Abstract
We identified a T-cell determinant of the 35-kDa antigen of Mycobacterium leprae which is discriminatory against cross-sensitization by its closely related homologue in Mycobacterium avium. From synthetic peptides covering the entire sequence, those with the highest affinity and permissive binding to purified HLA-DR molecules were evaluated for the stimulation of proliferation of peripheral blood mononuclear cells (PBMCs) from leprosy patients and healthy sensitized controls. Responses to the peptide pair 206–224, differing by four residues between M. leprae and M. avium, involved both species-specific and cross-reactive T cells. Lymph node cell proliferation in HLA-DRB1*01 transgenic mice was reciprocally species specific, but only the response to the M. leprae peptide in the context of DR1 was immunodominant. Of the cytokines in human PBMC cultures, gamma interferon production was negligible, while interleukin 10 (IL-10) responses in both patients and controls were more pronounced. IL-10 was most frequently induced by the shared 241–255 peptide, indicating that environmental cross-sensitization may skew the response toward a potentially pathogenic cytokine phenotype.
The study of the epitope specificity and interlinked function of responding T cells may help to improve the understanding of immunopathogenesis of nerve and skin lesions and may advance the search for immunodiagnostic and vaccine subunits. Considering the many antigens with structural homology among the pathogenic Mycobacterium leprae, Mycobacterium tuberculosis, and environmental mycobacteria (2) only a few have been studied by using synthetic peptides for the stimulation of cloned T cells (10) or blood lymphocytes (3, 12). Although most M. leprae antigens have close homologues in the M. tuberculosis complex (20), the 35-kDa antigen is an exception, with homologues in Mycobacterium avium only (21, 22). The 35-kDa antigen of M. leprae, a major membrane protein (8), has been cloned and sequenced (25). It contains an M. leprae-specific B-cell epitope of conformational nature (9), which is the target of serum antibodies (Ab), elevated in a very high proportion of multibacillary, but only few tuberculoid (TT/BT), leprosy patients (17, 19). The purified 35-kDa antigen of M. leprae has previously been found to be stimulatory for peripheral blood mononuclear cells (PBMCs) of TT/BT leprosy patients and Mycobacterium bovis BCG-vaccinated healthy subjects in California (15), and the purified recombinant protein was stimulatory of both proliferation and gamma interferon (IFN-γ) production in 65% of paucibacillary patients and 80% of healthy contacts in Nepal (21). These studies with the whole antigen indicated a strong immunogenicity but also its lack of M. leprae specificity, attributable to sensitization by the 88% homologous 35-kDa antigen of M. avium, since the gene encoding the 35-kDa protein is not found in the M. tuberculosis complex or BCG (21, 25). The purpose of this study was to identify immunogenic peptides and search for M. leprae-specific epitopes involving the 12% of residues that differ between M. leprae and M. avium. The mapping of T-cell epitopes is complicated in humans because of heterozygosity at three HLA class II loci (DR, DQ, and DP). Predictive motifs are only of partial value, and empirical mapping by PBMC proliferation is too elaborate for larger proteins. Therefore, for the analysis of the 35-kDa protein we opted for a strategy in which the initial screening of peptides was carried out by binding to purified HLA-DR molecules.
Affinity of peptide binding to purified HLA-DR molecules.
On the basis of the 35-kDa protein sequence (25), a total of 58 15-mer peptides overlapping by five residues were synthesized and characterized by previously described methods (23). HLA-DR molecules of 10 different haplotypes were purified (5, 6), and the binding assay was performed with mixtures containing purified HLA-DR protein (0.5 μM), N-terminally biotinylated reference peptide (1.8 μM class II-associated invariant chain peptide [CLIP], 1.6 μM influenza hemagglutinin 307–319, or 1.6 μM HLA-A3 153–168), and competitor peptide (0, 3, 30, or 300 μM) (11). The 50% inhibitory concentrations (IC50) for competitor peptides were calculated by regression analysis of the measured absorbencies.
Of the 58 synthesized peptides, all but two showed some binding (IC50, <200 μM) for at least one, but often a number, of the HLA-DR molecules tested, with large variation in the specificity, binding affinity, and the total number of DR alleles bound (results not shown). Of these, peptides 176–190, 206–219, 271–285, and 276–290 bound to three or more DR alleles with the highest affinity (Table 1). Peptides differing in four to five residues between M. leprae and M. avium showed high-affinity (206–220 and 271–285) or moderate-affinity (141–155 and 266–280) DR binding. The M. leprae sequence of 206–219 differs from that of M. avium by a frameshift due to the insertion of a tyrosine at position 209 in the M. avium sequence that comes back into frame at position 219 with an additional isoleucine. The apparent specificity of 271–285 is compromised by the N-terminal location of four of the distinct residues. Therefore, the best combination of high affinity and potential specificity was predicted to be the 206–219 [FGA(P)(T)QFIT WR(H)GIP(R)LI(−)P] M. leprae sequence (differences in the M. avium sequence are shown in parentheses).
TABLE 1.
Proliferative responses of leprosy patient and control PBMCs to peptides selected on the grounds of a distinct sequence and pronounced binding to HLA-DR molecules
| Peptide | Binding to purified proteins of HLA-DRb | No. of respondersc/total no. (%)
|
|
|---|---|---|---|
| Healthy subjects | TT/BT leprosy patients | ||
| 26–40 (0)a | 1, 15, 6, 7, 8 | NT | 3/14 (21) |
| 36–50 (1) | 4, 6, 9 | NT | 1/14 (7) |
| 41–55 (1) | B5, 6, 7 | 2/9 (22) | 5/29 (17) |
| 9/14 (64)d | 3/8 (38)d | ||
| 86–100 (2) | 6, 7, 8 | NT | 2/14 (14) |
| 141–155 (5) | 15 | 2/6 (33) | 3/10 (30) |
| 176–190 (1) | 1, B5, 4, 5, 6, 7, 8 | 1/3 (33) | 4/10 (40) |
| 206–219 (5) | 1, 15, B5, 6, 7, 8 | 5/9 (55) | 12/31 (39) |
| 9/18 (50)d | 4/8 (50)d | ||
| 216–230 (4) | B5 | 0/3 (0) | 1/10 (10) |
| 226–240 (3) | 6, 8 | NT | 1/15 (7) |
| 231–245 (1) | B5, 6, 8 | 2/9 (22) | 6/20 (30) |
| 241–255 (0) | 15, 8 | 1/6 (17) | 1/10 (10) |
| 266–280 (5) | 6, 8 | 3/6 (50) | 5/10 (50) |
| 8/12 (66)d | 5/8 (63)d | ||
| 271–285 (4) | 15, B5, 6, 7 | NT | 1/15 (7) |
| 276–290 (1) | B5, 4, 6, 8, 9 | 0/9 (0) | 6/20 (30) |
Number of residues differing between M. leprae and M. avium.
IC50 of ≤100 μM or <10 μM for those in bold.
SI, >2.0. NT, not tested.
Tested patients and controls were from London; all others were from India.
Peptide-stimulated proliferation of PBMCs from leprosy patients and controls.
All 55 Indian leprosy patients recruited from the Central Jalma Institute for Leprosy, Agra, India, and the Army Hospital, Lucknow, India, had received less than 4 weeks of World Health Organization multidrug chemotherapy, while healthy controls were recruited from staff working in the same institutes. Leprosy patients without microbiologically active disease were from the Hospital for Tropical Diseases in London, United Kingdom (UK). Healthy purified protein derivative-negative (4) and purified protein derivative-positive (18) controls, none of whom had been exposed to leprosy, were recruited from the tuberculosis contact clinic at Northwick Park Hospital, Harrow, England. The absence of any likely exposure to leprosy was ascertained by excluding those who had traveled to any area where leprosy was known to be endemic and by excluding any in a health service occupation. Proliferation and cytokine assays were performed as previously described (23). Although peptides were essentially lipopolysaccharide free, polymyxin B (10 μg/ml) was added to cultures as an additional precaution. The lymphocyte stimulation index (SI) was calculated as the average counts per minute in the presence of antigen/the average counts per minute in the absence of antigen. A positive response was defined by a lymphocyte SI of >2. A total of 4 of 105 subjects having values for background counts per minute of >3 standard deviations from the population mean were excluded from the analysis.
Fourteen peptides which bound with moderate to high affinities (IC50, <100 μM or <10 μM, respectively) to one or more DR molecules were selected for evaluation (Table 1). This part of the study was performed mainly with TT/BT leprosy patients and controls from areas where leprosy was endemic and where it was not. The most frequently stimulatory peptides, giving positive responses in at least 30% of tested subjects, were 41–55, 141–155, 206–219, 231–245, 266–280, and 271–285. The high frequency of recognition suggests a genetically permissive recognition (confirmed by PCR and sequence-specific primer-based HLA-DRB1* typing of the donors; data not shown). Response frequencies in patients and controls did not differ consistently for any of the tested peptides. The trend towards greater recognition of 276–290 by TT/BT patients was not statistically significant. However, responses to 41–55 were about three times more frequent for both patients and controls in London than for those in India (P < 0.01).
Response to pairs of homologous peptides with either the M. leprae or M. avium sequence.
In view of the abundant exposure to environmental M. avium in areas where leprosy is endemic, we investigated if cross-sensitization could be distinguished with a pair of 19-mer peptides (206–224) (rather than the original 14-mer, 206–219) containing five residues differing between M. leprae and M. avium. Proliferation was tested in PBMC cultures of leprosy patients and controls from India and the UK. In the latter group (excluding any contact with M. leprae infection) all but one subject (a total of five) responded only to the M. avium peptide (P = 0.019), thus suggesting a specific recognition of M. avium 206–224. In contrast, T cells from the UK leprosy patients recognized 206–224 in about equal proportions either alone or in conjunction with M. avium 206–224. These data suggest that T cells can recognize each of these two peptides specifically, but a contribution from cross-reactive T cells cannot be excluded. This conclusion is corroborated by the absence of any response to the M. avium peptide in all three responders of the Indian lepromatous (LL/BL) group, hence suggesting the presence of M. leprae 206–224-specific T cells. One problem of interpretation remains the initial finding of 50% responsiveness of UK controls to peptide 206–219 (Table 1). Although the four N-terminal residues of the extended 19-mer peptide used in the comparison shown in Table 2 were all conserved between the two species, the addition of these residues ostensibly improved specificity. Therefore, detailed analysis of the epitope localization (i.e., window pepscan, establishment of minimal epitope length, the role of flanking regions, and characterization of the epitope core) is required. Based on the lack of response in UK controls who were not exposed to M. leprae infection we conclude, tentatively, that peptide 206–224 carries M. leprae specificity.
TABLE 2.
Proliferation of PBMCs in response to peptide 206–224 of either M. leprae or M. avium
| Group of patients
|
No. of respondersa to peptide of:
|
||||
|---|---|---|---|---|---|
| Diagnosis | Country | No. tested | M. leprae only | M. leprae + M. avium | M. avium only |
| Healthy sensitized | India | 6 | 1 | 1 | 1 |
| United Kingdom | 8 | 0 | 1 | 5 | |
| TT/BT leprosy | India | 10 | 1 | 1 | 2 |
| United Kingdom | 8 | 2 | 2 | 2 | |
| LL/BL leprosy | India | 10 | 3 | 0 | 0 |
| United Kingdom | 13 | 2 | 3 | 3 | |
SI, >2.
Analysis of p206–224 specificity in HLA-DRB1*0101 transgenic and FVB/N (H2-Aq) control mice.
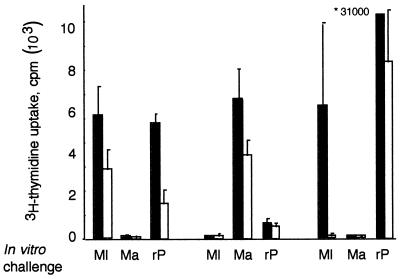
The transgenic mice express both DRB1*0101 and indigenous H2-Aq but only murine CD4 (1). Mice were immunized subcutaneously in each hind footpad with 50 μg of synthetic peptides of either the M. leprae or M. avium sequence or with 25 μg of recombinant 35-kDa protein (a gift from Warwick Britton, Centenary Institute, Sydney, Australia) (21) (rpL35) or phosphate-buffered saline emulsified in a 1:1 (vol/vol) ratio with incomplete Freund’s adjuvant (Difco, Detroit, Mich.). After 8 days, the draining popliteal lymph node (LN) cells in triplicate cultures of 4 × 105 cells/well were incubated in the presence of the synthetic peptides (50 μg/ml) or rpL35 (10 μg/ml), and [3H]thymidine uptake (on day 3 after 6 h) was determined. Reciprocal priming and in vitro stimulation of LN cells showed that LN cells from M. leprae 206–224-primed mice were stimulated by the homologous peptide but not by the M. avium peptide (Fig. 1); this response was higher in the DR1 transgenic mice than in the control FVB mice. Stimulation with rpL35 was also pronounced in the DR1 transgenic mice but was much weaker in the control mice, thus suggesting the immunodominance of M. leprae 206–224 in the context of DR1. Moreover, this immunodominant determinant appeared to be M. leprae-specific, since LN cells from M. avium 206–224-primed mice responded only to the homologous peptide and not the peptide or the whole antigen from M. leprae.
FIG. 1.
Proliferative responses of LN cells from HLA-DRB1*01-transgenic mice (black bars) and control FVB mice (white bars). Mice (two males per group) were primed by footpad inoculation with 50 μg of peptide 206–224 of M. leprae (Ml) or M. avium (Ma) or 25 μg of rpL35 (rP) in incomplete Freund’s adjuvant. [3H]thymidine uptake corrected for phosphate-buffered saline controls of pooled 8-day primed LN cells incubated in the presence of 50 μg of peptide or protein stimuli per ml was measured for transgenic and control mice. Anti-HLA-DR monoclonal Ab inhibited 53.3% of the M. leprae 206–224-specific response and not the rpL35 stimulation of DR1 transgenic LN cells (results not shown). *31000, counts per minute.
Interestingly, following priming with rpL35, the LN response to the M. leprae peptide was demonstrable exclusively in the DR1 transgenic mice and not in the control FVB mice. Thus, the response to M. leprae 206–224 was immunodominant in the context of DR1 but was cryptic in the control FVB mice. Further evidence for the role of DR1 was suggested by the finding of 53.3% specific inhibition of the peptide response in the LN cultures which contained the L243 anti-DR1 monoclonal Ab (results not shown). In contrast, the LN proliferation response to rpL35 was pronounced in both DR1 transgenic and control mice. The response of DR1 transgenic cells to rpL35 was not inhibited by the pan-HLA-DR-specific monoclonal Ab L243 (ATCC HB-55, mouse immunoglobulin G2a) at 10 μg/ml, when compared with the same concentration of isotype control (murine anti-mouse I-Ab+k, ATCC HB-163).
Cytokine production by peptide-stimulated PBMCs.
PBMCs (107 cells/ml) were cultured in 96F plates (Nunc, Roskilde, Denmark), and the supernatants were harvested at 48 h for enzyme-linked immunosorbent assay. Median values of nonparametric variables with the range were calculated. In cultures of 22 Indian controls and leprosy patients, concanavalin A (5 μg/ml) stimulated higher IFN-γ levels in the healthy controls (415.4 pg/ml; range, 409.1 to 642.9 pg/ml) in UK controls than in either the TT/BT group (344.9 pg/ml; range, 101.5 to 608.2 pg/ml) or the LL/BL group (281.8 pg/ml; range, 70.9 to 583.5 pg/ml) (P < 0.05 in both cases). Although M. leprae soluble extract, obtained from R. J. W. Rees, World Health Organization Bank, also stimulated higher IFN-γ levels in TT/BT patients (206.0 pg/ml; range, 33.8 to 415.0 pg/ml) than in healthy controls (48.3 pg/ml; range, 0 to 279.1 pg/ml), this difference was not statistically significant, while LL/BL patients produced, as expected, the lowest levels (9.1 pg/ml; range, 0 to 262.2 pg/ml; P < 0.05, by comparison with the TT/BT group). Most surprisingly, the IFN-γ responses of both healthy controls and TT/BT patients to the tested peptides were very low (results not shown). Considering the high sensitivity of the IFN-γ assay (≤1 pg/ml), the low background production (median value, 0 pg/ml; the highest value in the range was 11 pg/ml), and the high values in a previous analysis of tuberculosis patients (24), we are confident that the lack of significant peptide-stimulated IFN-γ production was not due to technical reasons. This is in contrast with the corresponding proliferation and IFN-γ responses to the recombinant whole 35-kDa protein in the Nepali study (21). It seems unlikely that immunogenic peptides per se are not stimulatory of IFN-γ, since at least one peptide (p-624), derived from the LSR/A15 antigen of M. leprae, was reported to be stimulatory in Indian leprosy patients and controls (13).
The interleukin 10 (IL-10) assay, which had a sensitivity of 20 pg/ml, showed considerable individual variation (0 to 1000 pg/ml) in the absence of antigen. Therefore, it was necessary to compensate for this problem by introducing an arbitrary cutoff point for grading a positive peptide-stimulated increase. The results (Table 3) showed that the peptide-induced IL-10 response, irrespective of peptide specificity, was more than twice as common among patients with LL/BL disease (37.1%) than in both TT/BT (14.6%) and control (14.7%) groups (P < 0.01). Moreover, the results also suggested a significant role for peptide specificity, since 241–255 induced IL-10 production more frequently (43.5%) than peptides 41–55, 141–155, or 206–219 (8.7 to 16.17%) (P < 0.01), while 231–245 had an intermediate capacity.
TABLE 3.
Disease associations and specificities of peptide-stimulated IL-10 responses
| Peptide sequence | No. of IL-10 positivea/total no. of Indian subjects (%)
|
|||
|---|---|---|---|---|
| Healthy controls | TT/BT leprosy | LL/BL leprosy | Total | |
| 41–55 | 1/7 | 1/10 | 1/7 | 3/24 (12.5) |
| 141–155 | 0/7 | 2/10 | 2/7 | 4/24 (16.7) |
| 206–220 | 0/6 | 0/10 | 2/7 | 2/23 (8.7) |
| 231–245 | 1/7 | 1/9 | 4/7 | 6/23 (26.1) |
| 241–255 | 3/7 | 3/9 | 4/7 | 10/23c (43.5) |
| Total | 5/34 (14.7) | 7/48 (14.6) | 13/35b (37.1) | |
Cutoff: increment of >100 pg/ml and at least twice above background (no peptide) value.
Higher than either controls or TT/LL patients (P < 0.01; Fisher’s exact test).
Higher than 41–55, 141–155, or 231–245 (P < 0.01; Fisher’s exact test).
We interpret the significant production of IL-10 in the absence of antigen in both TT/BT and LL/BL patients as a sign of macrophage activation, contributing to T anergy in LL disease (14, 18). Interestingly peptide 241–255, identical in sequence in both M. leprae and M. avium, stimulated an increase in IL-10 secretion, especially in LL/BL patients, whereas the M. leprae-specific 206–219 sequence induced a minimal IL-10 response. Although the protective effect of BCG against leprosy (16) suggests a role for cross-reactive T cells in protection, our observation suggests that sensitization by M. avium in this instance could lead to a nonprotective response. Downregulation of the expression of major histocompatibility complex class II and costimulatory molecules on antigen-presenting cells by IL-10 (4) could also explain the observed low IFN-γ response to peptides. It is of further interest that 241–255 binds with the highest affinity to the DR15 allele, which has been associated with leprosy (7).
Acknowledgments
This work was supported by the European Commission EC STD3 contract TS3*CT940304 and the Medical Research Council of the UK. R.J.W. is a Wellcome Trust Fellow in Clinical Tropical Medicine.
We thank Carlos Moreno for designing the peptides; Warwick Britton of the Centenary Institute in Sydney, Australia, for providing the recombinant 35-kDa antigen; and Rama Mukherjee and K. K. Sarin, who provided the laboratory facilities at the National Institute for Immunology in New Delhi. We also thank Belinda McGinty for help in performing and interpreting the PCR with sequence-specific primers, Hemlata Devchand and Martin Vordermeier for assistance with the DRB1*01 transgenic mice, Peter Byfield for performing mass spectroscopy on the peptides, and Mohammed Latif for assistance in recruiting healthy donors in Harrow, England, who had not been exposed to leprosy. We also thank W. H. Boom of Case Western Reserve University, Cleveland, Ohio, for helpful comments on the manuscript.
REFERENCES
- 1.Altmann D M, Douek D C, Frater A J, Hetherington C, Inoko H, Elliott J I. The T cell response of HLA-DR transgenic mice to human myelin basic protein and other antigens in the presence and absence of human CD4. J Exp Med. 1995;181:867–875. doi: 10.1084/jem.181.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booth R J, Williams D L, Moudgil K D, Noonan L C, Grandison P M, McKee J J, Prestidge R L, Watson J D. Homologs of Mycobacterium leprae 18-kilodalton and Mycobacterium tuberculosis 19-kilodalton antigens in other mycobacteria. Infect Immun. 1993;61:1509–1515. doi: 10.1128/iai.61.4.1509-1515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chua-Intra B, Peerapakorn S, Davey N, Jurcevic S, Busson M, Vordermeier H M, Pirayavaraporn C, Ivanyi J. T-cell recognition of mycobacterial GroES peptides in Thai leprosy patients and contacts. Infect Immun. 1998;66:4903–4909. doi: 10.1128/iai.66.10.4903-4909.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong J-H, Zhang M, Modlin R L, Linsley P S, Iyer D, Lin Y, Barnes P. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect Immun. 1996;64:913–918. doi: 10.1128/iai.64.3.913-918.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorga J C, Foran J, Burakoff S J, Strominger J L. Use of the HLA-DR antigens incorporated into liposomes to generate HLA-DR specific cytotoxic T lymphocytes. Methods Enzymol. 1984;108:607–613. doi: 10.1016/s0076-6879(84)08121-0. [DOI] [PubMed] [Google Scholar]
- 6.Gorga J C, Horejsi V, Johnson D R, Raghupathy R, Strominger J L. Purification and characterization of class II histocompatibility antigens from a homozygous human B cell line. J Biol Chem. 1987;262:16087–16094. [PubMed] [Google Scholar]
- 7.Hill A V S. Immunogenetics of human infectious diseases. Annu Rev Immunol. 1998;16:593–617. doi: 10.1146/annurev.immunol.16.1.593. [DOI] [PubMed] [Google Scholar]
- 8.Hunter S W, Rivoire B, Mehra V, Bloom B R, Brennan P J. The major native proteins of the leprosy bacillus. J Biol Chem. 1990;265:14065–14068. [PubMed] [Google Scholar]
- 9.Ivanyi J, Morris J A, Keen M. Studies with monoclonal antibodies to mycobacteria. In: Macario A J L, editor. Monoclonal antibodies against bacteria. Vol. 1. New York, N.Y: Academic Press; 1985. pp. 59–90. [Google Scholar]
- 10.Janson A A, Klatser P R, van der Zee R, Cornelisse Y E, de Vries R R, Thole J E, Ottenhoff T H. A systematic molecular analysis of the T cell-stimulating antigens from Mycobacterium leprae with T cell clones of leprosy patients. Identification of a novel M. leprae HSP 70 fragment by M. leprae-specific T cells. J Immunol. 1991;147:3530–3537. [PubMed] [Google Scholar]
- 11.Jurcevic S, Hills A, Pasvol G, Davidson R N, Ivanyi J, Wilkinson R J. T cell responses to a mixture of Mycobacterium tuberculosis peptides with complementary HLA-DR binding profiles. Clin Exp Immunol. 1996;105:416–421. doi: 10.1046/j.1365-2249.1996.d01-791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Sette A, Rodda S, Southwood S, Sieling P A, Mehra V, Ohmen J D, Oliveros J, Appell E, Higashmoto Y, Rea T H, Bloom B R, Modlin R L. Determinants of T cell reactivity to the Mycobacterium leprae GroES homologue. J Immunol. 1997;159:335–343. [PubMed] [Google Scholar]
- 13.Misra N, Murtaza A, Walker B, Narayanan N P, Misra R S, Ramesh V, Singh S, Colston M J, Nath I. Cytokine profile of circulating T cells of leprosy patients reflects both indiscriminate and polarized T-helper subsets: T-helper phenotype is stable and uninfluenced by related antigens of Mycobacterium leprae. Immunology. 1995;86:97–103. [PMC free article] [PubMed] [Google Scholar]
- 14.Misra N, Selvakumar M, Singh S, Bharadwaj M, Ramesh V, Misra R S, Nath I. Monocyte derived IL 10 and PGE2 are associated with the absence of Th 1 cells and in vitro T cell suppression in lepromatous leprosy. Immunol Lett. 1995;48:123–128. doi: 10.1016/0165-2478(95)02455-7. [DOI] [PubMed] [Google Scholar]
- 15.Mohagheghpour N, Munn M W, Gelber R H, Engleman E G. Identification of an immunostimulating protein from Mycobacterium leprae. Infect Immun. 1990;58:703–710. doi: 10.1128/iai.58.3.703-710.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poennighaus J M, Fine P E M, Sterne J A C, Wilson R J, Msosa E, Gruer P J K, Jenkins P A, Lucas S B, Liomba N G, Bliss L. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet. 1992;339:636–639. doi: 10.1016/0140-6736(92)90794-4. [DOI] [PubMed] [Google Scholar]
- 17.Roche P W, Britton W J, Failbus S S, Ludwig H, Theuvenet W J, Adiga W B. Heterogeneity of serological responses in paucibacillary leprosy; differential responses to protein and carbohydrate antigens and correlation with clinical parameters. Int J Lepr. 1990;58:319–327. [PubMed] [Google Scholar]
- 18.Sieling P A, Abrams J S, Yamamura M, Salgame P, Bloom B R, Rea T H, Modlin R L. Immunosuppressive roles for IL-10 and IL-4 in human infection. In vitro modulation of T cell responses in leprosy. J Immunol. 1993;150:5501–5510. [PubMed] [Google Scholar]
- 19.Sinha S, Sengupta U, Ramu G, Ivanyi J. Serological survey of leprosy and control subjects by a monoclonal antibody-based immunoassay. Int J Lepr Other Mycobact Dis. 1985;53:33–38. [PubMed] [Google Scholar]
- 20.Thole J E, Wieles B, Clark-Curtiss J E, Ottenhoff T H, de Wit T F. Immunological and functional characterization of Mycobacterium leprae protein antigens: an overview. Mol Microbiol. 1995;18:791–800. doi: 10.1111/j.1365-2958.1995.18050791.x. [DOI] [PubMed] [Google Scholar]
- 21.Triccas J A, Roche P A, Winter N, Feng C G, Butlin R, Britton W J. A 35-kilodalton protein is a major target of the human immune response to Mycobacterium leprae. Infect Immun. 1996;64:5171–5177. doi: 10.1128/iai.64.12.5171-5177.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Triccas J A, Winter N, Roche P W, Gilpin A, Kendrick K E, Britton W J. Molecular and immunological analyses of the Mycobacterium avium homolog of the immunodominant Mycobacterium leprae 35-kilodalton protein. Infect Immun. 1998;66:2684–2690. doi: 10.1128/iai.66.6.2684-2690.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkinson R J, Vordermeier H M, Wilkinson K A, Sjölund A, Moreno C, Pasvol G, Ivanyi J. Peptide specific response to M. tuberculosis: clinical spectrum, compartmentalization, and effect of chemotherapy. J Infect Dis. 1998;178:760–768. doi: 10.1086/515336. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson R J, Wilkinson K A, De Smet K A L, Haslov K, Pasvol G, Singh M, Svarcova I, Ivanyi J. Human T and B cell reactivity to the 16 kDa alpha crystallin protein of Mycobacterium tuberculosis. Scand J Immunol. 1998;48:403–409. doi: 10.1046/j.1365-3083.1998.00420.x. [DOI] [PubMed] [Google Scholar]
- 25.Winter N, Triccas J A, Rivoire B, Pessolani M C V, Eiglmeier K, Lim E M, Hunter S W, Brennan P J, Britton W J. Characterization of the gene encoding the immunodominant 35 kDa protein of Mycobacterium leprae. Mol Microbiol. 1995;16:865–876. doi: 10.1111/j.1365-2958.1995.tb02314.x. [DOI] [PubMed] [Google Scholar]