Abstract
Introduction
Epstein−Barr virus (EBV) contributes significantly to the development and occurrence of B-cell lymphomas. However, the association between EBV infection status and clinical outcomes in Hodgkin lymphoma (HL) patients has long been controversial. Therefore, we aimed to estimate the prognostic significance of EBV infection in HL survival.
Methods
We searched PubMed, Embase, Web of Science, and the Cochrane Library for relevant cohort studies from the date of their inception to February 20, 2022. Hazard ratios (HRs) and 95% confidence intervals (CIs) for overall survival (OS), Failure-free survival (FFS), Progression-free survival (PFS), Event-free survival (EFS) and disease-specific survival (DSS) were extracted from the studies or calculated. Subgroup analyses were conducted independently on the five survival outcomes to investigate the source of heterogeneity.
Results
A total of 42 qualified studies involving 9570 patients were identified in our meta-analysis. There was an association between EBV positivity and significantly poorer OS (HR=1.443, 95% CI: 1.250-1.666) and DSS (HR=2.312, 95% CI: 1.799-2.972). However, the presence of EBV in HL showed no effect on FFS, PFS or EFS. In subgroup analyses of OS, DSS and FFS stratified by age groups, EBV positivity was associated with poorer prognosis in elderly patients. Meanwhile, in children and adolescents with EBV-positive HL, we also observed a trend toward a better prognosis, though the results were not statistically significant.
Conclusions
EBV-positive status is associated with poor OS and DSS in HL patients. EBV infection should therefore be considered a valuable prognostic marker and risk-stratifying factor in HL, especially in older patients.
Systematic Review Registration
https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022328708.
Keywords: Epstein – Barr virus, Hodking's lymphoma, Meta - analysis, prognosis, Virus infection
Introduction
Hodgkin lymphoma (HL) is a malignant neoplasm derived from B lymphocytes, accounting for approximately 10% of all human lymphomas (1). HL is one of the most frequent neoplasms in young individuals aged 20 to 40 years, accounting for nearly one-third of all new diagnoses (2). After the advent of combination chemotherapy, HL is now a highly curable malignancy. Optimal treatment selected according to standard staging has led to a cure rate exceeding 90% for limited stage disease and 80% for advanced disease as the norm (3). Nevertheless, the implication behind this rather impressive success rate is inevitability over- and undertreatment of at least 10-20% of patients in all stages of the disease. The challenge under such circumstances is to maximize cure rates while minimizing long-term toxicity, such as the induction of a second malignancy, dysplasia, or cardiac dysfunction (4, 5). Therefore, the identification of factors indicating different survival outcomes is critical in guiding risk-adapted therapy for HL.
Currently, commonly used prognostic systems for HL are based mainly on clinical parameters such as Ann Arbor staging and tumor size (6). Clearly, it is necessary to improve the traditional prognostic factors in combination with immunological, biological, and functional imaging data (7). Research on immunohistochemical markers for HL prognosis is currently ongoing, with studies in which the expression of the anti-apoptotic protein B-cell lymphoma-2 (Bcl2), the tumor suppressor protein p53 and topoisomerase IIα are associated with poorer prognosis (8, 9). It is accepted that EBV has transforming potential and that latent infections contribute to the pathogenesis of HL (10). EBV-positive HL is defined as the presence of EBV in tumor cells, not in bystander reactive lymphocytes (11). Currently, EBV-encoded mRNA (EBER) in situ hybridization (ISH) is considered to be the “gold standard” for EBV status. Meanwhile, some studies have shown that immunohistochemistry with LMP-1 antibodies can also reliably indicate EBV infection in HL (12, 13).
To date, a large number of studies have reported the correlation between Epstein−Barr virus (EBV) infection and the prognosis of HL, but the results of the studies have been inconsistent (positive, negative or no association) (11, 14–54). The differences in the results of the studies may be explained by different population distributions, patient selection, statistical analysis techniques and outcome measures. Therefore, we conducted a meta-analysis of all eligible published studies to quantify the prognostic value of EBV infection in HL patients.
Materials and methods
We followed the PRISMA Statement guidelines to conduct and report this systematic review and meta-analysis (55). The study was registered in PROSPERO (Record Number CRD42022328708).
Literature search
We systematically searched PubMed, Embase, Web of Science and the Cochrane library for articles published from the date of their inception to February 20, 2022. We identified studies by using the following terms: (“Epstein−Barr Virus Infections” or “EBV Infections” or “Epstein−Barr Virus” or “Human Herpes Virus 4 Infections” or “HHV 4”) and (“Hodgkin Disease” or “Hodgkin Lymphoma” or “Hodgkin’s Disease” or “Hodgkin’s Granuloma”) and (“prognosis” or “prognostic factor” or “survival”). The reference lists of the identified articles were also searched manually to ensure that no studies were overlooked.
Study selection
Two investigators (J. Y. Hu, X. Zhang) independently screened each study based on titles and abstracts. When the studies met our inclusion criteria, the full text of the articles was retrieved. We resolved disagreements through discussions or negotiations with a third investigator (H. Tao). Studies that met the following criteria were included: (1) discussed the prognosis of EBV infection in HL whose infection status was detected by EBER in situ hybridization and/or LMP-1 immunohistochemistry; (2) outcomes were survival-related; (3) sufficient survival data were provided; (4) articles were published in English; and (5) cohort design. If the same author or institution published multiple articles, we selected the most informative article.
Studies were excluded if (1) they were reviews, letters, case reports, conference abstracts, or unpublished articles; (2) study subjects were animals; or (3) the study population was human immunodeficiency virus-associated lymphoma.
Data extraction and outcomes
The data we extracted from selected articles included the following: (1) baseline characteristics (first author, publication year, country, number of patients, median/mean age, histology, etc.); (2) EBV detection method and EBV status; (3) survival outcomes (including overall survival [OS], failure-free survival [FFS], progression-free survival [PFS], event-free survival [EFS], disease-specific survival [DSS]), definitions of the five survival endpoints are summarized in Table S1 ; and (4) statistical evaluations, including Cox regression analysis hazard ratios (HRs), 95% confidence intervals (CIs), and P values. When HR and 95% CIs were absent from the original article, we used the software designed by Tierney et al. (56) to indirectly estimate from Kaplan−Meier curve.
Quality assessment
The quality of each study was assessed independently by two investigators (J. Y. Hu, X. Zhang) using the Newcastle−Ottawa Scale (NOS) (57). This scale is an eight-item instrument used to assess the selection of participants, study comparability, and ascertainment of the outcome. The NOS scores ranged from 0 to 9, and high-quality studies were defined if the score was more than 6.
Statistical analysis
We used the HRs and corresponding 95% CIs to investigate the associations between EBV infection and HL survival outcomes (OS, FFS, PFS, EFS and DSS). For a more accurate estimation of the effect of EBV infection, we selected the results of the multivariate model when both multivariate and univariate Cox regression analyses were reported in the same article. Heterogeneity was assessed by the Cochran’s Q test and I2 index, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance (58). Statistically significant heterogeneity was defined as I2 statistic>50% and/or P value < 0.10 of Cochran’s Q test. When I2>50% and/or P<0.10, the random-effects model was used to estimate pooled HRs (59); otherwise, a fixed-effects model was used (60). To explore a potential source of heterogeneity, subgroup analyses were conducted based on variables including continent, histologic subtype, age, detection method, and whether a multivariate or univariate Cox regression was used. Sensitivity analyses were performed to assess the stability of pooled HR by sequentially excluding each study. Publication bias was evaluated by visual inspection of the symmetry of the funnel plot and assessment with Begg’s and Egger’s tests (P<0.05 was deemed strong publication bias) (61). All statistical analyses were performed using Stata Version 15.1. (Stata, College Station, TX, USA), and P<0.05 was considered statistically significant.
Result
Search results
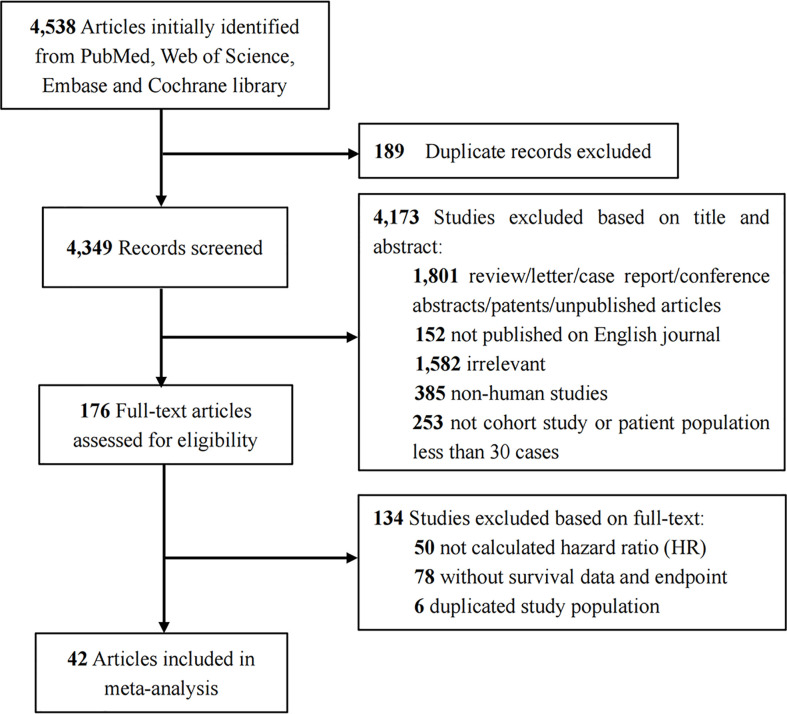
Figure 1 illustrates a flowchart describing the study inclusion process. We initially identified 4538 articles. After the removal of duplicates and screening of titles and abstracts, the full text of the 176 potentially qualified articles was reviewed. Finally, after excluding those with a duplicated study population (n=6), nonsurvival analysis data (n=78) and unable to obtain HR (n=50), 42 articles (11, 14–54) studying 9570 patients were included in our meta-analysis.
Figure 1.
Flow diagram for selection of studies.
Study characteristics and quality assessment
Table 1 shows a summary of the characteristics of the 42 included studies, most of which were retrospective cohort studies. The studies were conducted in Asia (37.7%), Europe (33.3%), North America (6.6%), South America (8.8%), Australia (2.2%) and Africa (6.6%) and published between 1997 and 2022. The sample size per study ranged from 47 to 922. The reported mean or median age for studies differed widely; five studies only included patients younger than 18 years old (16, 30, 33, 41, 48), and one study only included the elderly (20). Thirty studies (11, 14, 15, 17, 18, 20–25, 28–30, 32–36, 38, 41, 44–46, 48–51, 53, 54) reported the median or mean follow-up time, ranging from 25 to 130 months. In terms of methodological quality, all included studies scored more than six stars on the NOS. Details of the risk of bias assessment are shown in Additional file: Table S2 .
Table 1.
Main characteristics of the included studies.
| First author | Year | Country | Inclusion period | N | Median/Mean age (range) | Histology | Median follow-up (Month) | Detection method | EBV+/EBV- | Data source | Data extraction | Outcome | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yang, L. Q. | 2022 | China | 2010-2020 | 187 | 26 (2-82) | HL | 48 | EBER | 106/81 | Univariate | K-M curve | OS | 7 |
| Wang, C. | 2021 | China | 2012-2019 | 134 | 31 (5-74) | HL | 56.8 a | EBER | 62/72 | Univariate | K-M curve | OS/FFS | 8 |
| Santisteban-E, A. | 2021 | Spain | 2009-2020 | 88 | 39 (19-82) | cHL | NR | LMP1 | 36/52 | Univariate | K-M curve | OS/PFS | 7 |
| Qin, J. Q. | 2021 | China | 2013-2019 | 96 | 32 (12-79) | HL | 25 | EBER | 22/40 | Multivariate | Direct | OS/PFS | 8 |
| Antel, K. | 2021 | South Africa | 2004-2018 | 77 | 31 (25-43) | HL | 51 | EBER/LMP1 | 39/38 | Univariate | K-M curve | OS/PFS | 8 |
| Werner, L. | 2020 | Germany | 1991-2007 | 76 | 40 (4-84) | HL | 80.4 a | EBER/LMP1 | 26/50 | Univariate | K-M curve | OS | 8 |
| Cheriyalinkal P, B. | 2020 | India | 2013-2018 | 189 | NR (≤15) | cHL | 29 | LMP1 | 160/29 | Multivariate | Direct | OS/EFS | 7 |
| Wang, C. | 2018 | China | 2004-2013 | 86 | 31.5 (7-82) | HL | NR | EBER | 37/49 | Univariate | Direct | OS/PFS | 8 |
| Koh, Y. W. | 2018 | South Korea | 1990-2016 | 135 | 37 (15-78) | cHL | 58.92 a | EBER | 50/85 | Univariate | Direct | OS | 8 |
| Hollander, P. | 2018 | Sweden and Denmark | 1999-2002 | 459 | NR (18-74) | cHL | 154.8 a | EBER/LMP1 | 122/319 | Multivariate | Direct | OS | 8 |
| Myriam, B. D. | 2017 | Tunisia | 1998-2012 | 131 | 26 (4-83) | cHL | 40 | EBER | 62/69 | Univariate | K-M curve | OS/EFS | 8 |
| Chang, K. C. b | 2017 | Taiwan | 1985-2006 | 104 | 39 (6-78) | HL | NR | EBER | 48/52 | Multivariate | Direct | OS | 8 |
| LMP1 | 34/53 | ||||||||||||
| Park, J. H. | 2016 | South Korea | 2007-2013 | 70 | 39 (14–77) | cHL | NR | EBER | 32/38 | Univariate | K-M curve | OS/EFS | 8 |
| Tanyildiz, H. G. | 2015 | Turkey | 1997-2012 | 58 | 11 (3-16) | HL | 55 | LMP1 | 20/38 | Univariate | K-M curve | OS/FFS | 8 |
| Paydas, S. | 2015 | Turkey | NR | 87 | 35.3 (15-71) | HL | NR | EBER | 40/47 | Univariate | K-M curve | OS | 7 |
| Elsayed, A. A. | 2014 | Japan | 1981-2007 | 389 | 48 (4-89) | cHL | NR | EBER | 173/216 | Multivariate | Direct | OS/PFS | 9 |
| Koh, Y. W. | 2013 | South Korea | 1990-2011 | 167 | 35 (6-77) | cHL | 75.6 | EBER | 66/101 | Univariate | Direct | OS/EFS | 8 |
| Kanakry, J. A. | 2013 | USA | 1999-2006 | 794 | 32 (16-83) | HL | NR | EBER | 51/264 | Univariate | Direct | FFS | 8 |
| Koh, Y. W. c | 2012 | South Korea | 1990-2009 | 159 | 32 (4-77) | HL | 70 | EBER | 55/104 | Univariate | Direct | DSS | 8 |
| Kamper, Peter | 2011 | Denmark | 1990-2007 | 288 | 37 (6-86) | cHL | 84 | EBER/LMP1 | 95/193 | Univariate | Direct | OS/EFS | 8 |
| Souza, E. M. | 2010 | Brazil | 1994-2004 | 97 | 30 (18-75) | cHL | 80 | EBER/LMP1 | 51/46 | Univariate | K-M curve | OS/EFS | 8 |
| Barros, M. H. | 2010 | Brazil | 1999-2006 | 104 | 14 (3–18) | cHL | 68 | EBER/LMP1 | 43/55 | Univariate | K-M curve | EFS | 7 |
| Diepstra, A. | 2009 | Netherlands | 1989-2000 | 412 | 35 (7-91) | cHL | 85.2 a | EBER | 141/271 | Multivariate | Direct | FFS | 8 |
| Chetaille, B. | 2009 | France | NR | 146 | NR | cHL | NR | EBER/LMP1 | 30/115 | Univariate | Direct | OS/EFS | 7 |
| Chabay, P. A. d | 2008 | Argentina | 1990-2005 | 111 | 8 (2-18) | HL | 76 | EBER/LMP1 | 60/51 | Univariate | K-M curve | EFS | 8 |
| Chabay, P. A. d | 2008 | Brazil | 1998-2003 | 65 | 14 (3-18) | HL | 38 | EBER/LMP1 | 31/34 | Univariate | K-M curve | EFS | 8 |
| Keresztes, K. | 2006 | Hungary | NR | 109 | 31 (3-74) | HL | 83 | LMP1 | 47/62 | Univariate | K-M curve | OS/EFS | 7 |
| Jarrett, R. F. | 2006 | United Kingdom | 1993-1997 | 437 | NR (16-74) | cHL | 93 | EBER | 145/292 | Univariate | K-M curve | OS/DSS | 8 |
| Asano, N. | 2006 | Japan | NR | 324 | 48 (4-89) | cHL | NR | EBER | 149/165 | Univariate | Direct | DSS | 7 |
| Al-Kuraya, K. | 2006 | Saudi Arabia | 1991-2002 | 141 | NR | HL | NR | EBER/LMP1 | 24/55 | Univariate | K-M curve | OS | 6 |
| Keegan, T. H. e | 2005 | USA | 1988-1997 | 922 | NR | cHL | 97 | EBER/LMP1 | 246/676 | Multivariate | Direct | OS/DSS | 8 |
| Claviez, A. | 2005 | Germany | 1990-2001 | 842 | 13.7 (2.2-20.2) | HL | 58.5 | LMP1 | 263/579 | Univariate | K-M curve | OS/FFS | 8 |
| Krugmann, J. | 2003 | Austria | 1974-1999 | 119 | 37.6 (14-83) | cHL | 122 | LMP1 | 31/88 | Univariate | K-M curve | OS/FFS | 8 |
| Herling, M. | 2003 | USA, Italy, Greece | 1984-2000 | 577 | 30 (NR) | cHL | 65 | LMP1 | 61/242 | Univariate | K-M curve | OS/FFS | 8 |
| Flavell, K. J. | 2003 | UK | 1983-1996 | 273 | NR | HL | 60 | EBER | 78/195 | Multivariate | Direct | FFS | 7 |
| Stark, G. L. | 2002 | UK | 1991–1998 | 102 | 70 (60–91) | HL | 63 | LMP1 | 24/46 | Univariate | K-M curve | DSS | 8 |
| Glavina-D, M. | 2001 | Croatia | 1980-1990 | 100 | 40 (13–84) | HL | NR | LMP1 | 26/74 | Univariate | K-M curve | OS | 7 |
| Clarke, C. A. | 2001 | USA | 1988-1994 | 311 | NR (19-79) | HL | 73 | LMP1 | 53/258 | Univariate | K-M curve | OS | 7 |
| Naresh, K. N. | 2000 | India | 1984-1988 | 110 | 22 (4-61) | cHL | 57 | EBER/LMP1 | 86/24 | Univariate | K-M curve | OS | 6 |
| Engel, M. | 2000 | South Africa | NR | 47 | 8 (3-14) | HL | NR | EBER/LMP1 | 24/12 | Univariate | K-M curve | OS | 7 |
| Murray, P. G. | 1999 | UK | 1992-1996 | 190 | 33 (22-49) | HL | 86 | EBER/LMP1 | 51/139 | Univariate | K-M curve | OS/FFS | 6 |
| Enblad, G. | 1999 | Sweden | 1985-1988 | 117 | 45 (11-87) | HL | 130 | EBER/LMP1 | 32/85 | Univariate | K-M curve | DSS | 7 |
| Morente, M. M. | 1997 | Spain | NR | 140 | 37.2 (5-83) | HL | 65 | LMP1 | 72/68 | Multivariate | Direct | OS | 8 |
Mean follow-up time.
For article written by Chang, K. C. et al., two detection methods were used to define the EBV infection status and the HR were given respectively.
Koh, Y. W. et al. had another article published in 2013 with similar study population, but this article included for one more endpoint.
The same article, two patient groups gave HR values respectively.
For article written by Keegan, T. H., HR values of the total population were not given, and were divided into three age groups.
EBV, Epstein–Barr virus; HL, Hodgkin’s lymphoma; cHL, classical Hodgkin lymphoma; HR, hazard ratios; NOS, Newcastle-Ottawa Scale; EBER, Epstein–Barr virus-encoded small RNA; LMP1, latent membrane protein-1; OS, Overall survival; FFS, Failure-free survival; PFS, Progression-free survival; EFS, Event-free survival; DSS, Disease-specific survival; K-M curve, Kaplan-Meier curve; NR, Not reported.
Meta-analysis results
Overall survival
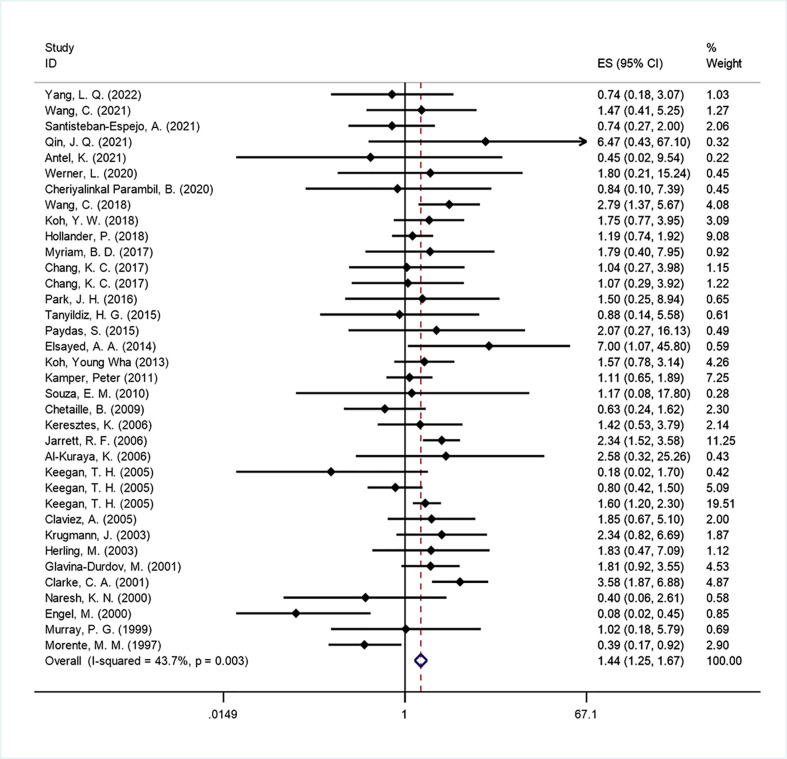
Thirty-three studies (11, 14, 16–19, 22–26, 28, 29, 31, 34, 35, 38–54) (corresponding to 36 sets) were included to analyze the impact of EBV infection on OS. Our meta-analysis showed that EBV positivity in HL was correlated with unfavorable outcomes for OS (HR=1.443, 95% CI: 1.250-1.666, P<0.001; Figure 2 ). Moderate heterogeneity was found across the studies (I2 = 43.7%, P=0.003) by employing a fixed effects model. Therefore, to explain the heterogeneity, we conducted subgroup analyses according to continents, histology, age groups, detection method, data source and data extraction ( Table 2 ). In the subgroup analysis by disease distribution on six continents, the African subgroup showed that EBV-positive patients had a borderline better OS (HR=0.408, 95% CI: 0.147-1.129; P =0.084). For age distribution, some articles (16, 18, 25, 28, 41, 48, 50, 52) had sufficient age-stratified survival data, so we combined their HR by a random effects model (I2 = 62.9%, P=0.003), which was 1.080 (95% CI: 0.657-1.776; P=0.762). In the subgroup of children and adolescents, the pooled HR showed that EBV positivity in HL was correlated with a favorable outcome for OS (HR=0.296, 95% CI: 0.085-1.034, P=0.056), while a significantly poorer OS was associated with EBV positivity in studies covering older adults (HR=1.905, 95% CI: 1.380–2.629; P<0.001). This may partly explain the heterogeneity observed when examining EBV infection as a prognostic factor in HL patients.
Figure 2.
Forest plot of the hazard rations for overall survival (OS) between patients with EBV-positive and EBV-negative HL.
Table 2.
Subgroup analysis of relationship between EBV infection and OS/FFS/PFS/EFS/DSS.
| Outcome | Subgroup | Data set (n) | Patients (n) | Model | HR (95%CI) | P | Heterogeneity | |
|---|---|---|---|---|---|---|---|---|
| I2 | Ph | |||||||
| OS | ALL | 36 | 7213 | Fixed | 1.443 (1.250-1.666) | <0.001 | 43.7% | 0.003 |
| Continent | Fixed | |||||||
| Asia | 15 | 2057 | 1.658 (1.205-2.282) | 0.002 | 0 | 0.696 | ||
| Europe | 11 | 2875 | 1.321 (1.065-1.638) | 0.011 | 51.4% | 0.024 | ||
| Africa | 3 | 255 | 0.408 (0.147-1.129) | 0.084 | 74.9% | 0.019 | ||
| South America | 1 | 97 | 1.170 (0.078-17.452) | 0.909 | – | – | ||
| North America | 4 | 1233 | 1.572 (1.209-2.045) | 0.001 | 78.8% | 0.003 | ||
| Australia | 1 | 119 | 2.340 (0.819-6.684) | 0.112 | – | – | ||
| Unclassified | 1 | 577 | 1.830 (0.471-7.108) | 0.383 | – | – | ||
| Histology | ||||||||
| HL | 18 | 2889 | 1.501 (1.151-1.958) | 0.003 | 55.1% | 0.003 | ||
| cHL | 18 | 4324 | 1.420 (1.197-1.684) | <0.001 | 29.7% | 0.114 | ||
| Detection method | Fixed | |||||||
| EBER | 12 | 1606 | 2.024 (1.551-2.642) | <0.001 | 0 | 0.749 | ||
| LMP1 | 11 | 2409 | 1.506 (1.121-2.022) | 0.007 | 51.6% | 0.024 | ||
| EBER/LMP1 | 13 | 2363 | 1.147 (0.930-1.414) | 0.200 | 47.9% | 0.027 | ||
| Data source | Fixed | |||||||
| Univariate | 27 | 4247 | 1.599 (1.328-1.926) | <0.001 | 39.2% | 0.021 | ||
| Multivariate | 9 | 2131 | 1.240 (0.989-1.555) | 0.062 | 51.7% | 0.035 | ||
| Data extraction | Fixed | |||||||
| K-M curve | 21 | 3389 | 1.740 (1.380-2.195) | <0.001 | 34.6% | 0.061 | ||
| Direct | 15 | 2989 | 1.285 (1.070-1.543) | 0.007 | 49.2% | 0.016 | ||
| Age subgroups | 11 | Random | 1.080 (0.657-1.776) | 0.762 | 62.9% | 0.003 | ||
| children and adolescent | 4 | 330 | 0.296 (0.085-1.034) | 0.056 | 41.4% | 0.163 | ||
| young adults | 4 | 995 | 0.882 (0.518-1.500) | 0.642 | 0 | 0.882 | ||
| older adults | 3 | 480 | 1.905 (1.380-2.629) | <0.001 | 23% | 0.273 | ||
| FFS | ALL | 9 | 3399 | Fixed | 1.030 (0.832-1.274) | 0.788 | 0 | 0.556 |
| Detection method | Fixed | |||||||
| EBER | 4 | 685 | 0.961 (0.723-1.277) | 0.785 | 35.7% | 0.198 | ||
| LMP1 | 4 | 1538 | 1.158 (0.821-1.632) | 0.403 | 0 | 0.703 | ||
| EBER/LMP1 | 1 | 190 | 0.910 (0.361-2.295) | 0.842 | – | – | ||
| Data source | Fixed | |||||||
| Univariate | 7 | 1728 | 1.168 (0.891-1.529) | 0.261 | 0 | 0.920 | ||
| Multivariate | 2 | 685 | 0.836 (0.591-1.183) | 0.312 | 61.7% | 0.106 | ||
| Data extraction | Fixed | |||||||
| K-M curve | 6 | 1728 | 1.158 (0.853-1.573) | 0.346 | 0 | 0.852 | ||
| Direct | 3 | 685 | 0.921 (0.685-1.240) | 0.589 | 46.4% | 0.155 | ||
| Age subgroups | Fixed | 1.487 (0.947-2.337) | 0.085 | 36.0% | 0.154 | |||
| children and adolescent | 1 | 58 | 0.910 (0.171-4.851) | 0.912 | – | – | ||
| young adults | 4 | 353 | 1.000 (0.563-1.775) | 1.000 | 0 | 0.733 | ||
| older adults | 2 | 128 | 3.726 (1.649-8.419) | 0.002 | 3.9% | 0.308 | ||
| PFS | ALL | 5 | 736 | Random | 1.365 (0.694-2.684) | 0.368 | 54.5% | 0.066 |
| EFS | ALL | 10 | 1477 | Fixed | 0.962 (0.755-1.227) | 0.756 | 0 | 0.543 |
| Continent | Fixed | |||||||
| Asia | 3 | 426 | 1.087 (0.710-1.664) | 0.702 | 65.5% | 0.055 | ||
| Africa | 1 | 131 | 0.720 (0.228-2.277) | 0.576 | – | – | ||
| Europe | 3 | 543 | 0.937 (0.677-1.298) | 0.697 | 0 | 0.400 | ||
| South America | 4 | 377 | 0.816 (0.332-2.005) | 0.657 | 0 | 0.906 | ||
| Detection method | Fixed | |||||||
| EBER | 3 | 368 | 1.238 (0.805-1.902) | 0.331 | 0 | 0.528 | ||
| LMP1 | 2 | 298 | 0.731 (0.346-1.548) | 0.413 | 74.9% | 0.046 | ||
| EBER/LMP1 | 6 | 811 | 0.880 (0.638-1.212) | 0.432 | 0 | 0.913 | ||
| Data source | Fixed | |||||||
| Univariate | 10 | 1288 | 1.018 (0.794-1.307) | 0.886 | 0 | 0.839 | ||
| Multivariate | 1 | 189 | 0.330 (0.112-0.975) | 0.045 | – | – | ||
| Data extraction | Fixed | |||||||
| K-M curve | 7 | 687 | 1.039 (0.595-1.815) | 0.893 | 0 | 0.881 | ||
| Direct | 4 | 790 | 0.945 (0.721-1.238) | 0.682 | 53.2% | 0.093 | ||
| DSS | ALL | 8 | 2061 | Fixed | 2.312 (1.799-2.972) | <0.001 | 26% | 0.221 |
| Continent | ||||||||
| Asia | 2 | 483 | 2.120 (1.315-3.418) | 0.002 | 71.7% | 0.060 | ||
| Europe | 3 | 656 | 2.629 (1.738-3.977) | <0.001 | 0 | 0.999 | ||
| North America | 3 | 922 | 2.165 (1.420-3.300) | <0.001 | 62.5% | 0.069 | ||
| Detection method | Fixed | |||||||
| EBER | 3 | 920 | 2.334 (1.644-3.313) | <0.001 | 48.3% | 0.144 | ||
| LMP1 | 1 | 102 | 2.660 (1.099-6.436) | 0.030 | – | – | ||
| EBER/LMP1 | 4 | 1039 | 2.223 (1.499-3.297) | <0.001 | 45.0% | 0.142 | ||
| Data source | Fixed | |||||||
| Univariate | 7 | 1902 | 2.189 (1.686-2.844) | <0.001 | 18.1% | 0.292 | ||
| Multivariate | 1 | 159 | 4.396 (1.792-10.785) | 0.001 | – | – | ||
| Data extraction | Fixed | |||||||
| K-M curve | 5 | 1542 | 2.504 (1.859-3.373) | <0.001 | 0 | 0.978 | ||
| Direct | 3 | 519 | 1.902 (1.192-3.033) | 0.007 | 75.2% | 0.018 | ||
| Age subgroups | Fixed | 2.094 (1.506-2.912) | <0.001 | 1.2% | 0.415 | |||
| children and adolescent | 1 | 36 | 0.180 (0.020-1.660) | 0.130 | – | – | ||
| young adults | 2 | 845 | 1.744 (0.736-4.131) | 0.206 | 0 | 0.965 | ||
| older adults | 4 | 501 | 2.308 (1.607-3.312) | <0.001 | 0 | 0.817 | ||
EBV, Epstein–Barr virus; HL, Hodgkin’s lymphoma; cHL, classical Hodgkin lymphoma; HR, hazard ratios, EBER, Epstein–Barr virus-encoded small RNA; LMP1, latent membrane protein-1; OS, Overall survival; FFS, Failure-free survival; PFS, Progression-free survival; EFS, Event-free survival; DSS, Disease-specific survival; K-M curve, Kaplan-Meier curve.
Failure-free survival
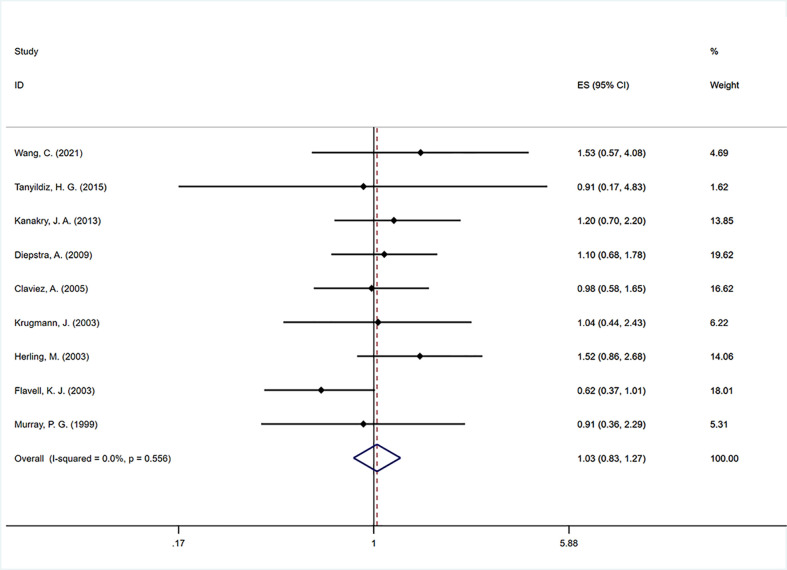
Nine studies (11, 21–24, 32, 37, 41, 53) were included to analyze the impact of EBV infection on FFS. The pooled estimate showed no significant association between EBV positivity and FFS (HR=1.030, 95% CI: 0.832–1.274, P=0.788; Figure 3 ). No significant heterogeneity was found across the studies (I2 = 0, P=0.556). In the subgroup analysis, we found that EBV positivity was strongly associated with poorer FFS (HR=3.726, 95% CI: 1.649–8.419, P=0.002) in older adults. In addition, the prognostic results of the three subgroups grouped according to detection method, data source and data extraction were similar, and all had no effect on FFS ( Table 2 ).
Figure 3.
Forest plot of the hazard rations for failure free survival (FFS) between patients with EBV-positive and EBV-negative HL.
Progression-free survival
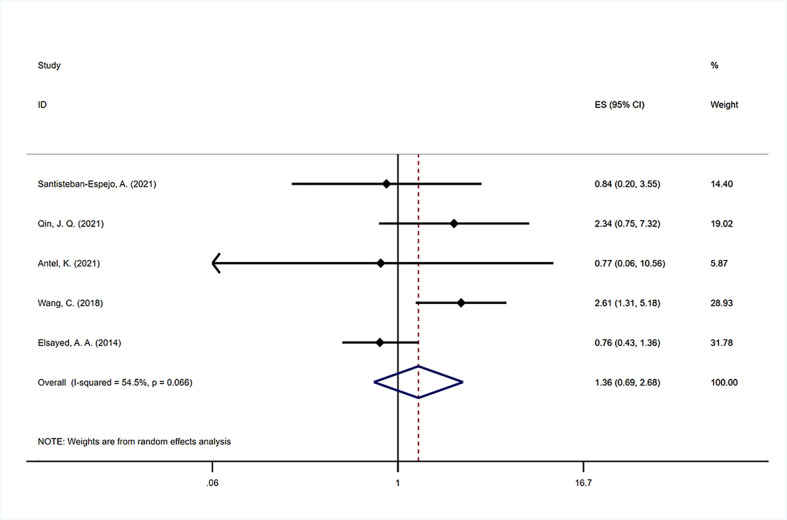
Five studies (39, 47, 50–52) were included to analyze the impact of EBV infection on PFS. There was significant between-study heterogeneity (I2 = 54.5%, P=0.066), and the pooled estimate by the random-effects model showed that no significant association was found between EBV positivity and PFS (HR=1.302, 95% CI: 0.881–1.926, P=0.186; Figure 4 ). Due to the lower number of analyzable studies, subgroup analysis was not performed for PFS.
Figure 4.
Forest plot of the hazard rations for progression free survival (PFS) between patients with EBV-positive and EBV-negative HL.
Event-free survival
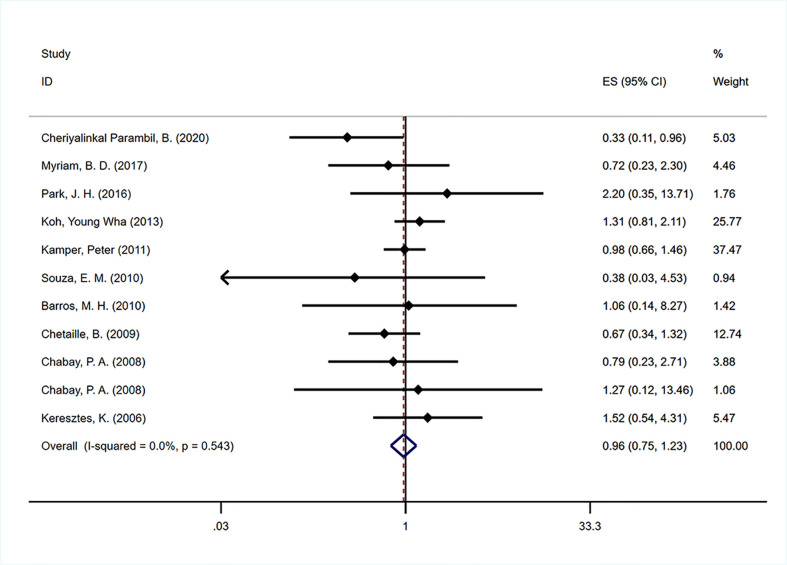
Ten studies (29–31, 33–35, 38, 42, 44, 48) were included to analyze the impact of EBV infection on EFS. The pooled estimate showed that no significant association was found between EBV positivity and EFS (HR=0.962, 95% CI: 0.755-1.227, P=0.756; Figure 5 ). No significant heterogeneity was found across the studies (I2 = 0, P=0.543). The prognostic effects were similar between the four predefined subgroups according to continent, detection method, data source and extraction ( Table 2 ).
Figure 5.
Forest plot of the hazard rations for event free survival (EFS) between patients with EBV-positive and EBV-negative HL.
Disease-specific survival
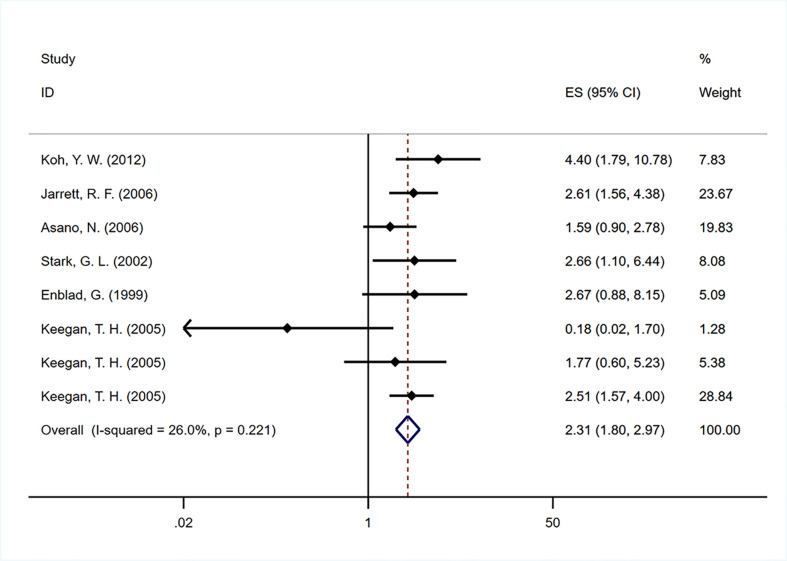
Six studies (15, 20, 25, 26, 28, 36) were included to analyze the impact of EBV infection on DSS. The pooled HR of 2.312 (95% CI: 1.799-2.972) was calculated on the basis of a fixed-effects model ( Figure 6 ), which showed a worse DSS among EBV-positive patients than EBV-negative patients. In subgroup analysis, a fixed-effects model was used for the age subgroup meta-analysis due to the heterogeneity among studies (I2 = 1.2, P=0.415). Interestingly, EBV-positive older adults had poorer DSS (HR=2.308, 95% CI: 1.607-3.312; P<0.001) than EBV-negative adults, whereas studies involving children, adolescents and young adults yielded no association between EBV infection and DSS ( Table 2 ).
Figure 6.
Forest plot of the hazard rations for disease-specific survival (DSS) between patients with EBV-positive and EBV-negative HL.
Sensitivity analysis and publication bias
We conducted a sensitivity analysis of the association between EBV infection and survival outcomes and demonstrated that the results were robust after omitting any of the included studies ( Figure S1 ).
Funnel plots with Begg’s test and Egger’s test were used to assess publication bias, and no evidence of bias was found in our meta-analysis of the selected studies. The p values were all >0.05, and details of the survival outcome publication bias can be seen in Figure S2 .
Discussion
The prognostic significance of EBV infection in HL patients remains controversial. Here, we conducted a meta-analysis involving 9570 patients from 42 studies to systematically explore the prognostic value of EBV infection in HL. Our results demonstrated that EBV positivity predicted short DSS and OS, but it had no significant effect on FFS, PFS or EFS. Moreover, subgroup analysis showed that in children and adolescent HL patients, EBV positivity allowed some survival advantage compared with the outcomes of EBV-negative patients, although the difference was not statistically significant. In contrast, EBV-positive elderly patients with HL have strongly poorer survival outcomes than EBV-negative patients.
To our knowledge, this study is an update of two meta-analyses published in 2014 (62) and 2015 (63) on EBV infection and HL OS. Chen, Y. P. et al. (63) found no significant association between EBV infection and overall survival, but their age-specific subgroup analyses showed that OS was significantly shorter when patients’ median/mean age was ≥40 years. In addition, they found that EBV positivity had a tendency for worse OS in patients in Europe and North America. Similar to the findings of Chen and colleagues, a meta-analysis by Lee, J. H. et al. (62) also failed to reveal an association between EBV infection status and cHL patient survival. The reason for the different results between the two meta-analyses above and ours may be that we included more studies published in recent years; moreover, the type of disease was not limited to cHL, and the detection of EBV infection was not restricted to LMP1.
For the survival endpoints of OS and DSS, our results are in line with 7 studies (20, 28, 36, 39, 46, 47, 49), and other reports describing clinical outcomes in relation to EBV status are conflicting. Many studies have not demonstrated that EBV status has an impact on prognosis (35, 38, 40–42, 44, 45, 51–54), whereas some studies have shown that EBV-positive status is associated with a favorable clinical outcome (14, 16, 17, 23, 48, 50). The discrepancies observed in these studies were generally due to the heterogeneous nature of the disease and the selection bias of study subjects, age groups, EBV detection, treatment regimens and the different outcome measures used. Additionally, since the distribution of EBV varies widely in the population, it may reflect racial or ethnic differences. In fact, our subgroup analyses showed that the HR of OS and DSS was not influenced by whether nodular lymphocytic predominant Hodgkin’s lymphoma (NLPHL) patients were excluded, whether the data were extracted from the KM curve and different detection methods. Only the pooled HR from Africa had a tendency to improve OS; interestingly, two of the three included studies enrolled populations younger than 50 years old (16, 50). This was in good agreement with the findings obtained from our subgroup analysis of different age groups. The effect of EBV status on OS and DSS is age dependent, and older adult patients with EBV-positive HL had a particularly poor prognosis, which was consistent with the findings of some population-based studies (18, 25, 28, 38, 50). Our study showed better survival trends for children and adolescents, although this trend was not statistically significant. The abovementioned differential effects on outcomes with respect to age and geography may be attributed to the following reason. EBV infection rates in patients with HL were significantly higher in African and South American countries than in other regions, according to an epidemiological survey (62); therefore, children had a relatively high risk of early exposure to a wide range of infectious agents. As LMP1 has antigenicity, LMP1 could activate cytotoxic T lymphocytes (CTLs) more effectively, resulting in a stronger antitumor immune response (64), which may in turn limit disease progression. However, cytotoxic T-cell responses have been observed to decline with age (65, 66), and another possibility is immunosenescence, in which the impaired immune system is unable to respond effectively to viral infection, allowing EBV reactivation and oncogenic transformation (67). In summary, the younger group has a beneficial EBV-specific immune response to the tumor cell population, whereas in older patients, this response may be less effective or other negative prognostic factors may outweigh any beneficial effect EBV may have. For example, elderly patients have poor treatment tolerance, with a subset unable to tolerate enough chemotherapy or combined radiotherapy and chemotherapy. Furthermore, elderly patients may have had complications that harmed their chances of survival (68).
Herling et al. (22) considered that selection of the study endpoint may be an important factor affecting EBV status and prognosis, and compared to OS and DSS, FFS is a better survival endpoint. OS and DSS are both affected by salvage management after relapse, which was not even mentioned in most studies. Meanwhile, the frequency of disease-unrelated deaths is relatively high in the elderly population, and the natural limitation of life expectancy, these deaths may obscure disease effects in older adult patients. Our meta-analysis concluded that EBV infection status did not affect FFS in the entire population, which is consistent with the results of many previous studies (11, 21, 22, 24, 32, 37, 41, 53). However, Wang, C. et al. (53) and Diepstra, A (32). illustrated that the prognosis was significantly worse for EBV-positive than EBV-negative patients when patients were older than 50 years. Because our age subgroup analysis included the same two papers as well, the results were similar. Only one article (23) reported that EBV-positive patients have a longer FFS than EBV-negative patients; this is the sole study from Australia, which contradicts my results and is probably related to geographical differences.
As with FFS, EFS and PFS were also unaffected by EBV infection status. The ending endpoint was PFS in a small number of articles (23, 39, 47, 50–52), and we did not perform further subgroup analyses. A child-based study from India (48) showed that EBV-positive children have longer EFS than EBV-negative children, which contradicted our finding and may be explained by the high prevalence of EBV infection in Indian children and the greater chemotherapy and radiotherapy sensitivity of infected tumor cells (17).
At present, the mechanism by which EBV acts on HL is still unclear (69). In EBV-positive HL, viral infection of malignant tumor cells is characterized by the consistent expression of three EBV-associated viral proteins (EBNA1, LMP1, and LMP2A) and two noncoding RNAs (EBERs and BARTs) (67), which are believed to play important roles in tumorigenesis, including the regulation of proliferation, metastasis, immune escape, and apoptosis (70, 71). EBNA1 enhanced the activity of the AP‐1 transcription factor, triggering the induction of VEGF and IL‐8 (72); meanwhile, this protein can inhibit the antigenic peptide bound to major histocompatibility complex 1 (MHC-1) to evade recognition by CTL (73). LMP1 stimulates the proliferation of B cells by activating nuclear factor-kappa B (NFκB) and the transcription factor AP-1 (74). Moreover, LMP1 can also immortalize resting B lymphocytes and turn them into latently infected lymphoblastoid cell lines (75, 76). Collectively, these mechanisms may explain why EBV positivity is associated with poor clinical outcomes in HL patients. There are certain limitations that must be considered when interpreting the results of our study. First, there was some heterogeneity across these included articles, and despite the use of subgroup analysis, it was not feasible to explore all of the variability. Treatment regimens are not clearly indicated in some studies, and many articles do not conduct age-stratified analysis. These limitations prevented us from fully tracing the origin of heterogeneity. Second, the quality of published data for our study was relatively low, and most of the included studies were retrospective in design. Third, the age cutoff between children, adults and elderly varied according to the published studies; thus, to include as many studies as possible, 15-18 years old and 45-50 years old were used as a vague distinction dividing patients into children and adolescents, young adults and older adults. To obtain more meaningful results, more research involving the unified age cutoff is needed. Finally, because this study was limited to studies published in English, publication bias cannot be ruled out. The prevalence of EBV is higher in developing countries, but our study embraces only a small number of studies in Africa and South America. In addition, although some studies have shown that EBV-DNA can be used as a prognostic marker for EBV-associated HL, the choice of compartments of peripheral blood and cut-off copies of EBV-DNA is different in various studies (37, 51, 77, 78). Given the different criteria in the related original studies, we did not include the studies of using PCR method to detect EBV infection.
Conclusion
Our findings suggest that EBV-positive status is associated with poor OS and DSS in HL patients. EBV infection should therefore be considered a valuable prognostic marker and risk-stratifying factor in HL, especially in older patients. More studies in the future should include a larger number of children and young adults to investigate the combined effects of age and EBV status with other prognostic factors to improve the therapeutic applicability of these findings.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author contributions
JH and YJ formulated the research questions and designed the study; JH, XZ, and HT conducted the literature search, selected the articles, and extracted the data; JH and HT analyzed the data and drafted the manuscript; and YJ critically revised the article. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (grant number 2020HXFX020).
Acknowledgments
Thanks to the authors of all the included articles that were used as data sources for this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1034398/full#supplementary-material
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin (2019) 69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2. Eichenauer DA, Engert A, André M, Federico M, Illidge T, Hutchings M, et al. Hodgkin’s lymphoma: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2014) 25(Suppl 3):iii70–5. doi: 10.1093/annonc/mdu181 [DOI] [PubMed] [Google Scholar]
- 3. Connors JM, Cozen W, Steidl C, Carbone A, Hoppe RT, Flechtner H-H, et al. Hodgkin Lymphoma. Nat Rev Dis Primers (2020) 6(1):61. doi: 10.1038/s41572-020-0189-6 [DOI] [PubMed] [Google Scholar]
- 4. Trotman J, Barrington SF. The role of pet in first-line treatment of Hodgkin lymphoma. Lancet Haematol (2021) 8(1):e67–79. doi: 10.1016/s2352-3026(20)30357-4 [DOI] [PubMed] [Google Scholar]
- 5. Ansell SM. Hodgkin Lymphoma: A 2020 update on diagnosis, risk-stratification, and management. Am J Hematol (2020) 95(8):978–89. doi: 10.1002/ajh.25856 [DOI] [PubMed] [Google Scholar]
- 6. Wang HW, Balakrishna JP, Pittaluga S, Jaffe ES. Diagnosis of Hodgkin lymphoma in the modern era. Br J Haematol (2019) 184(1):45–59. doi: 10.1111/bjh.15614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bröckelmann PJ, Angelopoulou MK, Vassilakopoulos TP. Prognostic factors in Hodgkin lymphoma. Semin Hematol (2016) 53(3):155–64. doi: 10.1053/j.seminhematol.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 8. Travaglino A, Russo D, Varricchio S, Picardi M, Mascolo M. Prognostic value of Bcl2 and P53 in Hodgkin lymphoma: A systematic review and meta-analysis. Pathol Res Pract (2021) 219:153370. doi: 10.1016/j.prp.2021.153370 [DOI] [PubMed] [Google Scholar]
- 9. Mottok A, Steidl C. Biology of classical Hodgkin lymphoma: Implications for prognosis and novel therapies. Blood (2018) 131(15):1654–65. doi: 10.1182/blood-2017-09-772632 [DOI] [PubMed] [Google Scholar]
- 10. Murray P, Bell A. Contribution of the Epstein-Barr virus to the pathogenesis of Hodgkin lymphoma. Curr Top Microbiol Immunol (2015) 390(Pt 1):287–313. doi: 10.1007/978-3-319-22822-8_12 [DOI] [PubMed] [Google Scholar]
- 11. Murray PG, Billingham LJ, Hassan HT, Flavell JR, Nelson PN, Scott K, et al. Effect of Epstein-Barr virus infection on response to chemotherapy and survival in hodgkin’s disease. Blood (1999) 94(2):442–7. doi: 10.1182/blood.V94.2.442.414a46_442_447 [DOI] [PubMed] [Google Scholar]
- 12. Gulley ML, Glaser SL, Craig FE, Borowitz M, Mann RB, Shema SJ, et al. Guidelines for interpreting eber in situ hybridization and Lmp1 immunohistochemical tests for detecting Epstein-Barr virus in Hodgkin lymphoma. Am J Clin Pathol (2002) 117(2):259–67. doi: 10.1309/mmau-0qyh-7bha-w8c2 [DOI] [PubMed] [Google Scholar]
- 13. Carbone A, Gloghini A. Epstein Barr Virus-associated Hodgkin lymphoma. Cancers (Basel) (2018) 10(6):163. doi: 10.3390/cancers10060163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morente MM, Piris MA, Abraira V, Acevedo A, Aguilera B, Bellas C, et al. Adverse clinical outcome in hodgkin’s disease is associated with loss of retinoblastoma protein expression, high Ki67 proliferation index, and absence of Epstein-Barr virus-latent membrane protein 1 expression. Blood (1997) 90(6):2429–36. doi: 10.1182/blood.V90.6.2429 [DOI] [PubMed] [Google Scholar]
- 15. Enblad G, Sandvej K, Sundström C, Pallesen G, Glimelius B. Epstein-Barr Virus distribution in hodgkin’s disease in an unselected Swedish population. Acta Oncol (1999) 38(4):425–9. doi: 10.1080/028418699431942 [DOI] [PubMed] [Google Scholar]
- 16. Engel M, Essop MF, Close P, Hartley P, Pallesen G, Sinclair-Smith C. Improved prognosis of Epstein-Barr virus associated childhood hodgkin’s lymphoma: Study of 47 south African cases. J Clin Pathol (2000) 53(3):182–6. doi: 10.1136/jcp.53.3.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naresh KN, Johnson J, Srinivas V, Soman CS, Saikia T, Advani SH, et al. Epstein-Barr Virus association in classical hodgkin’s disease provides survival advantage to patients and correlates with higher expression of proliferation markers in reed-sternberg cells. Ann Oncol (2000) 11(1):91–6. doi: 10.1023/a:1008337100424 [DOI] [PubMed] [Google Scholar]
- 18. Clarke CA, Glaser SL, Dorfman RF, Mann R, DiGiuseppe JA, Prehn AW, et al. Epstein-Barr Virus and survival after Hodgkin disease in a population-based series of women. Cancer (2001) 91(8):1579–87. doi: [DOI] [PubMed] [Google Scholar]
- 19. Glavina-Durdov M, Jakic-Razumovic J, Capkun V, Murray P. Assessment of the prognostic impact of the Epstein-Barr virus-encoded latent membrane protein-1 expression in hodgkin’s disease. Br J Cancer (2001) 84(9):1227–34. doi: 10.1054/bjoc.2001.1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stark GL, Wood KM, Jack F, Angus B, Proctor SJ, Taylor PR. Hodgkin’s disease in the elderly: A population-based study. Br J Haematol (2002) 119(2):432–40. doi: 10.1046/j.1365-2141.2002.03815.x [DOI] [PubMed] [Google Scholar]
- 21. Flavell KJ, Billingham LJ, Biddulph JP, Gray L, Flavell JR, Constandinou CM, et al. The effect of Epstein-Barr virus status on outcome in age- and sex-defined subgroups of patients with advanced hodgkin’s disease. Ann Oncol (2003) 14(2):282–90. doi: 10.1093/annonc/mdg065 [DOI] [PubMed] [Google Scholar]
- 22. Herling M, Rassidakis GZ, Medeiros LJ, Vassilakopoulos TP, Kliche KO, Nadali G, et al. Expression of Epstein-Barr virus latent membrane protein-1 in Hodgkin and reed-sternberg cells of classical hodgkin’s lymphoma: Associations with presenting features, serum interleukin 10 levels, and clinical outcome. Clin Cancer Res (2003) 9(6):2114–20. Available at: https://aacrjournals.org/clincancerres/article/9/6/2114/3120/Expression-of-Epstein-Barr-Virus-Latent-Membrane?searchresult=1. [PubMed] [Google Scholar]
- 23. Krugmann J, Tzankov A, Gschwendtner A, Fischhofer M, Greil R, Fend F, et al. Longer failure-free survival interval of Epstein-Barr virus-associated classical hodgkin’s lymphoma: A single-institution study. Mod Pathol (2003) 16(6):566–73. doi: 10.1097/01.Mp.0000071843.09960.Bf [DOI] [PubMed] [Google Scholar]
- 24. Claviez A, Tiemann M, Lüders H, Krams M, Parwaresch R, Schellong G, et al. Impact of latent Epstein-Barr virus infection on outcome in children and adolescents with hodgkin’s lymphoma. J Clin Oncol (2005) 23(18):4048–56. doi: 10.1200/jco.2005.01.701 [DOI] [PubMed] [Google Scholar]
- 25. Keegan TH, Glaser SL, Clarke CA, Gulley ML, Craig FE, Digiuseppe JA, et al. Epstein-Barr Virus as a marker of survival after hodgkin’s lymphoma: A population-based study. J Clin Oncol (2005) 23(30):7604–13. doi: 10.1200/jco.2005.02.6310 [DOI] [PubMed] [Google Scholar]
- 26. Al-Kuraya K, Narayanappa R, Al-Dayel F, El-Solh H, Ezzat A, Ismail H, et al. Epstein-Barr Virus infection is not the sole cause of high prevalence for hodgkin’s lymphoma in Saudi Arabia. Leuk Lymphoma (2006) 47(4):707–13. doi: 10.1080/10428190500286879 [DOI] [PubMed] [Google Scholar]
- 27. Asano N, Oshiro A, Matsuo K, Kagami Y, Ishida F, Suzuki R, et al. Prognostic significance of T-cell or cytotoxic molecules phenotype in classical hodgkin’s lymphoma: A clinicopathologic study. J Clin Oncol (2006) 24(28):4626–33. doi: 10.1200/jco.2006.06.5342 [DOI] [PubMed] [Google Scholar]
- 28. Jarrett RF, Stark GL, White J, Angus B, Alexander FE, Krajewski AS, et al. Impact of tumor Epstein-Barr virus status on presenting features and outcome in age-defined subgroups of patients with classic Hodgkin lymphoma: A population-based study. Blood (2006) 107(3):1241. doi: 10.1182/blood-2004-09-3759 [DOI] [PubMed] [Google Scholar]
- 29. Keresztes K, Miltenyi Z, Bessenyei B, Beck Z, Szollosi Z, Nemes Z, et al. Association between the Epstein-Barr virus and hodgkin’s lymphoma in the north-Eastern part of Hungary: Effects on therapy and survival. Acta Haematol (2006) 116(2):101–7. doi: 10.1159/000093639 [DOI] [PubMed] [Google Scholar]
- 30. Chabay PA, Barros MH, Hassan R, De Matteo E, Rey G, Carrico MK, et al. Pediatric Hodgkin lymphoma in 2 south American series: A distinctive epidemiologic pattern and lack of association of Epstein-Barr virus with clinical outcome. J Pediatr Hematol Oncol (2008) 30(4):285–91. doi: 10.1097/MPH.0b013e3181647bc3 [DOI] [PubMed] [Google Scholar]
- 31. Chetaille B, Bertucci F, Finetti P, Esterni B, Stamatoullas A, Picquenot JM, et al. Molecular profiling of classical Hodgkin lymphoma tissues uncovers variations in the tumor microenvironment and correlations with ebv infection and outcome. Blood (2009) 113(12):2765–3775. doi: 10.1182/blood-2008-07-168096 [DOI] [PubMed] [Google Scholar]
- 32. Diepstra A, van Imhoff GW, Schaapveld M, Karim-Kos H, van den Berg A, Vellenga E, et al. Latent Epstein-Barr virus infection of tumor cells in classical hodgkin’s lymphoma predicts adverse outcome in older adult patients. J Clin Oncol (2009) 27(23):3815–21. doi: 10.1200/jco.2008.20.5138 [DOI] [PubMed] [Google Scholar]
- 33. Barros MH, Scheliga A, De Matteo E, Minnicelli C, Soares FA, Zalcberg IR, et al. Cell cycle characteristics and Epstein-Barr virus are differentially associated with aggressive and non-aggressive subsets of Hodgkin lymphoma in pediatric patients. Leuk Lymphoma (2010) 51(8):1513–22. doi: 10.3109/10428194.2010.489243 [DOI] [PubMed] [Google Scholar]
- 34. Souza EM, Baiocchi OC, Zanichelli MA, Alves AC, Assis MG, Eiras DP, et al. Impact of Epstein-Barr virus in the clinical evolution of patients with classical hodgkin’s lymphoma in Brazil. Hematol Oncol (2010) 28(3):137–41. doi: 10.1002/hon.933 [DOI] [PubMed] [Google Scholar]
- 35. Kamper P, Bendix K, Hamilton-Dutoit S, Honoré B, Nyengaard JR, d’Amore F. Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical hodgkin’s lymphoma. Haematologica (2011) 96(2):269–76. doi: 10.3324/haematol.2010.031542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koh YW, Yoon DH, Suh C, Huh J. Impact of the Epstein-Barr virus positivity on hodgkin’s lymphoma in a Large cohort from a single institute in Korea. Ann Hematol (2012) 91(9):1403–12. doi: 10.1007/s00277-012-1464-8 [DOI] [PubMed] [Google Scholar]
- 37. Kanakry JA, Li H, Gellert LL, Lemas MV, Hsieh WS, Hong F, et al. Plasma Epstein-Barr virus DNA predicts outcome in advanced Hodgkin lymphoma: Correlative analysis from a Large north American cooperative group trial. Blood (2013) 121(18):3547–53. doi: 10.1182/blood-2012-09-454694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koh YW, Park C, Yoon DH, Suh C, Huh J. Prognostic significance of cox-2 expression and correlation with bcl-2 and vegf expression, microvessel density, and clinical variables in classical Hodgkin lymphoma. Am J Surg Pathol (2013) 37(8):1242–51. doi: 10.1097/PAS.0b013e31828b6ad3 [DOI] [PubMed] [Google Scholar]
- 39. Elsayed AA, Asano N, Ohshima K, Izutsu K, Kinoshita T, Nakamura S. Prognostic significance of Cd20 expression and Epstein-Barr virus (Ebv) association in classical Hodgkin lymphoma in Japan: A clinicopathologic study. Pathol Int (2014) 64(7):336–45. doi: 10.1111/pin.12175 [DOI] [PubMed] [Google Scholar]
- 40. Paydas S, Bağır E, Seydaoglu G, Ercolak V, Ergin M. Programmed death-1 (Pd-1), programmed death-ligand 1 (Pd-L1), and ebv-encoded rna (Eber) expression in Hodgkin lymphoma. Ann Hematol (2015) 94(9):1545–52. doi: 10.1007/s00277-015-2403-2 [DOI] [PubMed] [Google Scholar]
- 41. Tanyildiz HG, Yildiz I, Bassullu N, Tuzuner N, Ozkan A, Celkan T, et al. The role of Epstein-Barr virus lmp-1 immunohistochemical staining in childhood Hodgkin lymphoma. Iran J Pediatr (2015) 25(6):e2359. doi: 10.5812/ijp.2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Park JH, Yoon DH, Kim S, Park JS, Park CS, Sung H, et al. Pretreatment whole blood Epstein-Barr virus-DNA is a significant prognostic marker in patients with Hodgkin lymphoma. Ann Hematol (2016) 95(5):801–8. doi: 10.1007/s00277-016-2610-5 [DOI] [PubMed] [Google Scholar]
- 43. Chang KC, Chen PCH, Chang Y, Wu YH, Chen YP, Lai CH, et al. Epstein–Barr Virus latent membrane protein-1 up-regulates cytokines and correlates with older age and poorer prognosis in Hodgkin lymphoma. Histopathology (2017) 70(3):442–55. doi: 10.1111/his.13085 [DOI] [PubMed] [Google Scholar]
- 44. Myriam BD, Sonia Z, Hanene S, Teheni L, Mounir T. Prognostic significance of Epstein-Barr virus (Ebv) infection in hodgkin lymphoma patients. J Infect Chemother (2017) 23(3):121–30. doi: 10.1016/j.jiac.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 45. Hollander P, Rostgaard K, Smedby KE, Molin D, Loskog A, de Nully Brown P, et al. An anergic immune signature in the tumor microenvironment of classical Hodgkin lymphoma is associated with inferior outcome. Eur J Haematol (2018) 100(1):88–97. doi: 10.1111/ejh.12987 [DOI] [PubMed] [Google Scholar]
- 46. Koh YW, Han JH, Yoon DH, Suh C, Huh J. Epstein-Barr Virus positivity is associated with angiogenesis in, and poorer survival of, patients receiving standard treatment for classical hodgkin’s lymphoma. Hematol Oncol (2018) 36(1):182–8. doi: 10.1002/hon.2468 [DOI] [PubMed] [Google Scholar]
- 47. Wang C, Xia B, Wang T, Tian C, Yu Y, Wu X, et al. Pd-1, Foxp3, and csf-1r expression in patients with Hodgkin lymphoma and their prognostic value. Int J Clin Exp Pathol (2018) 11(4):1923–34. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6958205/. [PMC free article] [PubMed] [Google Scholar]
- 48. Cheriyalinkal Parambil B, Narula G, Dhamne C, Roy Moulik N, Shet T, Shridhar E, et al. Assessment of tumor Epstein-Barr virus status and its impact on outcomes in intermediate and high-risk childhood classic Hodgkin lymphoma treated at a tertiary cancer center in India. Leuk Lymphoma (2020) 61(13):3217–25. doi: 10.1080/10428194.2020.1800005 [DOI] [PubMed] [Google Scholar]
- 49. Werner L, Dreyer JH, Hartmann D, Barros MHM, Büttner-Herold M, Grittner U, et al. Tumor-associated macrophages in classical Hodgkin lymphoma: Hormetic relationship to outcome. Sci Rep (2020) 10(1):9410. doi: 10.1038/s41598-020-66010-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Antel K, Chetty D, Oosthuizen J, Mohamed Z, van der Vyver L, Verburgh E. Cd68-positive tumour associated macrophages, pd-L1 expression, and ebv latent infection in a high hiv-prevalent south African cohort of Hodgkin lymphoma patients. Pathology (2021) 53(5):628–34. doi: 10.1016/j.pathol.2020.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Qin JQ, Yin H, Wu JZ, Chen RZ, Xia Y, Wang L, et al. Pretreatment whole blood Epstein-Barr virus DNA predicts prognosis in Hodgkin lymphoma. Leuk Res (2021) 107:106607. doi: 10.1016/j.leukres.2021.106607 [DOI] [PubMed] [Google Scholar]
- 52. Santisteban-Espejo A, Perez-Requena J, Atienza-Cuevas L, Moran-Sanchez J, Fernandez-Valle MC, Bernal-Florindo I, et al. Prognostic role of the expression of latent-membrane protein 1 of Epstein–Barr virus in classical Hodgkin lymphoma. Viruses (2021) 13(12):2523. doi: 10.3390/v13122523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang C, Zou SP, Chen DG, Wang JS, Zheng YB, Chen XR, et al. Latent Epstein-Barr virus infection status and prognosis in patients with newly diagnosed Hodgkin lymphoma in southeast China: A single-center retrospective study. Hematology (2021) 26(1):675–83. doi: 10.1080/16078454.2021.1971864 [DOI] [PubMed] [Google Scholar]
- 54. Yang LQ, Wang L, Zuo LK, Ma ZP, Yan SF, Yang MH, et al. Expression and prognostic analysis of Stat6(Ye361) in Hodgkin lymphoma. Pathol Res Pract (2022) 231:153781. doi: 10.1016/j.prp.2022.153781 [DOI] [PubMed] [Google Scholar]
- 55. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. Syst Rev (2021) 10(1):89. doi: 10.1186/s13643-021-01626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-Event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 58. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 59. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials (2015) 45(Pt A):139–45. doi: 10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Barili F, Parolari A, Kappetein PA, Freemantle N. Statistical primer: Heterogeneity, random- or fixed-effects model analyses? Interact Cardiovasc Thorac Surg (2018) 27(3):317–21. doi: 10.1093/icvts/ivy163 [DOI] [PubMed] [Google Scholar]
- 61. Herrmann D, Sinnett P, Holmes J, Khan S, Koller C, Vassar M. Statistical controversies in clinical research: Publication bias evaluations are not routinely conducted in clinical oncology systematic reviews. Ann Oncol (2017) 28(5):931–7. doi: 10.1093/annonc/mdw691 [DOI] [PubMed] [Google Scholar]
- 62. Lee JH, Kim Y, Choi JW, Kim YS. Prevalence and prognostic significance of Epstein-Barr virus infection in classical hodgkin’s lymphoma: A meta-analysis. Arch Med Res (2014) 45(5):417–31. doi: 10.1016/j.arcmed.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 63. Chen YP, Zhang WN, Chen L, Tang LL, Mao YP, Li WF, et al. Effect of latent membrane protein 1 expression on overall survival in Epstein-Barr virus-associated cancers: A literature-based meta-analysis. Oncotarget (2015) 6(30):29311–23. doi: 10.18632/oncotarget.4906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shen Y, Zhang S, Sun R, Wu T, Qian J. Understanding the interplay between host immunity and Epstein-Barr virus in npc patients. Emerg Microbes Infect (2015) 4(3):e20. doi: 10.1038/emi.2015.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Murphy EA. Reevaluating the impact of Epstein-Barr virus noncoding rnas on the interferon response. mBio (2021) 12(4):e0070021. doi: 10.1128/mBio.00700-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bouvet M, Voigt S, Tagawa T, Albanese M, Chen YA, Chen Y, et al. Multiple viral micrornas regulate interferon release and signaling early during infection with Epstein-Barr virus. mBio (2021) 12(2):e03440-20. doi: 10.1128/mBio.03440-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zheng X, Huang Y, Li K, Luo R, Cai M, Yun J. Immunosuppressive tumor microenvironment and immunotherapy of Epstein-Barr virus-associated malignancies. Viruses (2022) 14(5):1017. doi: 10.3390/v14051017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jarrett RF, Stark GL, White J, Angus B, Alexander FE, Krajewski AS, et al. Impact of tumor Epstein-Barr virus status on presenting features and outcome in age-defined subgroups of patients with classic Hodgkin lymphoma: A population-based study. Blood (2005) 106(7):2444–51. doi: 10.1182/blood-2004-09-3759 [DOI] [PubMed] [Google Scholar]
- 69. Carbone A, Gloghini A, Caruso A, De Paoli P, Dolcetti R. The impact of ebv and hiv infection on the microenvironmental niche underlying Hodgkin lymphoma pathogenesis. Int J Cancer (2017) 140(6):1233–45. doi: 10.1002/ijc.30473 [DOI] [PubMed] [Google Scholar]
- 70. Mesri E, Feitelson M, Munger K. Human viral oncogenesis: A cancer hallmarks analysis. Cell Host Microbe (2014) 15(3):266–82. doi: 10.1016/j.chom.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Satou A, Takahara T, Nakamura S. An update on the pathology and molecular features of Hodgkin lymphoma. Cancers (Basel) (2022) 14(11):2647. doi: 10.3390/cancers14112647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Albanese M, Tagawa T, Hammerschmidt W. Strategies of Epstein-Barr virus to evade innate antiviral immunity of its human host. Front Microbiol (2022) 13:955603. doi: 10.3389/fmicb.2022.955603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Daskalogianni C, Pyndiah S, Apcher S, Mazars A, Manoury B, Ammari N, et al. Epstein-Barr Virus-encoded Ebna1 and zebra: Targets for therapeutic strategies against ebv-carrying cancers. J Pathol (2015) 235(2):334–41. doi: 10.1002/path.4431 [DOI] [PubMed] [Google Scholar]
- 74. Kieser A, Sterz KR. The latent membrane protein 1 (Lmp1). Curr Top Microbiol Immunol (2015) 391:119–49. doi: 10.1007/978-3-319-22834-1_4 [DOI] [PubMed] [Google Scholar]
- 75. Paydas S, Ergin M, Erdogan S, Seydaoglu G. Prognostic significance of ebv-Lmp1 and vegf-a expressions in non-hodgkin’s lymphomas. Leukemia Res (2008) 32(9):1424–30. doi: 10.1016/j.leukres.2008.01.008 [DOI] [PubMed] [Google Scholar]
- 76. Wang L, Ning S. New look of ebv Lmp1 signaling landscape. Cancers (Basel) (2021) 13(21):5451. doi: 10.3390/cancers13215451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Welch JJG, Schwartz CL, Higman M, Chen L, Buxton A, Kanakry JA, et al. Epstein-Barr Virus DNA in serum as an early prognostic marker in children and adolescents with Hodgkin lymphoma. Blood Adv (2017) 1(11):681–4. doi: 10.1182/bloodadvances.2016002618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shen Z, Hu L, Yao M, He C, Liu Q, Wang F, et al. Disparity analysis and prognostic value of pretreatment whole blood Epstein-Barr virus DNA load and Epstein-Barr encoding region status in lymphomas: A retrospective multicenter study in huaihai lymphoma working group. Int J Cancer (2022) 150(2):327–34. doi: 10.1002/ijc.33802 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.