Abstract
Background
Given the impact of new antiretroviral drugs on weight and metabolic parameters, their potential contribution to the development of liver steatosis is of concern. We investigated the determinants of liver steatosis in patients on antiretroviral therapy (ART) in the Swiss HIV Cohort Study (SHCS).
Methods
Between 2019 and 2021, we measured liver stiffness and controlled attenuation parameter (CAP) using transient elastography in consecutive SHCS participants at Bern University Hospital. Individuals with viral hepatitis coinfection and pregnant women were excluded. We used multivariable logistic regression to explore factors associated with steatosis.
Results
Of 416 participants, 113 (27.2%) were female, median age was 51 years (interquartile range [IQR], 43–59), 305 (73.3%) were of European origin, and 212 (51.0%) were overweight/obese (body mass index [BMI] ≥25 kg/m2). Liver steatosis (CAP ≥248 dB/m) was present in 212 (51.0%) participants, 11 (5.2%) of whom had significant fibrosis or cirrhosis. One hundred seventy-nine (43.0%) met the criteria for metabolic-associated fatty liver disease (MAFLD). Among 64 individuals with a BMI <25 kg/m2 and liver steatosis, 31 (48.4%) had MAFLD. In multivariable analyses, BMI ≥25 kg/m2 (adjusted odds ratio, 5.76; 95% confidence interval, 3.57–9.29), age ≥50 years (1.88, 1.14–3.09), European origin (3.16, 1.69–5.89), and current use of tenofovir alafenamide (1.70, 1.08–2.69) were associated with liver steatosis. Exposure to integrase inhibitors was not associated with liver steatosis (0.83, 0.51–1.37).
Conclusions
Our findings suggest a high prevalence of liver steatosis among people with HIV (PWH) on ART in Switzerland. In addition to established risk factors, the use of tenofovir alafenamide was associated with hepatic steatosis.
Keywords: antiretroviral therapy, liver steatosis, metabolic-associated fatty liver disease, metabolic comorbidities, people with HIV
We found a high prevalence of liver steatosis among people with HIV on antiretroviral therapy in Switzerland. In addition to well known risk factors such as age, ethnicity, and obesity, the use of tenofovir alafenamide was associated with hepatic steatosis.
Liver steatosis is a growing public health concern, with an estimated global prevalence of 25% [1]. It encompasses a spectrum of conditions ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), and it can progress to liver fibrosis, cirrhosis, and its complications [2]. Liver steatosis appears to be more frequent in people with human immunodeficiency virus (PWH) compared to the general population: recent cohort studies from Canada and Brazil showed that more than 1 in 3 participants had liver steatosis based on controlled attenuation parameter (CAP) measurements using transient elastography (TE) [3]. The progression of liver disease seems faster and its disease severity higher among PWH, potentially due to a combination of persistent immune activation, bacterial translocation, as well as the high prevalence of metabolic comorbidities in this population [4, 5].
The potential contribution of new antiretroviral therapy (ART) combinations to liver steatosis has been raised, given recent data on their metabolic side effects. For instance, integrase strand inhibitors (InSTIs) and tenofovir alafenamide (TAF) have been associated with weight increases [6–9]. Furthermore, in the Swiss HIV Cohort Study (SHCS), a switch from tenofovir disoproxil fumarate (TDF) to TAF was also associated with increases in serum lipids [10]. In a recently published study from Germany, 49% of PWH had liver steatosis and exposure to TAF, and InSTI was associated with an increased risk of de novo steatosis as well as progression to NASH [9].
Although liver steatosis is closely associated with overweight/obese and metabolic syndrome, it appears to be present in a significant proportion of lean PWH, defined as a body mass index (BMI) <25 kg/m2. Among individuals with biopsy-confirmed steatosis, the mean BMI was lower in PWH compared to HIV-negative participants, suggesting a higher prevalence of “lean steatosis” in PWH [11, 12]. In a recent multicountry study of PWH, liver steatosis was present in 24% of lean participants [13]. However, data regarding the impact of specific ART components on liver steatosis among PWH with normal BMI are lacking. We aimed (1) to describe the determinants of liver steatosis among PWH in a single center of the SHCS and (2) to describe the proportion of individuals with metabolic dysfunction-associated fatty liver disease (MAFLD), a concept recently endorsed by international liver diseases associations [14].
MATERIALS AND METHODS
Study Design and Population
Between November 2019 and August 2021, we tested consecutive participants from the SHCS at Bern University Hospital for the presence of liver steatosis and fibrosis. The SHCS (www.shcs.ch) is a nationally representative prospective cohort study and includes approximately 80% of all PWH receiving ART in Switzerland [15]. Demographic, clinical, and laboratory data as well as changes in ART regimens, comedications, and behavioral data are prospectively recorded at registration and every 6 months thereafter, using a standardized protocol. Patients with active or past viral hepatitis coinfection, defined as having a positive hepatitis C virus (HCV) antibody test or positive hepatitis B virus (HBV) surface antigen, as well as pregnant women were excluded from the study.
Transient Elastography With Controlled Attenuation Parameter
Transient elastography (Fibroscan 530; Echosens, Paris, France), including liver stiffness measurement (LSM) and CAP, was performed by a single, experienced operator. A minimum of 10 valid measurements with >60% success rate was required for each patient, and TE measurements were considered very reliable if the interquartile range divided by the median (IQR/M) was <0.1, reliable if IQR/M was 0.1–0.3, and poorly reliable if IQR/M was >0.3 [16]. In overweight/obese individuals with a skin-capsular distance of >25 mm, we used the XL probe instead of the M probe. Transient elastography was shown to have 68.8% sensitivity and 82.2% specificity for the diagnosis of liver steatosis grade S1–3 in an individual patient data meta-analysis on CAP accuracy for noninvasive grading of liver steatosis [17]. Liver steatosis was defined as follows: using the M probe, S1 (mild steatosis) if CAP 248–267 dB/m, S2 (moderate steatosis) if CAP 268–279 dB/m, and S3 (severe steatosis) if CAP ≥280 dB/m; or using the XL probe, S1 if 242–266 dB/m, S2 if 267–285 dB/m, and S3 if ≥286 dB/m [17–19]. Liver fibrosis was categorized according to METAVIR-equivalent stages: F0–1 (no or mild fibrosis) if LSM <7.1 kPa, F2–3 (significant fibrosis) if LSM 7.1–11 kPa, and F4 (cirrhosis) if LSM ≥11.1 kPa, as established previously [20].
Outcomes and Definitions
The primary study outcome was the prevalence of liver steatosis, defined as CAP ≥248 dB/m using the M probe and CAP ≥242 dB/m using the XL probe. As a recent study suggested a higher cutoff to define steatosis among PWH, we repeated the analysis using the definition of severe steatosis with a CAP cutoff of 280 dB/m [21]. For the categorization of MAFLD, data on clinical outcomes and laboratory values were collected at the same date as TE. Metabolic-associated fatty liver disease was diagnosed based on the evidence of hepatic steatosis by TE in addition to the presence of type 2 diabetes mellitus, and/or a BMI ≥25 kg/m2, and/or the presence of at least 2 of the following metabolic risk abnormalities: (1) waist circumference ≥102 cm in European men and ≥88 cm in women; ≥ 90 cm in Asian men and ≥80 cm in women; (2) blood pressure ≥130/85 mmHg or antihypertensive treatment; (3) plasma triglycerides ≥150 mg/dL or lipid lowering therapy; (4) plasma high-density lipoprotein (HDL) cholesterol <40 mg/dL (<1.0 mmol/L) for men and <50 mg/dL (<1.3 mmol/L) for women [14]. Elevated transaminases were defined as an alanine aminotransferase (ALT) ≥ 25 U/mL for women and ≥35 U/mL for men [22]. Participants with a BMI ≤24.9 kg/m2 were categorized as lean, and those with a value ≥25 kg/m2 were categorized as overweight/obese. Significant weight gain was defined as weight increase >10% within the last 3 years before TE measurement [23]. Arterial hypertension was defined as 2 measurements ≥140/90 mmHg within 1 year before TE or current antihypertensive treatment, diabetes mellitus was defined as HbA1c ≥ 6.5% or current treatment with antidiabetic medication, and dyslipidemia was defined as total cholesterol to HDL ratio >5 and/or currently being on lipid lowering therapy. A history of cardiovascular disease included the past occurrence of myocardial infarction, cerebral infarction, coronary angioplasty/stenting, coronary artery bypass grafting, or any procedure on peripheral arteries. Hazardous alcohol consumption was defined as an AUDIT-c > 4 points for men and >3 points of women [24].
Statistical Analysis
Continuous variables were expressed as medians and IQRs, and categorical variables were expressed as frequencies. Using logistic regression, we evaluated the association between liver steatosis and the following covariates: BMI (<25 vs ≥25 kg/m2), sex, age (<50 vs ≥50 years), ethnicity (European vs other origin), HIV transmission (men who have sex with men versus other transmission groups), CD4 nadir (<350 vs ≥350 cells/µL), diabetes, dyslipidemia, arterial hypertension, history of cardiovascular disease, hazardous alcohol consumption, current exposure to TAF, InSTI, protease inhibitors (PIs), and zidovudine (AZT)/didanosine (DDI)/stavudine (D4T). We further evaluated the association between liver steatosis and prior exposure to TAF, TDF, InsTI, PI, nonnucleoside reverse-transcriptase inhibitor, and AZT/DDI/D4T. Factors that were associated with liver steatosis (P < .05) in univariable analyses as well as predefined variables (age, sex, exposure to InSTI and TAF) were included in the multivariable model. In sensitivity analyses, we evaluated risk factors for liver steatosis among lean and overweight/obese participants separately. To assess the extent by which the association of ART with steatosis is mediated by changes in weight, we included weight increase >10% on ART as an additional covariate [25]. Statistical analyses were performed using STATA 16.0 (StataCorp, College Station, TX).
Patient Consent Statement
The local ethical committee of the participating center (Kantonale Ethikkommission Bern) approved this cohort study, and all patients provided a written consent to participate in the SHCS.
RESULTS
Study Population
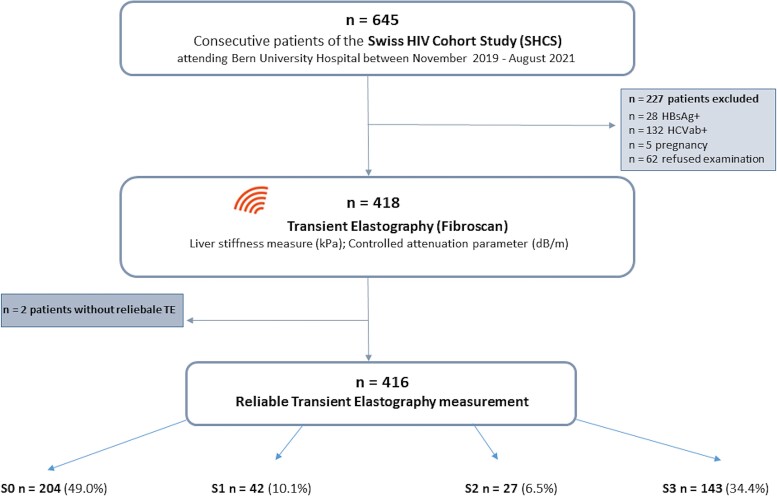
Between November 20, 2019 and August 31, 2021, 645 SHCS participants were evaluated. We excluded 160 patients due to HBV or HCV coinfections and 5 women who were pregnant, whereas 62 (9.6%) individuals declined to participate (Figure 1). Of 416 individuals with a reliable TE measurement, 113 (27.2%) were female and 212 (51.0%) were overweight or obese. The median age of the full study population was 51 years (IQR, 43–59) and median CD4+ count was 767 cells/µL (IQR, 546–935). The proportion of individuals of African origin was 13.2% in the lean and 24.5% in the obese/overweight group (Table 1). Cardiometabolic risk factors were common in the overweight/obese group: 46.2% had dyslipidemia, 34.0% arterial hypertension, and 13.2% diabetes mellitus. The median time on ART was 13 years in lean and 12 years in overweight/obese patients, and a similar proportion of participants was exposed to InSTI in both groups (72% and 69%, respectively). Among lean individuals, 51.0% were on a TAF-containing regimen for a mean duration of 20 months (standard deviation 12.47 months), whereas this proportion was 60.4% for a mean duration of 21 months (12.93 months) among overweight/obese. In both lean and overweight/obese participants, 22% of individuals reported hazardous alcohol consumption.
Figure 1.
Study flow chart. CAP, controlled attenuation parameter; HBsAg, hepatitis B antigen; HCVab+, hepatitis C antibodies; LSM, liver stiffness measurement; TE, transient elastography.
Table 1.
Demographic and Clinical Characteristics of Participants at the Time of Transient Elastography Measurement, Stratified by BMI Category
| Lean (BMI <25 kg/m2) | Overweight/Obese (BMI ≥25 kg/m2) | |
|---|---|---|
| Characteristics | N = 204 | N = 212 |
| Median age, years (IQR) | 51 (42–59) | 52 (46–59) |
| Female sex (%) | 52 (25.5) | 61 (28.8) |
| Region of origin (%) | ||
| Europe | 154 (75.5) | 151 (71.2) |
| Africa | 27 (13.2) | 52 (24.5) |
| Asia | 17 (8.3) | 5 (2.4) |
| Other | 6 (2.9) | 4(1.9) |
| HIV Transmission Group (%) | ||
| MSM | 112 (54.9) | 90 (42.5) |
| Heterosexual | 81 (39.7) | 106 (50.0) |
| PWID | 2 (1.0) | 2 (0.9) |
| Other/unknown | 9 (4.4) | 14 (6.6) |
| Arterial hypertension (%) | 49 (24.0) | 72 (34.0) |
| Diabetes (%) | 9 (4.4) | 28 (13.2) |
| Dyslipidemia (%) | 59 (28.9) | 98 (46.2) |
| History of cardiovascular disease (%) | 12 (5.9) | 16 (7.6) |
| Median BMI, kg/m2 (IQR) | 22.1 (20.8–23.9) | 29.6 (26.6–31.5) |
| Median ALT, U/L (IQR) | 28.0 (19.0–30.5) | 33.2 (22.0–40.0) |
| Median triglycerides, mmol/L (IQR) | 1.63 (0.9–1.9) | 2.30 (1.1–2.6) |
| Hazardous alcohol consumption (%) | 41 (22.2) | 42 (22.0) |
| Median current CD4+ count, cells/µL (IQR) | 740 (535–897) | 792 (577–985) |
| Median CD4 nadir in cells/µL (IQR) | 252 (100–347) | 245 (128–342) |
| HIV viral load <50 copies/mL (%) | 189 (92.6) | 198 (93.4) |
| Median eGFR in mL/min (IQR) | 91 (75–104) | 89 (71–100) |
| ART duration, years (IQR) | 13.3 (7.0–20.0) | 12.0 (6.0–19.0) |
| Current ART regimen (%) | ||
| 3TC/ABC/DTG | 52 (25.5) | 44 (20.8) |
| FTC/TAF/BIC | 27 (13.2) | 30 (14.2) |
| FTC/TAF/DTG | 22 (10.8) | 38 (17.9) |
| Other | 99 (48.5) | 97 (45.8) |
| No treatment | 4 (2.0) | 3 (1.4) |
| Exposed to TAF (%) | 104 (51.0) | 128 (60.4) |
| Exposed to InSTI (%) | 146 (71.6) | 146 (68.9) |
| Ever exposed to PI (%) | 112 (54.9) | 115 (54.3) |
| Ever exposed to AZT/DDI/D4T (%) | 95 (46.6) | 88 (41.5) |
Abbreviations: ABC, abacavir; ALT, alanine aminotransferase; ART, antiretroviral therapy; AST, aspartate aminotransferase; AZT/DDI/D4T, zidovudine/didanosine/stavudine; BIC, bictegravir; BMI, body mass index; CV, cardiovascular; DTG, dolutegravir; eGFR, estimated glomerular filtration rate; FTC, emtricitabine; HIV, human immunodeficiency virus; InSTI, integrase-strand transfer inhibitor; IQR, interquartile range; MSM, men who have sex with men; PI, protease inhibitor; PWID, patients who inject drugs; TAF, tenofovir alafenamide; 3TC, lamivudine.
Prevalence and Factors Associated With Liver Steatosis
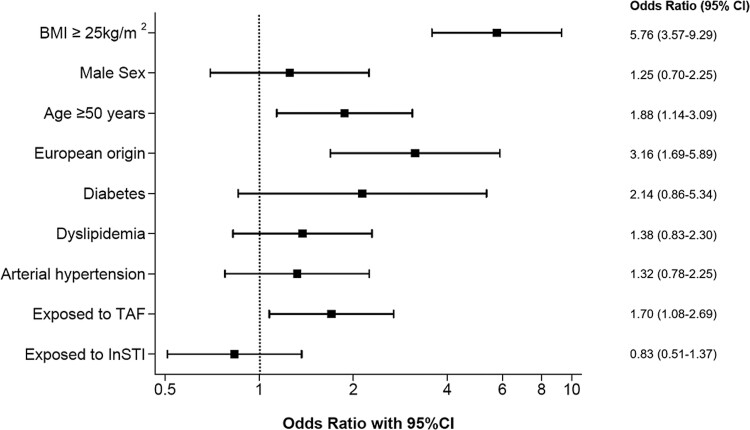
Overall, 212 (51.0%) participants had liver steatosis (S1–S3), including 143 (34.4%) with severe steatosis (S3). The proportion of individuals with liver steatosis was 69.8% in overweight/obese patients and 31.4% in the lean population. In multivariable analyses, BMI ≥25 kg/m2 (adjusted odds ratio [aOR], 5.76; 95% confidence interval [CI], 3.57–9.29), age ≥50 years (aOR, 1.88; 95% CI, 1.14–3.09), and European origin (aOR, 3.16; 95% CI, 1.69–5.89) were associated with liver steatosis (Figure 2, Supplementary Table 1). We found no association between InSTI exposure and liver steatosis. However, participants currently on a TAF-containing regimen were more likely to have liver steatosis than those not on TAF (aOR, 1.70; 95% CI, 1.08–2.69). The latter association did not depend on the time spent on a TAF-containing regimen: when TAF exposure was divided into 0–12 months and >12 months, the risk of liver steatosis was not higher in the group with longest exposure (0–12 months vs no TAF: aOR 2.06, 95% CI = 1.07–3.98; > 12 months vs no TAF: aOR 1.57, 94% CI = 0.95–2.59). When considering all patients who had ever been exposed to TAF (n = 262), we obtained similar results (aOR, 1.77; 95% CI, 1.10–2.85). Current or prior exposure to TDF or other drug classes, such as PI and AZT/DDI/D4T, were not associated with liver steatosis in univariable analyses. In a sensitivity analysis using a CAP cutoff of 280 dB/m to diagnose severe steatosis, the same factors remained associated with liver steatosis (Supplementary Table 2).
Figure 2.
Multivariable analysis of factors associated with liver steatosis. BMI, body mass index; CI, confidence interval; InSTI, integrase-strand transfer inhibitor; OR, odds ratio; TAF; tenofovir alafenamide.
Among 47 participants with a weight increase >10% during the past 3 years of ART, 33 (70.2%) had liver steatosis, 24 (72.7%) of whom were receiving a TAF-containing ART regimen. When we included weight increase of 10% into our multivariable model, both weight gain (aOR, 3.14; 95% CI, 1.43–6.88) and exposure to TAF (aOR, 1.71; 95% CI, 1.06–2.75) remained independently associated with liver steatosis (Supplementary Table 3).
Risk Factors of Liver Steatosis in Analyses Stratified by Body Mass Index Category
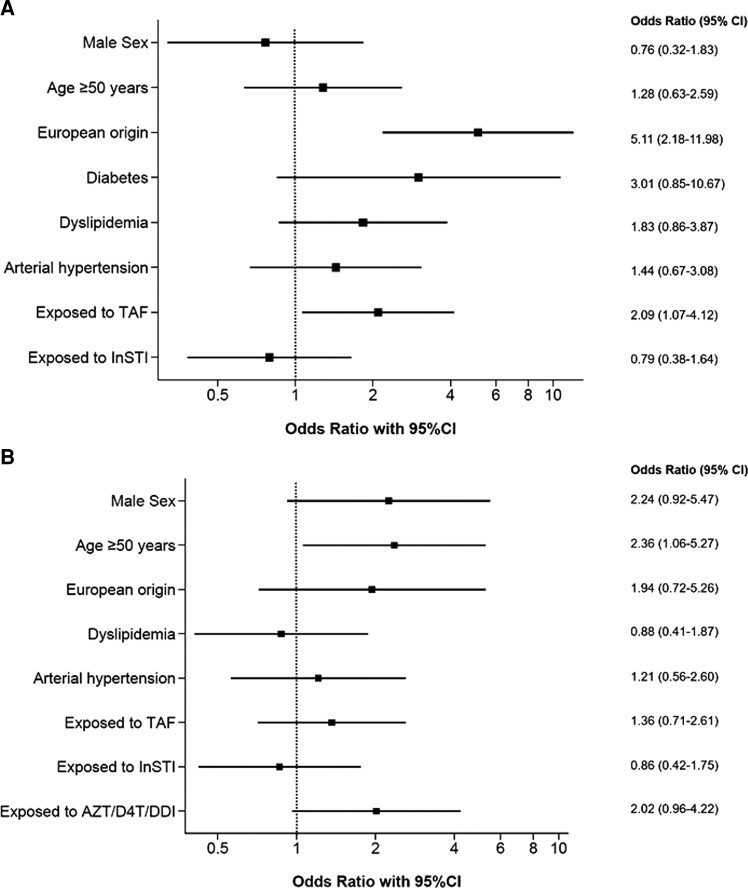
Results from multivariable analyses in the overweight/obese population were similar to the overall model, except for age >50 years, which was not associated with liver steatosis in this subpopulation (Figure 3A, Supplementary Table 4). In the lean population, age ≥50 years (aOR, 2.36; 95% CI, 1.06–5.27) was associated with the presence of hepatic steatosis, whereas we did not find any association with metabolic risk factors or TAF (Figure 3B, Supplementary Table 5). However, previous exposure to AZT/DDI/D4T was significantly associated with liver steatosis in univariable analysis (OR, 2.58; 95% CI, 1.40–4.74) but not after adjustment for covariables (aOR, 2.02; 95% CI, 0.96–4.22) in lean individuals. In a sensitivity analysis using a CAP cutoff of 280 dB/m, among the lean group, age ≥50 years (aOR, 5.04; 95% CI, 1.48–17.20) and hazardous alcohol consumption (aOR, 3.17; 95% CI, 1.22–8.25) were associated with severe liver steatosis (Supplementary Table 6).
Figure 3.
Multivariable analysis of factors associated with liver steatosis among overweight/obese (A) and lean participants (B). AZT/DDI/D4T, zidovudine/didanosine/stavudine; CI, confidence interval; InSTI, integrase-strand transfer inhibitor; TAF; tenofovir alafenamide.
Metabolic Dysfunction-Associated Fatty Liver Disease
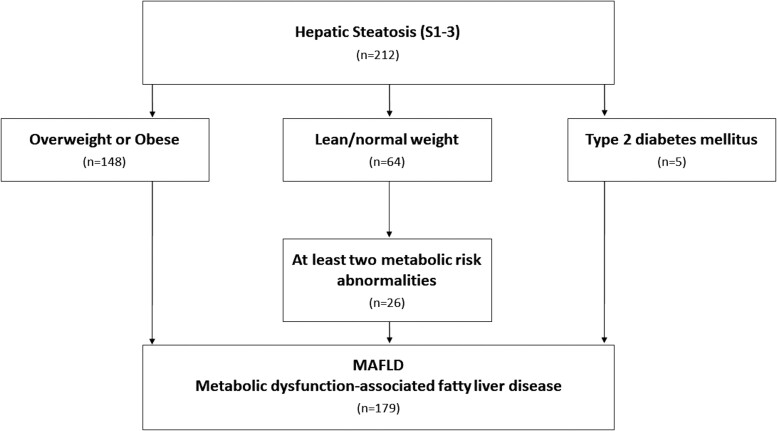
We diagnosed MAFLD in 179 (43.0%) participants. Among individuals with hepatic steatosis S1–S3, 84.4% fulfilled the consensus criteria for MAFLD (Figure 4). The proportion of patients with MAFLD was 69.8% among overweight/obese participants and 15.2% among lean participants. Among 64 lean individuals with liver steatosis, 31 (48.4%) met the criteria for MAFLD, whereas the rest did not show any metabolic dysregulation. Among patients with lean steatosis who did not have MAFLD, 90.9% were male.
Figure 4.
Flowchart for the diagnosis of metabolic-associated fatty liver disease (MAFLD). Twenty-four patients with type 2 diabetes mellitus and body mass index ≥ 25 kg/m2 simultaneously fulfil the criteria for MAFLD through the independent pathway of being overweight/obese.
Transaminase Elevation, Fibrosis, and Cirrhosis
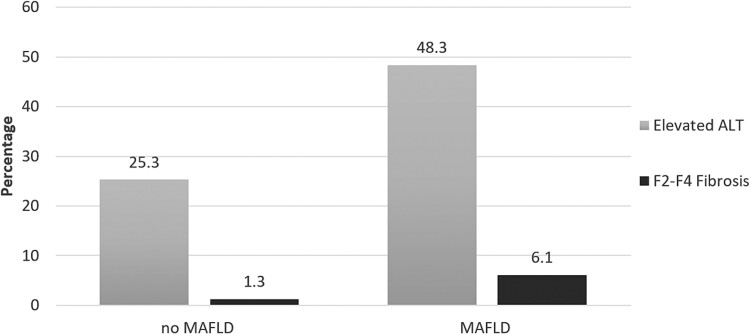
In the full study population, 146 (35.2%) patients had elevated ALT and 14 (3.4%) had significant liver fibrosis or cirrhosis (F2–F4). Patients with MAFLD were more likely to have elevated transaminases compared to those without (48.3% vs 25.3%) (Figure 5). Among PWH with MAFLD, 6 (3.4%) patients had a LSM compatible with significant fibrosis (F2–F3) and 5 (2.8%) with cirrhosis (F4), whereas among participant without MAFLD, only 3 (1.3%) had significant fibrosis or cirrhosis. Liver biopsy was performed in 4 patients, and steatohepatitis was confirmed in 3 of them. The remaining patients with fibrosis stage F2–3 will undergo a follow-up TE before further diagnostics are initiated.
Figure 5.
Distribution of significant fibrosis or cirrhosis and elevated alanine aminotransferase (ALT) by metabolic-associated fatty liver disease (MAFLD) category.
DISCUSSION
In our single-center assessment of liver steatosis using transient elastography, one half of PWH on ART without viral hepatitis coinfection had liver steatosis. In addition to classic risk factors, including age, BMI, and ethnicity, ART components were associated with this outcome. Participants currently exposed to TAF were twice as likely to have steatosis as those unexposed to this drug; however, this association was not observed among lean participants, and in both, lean and overweight/obese patients, no association was found with exposure to InSTI. Overall, MAFLD was diagnosed in 43% of participants, including in 17% of lean individuals, and it was associated with a high risk of having elevated transaminases. Taken together, our results highlight the need for continuous monitoring of liver steatosis in PWH, including lean PWH, and for further exploration of additional risk factors, as well as the mechanism leading to metabolic changes in this population.
Several large studies described the prevalence of liver steatosis in PWH, with estimates ranging from 28% to 48% [3, 18, 26]. In our study, the prevalence of steatosis was 51%, and the majority of cases diagnosed had a CAP measurement compatible with S3 steatosis (34.4% overall). Most previous studies showed that classic risk factors described in non-HIV populations remained the most important determinants for the development of steatosis in PWH [27]. Likewise, we found strong associations between liver steatosis and age, BMI, and European origin. The prevalence of diabetes mellitus in our cohort was low, which may explain why the association with steatosis was not statistically significant in multivariable analyses.
The high prevalence of steatosis in our cohort may in part reflect the rising steatosis trends in the general population, with worldwide yearly steatosis increases reaching almost 1% [28]. However, the high proportion of participants treated with ART components linked to weight increases could contribute to these findings. Among HIV-negative populations, body weight changes have been strongly associated with steatosis [29]. In our analyses adjusted for age, ethnicity, and metabolic conditions, both weight increase and the use of TAF were associated with liver steatosis. This observation suggests an independent effect of TAF on the development of liver steatosis, which could be driven by metabolic changes other than weight, such as changes in pathways of lipid or glucose metabolism. Furthermore, induced changes in the levels of endogenous and gut microbiota-produced metabolites may play a role. The lack of an association between InSTI and steatosis may be explained by the large proportion (45.9%) of them being on dolutegravir (DTG), whereas only 3.1% were treated with raltegravir (RGV), 2.1% were treated with elvitegravir (EVG), and 13.9% were treated with bictegravir. In a study from Denmark, the risk of moderate-to-severe hepatic steatosis was high with EVG and RGV, but not with DTG [30]. The low number of patients on the latter drugs precluded the evaluation of the potential associations between them and liver steatosis in our study. In another study, Bischoff et al [9] described a faster development or progression of steatosis in individuals on InSTI in Germany, but they did not report on specific drugs. To improve our understanding of the link between new ART combinations and liver steatosis, prospective cohorts with specific subgroup analyses are needed.
A subset of patients developed liver steatosis with a BMI <25 kg/m2. Lean patients may have a distinct metabolism, and changes in the gut microbiota or genetics might further influence the development of liver steatosis in this population. Furthermore, lifestyle, diet, and underreported alcohol use may also contribute to this clinical phenotype. A recent multicountry study of lean PWH reported a prevalence of liver steatosis of 24%, associated with older age and high levels of triglycerides and transaminases [12]. We found a slightly higher prevalence of liver steatosis (31.4%) in lean participants and strong associations with older age and hazardous alcohol consumption. However, we did not observe any association with metabolic risk factors in the lean subgroup. Given the limited sample size, our ability to draw any conclusions for this subgroup of participants is limited, and our findings need to be confirmed in dedicated studies.
In our cohort, 84% of individuals with hepatic steatosis also met the consensus criteria for the diagnosis of MAFLD. However, this was only the case for 31 (48.4%) lean individuals with steatosis. Compared to studies from the general population, we observed higher numbers of lean patients with steatosis who did not fulfil the MAFLD criteria [31, 32]. It is possible that subtle metabolic changes in patients with lean steatosis without MAFLD may not have an impact on clinical parameters besides liver steatosis, and this may constitute early warning signs. We found a low prevalence of a LSM compatible with significant liver fibrosis and cirrhosis among participants with MAFLD (6.1%), despite the high proportion of patients with elevated transaminases (48.3%). The prevalence of fibrosis in previous studies of PWH with steatosis was higher, varying between 8% and 15% [3, 18]. Considering the potential faster progression of liver disease among PWH and the potential link between steatosis and cardiovascular events, future studies will have to include liver biopsies to improve our understanding of the dynamics of fibrosis progression in this population [33].
We investigated the prevalence and factors associated with liver steatosis in a well characterized cohort of PWH under ART, and we were able to include 87% of eligible patients. The nearly complete data on clinical parameters, laboratory values, and cardiometabolic risk factors allowed us to perform one of the first assessments of the determinants of MAFLD among PWH. However, given the cross-sectional nature of this study, the temporal relationship between liver steatosis and associated factors remains unknown. Subgroup analyses stratified by BMI were based on small numbers, which limited our ability to draw any strong conclusions. For the assessment of prediabetes and diagnoses of MAFLD, we lacked data on parameters of insulin sensitivity other than fasting glucose levels and HbA1c in most participants. Finally, we did not have data from liver biopsies and could therefore not investigate the prevalence of NASH.
CONCLUSIONS
In conclusion, we showed a high prevalence of liver steatosis among PWH on ART in Switzerland. In addition to well established risk factors, the use of TAF was associated with the presence of hepatic steatosis. To what extent this association is mediated by weight increase or constitutes an independent effect of the drug remains incompletely understood. Our study highlights the need for hepatic phenotyping of patients treated with contemporary ART to provide insights into underlying mechanisms of their metabolic side effects.
Supplementary Material
Acknowledgments
We thank all patients, doctors, and nurses associated with the Swiss HIV Cohort Study (SHCS).
Author contributions. C. R., H. F., A. R., and G. W. designed the study. C. R. and G. W. drafted the first version of the manuscript. C. R. and G. W. performed the statistical analyses. A. B., B. S., H. F. G., and P. E. T. contributed to the conception of the study and revised the manuscript for substantial intellectual content. All authors read and approved the final manuscript.
Financial support. This work was funded by the framework of the SHCS, supported by the Swiss National Science Foundation (SNF Grant Number PP00P3_211025). G. W. was supported by a Professorship from the Swiss National Science Foundation (PP00P3_176944). C. R. is recipient of the Protected Research Time Grant for PhD students of the University of Bern.
The members of the SHCS are Anagnostopoulos A., Battegay M., Bernasconi E., Böni J., Braun D. L., Bucher H. C., Calmy A., Cavassini M., Ciuffi A., Dollenmaier G., Egger M., Elzi L., Fehr J., Fellay J., Furrer H., Fux C. A., Günthard H. F. (President of the SHCS), Haerry D. (Deputy of “Positive Council”), Hasse B., Hirsch H. H., Hoffmann M., Hösli I., Huber M., Kahlert C. R. (Chairman of the Mother and Child Substudy), Kaiser L., Keiser O., Klimkait T., Kouyos R. D., Kovari H., Ledergerber B., Martinetti G., Martinez de Tejada B., Marzolini C., Metzner K. J., Müller N., Nicca D., Paioni P., Pantaleo G., Perreau M., Rauch A. (Chairman of the Scientific Board), Rudin C., Scherrer A. U. (Head of Data Centre), Schmid P., Speck R., Stöckle M. (Chairman of the Clinical and Laboratory Committee), Tarr P., Trkola A., Vernazza P., Wandeler G., Weber R., and Yerly S.
Contributor Information
Carlotta Riebensahm, Department of Infectious Diseases, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland; Graduate School of Health Sciences, University of Bern, Bern, Switzerland.
Annalisa Berzigotti, Department for Visceral Surgery and Medicine, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland; Hepatology, Department of BioMedical Research, University of Bern, Bern, Switzerland.
Bernard Surial, Department of Infectious Diseases, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Huldrych F Günthard, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland; Institute of Medical Virology, University of Zurich, Zurich, Switzerland.
Philip E Tarr, University Department of Medicine and Infectious Diseases Service, Kantonsspital Baselland, University of Basel, Bruderholz, Switzerland.
Hansjakob Furrer, Department of Infectious Diseases, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Andri Rauch, Department of Infectious Diseases, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Gilles Wandeler, Department of Infectious Diseases, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland; Institute of Social and Preventive Medicine, University of Bern, Bern, Switzerland.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Younossi ZM. Non-alcoholic fatty liver disease—a global public health perspective. J Hepatol 2019; 70:531–44. [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64:73–84. [DOI] [PubMed] [Google Scholar]
- 3. Perazzo H, Cardoso SW, Yanavich C, et al. Predictive factors associated with liver fibrosis and steatosis by transient elastography in patients with HIV mono-infection under long-term combined antiretroviral therapy. J Int AIDS Soc 2018; 21:e25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pembroke T, Deschenes M, Lebouche B, et al. Hepatic steatosis progresses faster in HIV mono-infected than HIV/HCV co-infected patients and is associated with liver fibrosis. J Hepatol 2017; 67:801–8. [DOI] [PubMed] [Google Scholar]
- 5. Vodkin I, Valasek MA, Bettencourt R, Cachay E, Loomba R. Clinical, biochemical and histological differences between HIV-associated NAFLD and primary NAFLD: a case-control study. Aliment Pharmacol Ther 2015; 41:368–78. [DOI] [PubMed] [Google Scholar]
- 6. Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020; 71:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bourgi K, Jenkins CA, Rebeiro PF, et al. Weight gain among treatment-naive persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc 2020; 23:e25484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hasse B, Iff M, Ledergerber B, et al. Obesity trends and body mass index changes after starting antiretroviral treatment: the Swiss HIV cohort study. Open Forum Infect Dis 2014; 1:ofu040. doi: 10.1093/ofid/ofu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bischoff J, Gu W, Schwarze-Zander C, et al. Stratifying the risk of NAFLD in patients with HIV under combination antiretroviral therapy (cART). EClinicalMedicine 2021; 40:101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Surial B, Mugglin C, Calmy A, et al. Weight and metabolic changes after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in people living with HIV: a cohort study. Ann Intern Med 2021; 174(6):758–67. [DOI] [PubMed] [Google Scholar]
- 11. Mohammed SS, Aghdassi E, Salit IE, et al. HIV-positive patients with nonalcoholic fatty liver disease have a lower body mass index and are more physically active than HIV-negative patients. J Acquir Immune Defic Syndr 2007; 45:432–8. [DOI] [PubMed] [Google Scholar]
- 12. Denkmayr L, Feldman A, Stechemesser L, et al. Lean patients with non-alcoholic fatty liver disease have a severe histological phenotype similar to obese patients. J Clin Med 2018; 7:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cervo A, Milic J, Mazzola G, et al. Prevalence, predictors and severity of lean non-alcoholic fatty liver disease in HIV-infected patients. Clin Infect Dis 2020; 71(10):e694–e701. [DOI] [PubMed] [Google Scholar]
- 14. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020; 73:202–9. [DOI] [PubMed] [Google Scholar]
- 15. Scherrer AU, Traytel A, Braun DL, et al. Cohort profile update: the Swiss HIV cohort study (SHCS). Int J Epidemiol 2022; 51:33–4j. [DOI] [PubMed] [Google Scholar]
- 16. Boursier J, Zarski JP, de Ledinghen V, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013; 57:1182–91. [DOI] [PubMed] [Google Scholar]
- 17. Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol 2017; 66:1022–30. [DOI] [PubMed] [Google Scholar]
- 18. Vuille-Lessard E, Lebouche B, Lennox L, et al. Nonalcoholic fatty liver disease diagnosed by transient elastography with controlled attenuation parameter in unselected HIV monoinfected patients. AIDS 2016; 30:2635–43. [DOI] [PubMed] [Google Scholar]
- 19. de Ledinghen V, Hiriart JB, Vergniol J, Merrouche W, Bedossa P, Paradis V. Controlled attenuation parameter (CAP) with the XL probe of the fibroscan((R)): a comparative study with the M probe and liver biopsy. Dig Dis Sci 2017; 62:2569–77. [DOI] [PubMed] [Google Scholar]
- 20. Morse CG, McLaughlin M, Proschan M, et al. Transient elastography for the detection of hepatic fibrosis in HIV-monoinfected adults with elevated aminotransferases on antiretroviral therapy. AIDS 2015; 29:2297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ajmera VH, Cachay ER, Ramers CB, et al. Optimal threshold of controlled attenuation parameter for detection of HIV-associated NAFLD with magnetic resonance imaging as the reference standard. Clin Infect Dis 2020; 72(12):2124–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: aASLD 2018 hepatitis B guidance. Clin Liver Dis 2018; 12:33–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hill A, Waters L, Pozniak A. Are new antiretroviral treatments increasing the risks of clinical obesity? J Virus Erad 2019; 5:41–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998; 158:1789–95. [DOI] [PubMed] [Google Scholar]
- 25. Shah S, Hindley L, Hill A. Are new antiretroviral treatments increasing the risk of weight gain? Drugs 2021; 81:299–315. [DOI] [PubMed] [Google Scholar]
- 26. Maurice JB, Patel A, Scott AJ, Patel K, Thursz M, Lemoine M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS 2017; 31:1621–32. [DOI] [PubMed] [Google Scholar]
- 27. Price JC, Seaberg EC, Latanich R, et al. Risk factors for fatty liver in the multicenter AIDS cohort study. Am J Gastroenterol 2014; 109:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Le MH, Yeo YH, Li X, et al. Global NAFLD prevalence—a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2019; 2021. doi: 10.1016/j.cgh.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 29. Chang Y, Ryu S, Sung E, et al. Weight gain within the Normal weight range predicts ultrasonographically detected fatty liver in healthy Korean men. Gut 2009; 58:1419–25. [DOI] [PubMed] [Google Scholar]
- 30. Kirkegaard-Klitbo DM, Thomsen MT, Gelpi M, Bendtsen F, Nielsen SD, Benfield T. Hepatic steatosis associated with exposure to elvitegravir and raltegravir. Clin Infect Dis 2021; 73:e811–4. [DOI] [PubMed] [Google Scholar]
- 31. Niriella MA, Ediriweera DS, Kasturiratne A, et al. Outcomes of NAFLD and MAFLD: results from a community-based, prospective cohort study. PLoS One 2021; 16:e0245762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol 2021; 75:1284–91. [DOI] [PubMed] [Google Scholar]
- 33. Cervo A, Sebastiani G, Milic J, et al. Dangerous liaisons: NAFLD and liver fibrosis increase cardiovascular risk in HIV. HIV Med 2022; 23(8):911–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.