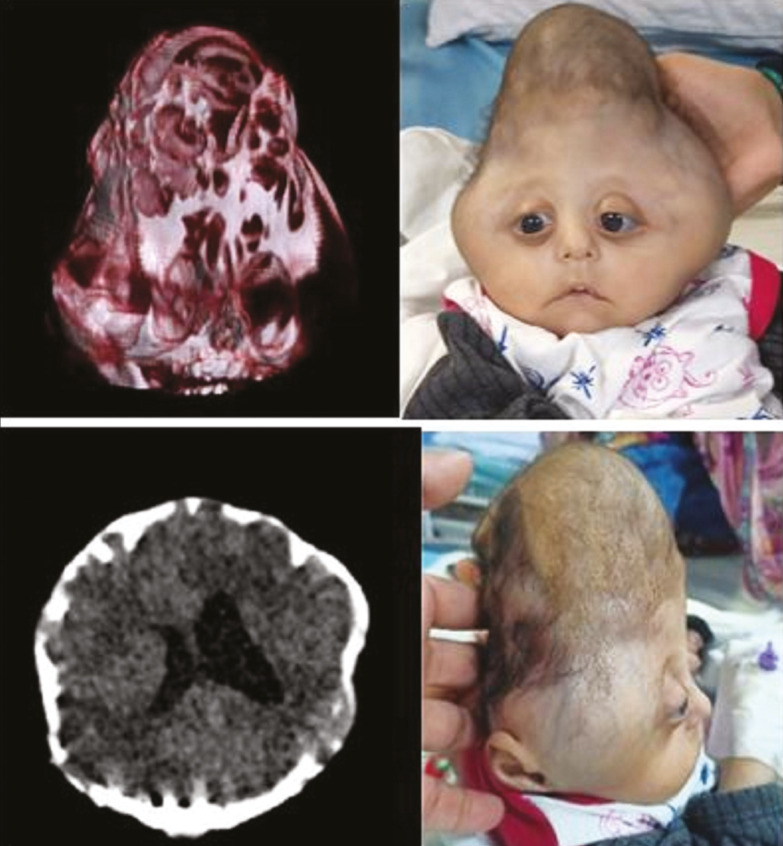
ABSTRACT
Nonsyndromic craniosynostosis (NSC) is more common than syndromic craniosynostosis and predominantly involves single suture. It affects sagittal, coronal, metopic, and lambdoid sutures in the decreasing order of frequency. A surgery for NSC is generally recommended to avoid potential neurodevelopmental delays and sequelae of raised intracranial pressure. Open calvarial vault reconstruction, strip craniectomy with/without the use of a postoperative molding helmet, strip craniectomy with spring implantations, endoscopic suture release, and cranial distraction osteogenesis are various surgical options used for NSC cases. The ideal age for intervention is 6–12 months for open procedures and 3–4 months for endoscopic approaches. The management is directed toward minimizing operative trauma and improving the neurocognitive outcome. The role of nonsurgical intervention by the use of genetic manipulation is still not a reality because of the nature of disease and time of presentation.
KEYWORDS: Barrel stave craniotomy, endoscopic-assisted, nonsyndromic craniosynostosis, strip craniectomy, suturectomy
INTRODUCTION
Craniosynostosis occurs in one in 2500 births. Craniosynostosis may present with single versus multiple sutures involvement and/or syndromic versus nonsyndromic craniosynostosis (NSC).[1] Syndromic craniosynostosis can be associated with other anomalies of the face, trunk, or limbs and generally involves multiple cranial sutures. In this article, we intend to discuss the NSC and its clinical presentation and state the management options based on our two-decade long experience.
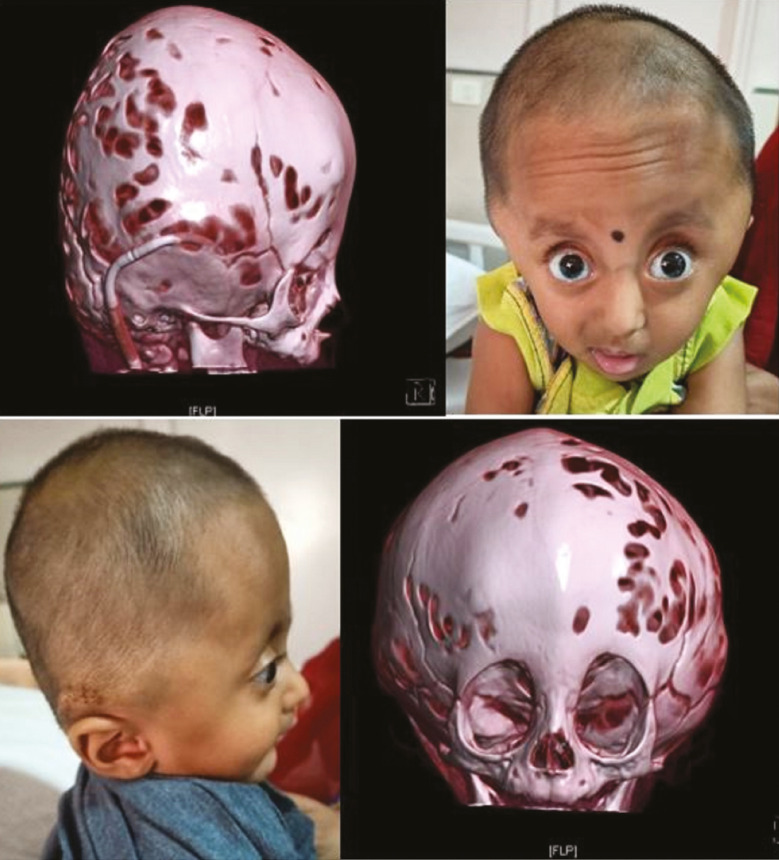
NSC usually involves only single suture; however, it may involve multiple sutures (complex NSC) [Figure 1]. NSC cases warrant timely diagnosis and interventions to prevent irreversible neurodevelopmental delays.
Figure 1.
A case of 6-month-old female child with complex nonsyndromic craniosynostosis (bicoronal, metopic, and partial sagittal synostosis)
EPIDEMIOLOGY
Sagittal synostosis (the premature fusion of sagittal suture) is the commonest subtype of NSC and occurs in 190–200 per million population with male and female ratio as 3:1. Coronal synostosis is present in 90–100 per million population with female preponderance, two females per each male. Metopic synostosis is the least common among all involving 60–70 per million population with male predominance. Of 150 patients (year 2000–2015) in our series, 88% of the patients were NSC with 61% of being males.[2]
The underlying genetic origin of pathologic premature suture fusion continues to be researched. Mutations in both the FGF-2 and TGF-b genes have been implicated in craniosynostosis of murine models. These genes are often specifically tested for when syndromic craniosynostosis is suspected, but they have also been associated with nonsyndromic disease.[3,4]
CLINICAL PRESENTATION
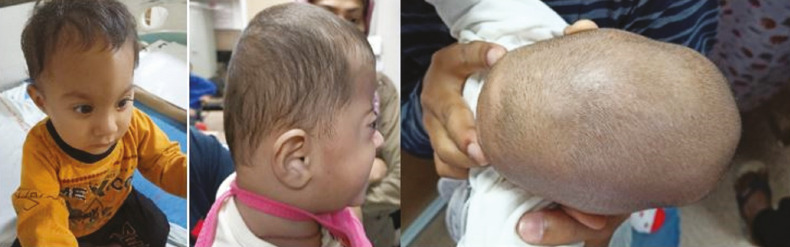
Abnormal head shape (cosmetic head deformity) is the commonest presentation in NSC cases [Figure 2]. On examination, there may be subtle or obvious signs of intracranial hypertension, vision involvement, age-inappropriate brain growth, hydrocephalus, and neuropsychiatric disorders. The most recent studies indicate a 15% incidence of intracranial hypertension.[5] The primary indication for intervention is to prevent the sequela of intracranial hypertension. All children need to be assessed for developmental scoring [Appendix 1]. The neurodevelopmental delay in these children is more likely multifactorial with associated hydrocephalus, brain deformity, premature delivery, and familial. We observed 8% of our NSC patients had visual impairment and 3% of the patients had seizures as presenting symptoms. Nearly half of the patients had delayed milestones. The various calvarial deformities are discussed in detail elsewhere in the article.[6]
Figure 2.
Abnormally shaped head as a presentation of craniosynostosis in a child of trigonocephaly, A; brachycephaly, B; scaphocephaly, C
INITIAL EVALUATION
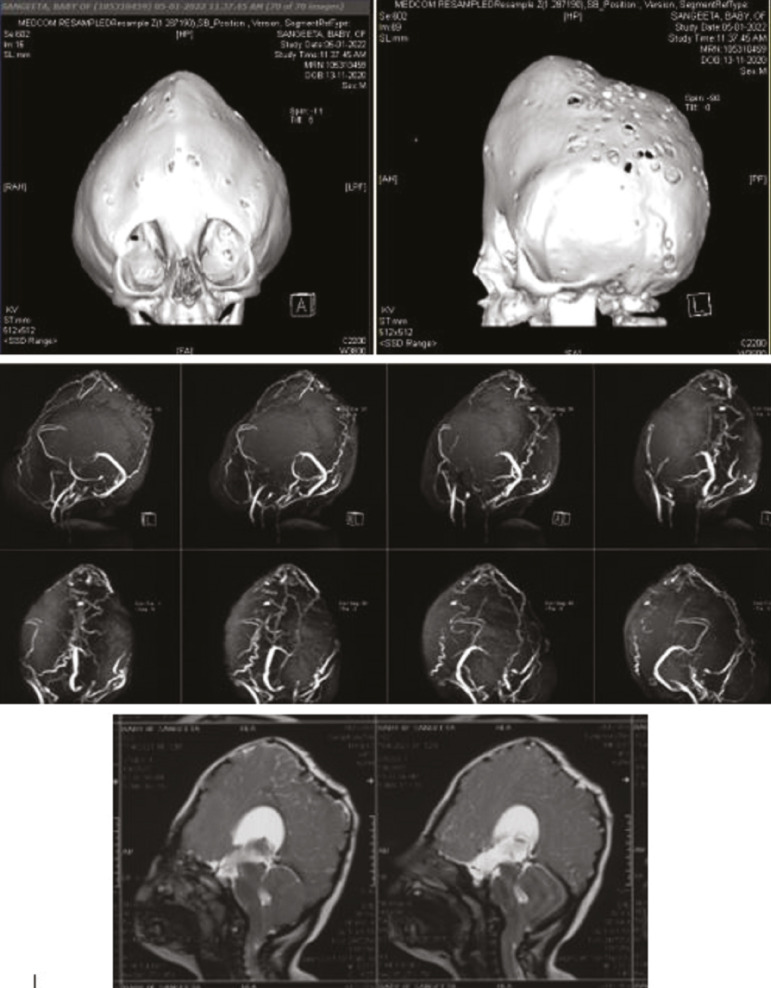
The current rapid low-dose craniofacial computed tomography (CT) with 3D reconstruction protocols has largely reduced the risk of radiation exposure by as much as 89%, and we do it routinely in all cases (single-time study only).[7] It offers several benefits, including the preoperative planning with anthropometric measurements and the assessment of the brain parenchyma and ventricles[8] [Figure 3]. We routinely perform magnetic resonance imaging (MRI) brain as well to rule out any associated brain parenchymal anomalies and magnetic resonance venography (MRV) brain to look for any abnormal draining veins in the midline, which could be of surgical consequences [Figure 4]. In our series of cases, MRI revealed Arnold-Chiari malformation in seven (4.8%), occipital encephalocele in one (0.7%), and type 1 split cord malformation at C7-D1 level. We also performed single photon emission tomography scan of brain using 99mTc-ethylene cysteine diethylester to assess the brain parenchyma perfusion in 51 (34%) patients and 28 of them had hypoperfusion in cerebral cortex in various distributions, most commonly in bilateral temporal lobes followed by frontal lobes.[9]
Figure 3.
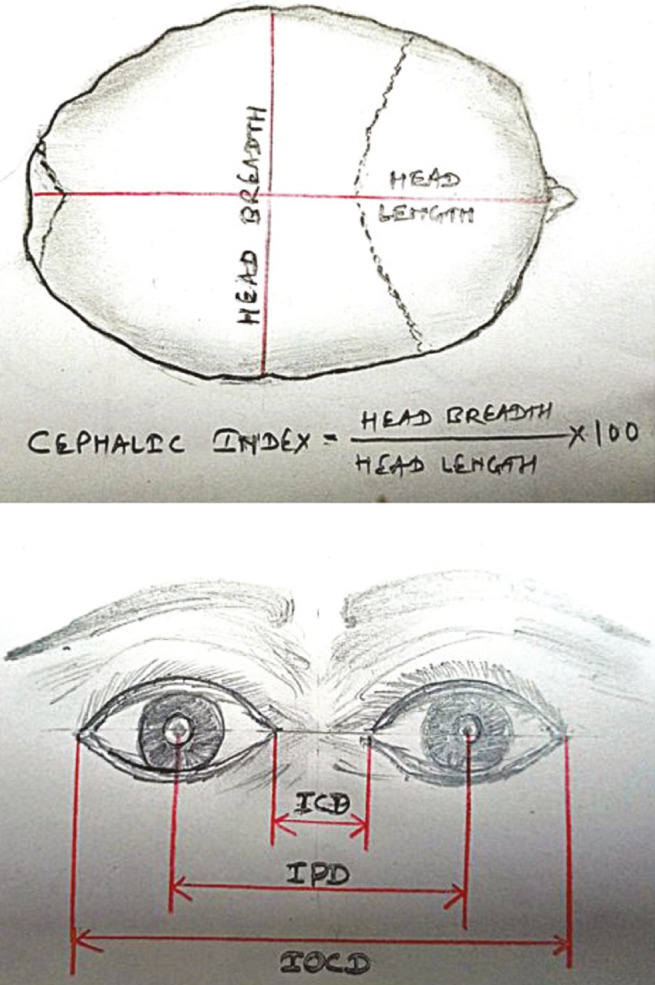
Schematic diagrams showing how to measure cephalic index, A, and different canthal distances, B, as a part of anthropometric parameters in craniosynostotic child. Head breadth = biparietal diameter, head length = occipitofrontal diameter, ICD = intercanthal distance, IOCD = outer canthal distance, IPD = interpupillary distance
Figure 4.
A case of complex NSC with MRI brain showing Chiari malformation and MR venogram showing hypoplastic sagittal and B/L transverse sinus with multiple collateral venous channels with prominent straight sinus
Neuroophthalmology examination is conducted for visual assessment including fundus examination, optical coherence test, and optic nerve sheath diameter in all cases preoperatively. Pediatric neurology assessment for the developmental growth of child that impacts long-term follow-up is also desired. Genetic evaluation is also a part of an initial workup to rule out other anomalies and associated genetic disorders [Table 1].
Table 1.
Battery of tests conducted as preoperative workup of NSC cases
| Name of tests | Purpose | |
|---|---|---|
| NCCT head with 3D reconstruction | For characterization of synostosis | |
| MRI brain with CVJ | To look for brain parenchymal changes and associated hindbrain malformations | |
| MR venogram | To look for abnormal cortical venous anatomy that required for preoperative planning | |
| Neuroopthalmological evaluation | Fundus examination | For papilledema |
| Optical coherence tomography | To look for retinal thickness changes due to raised ICP | |
| Optic nerve sheath diameter | To look for thinning due to raised ICP | |
| Visual acuity | For preoperative documentation | |
| ENT evaluation | To find out associated nasal and oropharyngeal malformation | |
| Pediatric neurological evaluation | For developmental and psychiatric assessment | |
| Genetic evaluation | To find out association with some genetic syndromes | |
The current management protocol recommends surgical correction of the condition in all children. Close follow-up with serial fundus examinations to detect the presence of papilledema and associated intracranial hypertension may be offered only to those unwilling for surgery. The aim of the surgery is to correct and prevent the progression of the deformity and raised intracranial pressures. In cases with severe craniofacial abnormality, the airway, eyes, and oral health care also need to be taken care of. A surgery is defined as early if performed within 1 year of life or late if performed beyond 1 year of life. Only 40 out of 150 patients underwent surgery at less than 6 months of age in our series. The median age at the time of surgery was 34 months with a range of 1.5–132 months in our series.[9] It is essential to explain the risks involved with the procedure to the parents before going ahead with the procedure. The risk of perioperative blood loss and hypothermia and dural sinus injury exists during surgery.
Various surgical options for NSC cases include open calvarial reconstructions, strip craniectomy with the use of a postoperative molding helmet, strip craniectomy with spring implantation, endoscopic suture release, and cranial distraction osteogenesis.
NONSYNDROMIC SAGITTAL CRANIOSYNOSTOSIS
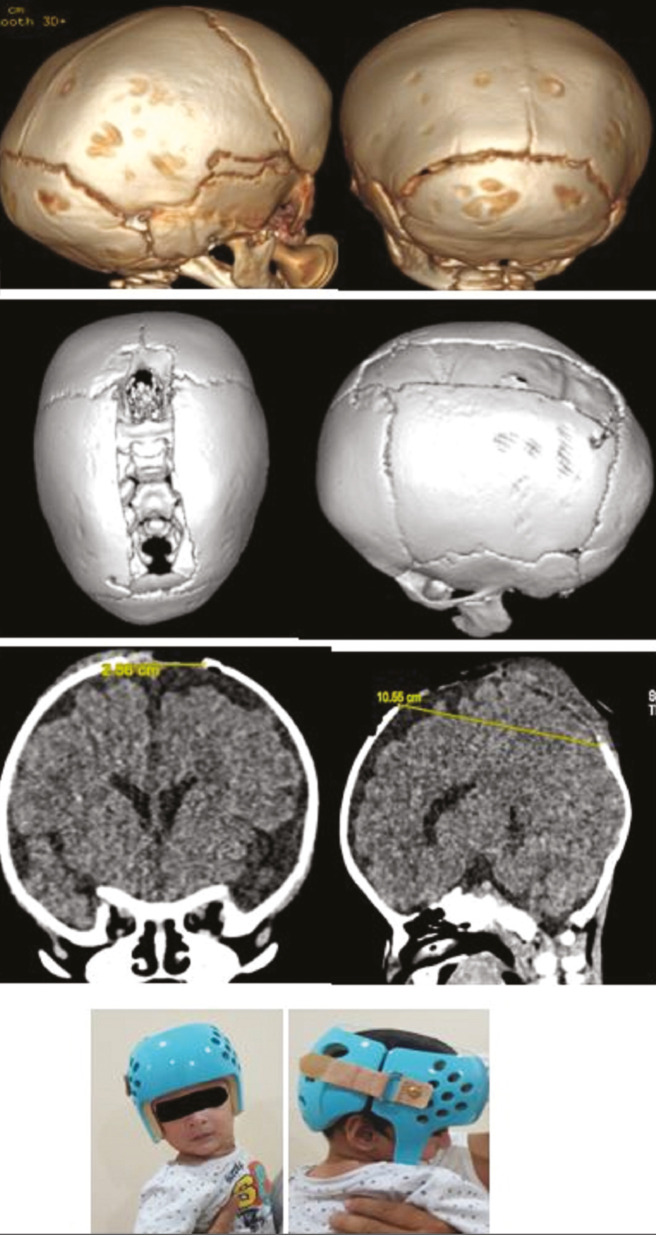
Sagittal suturectomy can be performed either through open or endoscopic technique. Early intervention within 3–4 months followed by helmet therapy for the next 12 months for desired outcome is recommended [Figure 5]. The thinner and more malleable cranial bones at this age facilitate easier contouring of the parietal skull, the correction of occipital bulleting, and anteroposterior shortening without the need of total vault remodeling procedures. In recent years, few authors advocate intermediate timed (usually between 3 and 8 months of age) intervention using spring assistance.[10,11] The spring facilitates biparietal vault widening after the window of exponential brain growth used during molding helmet therapy has closed. This intervention can be performed in a minimally invasive fashion with reduced hospital stay and need for blood transfusion but it does require a second surgery for spring device removal.[12,13] Other options are to perform more extensive strip craniectomies in combination with anterior-posterior (AP) shortening or total vault remodeling and barrel staving of parietotemporal bones.[14,15]
Figure 5.
A 5-month-old male child has undergone endoscopic suturectomy for sagittal synostosis, and postoperative molding helmet therapy, D, is offered. Pre- and postoperative CT films, A–C, showing the extent of suturectomy
OPERATIVE TECHNIQUE (MODIFIED PI)
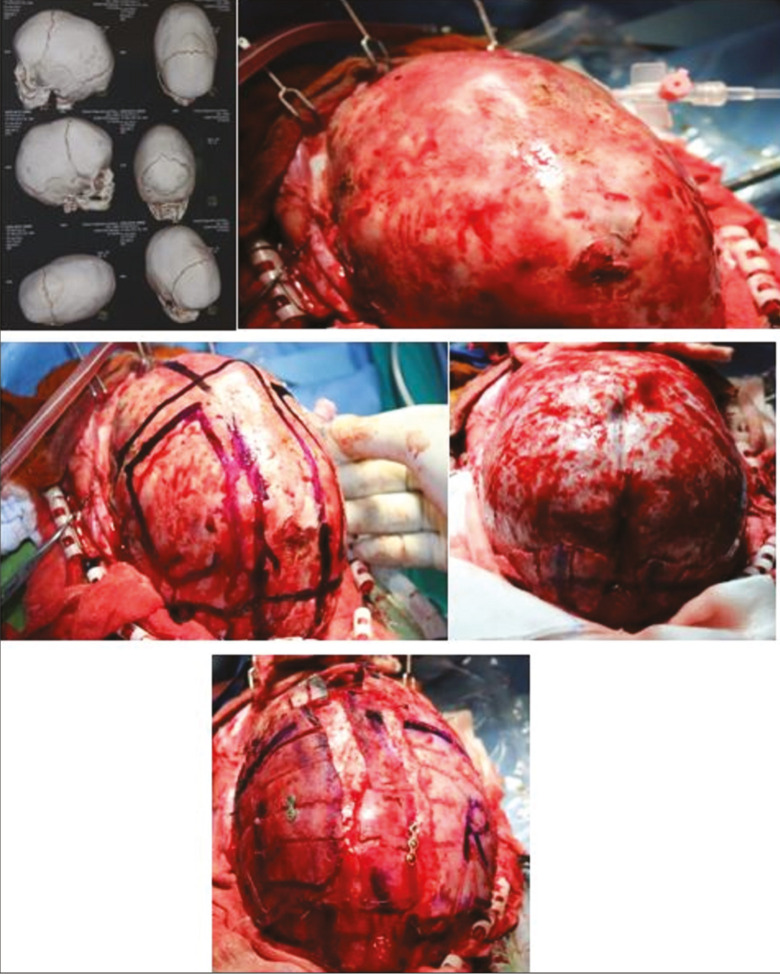
A child is placed in a prone position. Ensuring the airway and eyes are protected during this manoeuvre is of paramount importance, and the endotracheal tube is secured well. A bicoronal sawtooth-shaped incision is then planned within the hair bearing scalp over the vertex of the skull. Either a thin strip of hair or the entire head is then shaved and the scalp widely infiltrated with epinephrine containing local anesthesia. Dissection is carried down to the level of the periosteum, which is left intact over the calvarium. Osteotomies are then designed to remove a 2- to 3-cm narrow strip of bone including the entire sagittal suture. Osteotomies both anterior and posterior to the coronal and lambdoid sutures are made to “float” in addition to several lateral barrel stave osteotomies inferiorly to the level of the squamosal suture. This facilitates contouring of the temporal, parietal, and occipital regions. In severely elongated heads, AP shortening is achieved through bilateral posteriorly based lateral closing wedge osteotomies [Figure 6]. The sagittal strip is shortened accordingly (usually 1–2 cm) and then inset with polydioxanone (PDS) suspension sutures for neural protection. A single 10F closed suction drain is left exiting behind the ear, which allows for the egress of reactive fluid accumulation from the more extensive dissection compared with early intervention.
Figure 6.
Modified pi technique for sagittal synostosis (3D CT head showing isolated sagittal synostosis), A. After placing a patient in a prone position, adequate calvaria is exposed, B; craniotomy markings being done, indicating midline strip craniotomy involving sagittal suture and bilateral parietal bone craniotomy for barrel staving, C; complete exposure after craniotomy, D; midline strip craniotomy with barrel staving of bilateral parietal bone and fixed with miniplates and sutures, E
DISCHARGE AND FOLLOW-UP
In children undergoing only strip craniectomy at less than 6 months of age, overnight monitoring is done and child can be discharged the following morning if taking feeding well. They are then fitted with their molding helmet, which is applied after 3 weeks to allow the scalp incisions to heal. Children undergoing more extensive dissections need a longer hospital stay ranging from 3 to 5 days. Residual nonossified areas of skull that are less than 2 cm do not require any intervention. All children are followed at 3-, 6-, and 12-month intervals thereafter until the age of 6 years. During these visits, the symptoms of recurrent synostosis are screened (headache, nausea, disordered sleep, abnormal neurodevelopment, and yearly dilated eye examinations to evaluate for papilledema).
NONSYNDROMIC CORONAL CRANIOSYNOSTOSIS
Unilateral coronal craniosynostosis occurs four to seven times as often as bilateral coronal synostosis. The right side is affected twice as often as the left side [Figure 7]. Depending on the extent of bony anomalies, Di Rocco et al. (1988)[16] classified anterior plagiocephaly into three types [Figure 8]: type I characterized by unilateral flattening of the frontal bone and elevation of the superior orbital ridge without the deviation of the nasal pyramid; type II, in which frontal and orbital abnormalities are accompanied by contralateral deviation of the nasal pyramid and homolateral anterior displacement of the petrous bone; and type III, defined by the occurrence of severe deviation of the sphenobasilar bone besides the aforementioned anomalies, with secondary asymmetry of the craniovertebral junction. Bilateral coronal craniosynostosis on the other hand creates uniform AP growth restriction and shortening with bitemporal widening, referred to as brachycephaly. Instead of the characteristic facial twist observed in unilateral cases, there is a compensatory craniocaudal skull elongation, referred to as turricephaly. Therefore, the correction of either unicoronal or bicoronal deformity is focused not only on the forehead, but also on the superolateral orbits. This correction is accomplished through the removal of the frontal bones and orbital bandeau for recontouring and repositioning, known as frontoorbital advancement. This procedure requires a fairly extensive dissection extending from the vertex of the scalp posteriorly to the frontonasal suture anteriorly and laterally to the squamosal and zygomaticofrontal sutures, respectively. Consequently, intervention is deferred to approximately 9–12 months of age to be performed in an open fashion.
Figure 7.
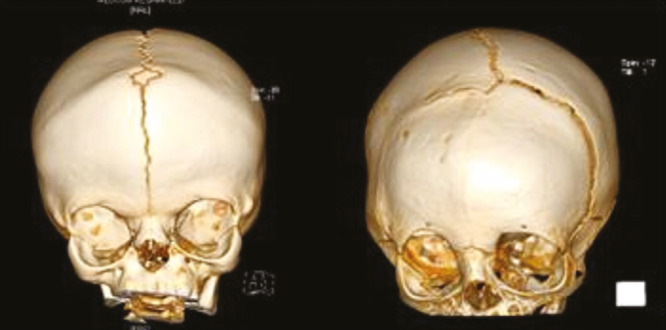
3D CT head showing bicoronal synostosis, A, and unilateral coronal synostosis, B
Figure 8.
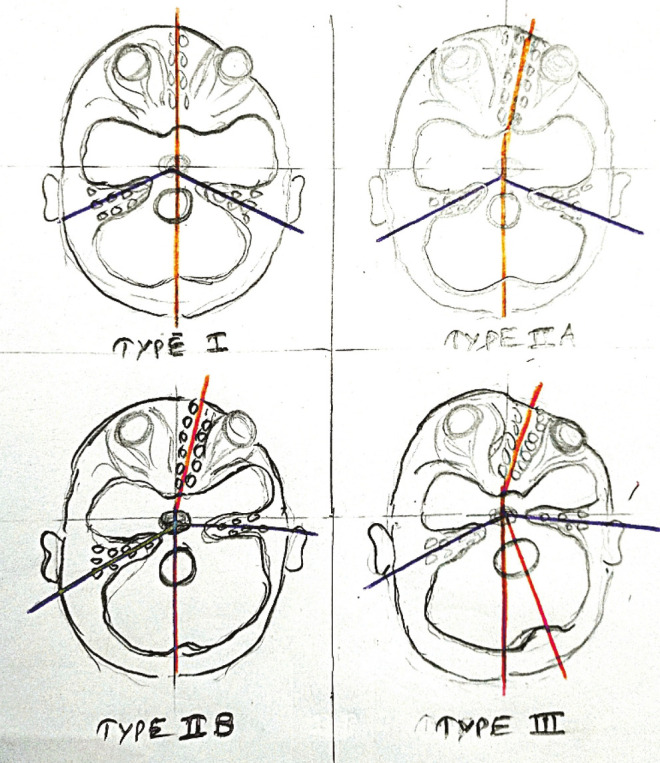
Di Rocco et al. (1988) classified anterior plagiocephaly into three types (schematic representation)
SURGICAL TECHNIQUE OF FRONTOORBITAL ADVANCEMENT
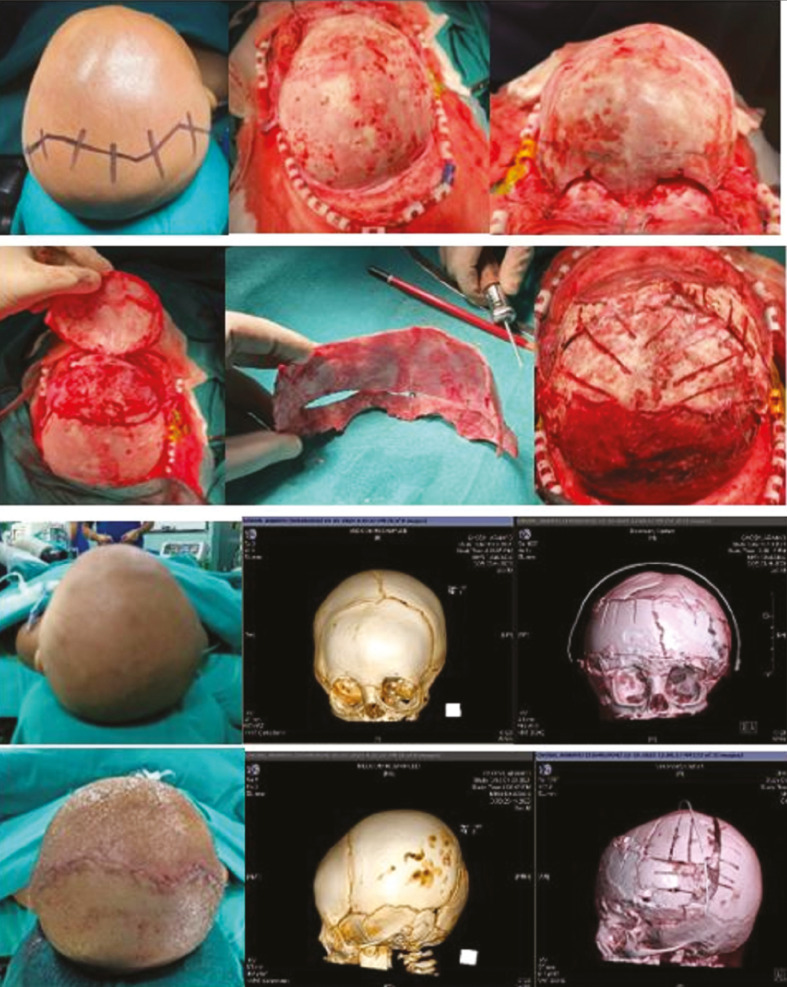
A patient is positioned supine to facilitate access to the forehead and orbital bandeau. A bicoronal sawtooth incision is made within the hair-bearing scalp. Additionally, dissection is carried in a subperiosteal plane to the limits described previously. A bifrontal craniotomy is then performed leaving approximately 20–25 mm of bone stock above the superior orbital rim to provide adequate rigidity of the orbital bandeau to be removed later. The bifrontal craniotomy may be performed in either one or two pieces. The orbital bandeau is then removed en bloc using a C-shaped osteotomy through the zygoma and orbital roof [Figure 9]. In unicoronal cases, additional temporal bone is often included on the affected side. The recontouring of the bandeau requires focal weakening at the midline to allow forward rotation of the affected side into a symmetric curve. This manoeuvre invariably widens the lateral temporal wing of the affected side, which is then narrowed through a closing wedge osteotomy on the inner table of the lateral orbital wall. The curve of the newly contoured bandeau is stabilized with absorbable plates on the inner table. Additionally, inner table bone shavings are harvested from the frontal craniotomy to fill any residual large bony gaps. The bandeau is then reinserted in a newly advanced and rotated position to optimize brow position. This is secured to the pterion with absorbable plates. The frontal bones are then recontoured to correct the flattened synostotic side and match the newly contoured bandeau. This is sometimes facilitated by splitting the frontal bones into two pieces if removed as a single unit originally. Appropriate orientation and position of the frontal bones are then chosen to optimize forehead shape, which may favor a switch cranioplasty. The frontal bones are then set with multiple PDS/nylon sutures placed through holes to the bandeau. The large single-piece biparietal bone flap can be rotated 360° and positioned above bandeau to acquire desired forehead contour and posterior parietal defect filled with frontal bone pieces, which are sutured with multiple PDS/nylon sutures placed through small wire pass holes. In general, overcorrection is favored to accommodate minor relapse and future growth. Additional contouring of the restricted parietal bones is achieved without fracture of the posteriorly based barrel stave osteotomies. The temporal bony gaps created by the advancement are filled with the previously mentioned inner table shaving bone graft. The scalp is then closed over with or without a subgaleal drain.
Figure 9.
A 9-month-old child with the right anterior plagiocephaly undergone frontoorbital advancement and anterior 2/3 cranial vault remodeling (classical zigzag bicoronal incision extending behind the ear), A; the exposure of cranium anteriorly up to frontozygomatic and frontonasal suture and posteriorly up to lambdoid suture, B; marking frontoorbital bandeau (20–25 mm) involving temporal squama after retracting both periorbita, C; single-piece bifrontal craniotomy above bandeau to switch by 360°, D; newly formed forehead with orbital bandeau with biparietal bone, E; frontoorbital bandeau fixed in an advanced manner (in case of unilateral coronal synostosis more advancement done on affected side), F; and barrel staving of bifrontal bone switched posteriorly and fixed loosely with PDS sutures hanging freely, pre- and postoperative images of head and 3D CT head showing newly formed frontoorbital contour, G
Jeyaraj modified the technique of “bilateral frontal-unilateral orbital advancement” spares osteotomy of the contralateral unaffected orbit.[17] Pellerin et al.[18] have suggested asymmetrical harvesting of the frontoorbital bandeau. The bandeau bone cut in the lateral orbital rim is extended to the frontozygomatic suture on the synostotic side and is shorter on the unaffected side. Also, they have suggested the role of hypercorrection through which the frontoorbital bandeau may be fixed a fewer millimeters more anteriorly than required on the diseased, plagiocephalic side in comparison to the opposite, unaffected side, thus reducing its long-term recurrence. It is important to note and counsel the family that the facial twist seen in unicoronal craniosynostosis is not fully corrected with this procedure. No procedure has been found to correct the deformity satisfactorily. The main aim remains to stop the progression of the deformity.[19,20] The postoperative course is similar to the sagittal craniosynostosis children. However, expected hospital stay is usually 3–5 days owing to inevitable periorbital swelling, preventing eye opening.
METOPIC CRANIOSYNOSTOSIS
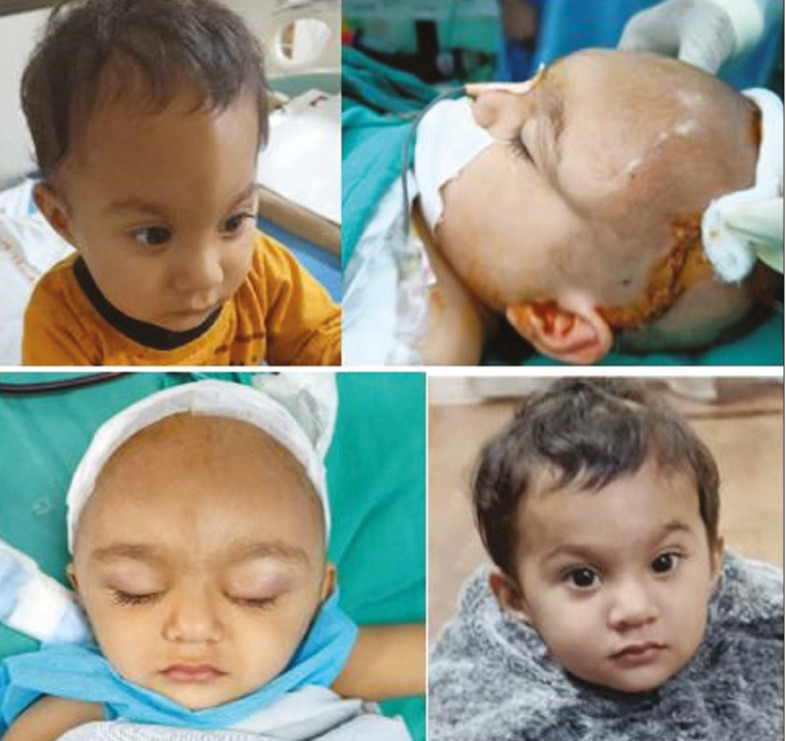
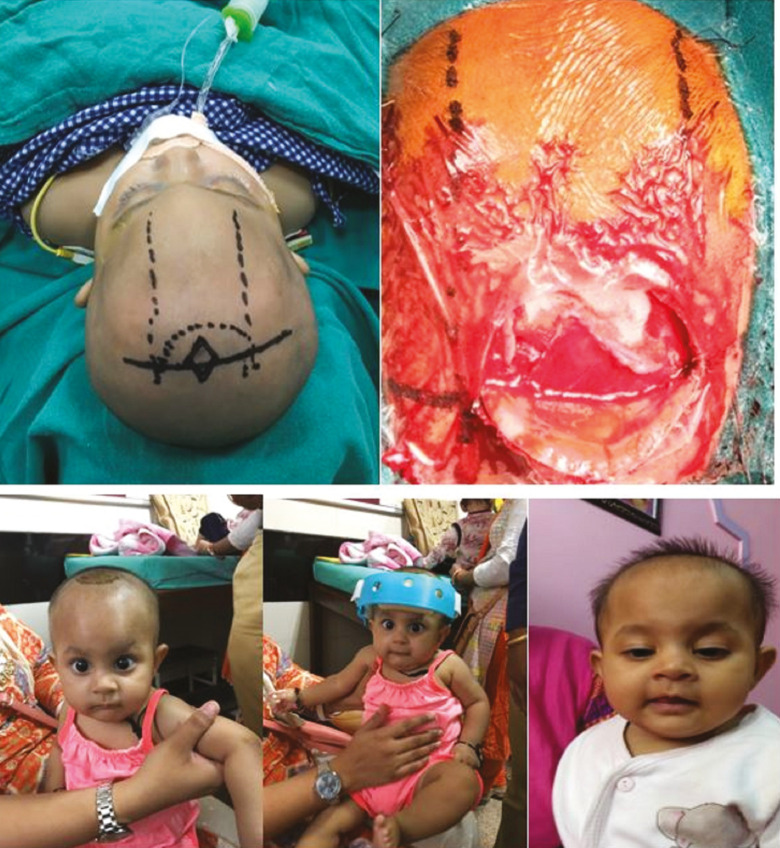
The metopic suture is the first to fuse, within the first 6–12 months of life, and therefore differentiating between a normal overriding suture ridge and pathologic synostosis is important.[21] Metopic craniosynostosis results from suture fusion either in utero or shortly after birth, which produces trigonocephaly with a characteristic triangular-shaped forehead with associated bifrontal and temporal narrowing and hypotelorism [Figure 10]. Indications of a surgery in trigonocephaly include prominent metopic ridge, smaller frontal area and anterior fossa, abnormally wide sphenoid ridges, and the marked digital markings. Early intervention can be performed at 3 months of age through a minimally invasive endoscopic approach [Figure 11]. A limited V-shaped incision is made behind the hairline of the anterior scalp in the midline. Subgaleal dissection is then carried inferiorly to the level of the frontonasal suture and posteriorly up to anterior fontanel. A wedged strip suturectomy is then performed to include the metopic suture. Oblique barrel stave osteotomies are also performed laterally to allow recontouring of the frontal bones from the anterior fontanel to the superior orbital rim. This incision is closed without a drain, and the postoperative course is similar to that of the endoscopic-assisted sagittal suturectomy patients, including a course of molding helmet therapy. Late intervention is performed at 10–12 months of age and similar technique advocated as in coronal craniosynostosis via frontoorbital advancement. There are some key differences while remodeling the bandeau. Here the bandeau requires glabellar widening to correct hypotelorism. This is accomplished by splitting the bandeau ex vivo and the placement of an 8–10 mm interposition bone graft obtained from the exposed parietal bone. The new bandeau construct is then secured and reinset on the skull with absorbable plates in an advanced position. The frontal bones are also similarly replaced, posteriorly based parietal barrel stave osteotomies performed, and large bony gaps filled with harvested inner table bone graft shavings mixed with fibrin glue. The postoperative course is identical for both frontoorbital advancement procedures.
Figure 10.
A 1-year-old child with trigonocephaly managed with frontoorbital advancement and anterior 2/3 cranial vault remodeling and after 8-month follow-up
Figure 11.
Endoscopic-assisted approach for metopic craniosynostosis in a 4-month-old male child, A, and molding helmet therapy, B, was offered postoperatively and being followed up
LAMBDOID CRANIOSYNOSTOSIS
It is rare, accounting for less than 5% of all cases. Mostly it is a unilateral form; the deformity classically seen is bossing of the ipsilateral mastoid process and downward displacement of the cranial base on the affected side creating an apparent tilt. There is also compensatory bossing of the contralateral parietal skull. Lambdoid craniosynostosis and positional plagiocephaly are differentiated by looking the position of the ear with anterior displacement in positional and posterior displacement of the ear in lambdoid craniosynostosis. However, owing to the downward tilt of the cranial base on the affected side, the entire ear is subsequently inferiorly displaced. These two findings have proven significantly more reliable in identifying children with surgically correctable synostosis.[22] Surgical intervention mandates remodeling of the posterior vault at approximately 6 months of age. Surgical technique involves prone positioning and surgical access similar to the open approach described in the sagittal craniosynostosis section. The posterior parietal and occipital bones are then removed, taking care to avoid injury to the torcula. Removed occipital and parietal bone remodeled ex vivo and placed back. The hallmark of the operation is switching the right and left newly contoured occipital bones when placed back on the skull. This switch cranioplasty provides increased volume to the affected side, which is then secured with absorbable plates. Additional parietal barrel staving of the bossed unaffected side is added to create a normal contour. The scalp is closed with or without a closed suction drain. This technique for open posterior vault remodeling through switch cranioplasty is very effective at correcting the flattened occiput on the affected side and contralateral parietal bossing. However, it does not address the ipsilateral mastoid bossing or cranial base tilt, and no adjuvant procedure has been found to be able to correct the abnormality reliably. Earlier intervention does seem to stop the progression of the deformity.
OXYCEPHALY (PANSUTURAL SYNOSTOSIS)
The name oxycephaly meaning “pointed head” refers to the “clown’s hat” found at the site of the ossified bulging bregmatic fontanel. In oxycephaly, the sagittal suture is also fused, and lambdoid sutures may or may not be involved. The continuation of the coronal suture into the cranial base (i.e., the sphenofrontal suture) may also be prematurely fused, whereas the synchondroses of the cranial base appear to be normal. The calvaria is characterized with a high, steep forehead often with a depression of the supraorbital region. The sagittal diameter is short, and the head may appear pointed in the bregmatic region. The cephalic index is increased and the head circumference decreased. The anterior cranial base is short. There is a frontalization of the orbital roof. The two characteristic, virtually diagnostic features of this condition include the absence of the frontonasal angle and the backward slope of the forehead [Figure 12]. The slope of the nose, if followed up into the forehead, runs in a straight line nearly up to the vertex. These result in a recession of the supraorbital rims leading to a situation of pseudoproptosis. The differential diagnosis is turricephaly that results from bicoronal fusion. Here the head is “tower” like, and the conical shape is not present. Additionally, the forehead tends to “overhang” the midface. The most marked feature in plain films is the thumb printing due to raised ICP. This condition is the result of premature fusion of bicoronal and sagittal sutures. The part of the metopic suture is also involved. The anterior fontanelle is ossified over, and it may need removal during surgery. Anterior 2/3 cranial vault remodeling with frontoorbital advancement is offered. After frontoorbital bandeau advancement, posterior forehead flap is rotated 180° and fixed to the bandeau. The anterior forehead flap is fixed to this posterior flap and fixed with sutures. The prominent fused-over fontanelle and the sagittal suture are drilled off or excised. Bilateral tarsorrhaphy is done to prevent conjunctival chemosis.
Figure 12.
A case of oxycephaly with severe copper beaten skull appearance in CT head
COMPLICATION OVERVIEW AND AVOIDANCE
The mortality rates in pediatric craniofacial surgery have come down dramatically, from 1.6% in the 1970s[23,24] to less than 0.1% more recently.[25] Perioperative complications include anesthesia-related complications. Supine positioning in reverse Trendelenburg has the risk of air embolism. During surgery, any retraction or pressure on the orbits causes changes in pulse rate and blood pressure by oculocardiac reflex. Cortical damage secondary to brain handling may rarely cause contusion and seizure. Several measures are taken to limit blood loss in view of less blood reserve in pediatric patients. Two randomized, placebo-controlled prospective trials have shown that tranexamic acid (TXA) decreases blood loss and the need for transfusion.[26,27] A dosing regimen of 50 mg/kg TXA initially followed by a 5-mg/kg/h infusion is effective and may be planned in extensive surgery.[27] Cell saver is also an option, yet its utilization was relatively low, perhaps because of costs and availability of equipment and personnel.[28] In immediate postoperative period, hemodynamic instability may be present because of ongoing blood loss. Czerwinski et al.[29] in their study of more than 8000 craniofacial cases reported that 50% of all intracranial mortalities were the direct result of excessive blood loss. Fluid and electrolyte imbalances are related to the large volume fluid exchange occurring in these surgeries. Airway issues are common. Postoperative fever is very common occurrence in these children. Cerebrospinal fluid leaks that persist for more than 5 days require intervention. The insertion of a lumbar drain is an option if hydrocephalus is not present. Wound infection is usually associated with contaminated surgeries and whenever distractors are used. Long-term complications include nonhealing bone gaps. The protrusion of metal implants or embedding of metal implants and wires into the brain is common in older children. Planning and anticipating complication is a key to avoid complications. Seizure prophylaxis may be an option, and we prefer to use it perioperatively. Thromboelastography can be used to assess specific components of coagulation function and guide hemostatic therapy.[30,31] Strict protocol for blood replacement and investigation of blood loss are keys to reducing adverse events. Blood products in operating room are mandatory, and their use can be situation based. Temperature monitoring and avoidance of hypothermia are very important. The preservation of bone dust and bone chips and refilling the gaps with same may avoid persistent bone gaps. The avoidance of wound infection depends on prophylactic antibiotic protocol, meticulous asepsis, and optimal surgical technique and tissue handling. Careful preoperative airway assessment is essential.
SUMMARY
The abnormal head shape along with facial deformity is a common reason for the referral of NSC cases to the craniofacial surgeon. NSC can be safely and reliably corrected through single or multiple timely surgical interventions. Future research is needed to continue to refine our surgical techniques into increasingly minimally invasive approach and aim at decreasing perioperative risks. Proper preoperative evaluation with meticulous perioperative management with interdepartment involvement is the key to better surgical outcomes. Additionally, further investigation is warranted into the underlying genetic causes of craniosynostosis, which may eventually provide nonoperative treatment options to the developing synostosis child.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
APPENDIX 1
Developmental milestone chart for the assessment of pediatric development
REFERENCES
- 1.Persing JA. MOC-PS(SM) CME article: management considerations in the treatment of craniosynostosis. Plast Reconstr Surg. 2008;121:1–11. doi: 10.1097/01.prs.0000305929.40363.bf. [DOI] [PubMed] [Google Scholar]
- 2.Garrocho-Rangel A, Manríquez-Olmos L, Flores-Velázquez J, Rosales-Berber M-Á, Martínez-Rider R, Pozos-Guillén A. Non-syndromic craniosynostosis in children: scoping review. Med Oral Patol Oral Cir Bucal. 2018;23:e421. doi: 10.4317/medoral.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunenko O, Karmacharya J, Ong G, Kirschner RE. Toward an understanding of nonsyndromic craniosynostosis: altered patterns of TGF-beta receptor and FGF receptor expression induced by intrauterine head constraint. Ann Plast Surg. 2001;46:546–53; discussion 553-4. doi: 10.1097/00000637-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Boyadjiev SA International Craniosynostosis Consortium. Genetic analysis of non-syndromic craniosynostosis. Orthod Craniofac Res. 2007;10:129–37. doi: 10.1111/j.1601-6343.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- 5.Thompson DN, Harkness W, Jones B, Gonsalez S, Andar U, Hayward R. Subdural intracranial pressure monitoring in craniosynostosis: its role in surgical management. Childs Nerv Syst. 1995;11:269–75. doi: 10.1007/BF00301758. [DOI] [PubMed] [Google Scholar]
- 6.Persing JA, Jane JA, Shaffrey M. Virchow and the pathogenesis of craniosynostosis: a translation of his original work. Plast Reconstr Surg. 1989;83:738–42. doi: 10.1097/00006534-198904000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Harshbarger R, Kelley P, Leake D, George T. Low dose craniofacial CT/rapid access MRI protocol in craniosynostosis patients: decreased radiation exposure and cost savings. Plast Reconstr Surg. 2010;126:4. [Google Scholar]
- 8.Fearon JA, Singh DJ, Beals SP, Yu JC. The diagnosis and treatment of single-sutural synostoses: are computed tomographic scans necessary? Plast Reconstr Surg. 2007;120:1327–31. doi: 10.1097/01.prs.0000279477.56044.55. [DOI] [PubMed] [Google Scholar]
- 9.Verma S, Mahapatra AK, Gupta D. Tenets of Craniosynostosis. Delhi: Thieme Publisher; 2018. Craniosynostosis: Experience over 15 years at the All India Institute of Medical Sciences, New Delhi, a tertiary care center; pp. 66–74. [Google Scholar]
- 10.David LR, Plikaitis CM, Couture D, Glazier SS, Argenta LC. Outcome analysis of our first 75 spring-assisted surgeries for scaphocephaly. J Craniofac Surg. 2010;21:3–9. doi: 10.1097/SCS.0b013e3181c3469d. [DOI] [PubMed] [Google Scholar]
- 11.David LR, Proffer P, Hurst WJ, Glazier S, Argenta LC. Spring-mediated cranial reshaping for craniosynostosis. J Craniofac Surg. 2004;15:810–6 [discussion 817-8]. doi: 10.1097/00001665-200409000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Gerety PA, Basta MN, Fischer JP, Taylor JA. Operative management of nonsyndromic sagittal synostosis: a head-to-head meta-analysis of outcomes comparing 3 techniques. J Craniofac Surg. 2015;26:1251–7. doi: 10.1097/SCS.0000000000001651. [DOI] [PubMed] [Google Scholar]
- 13.Sun J, Ter Maaten NS, Mazzaferro DM, Wes AM, Naran S, Bartlett SP, et al. Spring-mediated cranioplasty in sagittal synostosis: does age at placement affect expansion? J Craniofac Surg. 2018;29:632–5. doi: 10.1097/SCS.0000000000004233. [DOI] [PubMed] [Google Scholar]
- 14.Fearon JA, McLaughlin EB, Kolar JC. Sagittal craniosynostosis: surgical outcomes and long-term growth. Plast Reconstr Surg. 2006;117:532–41. doi: 10.1097/01.prs.0000200774.31311.09. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SR, Pryor L, Mittermiller PA, Meltzer HS, Levy ML, Broder KW, et al. Nonsyndromic craniosynostosis: current treatment options. Plast Surg Nurs. 2008;28:79–91. doi: 10.1097/01.PSN.0000324781.80590.f1. [DOI] [PubMed] [Google Scholar]
- 16.Di Rocco C, Paternoster G, Caldarelli M, Massimi L, Tamburrini G. Anterior plagiocephaly: epidemiology, clinical findings, diagnosis, and classification. A review. Childs Nerv Syst. 2012;28:1413–22. doi: 10.1007/s00381-012-1845-2. [DOI] [PubMed] [Google Scholar]
- 17.Jeyaraj P. A modified approach to surgical correction of anterior plagiocephaly. J Maxillofac Oral Surg. 2012;11:358–63. doi: 10.1007/s12663-011-0309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellerin P, Calibre C, Vinchon M, Dhellemmes P, Wolber A, Guerreschi P. Unicoronal synostotic plagiocephaly: surgical correction: Lille’s technique. Childs Nerv Syst. 2012;28:1433–8. doi: 10.1007/s00381-012-1793-x. [DOI] [PubMed] [Google Scholar]
- 19.Miri S, Mittermiller P, Buchanan EP, Khosla RK. Facial twist (asymmetry) in isolated unilateral coronal synostosis: does premature facial suture fusion play a role? J Craniofac Surg. 2015;26:655–7. doi: 10.1097/SCS.0000000000001436. [DOI] [PubMed] [Google Scholar]
- 20.Mundinger GS, Skladman R, Wenger T, Birgfeld CC, Gruss JS, Lee A, et al. Defining and correcting asymmetry in isolated unilateral frontosphenoidal synostosis: differences in orbital shape, facial scoliosis, and skullbase twist compared to unilateral coronal synostosis. J Craniofac Surg. 2018;29:29–35. doi: 10.1097/SCS.0000000000004052. [DOI] [PubMed] [Google Scholar]
- 21.Weinzweig J, Kirschner RE, Farley A, Reiss P, Hunter J, Whitaker LA, et al. Metopic synostosis: defining the temporal sequence of normal suture fusion and differentiating it from synostosis on the basis of computed tomography images. Plast Reconstr Surg. 2003;112:1211–8. doi: 10.1097/01.PRS.0000080729.28749.A3. [DOI] [PubMed] [Google Scholar]
- 22.Ploplys EA, Hopper RA, Muzaffar AR, Starr JR, Avellino AM, Cunningham ML, et al. Comparison of computed tomographic imaging measurements with clinical findings in children with unilateral lambdoid synostosis. Plast Reconstr Surg. 2009;123:300–9. doi: 10.1097/PRS.0b013e31819346b5. [DOI] [PubMed] [Google Scholar]
- 23.Poole MD. Complications in craniofacial surgery. Br J Plast Surg. 1988;41:608–13. doi: 10.1016/0007-1226(88)90168-3. [DOI] [PubMed] [Google Scholar]
- 24.Whitaker LA, Munro IR, Salyer KE, Jackson IT, Ortiz-Monasterio F, Marchac D. Combined report of problems and complications in 793 craniofacial operations. Plast Reconstr Surg. 1979;64:198–203. doi: 10.1097/00006534-197908000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Arnaud E, Marchac D, Renier D. Reduction of morbidity of the frontofacial monobloc advancement in children by the use of internal distraction. Plast Reconstr Surg. 2007;120:1009–26. doi: 10.1097/01.prs.0000278068.99643.8e. [DOI] [PubMed] [Google Scholar]
- 26.Dadure C, Sauter M, Bringuier S, Bigorre M, Raux O, Rochette A, et al. Intraoperative tranexamic acid reduces blood transfusion in children undergoing craniosynostosis surgery: a randomized double-blind study. Anesthesiology. 2011;114:856–61. doi: 10.1097/ALN.0b013e318210f9e3. [DOI] [PubMed] [Google Scholar]
- 27.Goobie SM, Meier PM, Pereira LM, McGowan FX, Prescilla RP, Scharp LA, et al. Efficacy of tranexamic acid in pediatric craniosynostosis surgery: a double-blind, placebo-controlled trial. Anesthesiology. 2011;114:862–71. doi: 10.1097/ALN.0b013e318210fd8f. [DOI] [PubMed] [Google Scholar]
- 28.Stricker PA, Goobie SM, Cladis FP, Haberkern CM, Meier PM, Reddy SK, et al. Pediatric Craniofacial Collaborative Group. Perioperative outcomes and management in pediatric complex cranial vault reconstruction: a multicenter study from the pediatric craniofacial collaborative group. Anesthesiology. 2017;126:276–87. doi: 10.1097/ALN.0000000000001481. [DOI] [PubMed] [Google Scholar]
- 29.Czerwinski M, Hopper RA, Gruss J, Fearon JA. Major morbidity and mortality rates in craniofacial surgery: an analysis of 8101 major procedures. Plast Reconstr Surg. 2010;126:181–6. doi: 10.1097/PRS.0b013e3181da87df. [DOI] [PubMed] [Google Scholar]
- 30.American Society of Anesthesiologists Task Force on Perioperative Blood Management. Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management. Anesthesiology. 2015;122:241–75. doi: 10.1097/ALN.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 31.Kozek-Langenecker SA, Afshari A, Albaladejo P, Santullano CA, De Robertis E, Filipescu DC, et al. Management of severe perioperative bleeding: guidelines from the European society of anaesthesiology. Eur J Anaesthesiol. 2013;30:270–382. doi: 10.1097/EJA.0b013e32835f4d5b. [DOI] [PubMed] [Google Scholar]