2RB genes that encode the α and β chains of the GM-CSF receptor, respectively, and mutations in the gene encoding the methionyl tRNA synthetase. Then, genetic immune deficiencies and metabolic diseases that often display PAP features will be described. Finally, surfactant homeostasis disorders that can be revealed by PAP will then be discussed.
Mutations in CSF2RA and CSF2RB genes
Pathogenesis
PAP related to CSF2RA or CSF2RB mutations is an autosomal recessive disease. CSF2RA and CSF2RB encode the α and β chains of the GM-CSF receptor and are located in the pseudo-autosomal region of chromosome X and in chromosome 22, respectively. The identification of their role in hereditary PAP came from animal studies. Transgenic mice deficient for GM-CSF [4] or the α [5] or β chain of its receptor [6, 7] develop PAP. In those mice, PAP phenotype results from a reduced catabolism of surfactant by alveolar macrophages. Pulmonary phenotype is rescued by wild-type bone marrow transplantation [8], thus confirming that alveolar macrophages are the cellular component in its pathogenesis. Mechanisms responsible for the development of PAP in patients with CSF2RA and CSF2RB mutations were recently reviewed by Trapnell et al. [9]. In alveolar surfactant homeostasis, alveolar macrophages remove approximately 50% of the expelled surfactant by catabolism of phospholipids and efflux and reverse transport of cholesterol to the liver. GM-CSF binding to its receptor leads to the activation of JAK2 and initiation of signalling via multiple pathways, including activation of signal transducer and activator of transcription 5 (STAT5) [10], transcription factor PU.1 (encoded by SPI1) [11] and peroxisome proliferator-activated receptor (PPAR)-γ [12]. GM-CSF signalling via PU.1 [12], PPARγ [13, 14] and its downstream effector ATP-binding cassette subfamily G member 1 (ABCG1) is required for cholesterol efflux and surfactant clearance [15, 16]. When GM-CSF signalling is deficient, the expression of ABCG1 is reduced, resulting in a primary reduction in cholesterol efflux from alveolar macrophages with the accumulation of esterified-cholesterol-rich intra-cytoplasmic lipid droplets, resulting in the formation of foamy cells. The reduction in the clearance of surfactant from the alveolar surface occurs as a consequence of reduced surfactant uptake by foamy alveolar macrophages and leads to PAP [9]. Interesting results also came from studies performed on induced pluripotent stem cells (iPSCs) [17, 18]. Suzuki et al. [17] developed iPSCs from peripheral blood mononuclear cells (PBMCs) from two patients with PAP and CSF2RA mutations and three healthy controls. The obtained iPSCs were then differentiated into iPSC-derived macrophages. Compared with normal iPS cell-derived macrophages, patient's iPS cell-derived macrophages (human pulmonary alveolar proteinosis iPS cell-derived macrophages; hPAP-iPS-Mfs) had impaired GM-CSF receptor signalling and reduced GM-CSF-dependent gene expression and surfactant clearance, as shown by incubation of cells with the abnormal BALF of the patients. Restoration of GM-CSF receptor signalling by wild-type CSF2RA gene transfer corrected the surfactant clearance abnormality in hPAP-iPS-Mfs. Lachman et al. [18] also developed iPS-derived macrophages from CD34+ bone marrow cells of a CSF2RA-deficient patient with PAP and showed that those cells exhibited distinct defects in GM-CSF-dependent functions and that these defects were fully repaired on lentiviral wild-type CSF2RA gene transfer.
Clinical presentation and diagnosis of PAP
To date, 23 patients with CSF2RA mutations [19–24] and two patients with CSF2RB mutations [25, 26] have been reported in the literature.
19 CSF2RA cases were reviewed in 2014 and four more patients were reported in 2017. We added a new patient into this, leading to 24 reported patients. Clinical characteristics of the patients are described in table 1. Initial symptoms usually occurred in the first years of life, with a median age of 3 years and were characterised by progressive dyspnoea and/or tachypnoea, dyspnoea on exertion and poor weight gain. More than 50% of the patients were hypoxaemic at diagnosis. In all symptomatic patients, PAP diagnosis was evoked on typical aspects on chest CT scan with crazy paving (figure 1a–f) and confirmed by PAS staining on BALF examination and/or lung biopsy. Genetic diagnosis was made in one young adult at age 19 years. She was the sister of one patient diagnosed at the age of 6 years, and the diagnosis was suggested when she developed a cough [19]. Five individuals, aged <3, 3, 5, 7 and 8 years, were asymptomatic and genetically tested during the familial screening of their affected sibling [20, 23, 24]. One of them underwent a chest CT scan and bronchoalveolar lavage that confirmed the diagnosis of PAP, one only underwent a chest CT scan and the other three had no investigation other than molecular biology. Length of follow-up for those asymptomatic patients ranged from 1 to 3 years. As one patient was diagnosed at the age of 19 years, those asymptomatic carriers may develop symptoms later in life. However, this variability in disease severity across family members with identical mutations suggests that other factors in addition to GM-CSF signalling may be important. Finally, Turner syndrome was associated with PAP in two girls who presented with complex X chromosome abnormalities (table 2) [20, 22].
TABLE 1.
Clinical characteristics of patients harbouring CSF2RA mutations
| Characteristics | |
| Female | 18/24 (75) |
| Consanguinity | 15/19# (79) |
| Age at onset years | 3 (0.2–19) |
| Age at diagnosis years | 5 (2.3–19) |
| Diagnostic latency years | 1 (0–5.8) |
| Signs at diagnosis | |
| Dyspnoea/tachypnoea | 17/24 (71) |
| Hypoxaemia | 13/24 (54) |
| Failure to thrive | 15/24 (63) |
| Asymptomatic (familial screening) | 5/24 (21) |
| Outcome | |
| Length of follow-up | 2.5 (0–12.5) |
| Age at last follow-up years | 11 (4.2–19) |
| Death | 1/24 (4) |
| Alive and asymptomatic | 11/24 (46) |
| Alive and symptomatic without O2 | 7/24 (29) |
| Alive and CRF | 5/24 (21) |
| Management | |
| WLL | 17/24 (72) |
| BMT | 2/23 (8.7) |
Data are presented as n/N (%) or median (min–max). CRF: chronic respiratory failure; WLL: whole-lung lavage; BMT: bone marrow transplantation. #: information missing for five patients. Data from [19–24].
FIGURE 1.
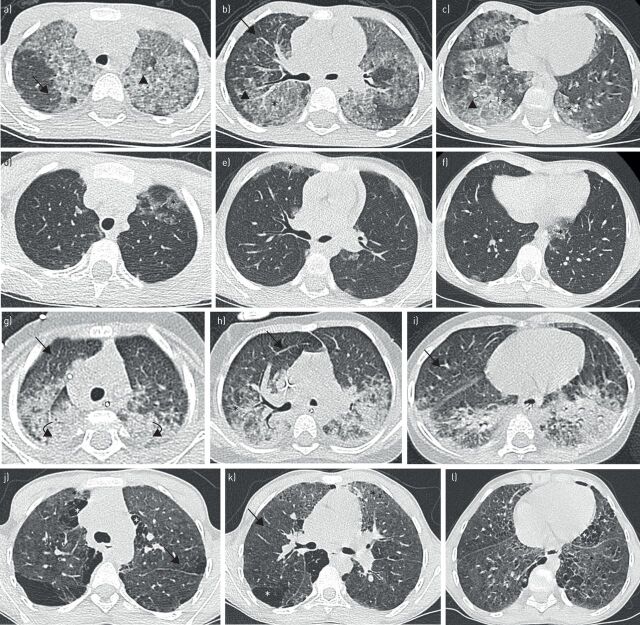
Chest computed tomography (CT) scan in CSF2RA and MARS patients. a–f) Patient with a complete deletion of CSF2RA. a–c) Chest CT scan at diagnosis in a 6-year-old girl showing diffuse ground-glass opacities (black asterisks) superimposed with interlobular septa thickening (black arrows) and intra-lobular lines (black arrowheads). d–f) Chest CT scan in the same patient at the age of 9 years and 1 month after a programme of bi-annual whole-lung lavage, showing a great improvement with only moderate and patchy ground-glass opacities (black asterisks). g–l) Patient from Comoros, Africa, harbouring the biallelic Ala393Thr/Ser567Leu mutations of MARS. g–i) Chest CT scan at diagnosis in an 8-month-old boy showing both ground-glass opacities (black asterisks) with interlobular septa thickening (black arrows) and important postero-basal consolidations (black curved arrows). j–l) Chest CT scan in the same patient at 10 years. Regular whole-lung lavages were performed on a weekly, then monthly and finally 3-monthly basis from diagnosis until the age of 3 years and 4 months. The patient had no specific treatment at the time of the CT that shows persistent ground-glass opacities (black asterisks) and septal thickening (black arrows) associated with the appearance of subpleural cystic lesions and signs of fibrosis with honey-combing (white asterisks).
TABLE 2.
Mutations described in patients with CSF2RA and CSF2RB and their biological features
| Gene | Mutation (colloquial nomenclature) | Type of mutation(s) | Protein (western blot) | GM-CSF serum | GM-CSF BALF | CD11b stimulation index | STAT5 phosphorylation | [Ref.] |
| CSF2RA | R217X | Nonsense | Absent | Increased | Increased | Reduced | Absent | [20] |
| 920dupGC | Duplication and frameshift | Absent | Increased | Increased | Reduced | Absent | [20] | |
| ΔEx7 | Deletion and frameshift | Absent | Increased | Increased | Reduced | Absent | [20] | |
| ΔEx7-8 | Deletion in frame | Absent | Increased | Increased | Reduced | Absent | [20] | |
| G196R | Missense | Present | Increased | Increased | Reduced | Reduced | [19–21] | |
| XpΔ1.6 | Deletion | Absent | Increased | Increased | Absent | Absent | [20] | |
| XpΔ0.41 | Deletion | Absent | Normal | ND | Absent | ND | [20] | |
| R199X | Nonsense | ND | # | # | ND | Absent | [19] | |
| G>A Ex12/Int12 border | Donor splice site mutation | ND | # | # | ND | Reduced | [19] | |
| dupEx8 | Duplication | ND | # | # | ND | ND | [19] | |
| ΔEx2-13 | Deletion | ND | # | # | ND | Reduced | [19] | |
| Xp22.3, Yp11.3 | Deletion | ND | # | # | ND | Absent | [19] | |
| S25X | Nonsense | ND | # | # | ND | Reduced | [19] | |
| ΔCSF2RA, Xp22.33p22.2 | Deletion | ND | ND | ND | ND | ND | [22] | |
| C178Y | Missense | ND | Increased | ND | ND | ND | [24] | |
| XpΔ0.425 | Deletion | Absent | ND | ND | ND | Reduced | [23] | |
| ΔEx1-13 | Deletion | Absent | Increased | ND | ND | Reduced | New patient | |
| CSF2RB | S271L | Missense | Present | Increased | ND | ND | Reduced | [25] |
| 631delC | Frameshift and stop | Absent | Increased | ND | Reduced | Absent | [26] |
GM-CSF: granulocyte–macrophage colony-stimulating factor; BALF: bronchoalveolar lavage fluid; ND: not done. #: GM-CSF levels in serum and BALF were determined in three and four patients, respectively, and were constantly elevated as compared to controls but the data are not given by patient and/or mutation.
Regarding CSF2RB, two patients were reported in the literature [25, 26]. One was a 9-year-old girl that developed progressive dyspnoea after pneumonia [25]. The diagnosis of PAP was suggested by chest radiography findings, chest CT scan and BALF cytology, and was confirmed by surgical lung biopsy. The other patient was a 36-year-old woman who gradually developed dyspnoea on exertion [26]. She was diagnosed as having PAP by typical findings on chest radiography (figure 1a–f), BALF and lung pathological examination.
Functional studies and molecular diagnosis
Mutations and biological features associated with each variant are presented in table 2. Patients harbouring pathogenic single-nucleotide variants and/or deletions in CSF2RA or CSF2RB genes share some biological characteristics that can be studied by ELISA from serum and BALF and by flow cytometry on peripheral blood leukocytes. GM-CSF auto-antibodies are negative. GM-CSF levels are increased in serum and BALF. Flow cytometry studies show a reduced or absent GM-CSF-stimulated increase in phosphorylated STAT5 and in cell surface CD11b levels, and a decreased or undetectable expression of GM-CSF-Rα or Rβc.
In 21 patients, molecular diagnosis was made by direct Sanger sequencing of the two genes because PAP was associated with one or several abnormalities described above, or during familial screening. In the two patients reported by Chiu et al. [23] (one boy with PAP and his asymptomatic brother) diagnosis was made by whole-genome sequencing of all family members. In the patient reported by Al-Haidary et al. [24], diagnosis was made by next-generation sequencing of a panel that contains the genes involved in surfactant disorders. His family members were then screened for the mutation that was identified by Sanger sequencing and his sister, albeit asymptomatic, was homozygous for the same mutation. 17 different mutations were described in CSF2RA and two in CSF2RB. One mutation (G196R) was described in three different families. No recurrence was observed for the other mutations.
Disease course and management
17 patients underwent repetitive whole-lung lavages (WLLs). This treatment allows a complete clinical recovery, with no respiratory symptoms noted at last follow-up for five patients. Seven patients evolved towards chronic respiratory failure requiring oxygen therapy despite this treatment. Two of them underwent bone marrow transplantation (BMT). One died of uncontrolled respiratory infection 4 weeks after BMT before reconstitution of the donor marrow. The other is still alive and is 12 years-old [27]. BMT was performed at the age of 6 years and allowed a complete recovery of PAP and chronic respiratory failure with a normal chest CT scan and lung function tests in 6 months. 14 months after BMT, she developed pulmonary graft-versus-host disease with bronchiolitis obliterans, but is currently doing well with minor dyspnoea on exertion.
More specific treatments are currently being developed in animal models with promising results [5, 28, 29]. Indeed, studies in mice gave successful results with a cell transplantation approach. Wild-type or gene-corrected bone-marrow-derived macrophages were administered directly to the lungs of Csf2ra or Csf2rb null mice. The procedure was well tolerated. Macrophages persisted for >1 year after a single administration and allowed persistent phenotype correction. It is possible to generate genetically corrected macrophages from patient-derived iPSCs [17, 18]. According to a recent review published by Trapnell et al. [9], a clinical trial employing these techniques to conduct pulmonary macrophage transplantation in human patients should be planned in the near future.
MARS mutations
ILD related to mutations in the MARS gene was first described in 2013 in a 6-month-old female presenting with failure to thrive, liver disease, hypothyroidism, anaemia, thrombocytopenia, developmental delay and hypotonia (table 3) [30]. Pulmonary phenotype was only succinctly described in this report with no imaging or pathological data. In 2015, Hadchouel et al. [31] identified recurrent biallelic mutations in MARS that cause a specific type of PAP prevalent on Réunion. They also reported two other mutations that displayed the same phenotype [31]. Since then, five other patients with four different genotypes were reported [33–36]. Transmission is autosomal recessive, with patients being homozygous or compound heterozygous. MARS mutations were also involved in Charcot-Marie-Tooth type 2U, which is a slowly progressive autosomal dominant neurological disorder, in seven patients reviewed previously [37].
TABLE 3.
Characteristics of patients harbouring MARS mutations
| Van Meel [ 30 ] | Hadchouel [ 31, 32 ] | Sun [ 33 ] | Rips [ 34 ] | Abuduxikuer [ 35 ] | Alzaid [ 36 ] | |
| Mutations# and location on the protein structure | F370L/I523T CAT |
Y344C/A393T/ S567L/D605V CAT |
D145N/F802S ABD/PBD |
Y307C/R618C CAT |
R299_S300insR/Q720# CAT/ABD |
I285Y CAT |
| Subjects | 1 male, age 1 month | 15 males and 7 female, age 2.8 (0.5–72) months 3 males and 1 female, ages 2 (1–2.5) months 1 male, age 10 months 1 male and 1 female, age 3.6 years and 3.9 years |
2 males, age 1 month | 1 male, neonatal period | 1 female, age 5 months | 1 male, neonatal period |
| Country | USA | Réunion, n=22 Comoros n=4 Caucasian/Réunion n=1 Tunisia n=2 |
China | Israel from Jewish Moroccan/Tunisian/Persian descent | China | Saudi Arabia |
| Lung involvement | ILD¶ | PAP | ILD, fibrosis¶ | ILD with foamy macrophages | ILD, compatible with PAP on chest CT scan, no BAL | PAP |
| Other features | FTT, HMG, cholestasis, liver failure, hypothyroidism, anaemia, hypotonia, developmental delay, acidosis, aminoaciduria | FTT, HMG, cholestasis, anaemia, inflammation with hyperleukocytosis, thrombocytosis, and high IgG level, hypoalbuminaemia | FTT, anaemia HMG, liver failure, acidosis, hypotonia, developmental delay | Anaemia, hypothyroidism, HMG, cholestasis, liver failure, developmental delay, aminoaciduria | FTT, cholestasis, liver failure, HMG, inflammation, anaemia, thrombocytosis, hyperleukocytosis, prolonged fever, kidney stones, developmental delay, acetabular dysplasia | FTT, intermittent fever, hypotonia, HMG, hypoglycaemia, hypothyroidism, anaemia, thrombopenia |
| Last follow-up | Age 3.5 years, stable on TPN and nasal oxygen | Death: n=13, 1.5 (0.4–25.2) years Asymptomatic: n=6, 6.3 (4.2–18.1) years Symptomatic no oxygen: n=2, 5.2 and 22.3 years CRF+: n=8, 10.6 (1.1–24.9) years |
Death: n=1 at 9 months Stable: n=1 at 4.2 years |
Age 1 year, improvement under methionine supplementation | Death at 11 months | Death at 6 months |
| Functional studies | Reduced aminoacylation activity, normal association with MSC | Reduced aminoacylation activity, normal association with MSC | None | Growth arrest in mutated yeast | None | None |
Data are presented as median (min–max), unless otherwise stated. CAT: catalytic domain; ABD: anticodon-binding domain; PBD: protein-binding domain; ILD: interstitial lung disease; CT: computed tomography; PAP: pulmonary alveolar proteinosis; BAL: bronchoalveolar lavage; FTT: failure to thrive; HMG: hepatomegaly; Ig immunoglobulin; MSC: multiaminoacyl-tRNA synthetase complex; TPN: total parenteral nutrition; CRF: chronic respiratory failure. #: protein nomenclature is given; ¶: no imaging or pathological data given; +: chronic respiratory failure with oxygen therapy either continuous, nocturnal or on exertion.
Pathogenesis
MARS encodes the cytosolic methionine tRNA synthetase (MetRS), which belongs to the class 1 family of aminoacyl-tRNA synthetases (ARSs). These enzymes play a critical role in protein biosynthesis by charging tRNAs with their cognate amino acids, leading to the formation of aminoacyl-tRNA. As with other several ARSs, MetRS has also an editing and proofreading function in order to ensure translational fidelity. Indeed, MetRS is able to discriminate its native methionine substrate from mis-activated amino acids, such as the nonstandard and highly toxic amino acid homocysteine, and its oxygen analogue, homoserine, by a substrate-assisted and enzymatic pre-transfer editing [38]. Finally, MetRS is also, with other ARSs, a component of a cytosolic multiprotein complex (multi-aminoacyl-tRNA synthetase complex (MSC)) with multiple roles described in immune response, inflammation, tumorigenesis, angiogenesis and neuronal homeostasis [39, 40]. The mechanisms that lead from mutations in MARS to a PAP phenotype are not yet completely understood. Regarding the mutations identified by Hadchouel et al. [31], all the variants are located in the catalytic domain of the protein and their functional consequences were first assessed by growth of wild-type and mutant strains and methionine incorporation assays in yeast. Yeast growth and enzyme activity were significantly reduced in yeast transfected with the mutated alleles compared to wild-type when cultured in a liquid medium without methionine [31]. Growth and enzyme activity were restored by methionine supplementation in the culture medium [31]. Functional studies were recently completed by catalytic parameters measurements and structural studies of the MSC [32]. This work confirmed the significant impact of the mutants on the rate of the aminoacylation reaction (reduction of the catalytic constant (kcat) by 5- to 6-fold relative to wild-type), especially at the level of methionine recognition, as shown by a significant increase in the Michaelis constant (KM) for methionine for all the mutants [32]. In addition, co-immunoprecipitation experiments showed that these mutations do not alter the ability of MetRS to associate with the other components of the MSC [32].
Regarding the other reports, mutations reported by Van Meel et al. [30] led to a reduced aminoacylation activity but did not affect the association of MetRS with the MSC. One of the two mutations reported by Rips et al. [34] led to an arrest in yeast growth. No functional studies were performed for the other published mutations [33, 35, 36]. Taken together, these results suggest that a deficiency of MetRS activity, an enzyme essential for protein synthesis at the levels of initiation and elongation of translation, would result in PAP, putatively through reduced aminoacylation and deficient translation to ensure adequate surfactant composition or homeostasis. In addition, two other diseases argue for the importance of intracellular amino acids contents and ARSs in lung homeostasis. First, PAP may also occur in lysinuric protein intolerance (described below), where a defective cationic amino acid transporter results in leakage of cationic amino acids [41]. Secondly, auto-antibodies against ARSs are responsible for the anti-synthetase syndrome that associates with variable degrees of ILD, myositis, inflammatory arthritis, mechanic's hands, Raynaud's phenomenon and fever, with pulmonary involvement being the major prognostic factor [42].
Clinical presentation and diagnosis of PAP
Characteristics of the patients are summarised in table 3. Mutations in MARS are responsible for multisystemic disease, often referred to as the interstitial lung and liver disease syndrome (OMIM 615486). First symptoms occurred very early in infancy, with a median age of 2months. Patients share common features, including failure to thrive, which is often the first manifestation, liver involvement and anaemia. Frequent but nonconstant features are developmental delay, hypotonia and hypothyroidism. Lung involvement is constant but not always precisely described in reports. PAP was confirmed by Hadchouel et al. [31], who reported the largest series with four different mutations with typical aspects on chest CT scan and bronchoalveolar lavage (figure 1g–l), and in the case report from Alzaid et al. [36]. Chest CT scan of the patient reported by Abuduxikuer et al. [35] showed typical crazy-paving pattern that was very suggestive of PAP. Although Rips et al. [34] mentioned that their patient had ILD without PAP, analysis of the BALF showed foamy macrophages. Regarding the other organs involved, bone marrow aspiration, when performed, showed an arrest of red blood cells maturation in two cases [30, 33]. Liver biopsy, performed in all but two cases [33, 36], showed cholestasis, steatosis and variable degrees of fibrosis with six patients reported by Enaud et al. [43] having cirrhosis.
Molecular diagnosis
Known mutations are listed in table 3. A total of 13 different mutations was identified and all the mutations were discovered by exome sequencing. 11 were missense mutations, one was a nonsense mutation and the last one was an insertion of an arginine at position 299. 10 mutations were located in the catalytic domain, two in the anticodon binding domain and one in the protein-binding domain.
Disease course and management
Repetitive WLLs were performed in 26 out of 29 patients reported by Hadchouel et al. [31]. In a previous report of 34 patients from Réunion, Enaud et al. [43] showed that, even if WLLs allowed the younger patients to reach childhood, this procedure did not significantly change global survival rates. In the series by Hadchouel et al. [31], systemic steroids were used in 14 patients, and other immunosuppressive drugs (hydroxychloroquine, cyclophosphamide, azathioprine and mycophenolate mofetil) were used in four patients. The patient reported by Rips et al. [34] was treated by hydroxychloroquine and methionine supplementation. Efficacy of steroids and immunosuppressive drugs was highly variable but never led to a complete remission of the disease. Methionine supplementation was associated with a global improvement with weaning off daytime oxygen, less frequent hospitalisations and weight gain [34]. This treatment was decided based on the results obtained in yeast where growth and enzymatic activity were restored by methionine supplementation in the culture medium [31]. A trial of methionine supplementation in patients harbouring the biallelic mutations Ala393Thr/Ser567Leu is currently underway (ClinicalTrials.gov identifier: NCT03887169).
The other reported patients did not receive any specific treatment. Many patients required nutritional support (enteral feeding with gastrostomy and/or parenteral nutrition) and repetitive blood transfusions. Five patients from Réunion underwent lung transplantation: four died (three shortly after surgery) and the other 18 months after transplantation. One is still alive with a current follow-up of 6 months (personal communication).
Disease is severe with an overall mortality rate of 46% (16 out of 35), with most of deaths occurring in the first years of life. The recurrent biallelic mutations isolated in patients originated from Réunion were associated with an evolution toward lung fibrosis that was histologically documented in 19 patients [43].
PAP and ILD in other ARS mutations
Two other ARSs were recently identified in infants and children presenting with a multisystemic disorder including PAP: the isoleucine tRNA synthetase (IARS gene) and the β-subunit of the phenylalanine tRNA synthetase (FARSB gene) [44, 45]. FARSB mutations were also identified in patients with a multisystemic phenotype that included ILDs different from PAP [46, 47]. Others ILDs were also described in other ARS gene mutations, namely YARS (tyrosine tRNA synthetase) [48, 49], LARS (leucine tRNA synthetase) [44] and FARSA (α-subunit of the phenylalanine synthetase) [50].
Monogenic immune deficiencies associated with PAP
Monogenic immune deficiencies that can be frequently associated with PAP are listed in table 4. GATA2 deficiency is responsible for the so-called MonoMAC syndrome [51]. GATA2 is a transcription factor that acts as a critical regulator of gene expression in haematopoietic cells. In vitro studies showed that GATA2 regulates alveolar macrophage phagocytosis [56]. Therefore, PAP in GATA2 deficiency must reflect alveolar macrophage dysfunction rather than a quantitative deficit. Adenosine deaminase deficiency leads to an accumulation of toxic purine degradation by-products [52, 53]. The mechanisms responsible for PAP in this disease are still undetermined. Recently, Cho et al. [54] described a novel form of inherited PAP associated with hypogammaglobulinaemia in three siblings and two unrelated infants due to heterozygous OAS1 gain-of-function variants. The OAS1 protein is a member of the 2-5A synthetase family, involved in the innate immune response to viral infections. The mechanisms by which those mutations cause PAP are unknown, but the authors speculated that those gain-of-function mutations might be associated with exaggerated immune response, especially in alveolar macrophages in response to viral infections, leading to dysfunction of alveolar macrophages and impaired catabolism of lung surfactant. This hypothesis was, in part, suggested by the fact that the onset of PAP in those patients was triggered by viral infections [54].
TABLE 4.
Monogenic immune deficiencies and metabolic disorders having pulmonary alveolar proteinosis (PAP) as a frequent pulmonary manifestation
| Disease | Gene | Transmission | PAP frequency | Other features | |
| Immune deficiency | GATA2 deficiency/MonoMAC syndrome | GATA2 | AD, haploinsufficiency | 18% in one series [51] | Monocytopenia, mycobacterial infections, increased susceptibility to myelodysplastic syndromes and acute myeloid leukaemia, lymphedema, |
| Adenosine deaminase deficiency [52] | ADA | AR | 43.8% in one series [53] | SCID, neurodevelopmental deficits, sensorineural deafness and skeletal abnormalities | |
| Infantile-onset pulmonary alveolar proteinosis with hypogammaglobulinaemia [54] |
OAS1 | AD | Unknown | Hypogammaglobulinaemia, splenomegaly, recurrent bacterial and viral infections | |
| Metabolic disorder | Lysinuric protein intolerance | SLC7A7 | AR | 62.5% in one series [55] | Failure to thrive, hepato-splenomegaly, renal, neurological, musculoskeletal and haematological involvements |
AD: autosomal dominant; AR: autosomal recessive; SCID: severe combined immunodeficiency.
PAP was also reported in case reports in other monogenic immune deficiencies and haematological disorders such as agammaglobulinaemia, Di George syndrome, selective immunoglobulin (Ig)A deficiency, X-linked hyper IgM syndrome and Fanconi's anaemia.
Genetic metabolic disorders associated with PAP
Monogenic metabolic disorders that can display PAP features are listed in table 4. Lysinuric protein intolerance is characterised by a defective cationic amino acid transport in the intestine and kidney, leading to aminoaciduria with high arginine, ornithine and lysine urinary excretion [55]. Pulmonary involvement is not constant but always presents as PAP that may be life threatening [42]. The SLC7A7 transporter is also expressed in macrophages and its expression is induced by GM-CSF. A severe impairment of the phagocytic activity was shown in macrophages from lysinuric protein intolerance patients [57] and may be the mechanism leading to PAP by deficient surfactant clearance from alveolar macrophages. PAP was also reported in some cases of Niemann–Pick disease type C2 [58, 59] and B [60].
Surfactant protein disorders
Mutations in SFTPB, SFTPC, ABCA3 and NKX2.1 disrupt the production and function of surfactant and are responsible for ILDs. Those disorders are associated with varying levels of surfactant accumulation and may be revealed by a PAP pattern, often accompanied by marked parenchymal distortion and fibrosis. As a consequence, PAP diagnostic work-up in children should include screening for mutations in genes required for production of surfactant.
Diagnostic algorithm
PAP is suspected when a patient presents with respiratory symptoms and a crazy-paving appearance on a chest CT. Diagnosis is confirmed by PAS staining of BALF or rarely, histological examination of lung biopsy specimens. The age of the patient and the presence of extra-respiratory symptoms may help the clinician and orientate towards specific causes. Autoimmune PAP will be diagnosed, especially in adults and is confirmed by the presence of auto-antibodies against GM-CSF in the serum and BALF. Secondary causes such as immune deficiencies, infections, haematological disorders, metabolic diseases or toxic aetiologies will be suspected according to the past medical history of the patient and the presence of specific extra-respiratory symptoms. Genetic mutations will be suspected, especially in children. Surfactant protein disorders (SFTPB, SFTPC, ABCA3 and NKX2.1) must be screened. Then, when PAP is isolated, mutations in CSF2RA and CSF2RB are initially suspected. When PAP is associated with liver involvement and systemic inflammation, MARS mutations are suspected. Genetic diagnostic strategy can rely on targeted gene Sanger sequencing, starting from the most suspected gene and progressing towards other known genes. However, nowadays, next-generation sequencing panels represent an efficient diagnostic tool and, for PAP, such panels include all the genes that may be involved.
Conclusion
PAP is a rare respiratory disorder for which several monogenic causes have been identified in recent decades. Genetic PAP occurs in young children and is often associated with a poor prognosis. Next-generation sequencing panels represent an efficient diagnostic tool. Exome sequencing studies already allow the discovery of new variants and genes in this setting and will surely continue to bring new insights in this rare disease in the future. Current treatment relies on WLLs but their efficacy largely depends on the underlying gene involved. In some cases, BMT may be an option. Innovative treatments are currently under development with promising results in animal models for transplantation of mature alveolar macrophages in patients with CSF2RA and CSF2RB mutations, and solid preliminary basic science data for methionine supplementation in MARS patients.
Footnotes
Number 2 in the Series “Rare genetic interstitial lung diseases” Edited by Bruno Crestani and Raphaël Borie
Previous articles in the Series: No. 1: Daccord C, Good J-M, Morren M-A, et al. Brit–Hogg–Dubé syndrome. Eur Respir Rev 2020; 29: 200042.
Provenance: Commissioned article, peer reviewed.
Conflict of interest: A. Hadchouel reports personal fees from AstraZeneca, Chiesi and Novartis, outside the submitted work.
Conflict of interest: D. Drummond has nothing to disclose.
Conflict of interest: R. Abou Taam has nothing to disclose.
Conflict of interest: M. Le Bourgeois has nothing to disclose.
Conflict of interest: C. Delacourt has nothing to disclose.
Conflict of interest: J. de Blic has nothing to disclose.
References
- 1.Rosen SH, Castleman B, Liebow AA. Pulmonary alveolar proteinosis. N Engl J Med 1958; 258: 1123–1142. doi: 10.1056/NEJM195806052582301 [DOI] [PubMed] [Google Scholar]
- 2.Holbert JM, Costello P, Li W, et al. CT features of pulmonary alveolar proteinosis. AJR Am J Roentgenol 2001; 176: 1287–1294. doi: 10.2214/ajr.176.5.1761287 [DOI] [PubMed] [Google Scholar]
- 3.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med 2003; 349: 2527–2539. doi: 10.1056/NEJMra023226 [DOI] [PubMed] [Google Scholar]
- 4.Stanley E, Lieschke GJ, Grail D, et al. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA 1994; 91: 5592–5596. doi: 10.1073/pnas.91.12.5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arumugam P, Suzuki T, Shima K, et al. Long-term safety and efficacy of gene-pulmonary macrophage transplantation therapy of PAP in Csf2ra-/- mice. Mol Ther 2019; 27: 1597–1611. doi: 10.1016/j.ymthe.2019.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robb L, Drinkwater CC, Metcalf D, et al. Hematopoietic and lung abnormalities in mice with a null mutation of the common β subunit of the receptors for granulocyte-macrophage colony-stimulating factor and interleukins 3 and 5. Proc Natl Acad Sci USA 1995; 92: 9565–9569. doi: 10.1073/pnas.92.21.9565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishinakamura R, Nakayama N, Hirabayashi Y, et al. Mice deficient for the IL-3/GM-CSF/IL-5 β c receptor exhibit lung pathology and impaired immune response, while β IL3 receptor-deficient mice are normal. Immunity 1995; 2: 211–222. doi: 10.1016/1074-7613(95)90046-2 [DOI] [PubMed] [Google Scholar]
- 8.Nishinakamura R, Wiler R, Dirksen U, et al. The pulmonary alveolar proteinosis in granulocyte macrophage colony-stimulating factor/interleukins 3/5 β c receptor-deficient mice is reversed by bone marrow transplantation. J Exp Med 1996; 183: 2657–2662. doi: 10.1084/jem.183.6.2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trapnell BC, Nakata K, Bonella F, et al. Pulmonary alveolar proteinosis. Nat Rev Dis Primer 2019; 5: 16. doi: 10.1038/s41572-019-0066-3 [DOI] [PubMed] [Google Scholar]
- 10.Lehtonen A, Matikainen S, Miettinen M, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF)-induced STAT5 activation and target-gene expression during human monocyte/macrophage differentiation. J Leukoc Biol 2002; 71: 511–519. [PubMed] [Google Scholar]
- 11.Shibata Y, Berclaz PY, Chroneos ZC, et al. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 2001; 15: 557–567. [DOI] [PubMed] [Google Scholar]
- 12.Schneider C, Nobs SP, Kurrer M, et al. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat Immunol 2014; 15: 1026–1037. doi: 10.1038/ni.3005 [DOI] [PubMed] [Google Scholar]
- 13.Bonfield TL, Raychaudhuri B, Malur A, et al. PU.1 regulation of human alveolar macrophage differentiation requires granulocyte-macrophage colony-stimulating factor. Am J Physiol Lung Cell Mol Physiol 2003; 285: L1132–L1136. [DOI] [PubMed] [Google Scholar]
- 14.Moore KJ, Rosen ED, Fitzgerald ML, et al. The role of PPAR-γ in macrophage differentiation and cholesterol uptake. Nat Med 2001; 7: 41–47. doi: 10.1038/83328 [DOI] [PubMed] [Google Scholar]
- 15.Sallese A, Suzuki T, McCarthy C, et al. Targeting cholesterol homeostasis in lung diseases. Sci Rep 2017; 7: 10211. doi: 10.1038/s41598-017-10879-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy MA, Barrera GC, Nakamura K, et al. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab 2005; 1: 121–131. doi: 10.1016/j.cmet.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 17.Suzuki T, Mayhew C, Sallese A, et al. Use of induced pluripotent stem cells to recapitulate pulmonary alveolar proteinosis pathogenesis. Am J Respir Crit Care Med 2014; 189: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lachmann N, Happle C, Ackermann M, et al. Gene correction of human induced pluripotent stem cells repairs the cellular phenotype in pulmonary alveolar proteinosis. Am J Respir Crit Care Med 2014; 189: 167–182. [DOI] [PubMed] [Google Scholar]
- 19.Hildebrandt J, Yalcin E, Bresser H-G, et al. Characterization of CSF2RA mutation related juvenile pulmonary alveolar proteinosis. Orphanet J Rare Dis 2014; 9: 171. doi: 10.1186/s13023-014-0171-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki T, Sakagami T, Young LR, et al. Hereditary pulmonary alveolar proteinosis: pathogenesis, presentation, diagnosis, and therapy. Am J Respir Crit Care Med 2010; 182: 1292–1304. doi: 10.1164/rccm.201002-0271OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santiago-Burruchaga M, Zalacain-Jorge R, Alvarez-Martinez J, et al. Hereditary pulmonary alveolar proteinosis. Could it be triggered by Mycoplasma pneumoniae pneumonia? Respir Med 2013; 107: 134–138. doi: 10.1016/j.rmed.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 22.Auger J, Bonnet C, Valduga M, et al. De novo complex X chromosome rearrangement unmasking maternally inherited CSF2RA deletion in a girl with pulmonary alveolar proteinosis. Am J Med Genet A 2013; 161A: 2594–2599. [DOI] [PubMed] [Google Scholar]
- 23.Chiu C-Y, Su S-C, Fan W-L, et al. Whole-genome sequencing of a family with hereditary pulmonary alveolar proteinosis identifies a rare structural variant involving CSF2RA/CRLF2/IL3RA gene disruption. Sci Rep 2017; 7: 43469. doi: 10.1038/srep43469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Haidary AS, Alotaibi W, Alhaider SA, et al. A newly identified novel variant in the CSF2RA gene in a child with pulmonary alveolar proteinosis: a case report. J Med Case Reports 2017; 11: 122. doi: 10.1186/s13256-017-1285-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki T, Maranda B, Sakagami T, et al. Hereditary pulmonary alveolar proteinosis caused by recessive CSF2RB mutations. Eur Respir J 2011; 37: 201–204. doi: 10.1183/09031936.00090610 [DOI] [PubMed] [Google Scholar]
- 26.Tanaka T, Motoi N, Tsuchihashi Y, et al. Adult-onset hereditary pulmonary alveolar proteinosis caused by a single-base deletion in CSF2RB. J Med Genet 2011; 48: 205–209. doi: 10.1136/jmg.2010.082586 [DOI] [PubMed] [Google Scholar]
- 27.Frémond M-L, Hadchouel A, Schweitzer C, et al. Successful haematopoietic stem cell transplantation in a case of pulmonary alveolar proteinosis due to GM-CSF receptor deficiency. Thorax 2018; 73: 590–592. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki T, Arumugam P, Sakagami T, et al. Pulmonary macrophage transplantation therapy. Nature 2014; 514: 450–454. doi: 10.1038/nature13807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhn A, Ackermann M, Mussolino C, et al. TALEN-mediated functional correction of human iPSC-derived macrophages in context of hereditary pulmonary alveolar proteinosis. Sci Rep 2017; 7: 15195. doi: 10.1038/s41598-017-14566-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Meel E, Wegner DJ, Cliften P, et al. Rare recessive loss-of-function methionyl-tRNA synthetase mutations presenting as a multi-organ phenotype. BMC Med Genet 2013; 14: 106. doi: 10.1186/1471-2350-14-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hadchouel A, Wieland T, Griese M, et al. Biallelic mutations of methionyl-tRNA synthetase cause a specific type of pulmonary alveolar proteinosis prevalent on Réunion Island. Am J Hum Genet 2015; 96: 826–831. doi: 10.1016/j.ajhg.2015.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Comisso M, Hadchouel A, de Blic J, et al. Mutations in MARS identified in a specific type of pulmonary alveolar proteinosis alter methionyl-tRNAsynthetase activity. FEBS J 2018; 285: 2654–2661. doi: 10.1111/febs.14510. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Hu G, Luo J, et al. Mutations in methionyl-tRNA synthetase gene in a Chinese family with interstitial lung and liver disease, postnatal growth failure and anemia. J Hum Genet 2017; 62: 647–651. doi: 10.1038/jhg.2017.10 [DOI] [PubMed] [Google Scholar]
- 34.Rips J, Meyer-Schuman R, Breuer O, et al. MARS variant associated with both recessive interstitial lung and liver disease and dominant Charcot-Marie-Tooth disease. Eur J Med Genet 2018; 61: 616–620. doi: 10.1016/j.ejmg.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abuduxikuer K, Feng J-Y, Lu Y, et al. Novel methionyl-tRNA synthetase gene variants/phenotypes in interstitial lung and liver disease: a case report and review of literature. World J Gastroenterol 2018; 24: 4208–4216. doi: 10.3748/wjg.v24.i36.4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alzaid M, Alshamrani A, Al Harbi A, et al. Methionyl-tRNA synthetase novel mutation causes pulmonary alveolar proteinosis. Saudi Med J 2019; 40: 195–198. doi: 10.15537/smj.2019.2.23908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillespie MK, McMillan HJ, Kernohan KD, et al. A novel mutation in MARS in a patient with Charcot-Marie-Tooth Disease, axonal, type 2U with congenital onset. J Neuromuscul Dis 2019; 6: 333–339. doi: 10.3233/JND-190404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fortowsky GB, Simard DJ, Aboelnga MM, et al. Substrate-assisted and enzymatic pretransfer editing of nonstandard amino acids by methionyl-tRNA synthetase. Biochemistry 2015; 54: 5757–5765. doi: 10.1021/acs.biochem.5b00588 [DOI] [PubMed] [Google Scholar]
- 39.Guo M, Schimmel P. Essential nontranslational functions of tRNA synthetases. Nat Chem Biol 2013; 9: 145–153. doi: 10.1038/nchembio.1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao P, Fox PL. Aminoacyl-tRNA synthetases in medicine and disease. EMBO Mol Med 2013; 5: 332–343. doi: 10.1002/emmm.201100626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valimahamed-Mitha S, Berteloot L, Ducoin H, et al. Lung involvement in children with lysinuric protein intolerance. J Inherit Metab Dis 2015; 38: 257–263. doi: 10.1007/s10545-014-9777-5. [DOI] [PubMed] [Google Scholar]
- 42.Lega J-C, Fabien N, Reynaud Q, et al. The clinical phenotype associated with myositis-specific and associated autoantibodies: a meta-analysis revisiting the so-called antisynthetase syndrome. Autoimmun Rev. 2014; 13: 883–891. [DOI] [PubMed] [Google Scholar]
- 43.Enaud L, Hadchouel A, Coulomb A, et al. Pulmonary alveolar proteinosis in children on La Réunion Island: a new inherited disorder? Orphanet J Rare Dis 2014; 9: 85. doi: 10.1186/1750-1172-9-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuchs SA, Schene IF, Kok G, et al. Aminoacyl-tRNA synthetase deficiencies in search of common themes. Genet Med 2019; 21: 319–330. doi: 10.1038/s41436-018-0048-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Z, Lo W-S, Beck DB, et al. Bi-allelic mutations in Phe-tRNA synthetase associated with a multi-system pulmonary disease support non-translational function. Am J Hum Genet 2018; 103: 100–114. doi: 10.1016/j.ajhg.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zadjali F, Al-Yahyaee A, Al-Nabhani M, et al. Homozygosity for FARSB mutation leads to Phe-tRNA synthetase-related disease of growth restriction, brain calcification, and interstitial lung disease. Hum Mutat 2018; 39: 1355–1359. doi: 10.1002/humu.23595 [DOI] [PubMed] [Google Scholar]
- 47.Antonellis A, Oprescu SN, Griffin LB, et al. Compound heterozygosity for loss-of-function FARSB variants in a patient with classic features of recessive aminoacyl-tRNA synthetase-related disease. Hum Mutat 2018; 39: 834–840. doi: 10.1002/humu.23424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nowaczyk MJM, Huang L, Tarnopolsky M, et al. A novel multisystem disease associated with recessive mutations in the tyrosyl-tRNA synthetase (YARS) gene. Am J Med Genet A 2017; 173: 126–134. doi: 10.1002/ajmg.a.37973 [DOI] [PubMed] [Google Scholar]
- 49.Williams KB, Brigatti KW, Puffenberger EG, et al. Homozygosity for a mutation affecting the catalytic domain of tyrosyl-tRNA synthetase (YARS) causes multisystem disease. Hum Mol Genet 2019; 28: 525–538. doi: 10.1093/hmg/ddy344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krenke K, Szczałuba K, Bielecka T, et al. FARSA mutations mimic phenylalanyl-tRNA synthetase deficiency caused by FARSB defects. Clin Genet 2019; 96: 468–472. doi: 10.1111/cge.13614 [DOI] [PubMed] [Google Scholar]
- 51.Spinner MA, Sanchez LA, Hsu AP, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood 2014; 123: 809–821. doi: 10.1182/blood-2013-07-515528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flinn AM, Gennery AR. Adenosine deaminase deficiency: a review. Orphanet J Rare Dis 2018; 13: 65. doi: 10.1186/s13023-018-0807-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grunebaum E, Cutz E, Roifman CM. Pulmonary alveolar proteinosis in patients with adenosine deaminase deficiency. J Allergy Clin Immunol 2012; 129: 1588–1593. doi: 10.1016/j.jaci.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 54.Cho K, Yamada M, Agematsu K, et al. Heterozygous mutations in OAS1 cause infantile-onset pulmonary alveolar proteinosis with hypogammaglobulinemia. Am J Hum Genet 2018; 102: 480–486. doi: 10.1016/j.ajhg.2018.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mauhin W, Habarou F, Gobin S, et al. Update on lysinuric protein intolerance, a multi-faceted disease retrospective cohort analysis from birth to adulthood. Orphanet J Rare Dis 2017; 12: 3. doi: 10.1186/s13023-016-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lasbury ME, Tang X, Durant PJ, et al. Effect of transcription factor GATA-2 on phagocytic activity of alveolar macrophages from Pneumocystis carinii-infected hosts. Infect Immun 2003; 71: 4943–4952. doi: 10.1128/IAI.71.9.4943-4952.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barilli A, Rotoli BM, Visigalli R, et al. Impaired phagocytosis in macrophages from patients affected by lysinuric protein intolerance. Mol Genet Metab 2012; 105: 585–589. doi: 10.1016/j.ymgme.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 58.Sheth J, Joseph JJ, Shah K, et al. Pulmonary manifestations in Niemann–Pick type C disease with mutations in NPC2 gene: case report and review of literature. BMC Med Genet 2017; 18: 5. doi: 10.1186/s12881-017-0367-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griese M, Brasch F, Aldana VR, et al. Respiratory disease in Niemann–Pick type C2 is caused by pulmonary alveolar proteinosis. Clin Genet 2010; 77: 119–130. doi: 10.1111/j.1399-0004.2009.01325.x [DOI] [PubMed] [Google Scholar]
- 60.Sideris GA, Josephson M. Pulmonary alveolar proteinosis and Niemann–Pick disease type B: an unexpected combination. Respir Med Case Rep 2016; 19: 37–39. [DOI] [PMC free article] [PubMed] [Google Scholar]