ABSTRACT
Over the past 30 years, advances in endoscopic technology and advancing interest in the benefits of minimally invasive approaches for craniofacial surgery have resulted in these techniques becoming a part of the standard of care in the treatment of craniosynostosis. In this review, we discuss the evolution and adoption of endoscopic-assisted strip craniectomy procedures. In addition to reviewing the studies describing various nuances and modifications to minimally invasive strip craniectomy, attention to comparisons in outcomes between traditional or open cranial vault reconstructions and endoscopic-assisted techniques is highlighted for different craniosynostosis diagnoses.
KEYWORDS: Craniofacial, craniosynostosis, endoscope, endoscopic assisted, strip craniectomy, suturectomy
HISTORY OF ENDOSCOPIC SURGERY FOR CRANIOSYNOSTOSIS/CRANIOFACIAL ABNORMALITIES
Surgical management of craniosynostosis has evolved over the past century in line with technology. The use of endoscopic techniques for cranial reshaping has and continues to gain popularity for both single-suture craniosynostosis and for stages approaches for multi-suture or syndromic craniosynostosis.
Virchow first formally described craniosynostosis in 1851.[1,2] He described “craniostenosis” as abnormal ossification and synostosis, or early fusion of sutures, but believed abnormal head shape resulted from the unfused skull making up for deformity so as not to decrease the overall cranial cavity size.[3] It was not until 1880 when surgical intervention for this disorder was described by Lannelongue in Paris[4] and in 1892 by Lane in Seattle.[5] Lane described a strip craniectomy for a “nine month old with signs of mental imbecility” who expired 14 h after the procedure.[5] At initial adoption of this technique, the procedure was only done on patients with cognitive deficits and was met with poor outcomes, presumed to be secondary to late intervention as well as intervention on microcephaly rather than true synostosis.[6] The intervention was met with harsh criticism due to the high rates of mortality and was subsequently abandoned prior to the turn of the century.[7]
The technique of strip craniectomy remerged in the 1920s when Faber and Towne identified early intervention as the key to success.[8] In the 1960s, Shillito and Matson indicated cosmesis as a primary indication.[9] Yet, clinical data suggested high rates of reossification in these initial procedures, resulting in the emergence of whole calvarial remodeling as first described by Tessier in 1971.[10,11] Total calvarial reshaping was reported to produce superior cosmetic outcomes, but the procedure was associated with high rates of blood loss requiring subsequent transfusion requirements, increased operating time and thus anesthesia time, and long postoperative hospital stays.[12,13] Citing this as an impetus, Jimenez and Barone pioneered an endoscopic technique for strip craniectomy that was paired with subsequent helmet therapy for the treatment of craniosynostosis. They published their first series of four patients with sagittal synostosis in 1998.[14] The authors found that their new surgical technique was associated with less blood loss, shorter postoperative hospital stay, and lower cost as compared to standard strip craniectomy and total calvarial remodeling. Additionally, they utilized a cranial orthosis for 3–4 months postoperatively in an attempt to further mold and optimize the head shape; all four patients achieved normocephaly.[14] Since then, many authors have published modifications and nuances to endoscopic techniques in the management of craniosynostosis. This review provides an overview of the history and current trends in endoscopy for craniofacial conditions.
SAGITTAL CRANIOSYNOSTOSIS
For sagittal synostosis, Jimenez and Barone[14] first described in 1998 their endoscopic technique, which involved two 2 cm incisions, one directly over the anterior fontanelle and the second over the midpoint of the lambdoid suture. Burr holes were placed paramedian to lambda and in front of the lambdoid suture. After a thin strip of bone was removed in the midline, anterior to the lambdoid sutures, lateral paramedian osteotomies were made and a midline strip 3–4 cm wide and 6–9 cm long was removed through the anterior incision. Three wedges of bone were removed from either side (barrel stave) with the osteotomy extending laterally to the squamosal suture. Postoperative molding helmets were used to minimize anterior–posterior (A–P) growth and maximize bitemporoparietal growth.
This small but initial patient series provided evidence that endoscopic intervention, as compared to calvarial vault reconstruction, provided decreased time in the operating room and anesthesia, decreased blood volume loss, decreased need for blood transfusion, lower costs, and achievement of normocephaly. The study also highlighted the role of orthosis in optimizing aesthetic outcomes after endoscopic suturectomy. Cephalic index (CI) was measured by euryon–euryon distance divided by glabellar–opisthocranion distance × 100. Their subsequent series published in 2012[15] highlighted their longitudinal experience with sagittal synostosis. The updated technique also consisted of two small incisions across the midline, one behind the anterior fontanelle and one in front of the lambda. A single burr hole was placed on the side of each incision with osteotomies made across the stenosed sutures. A wedge was removed behind the anterior fontanelle and the anterior osteotomy to allow for endoscope insertion toward lambda. Following dissection of the bone from the dura with the endoscope, lateral paramedian osteotomies were made to develop the midline strip, with the width of the strip being inversely proportional to baby’s age with young infants having 5–6 cm bone removed and an older child having 2–3 cm removed.[15]
After the midline strip was removed, wedge osteotomies were made bilaterally in front of lambdoid and behind the coronal sutures for a “clam shell” expansion of the temporal bones to increase the CI. In their 2010 review, the authors stated that the “bilateral barrel stave osteotomies allow for immediate increase in euryon-euryon distance” and thus CI.[16] This technique has been adopted by others for the potentially better CI achieved in single-suture craniosynostosis, particularly sagittal synostosis, as a result of the wedge osteotomies made posterior to the coronal sutures and anterior to the lambdoid sutures, which allow for increase in biparietal width.[6] Helmet therapy was started by postoperative day 5 and restricted growth in the A–P direction, allowing bitemporal–biparietal expansion to occur. Optimal correction of head shape was typically achieved within 6 weeks of surgery, and helmets were used for 12 months to ensure maintenance of head shape.[15] By about 18 months, the child’s head shape has likely reached its final form.[15]
Mean estimated blood loss (EBL) was 27 cc, mean transfusion rate 7%, mean surgical time 57 min, and mean length of stay (LOS) 1.1 days. By using CI with anthropometric measurement as a qualifier for head shape (CI >80 was deemed excellent, 80–70 good, and <70 poor), they concluded that 87% of patients were excellent, 9% good, and 4% poor with their endoscopic-assisted technique. For endoscopic-assisted management of craniosynostosis, the suggested target patient age is less than 3 months. Although age of intervention may be a limitation of endoscopic intervention, strip craniectomy through a small incision can only be performed if the bone is thin enough to be easily cut or rongeured.
The Jimenez and Barone technique differs from that described by the group at Boston Children’s Hospital.[17,18] Their technique involved two 1.5–2.5 cm incisions, one placed behind the anterior fontanelle and the second at the junction of the lambdoid and sagittal sutures with subsequent burr holes at the midline of each incision. To reduce bone resection and the invasiveness of the procedure, an endoscopic-assisted 1 cm strip craniectomy was performed after dissection and lateral barrel stave osteotomies were omitted. Molding helmet was used until 1 year of age unless the CI exceeded 0.8. Following the same CI grading scale published by Jimenez and Barone, the Proctor’s group found similar outcome results, with excellent results in 75% of patients, good results in 21.4% of patients, and poor results in 3.6% of patients. CI index increased on average from 0.69 to 0.76. With or without lateral wedge osteotomies, endoscopic-assisted management of craniosynostosis produces superior cosmetic results in patients less than 3 months of age. Despite the technical differences, similar long-term aesthetic results were achieved by each group.[15,17,18]
Isaac et al. compared endoscopic-assisted suturectomy (EAS) to total cranial vault remodeling (CVR).[19] In addition to CI and head circumference as outcome measures, the Whitaker classification system was used to assess desired aesthetic outcome and any subsequent requirement of secondary operation. Class I is defined by good outcome without need for revision, class II with soft-tissue revision desirable, class III with major bony alterations needed, and class IV with major repeat procedure necessary.[20] Although this system has low interrater reliability, the authors observed that operative time, EBL, rate and volume of blood transfusions, and length of hospital stay were significantly lower in endoscopic interventions compared to CVR.[20] Head circumference was lower in the CVR patients preoperatively, but both groups showed no statistically significant difference in postoperative head circumference and both declined by 3 years. CI z scores were statistically significantly further from normal prior to intervention in the CVR group, but postoperatively, the EAS group had statistically superior outcome at 1 and 2 years, but by 3 years, there was no difference. In the CVR group, 95% were in Whitaker class I and 5% were in class II compared to 99% in class I and 1% in class III in the EAS group as a single patient required revision of a bone defect adjacent to the suturectomy. The authors concluded that the two surgical approaches are comparable for aesthetic outcome although EAS remains superior for perioperative morbidity but should only be pursued in patients less than 5 months of age.[19]
Ghenbot et al. described a similar technique to that of Jimenez and Barone with a wide vertex osteotomy accompanied by lateral temporal and parietal osteotomies.[21] Their analysis of cranial vault volume and CI did not demonstrate any statistically significant difference between pre- and postoperation or between open versus endoscopic approach, suggesting no growth restriction from molding helmet use and equivalent results with endoscopic correction when compared to open procedures without concern for elevated intracranial pressure (ICP).
Honeycutt argued that a 4–5 cm strip craniectomy allowed for continued calvarial expansion as the brain grows with reossification without any bony defect.[22] Han et al. described beginning their technique with a wide vertex craniectomy and parietal wedge osteotomies; however, they later adopted a narrow strip craniectomy as described by Ridgway et al.[17,23] In this large cohort study, minimally invasive endoscopic approaches had less blood loss, lower rates of transfusion, and decreased operative time and LOS compared to open approaches.[23]
The ideal width of the suturectomy strip continues to be debated and influenced by surgeon experience and opinion. Data suggest that the wide strip craniotomy is no more beneficial than a narrow one. In fact, in four patients in whom the molding helmet was used without strip craniectomy, the CI improved from a mean of 67 to 75.[24] Furthermore, in another 24 patients, the authors demonstrated improvement in the CI without an adverse effect on ICP from the use of helmet without craniectomy.[25] Hashmi et al. concluded that using a molding helmet preoperatively can decrease bathrocephaly and forehead bossing and improve posterior vertex as well as CI, and thus can be a valuable adjunct in patients with sagittal craniosynostosis.[26]
In general, surgeons tend to be more conservative with transfusions in their endoscopically managed patients. This combined with older and more complex synostoses being treated with open procedures contributes to a somewhat skewed lower rate of transfusion with endoscopy. In our experience, the endoscope’s primary function is for visualization, a function of the endoscope’s light, so that dural and brain injury can be prevented.
METOPIC CRANIOSYNOSTOSIS
As the second most common form of synostosis, metopic craniosynostosis results in trigonocephaly as a result of decreased frontal volume and has been historically treated via frontoorbital advancement (FOA).[27,28] Not only is achievement of good aesthetic outcome a primary indication for intervention, cognitive function is also of concern in metopic synostosis.[29] The hallmark of trigonocephaly includes hypotelorism, which can be difficult to correct and is associated with a higher recurrence rate.[29] Various parameters have been suggested for standardized measurement of metopic synostosis, including assessment of hypotelorism. The teloric ratio, a quantification of lateral orbital movement, is determined by the interpupillary distance divided by the inter-frontozygomatic distance.[29] Normal teloric ratio is defined as a ratio of 40–55, hypotelorism is <40, and hypertelorism is >55. Other outcome measurements include the distance between the zygomaticofrontal (ZF) sutures and the straight-line intercanthal distance, the angle between the ZF sutures bilaterally and the glabella, and the interfrontal angle (IFA).[30] Evaluation of pre- and postoperative cranial vault volumes, metopic angle, and two-dimensional (2D)/three-dimensional (3D) laser scans of head shape comparisons has also been utilized.[31]
Keshavarzi et al. described their metopic synostosis operative experience with three separate endoscopic techniques.[32] Each approach utilizes a transverse “W” incision over the anterior fontanelle. The first is described as an endoscopic suturectomy with entry through the anterior fontanelle or a single burr hole to perform a metopic suturectomy down to the nasion. The second technique described includes bilateral osteotomies anterior to the coronal sutures down to the orbital rim after completing the metopic suturectomy. The third technique described is an endoscopic-assisted fronto-orbital advancement that builds on the last two techniques by adding bilateral upper blepharoplasty incisions and corresponding superior orbital osteotomies from the orbital roof inward through the sphenoid wing. The fronto-orbital segments are then fractured to create the fronto-orbital advancement and bioresorbable plates are placed.
In Jimenez and Barone’s 19-year series of patients undergoing endoscopic-assisted surgical management of metopic synostosis with subsequent helmet therapy, the skin incision was placed posterior to the hairline and anterior to the anterior fontanelle. They removed a 6 mm wide strip of bone from the anterior fontanelle to the nasion by placing a burr hole directly over the stenosed metopic suture, enlarging it toward the anterior fontanelle, and then completing the dissection toward the nasofrontal suture. The wedge 6 of bone is cut and removed down to the nasofrontal sublevel to ensure release of the frontal bone. This intervention was determined to be optimal for children less than 3 months of age in order to achieve forehead normalization by 6 months of age and complete normalization by 3 years of age.[29] The authors reported a mean age at surgery of 4.1 months, EBL of 33 cc, 4.3% transfusion rate, 56 min of average operative time, and a 99% discharge rate by postoperative day 1. Forehead correction was achieved in 94% of patients. Preoperatively, hypotelorism was present in 76.6% of patients and 23.4% of patients were normal with 93% of patients achieving normalization postoperatively. No secondary procedures were required in any of the patients.[29]
Gociman et al.[31] described a modified approach to the Jimenez and Barone’s technique. They placed a burr hole anterior to the fontanelle and performed the metopic suturectomy from the fontanelle to the nasofrontal junction with a 1 cm width osteotomy. In some cases, two triangular transverse bifrontal osteotomies measuring 2.5 × 2.5 × 1 cm were made between the anterior fontanelle and the nasofrontal junction to better shape the frontal vault. They evaluated pre- and postoperative cranial head shapes with metopic angle measurements and 2D/3D head shape comparisons with laser scanning. They found that the mean preoperative metopic angle was 104.9° and changed to 111.3° at 3 months postoperatively and 114.9° at 1-year follow-up. Laser scanning comparison showed smooth cranial contour without significant deformities and was confirmed by physical examination.[31]
A comparison of endoscopic and open-approach techniques for treating metopic synostosis found that based on outcome measurements (i.e. ZF–ZF difference and IFA measurement), the two approaches were equivalent in outcomes.[30] Their endoscopic technique involved a metopic suture resection width of 1–1.5 cm width from the anterior fontanelle to the nasofrontal junction.
UNICORONAL CRANIOSYNOSTOSIS
Unicoronal synostosis is also traditionally treated by FOA.[27] The primary concern is facial asymmetry. Skull base changes occur by 9 months of age in unicoronal synostosis, including vertical dystopia, craniofacial scoliosis, and nasal deviation.[33] Therefore, intervention that solely focuses on the supraorbital rim, as in FOA, will not correct the underlying deformity of the orbit.[33,34] Assessment of dystopia, facial symmetry, and supraorbital rim advancement have been used as measures of success.[33,34,35]
Jimenez and Barone managed unicoronal craniosynostosis endoscopically with an incision posterior to the hairline at the junction of the coronal suture and superior temporal crest.[33] A single burr hole was enlarged from the anterior fontanelle toward the pterion. After dissection, a wedge osteotomy was made, extending from the burr hole to the squamosal suture. Helmets were designed with extra space at the ipsilateral frontoorbital complex to allow for expansion in this plane. Best results were found in patients less than 3 months of age with inconsistent results in patients aged 6–9 months.[33]
Alternatively, an incision and burr hole were placed along the ridge of the stenosed suture, halfway between the midline and the lateral canthus, as described by Tan et al.[34] The burr hole was widened and then endoscopic-assisted resection was completed toward the midline along the suture as well as inferiorly along the sphenoid wing, posteriorly toward the affected eye, to the level of the lateral canthus. In comparing their open FOA approach to endoscopic treatment of unicoronal synostosis, anthropometric analyses found that patients who underwent endoscopic suturectomy exhibited better (i.e. less deviation from the midline) postoperative measurements in facial symmetry including facial deviation, nasal tip deviation, and middle facial depth. These improved outcomes may be a result of earlier intervention done in the endoscopically treated patients.[34]
Isaac et al. also compared FOA and endoscopic suturectomy with helmeting in unicoronal synostotic patients, using strabismus as an outcome measure.[35] They performed an endoscopic suturectomy, a 1 cm strip craniectomy, along the stenosed coronal suture from the vertex and extending beyond the sphenoidal fontanelle at the level of the lateral canthus. Postoperative helmet therapy was used until normalization of head shape or 1 year of age. After intervention, 31% of endoscopically treated patients and 65% of FOA-treated patients required a surgery for strabismus. Reoperation and need for secondary procedures in patients were more common in the FOA group. Overall, perioperative ophthalmic and aesthetic outcomes were superior in the endoscopically treated group.[35,36]
UNILATERAL LAMBDOID CRANIOSYNOSTOSIS
The rarest of the nonsyndromic craniosynostoses, i.e. unilateral lambdoid synostosis, is characterized by prominent ipsilateral mastoidal bossing downward and laterally, deviation of the foramen magnum to the stenosed side, asymmetry of the petrous ridges and external acoustic meatus, and mastoid bulge ipsilateral to the stenosis.[37,38] Owing to the wide variability in severity of lambdoid synostosis, it is more challenging to consistently achieve an aesthetic correction.[38,39] Usually, unilateral lambdoid synostosis is treated with posterior vault CVR.[27,40]
Zubovic et al.[38] compared open cranial vault repair and endoscopic repair with postoperative helmeting for lambdoid synostosis. They analyzed cranial base asymmetry with posterior fossa deflection angle, petrous ridge angle, mastoid cant angle, vertical and A–P displacement of external acoustic meatus, and posterior cranial vault asymmetry quantified by volumetric analysis. At 1 year follow-up from the corrective procedure, no difference was seen in outcome measures between the open and endoscopic techniques. Some degree of asymmetry remained regardless of the procedural approach. Posterior fossa deflection angle, mastoid cant angle, and vertical external acoustic meatus displacement were significantly improved in all patients. Petrous ridge angle asymmetry and A–P external acoustic meatus displacement did not change significantly. As in other series comparing open to endoscopic-assisted procedures, all CVR procedures required blood transfusion, whereas endoscopic procedures did not.[38]
More recently, Rattani et al. also compared endoscopic lambdoid suturectomy with helmeting and CVR.[39] With their technique, they placed incisions and burr holes at the contralateral lambdoid and sagittal suture junction as well as at the mastoid bulge. With endoscopic guidance, the superior portion of the craniectomy was taken up to the contralateral sagittal and lambdoid suture junction with the inferior extent down to the mastoid bone. A 1.5 cm wide strip was removed from the sagittal suture to the mastoid bulge, separating the occipital and parietal bones. There were no significant differences in pre- and postoperative head circumference percentiles or z scores up to 36 months after surgery. The authors noted that the “windswept” appearance of the patients appeared better in the endoscopically treated patients.
Mittermiller et al. described a novel endoscopic spring-assisted technique via an incision lateral to the lambdoid suture followed by burr holes on either side connected with suturectomy.[41] Two springs were bent, at a median force of 7.0 N, and placed in notches across the suturectomy. The spring tension allowed for gradual distraction of bone with concurrent soft tissue expansion. The average age of this cohort was older, 9.4 months, compared to other endoscopic series.[39] Outcomes were evaluated by measuring deviation of skull base as defined by the angle between long axis of foramen magnum and axis of cribiform plate, petrous ridge angles, and mastoid cant. The springs were able to extend up to 15.3 mm, and the angle of the cranial base improved by 5.8°. The procedure was amenable up to 1.5 years of age.[41]
An average savings of US$45,000 was found when metopic, unicoronal, and unilateral lambdoid were intervened upon endoscopically compared to traditional open treatment (including CVR and FOA).[27]
NUANCES OF ENDOSCOPIC-ASSISTED SURGERY FOR CRANIOSYNOSTOSIS
In current practices, the various techniques for endoscopic-assisted strip craniectomy can be categorized into two common methods: 1) simple suturectomy and 2) suturectomy with barrel staves and/or wedge osteotomies. With either method, typically 2–4 cm of bone encompassing the prematurely fused suture is removed.
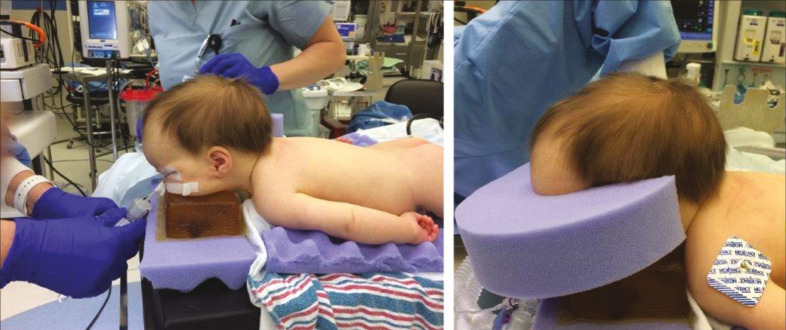
At our institutions, we have utilized both suturectomy alone and with wedge osteotomies for infants less than 4 months of age. Depending on the suture involved, we may position the patient in a modified “sphinx” position for the sagittal suture, prone position for the lambdoid suture, and supine for metopic or coronal sutures. We place the patient in a slight reverse Trendelenburg position [Figure 1]. This minimizes the venous pressure in the sinus and reduces the blood loss.
Figure 1.
Modified “sphinx” position for endoscopic-assisted sagittal suturectomy. The patient is placed in a slight reverse Trendelenburg position to minimize venous sinus pressure and thereby reduce potential blood loss
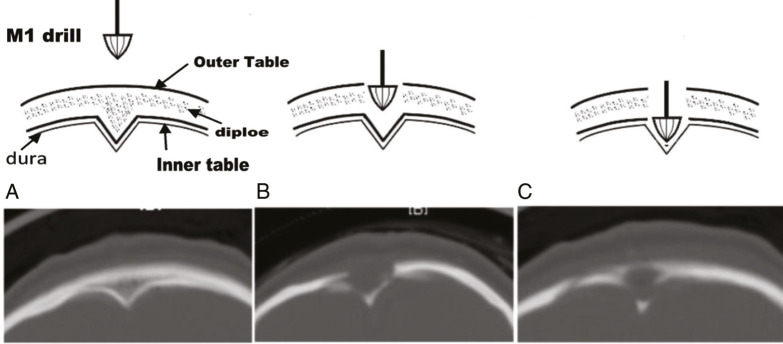
A single transverse incision is made for metopic and unicoronal synostosis cases. Two transverse incisions are made for sagittal synostosis, with one incision 1 cm posterior to the anterior fontanelle and a second incision 1 cm anterior to lambda. Alternatively, one of the authors (SS) divides the anterior fontanelle to posterior fontanelle distance into four parts. The anterior incision is placed one-fourth the distance behind the anterior fontanelle and the posterior incision one-fourth the distance in front of the posterior fontanelle. In this way, the exposure to bone removal distance is minimized from the incision. Subgaleal dissection is completed between the anterior fontanelle and lambda. Burr holes are made 1.5 cm lateral to the midline at each incision followed by transverse osteotomies with Kerrison rongeurs across the sagittal sinus. We have also found it safe to make the burr hole directly in the midline using an acorn-shaped drill bit [Figure 2]. This eliminates the risk entailed in separating the dura from the prominent keel that is often present posteriorly. Because the shape of the acorn matches that of the keel, we have not had any instance of sinus injury. The endoscope is inserted into the epidural space to visualize the dissection of the dural and sagittal sinus from the overlying fused suture. With the aid of the endoscope, a 3–4 cm wide suture craniectomy is performed with angled or straight scissors. The osteotomies are then extended anteriorly on either side of the midline from the anterior incision, connecting to and removing the bone up to the anterior fontanelle. If needed, further posterior extension of the osteotomy is completed toward the lambda from the posterior incision.
Figure 2.
A burr hole made in the midline. Using high-speed drill and acorn-shaped drill bit, a burr hole can be placed at the midline. Because the shape of the acorn drill bit matched the midline keel, this technique helps minimize risks of dural injury when coming across the keel with a scissor or rongeur
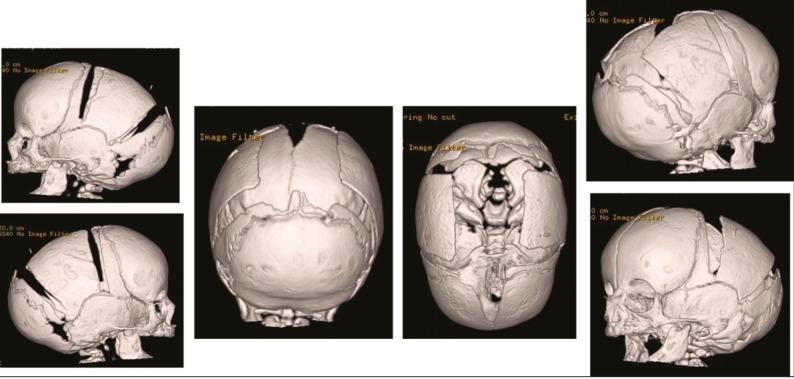
If an extended strip craniectomy procedure is performed in cases of sagittal craniosynostosis, further endoscopic-assisted dural dissection is done just posterior to the coronal suture bilaterally and anterior to the lambdoid suture bilaterally. Triangular wedge-shaped osteotomies are performed using scissors posterior to each coronal suture and anterior to each lambdoid suture, down to the squamosal suture inferiorly. This allows for out fracturing and reshaping of the frontoparietal bones, thereby achieving immediate biparietal widening [Figure 3]. The endoscope is used to visualize cut bone edges for coagulation using a suction and cautery device. By extending the cut down to or just beyond the squamosal suture, immediate improvement is noted in the CI and overall head shape due to the immediate biparietal widening [Figure 4]. In metopic synostosis, after endoscopic strip craniectomy is performed from the anterior fontanelle/coronal suture to the nasion, the edges of the bone are contoured with a Tessier bone bender [Figure 5].
Figure 3.
Endoscopic-assisted strip craniectomy and bifrontal and biparietal wedge osteotomies for sagittal craniosynostosis. In addition to the sagittal suturectomy, triangular wedge-shaped osteotomies are performed bilaterally, posterior to the coronal suture and anterior to the lambdoid suture. The osteotomies are made down to the squamosal suture inferiorly to achieve biparietal widening
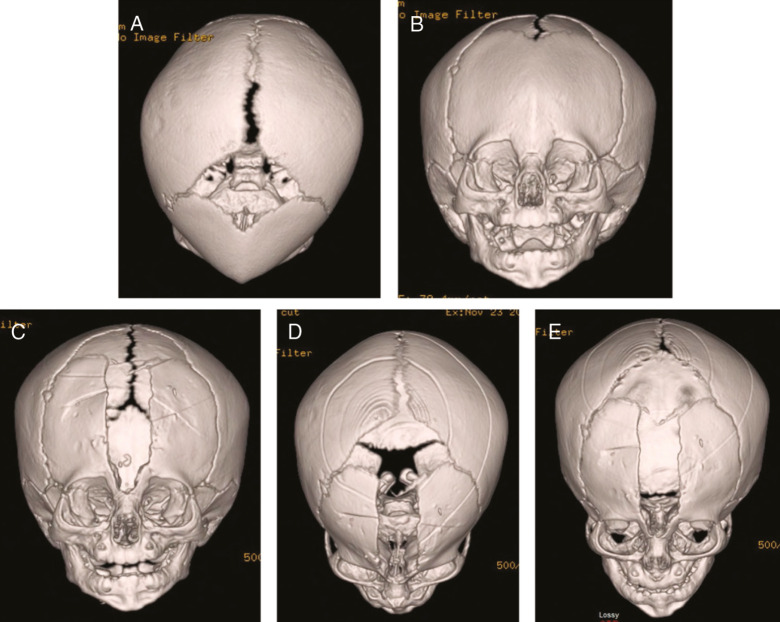
Figure 4.
Immediate improvements in cranial index with wedge osteotomies. A–C: Preoperative top and lateral views. D–E: Immediate postoperative top views showing significant immediate biparietal widening. By extending the bifrontal and biparietal wedge osteotomy cuts down to or just beyond the squamosal suture, immediate improvement is noted in the CI and overall head shape due to the immediate biparietal widening
Figure 5.
Endoscopic-assisted metopic suturectomy. A and B: Preoperative 3D reconstruction of the patient with metopic craniosynostosis. C–E: Postoperative 3D reconstruction. Metopic suturectomy performed from the anterior fontanelle to the level of the nasion. The frontal bones are contoured intraoperatively with a bone bender
Although the use of an endoscope is heralded for a minimally invasive approach in craniosynostosis surgery, the endoscope is really a light source that provides visualization under the bone. In craniofacial cases, particularly in young infants, the endoscope is an excellent tool for blunt dissection for separating the dura from the inner table. In our practice, we use a zig-zag style incision and mobilize the incision with subgaleal dissection to minimize the length of incision. We do not typically perform bone grafting or replace morselized bone into the craniectomy sites, although we acknowledge that other groups achieve equally aesthetic outcomes with grafting techniques.
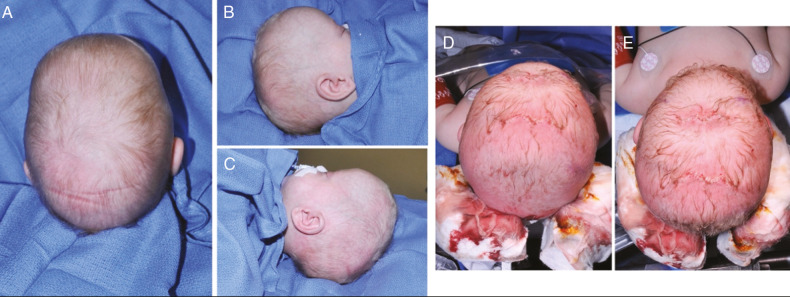
Postoperatively, the infant is fitted for the first helmet in a series of two to three cranial molding helmets. We emphasize that the child wears the cranial molding helmet 23 h/day. The skill of the orthotist is critical in optimizing the head shape. This is further supported by our findings in patients treated with a molding helmet preoperatively, where improvements in the head shape and cranial index were achieved without adverse effects on ICP.[25,26] The child is followed monthly in our craniofacial clinic until termination of helmet therapy at approximately 1 year of age. We typically see normalization of head shape by 3 months post endoscopic strip craniectomy for sagittal synostosis [Figure 6]. In our experience, performing endoscopic-assisted triangular wedge osteotomies for sagittal craniosynostosis in addition to the sagittal suturectomy results in a more immediate and earlier overall improvement in cranial index and head shape.
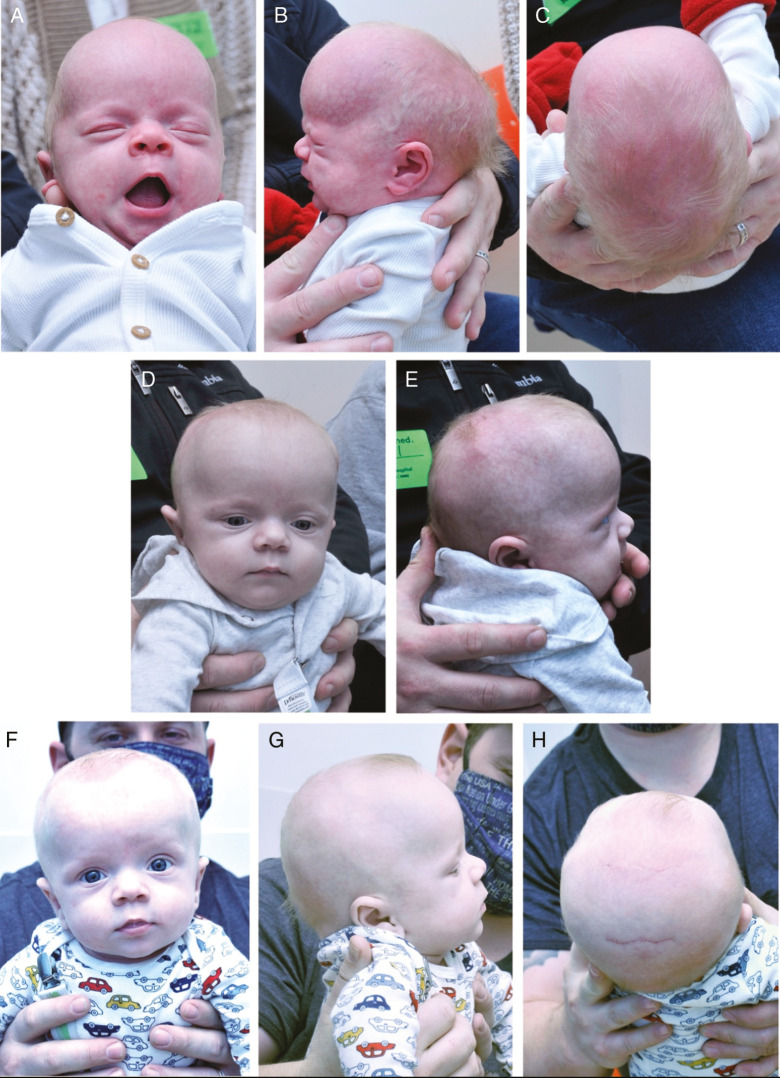
Figure 6.
Preoperative and postoperative images of infant with sagittal craniosynostosis who underwent endoscopic-assisted strip craniectomy with bifrontal and biparietal wedge osteotomies. A–C: Preoperative views of sagittal craniosynostosis at 1 month of age. D–E: Postoperative images 1 month after endoscopic-assisted sagittal suturectomy and wedge osteotomies. F–H: Postoperative images at 3-month follow-up
SUMMARY/CONCLUSION
The use of an endoscope in the management of craniofacial anomalies decreases the extent of the surgical incision, limits the need for major skull bone manipulation, and allows for surgery at a younger age, which may optimize long-term outcomes both aesthetically and neurocognitively. The benefits of endoscopic-assisted craniofacial surgery include decreased blood loss, shorter operating and anesthesia time, and smaller incisions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Virchow R. Uber den Cretinismus, namentlich in Franken, und uber pathologische Schadelformen. Verh Phys Med Gesell Wurzburg. 1851;2:230–71. [Google Scholar]
- 2.Persing JA, Jane JA, Shaffrey M. Virchow and the pathogenesis of craniosynostosis: a translation of his original work. Plast Reconstr Surg. 1989;83:738–42. doi: 10.1097/00006534-198904000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Delashaw JB, Persing JA, Broaddus WC, Jane JA. Cranial vault growth in craniosynostosis. J Neurosurg. 1989;70:159–65. doi: 10.3171/jns.1989.70.2.0159. [DOI] [PubMed] [Google Scholar]
- 4.Lannelongue M. De la craniectomie dans la microcéphalie. Compt Rend Seances Acad Sci. 1890;50:1382–85. [Google Scholar]
- 5.Lane LC. Pioneer craniectomy for relief of mental imbecility due to premature sutural closure and microcephalus. JAMA. 1892;XVIII:49–50. [Google Scholar]
- 6.Delye HHK, Borstlap WA, van Lindert EJ. Endoscopy-assisted craniosynostosis surgery followed by helmet therapy. Surg Neurol Int. 2018;9:59. doi: 10.4103/sni.sni_17_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobi A. Non nocere. Med Rec. 1894;45:609–18. [Google Scholar]
- 8.Faber H, Towne E. Early craniectomy as a preventive measure in oxycephaly and allied conditions: with special reference to the prevention of blindness. The American Journal of the Medical Sciences. 1927;173:701–11. [Google Scholar]
- 9.Shillito J, Jr, Matson DD. Craniosynostosis: a review of 519 surgical patients. Pediatrics. 1968;41:829–53. [PubMed] [Google Scholar]
- 10.Tessier P. Relationship of craniostenoses to craniofacial dysostoses, and to faciostenoses: a study with therapeutic implications. Plast Reconstr Surg. 1971;48:224–37. doi: 10.1097/00006534-197109000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Tessier P. The definitive plastic surgical treatment of the severe facial deformities of craniofacial dysostosis. Crouzon’s and Apert’s diseases. Plast Reconstr Surg. 1971;48:419–42. doi: 10.1097/00006534-197111000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Kearney RA, Rosales JK, Howes WJ. Craniosynostosis: an assessment of blood loss and transfusion practices. Can J Anaesth. 1989;36:473–7. doi: 10.1007/BF03005352. [DOI] [PubMed] [Google Scholar]
- 13.Faberowski LW, Black S, Mickle JP. Blood loss and transfusion practice in the perioperative management of craniosynostosis repair. J Neurosurg Anesthesiol. 1999;11:167–72. doi: 10.1097/00008506-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez DF, Barone CM. Endoscopic craniectomy for early surgical correction of sagittal craniosynostosis. J Neurosurg. 1998;88:77–81. doi: 10.3171/jns.1998.88.1.0077. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez DF, Barone CM. Endoscopic technique for sagittal synostosis. Childs Nerv Syst. 2012;28:1333–9. doi: 10.1007/s00381-012-1768-y. [DOI] [PubMed] [Google Scholar]
- 16.Jimenez DF, Barone CM. Endoscopic techniques for craniosynostosis. Atlas Oral Maxillofac Surg Clin North Am. 2010;18:93–107. doi: 10.1016/j.cxom.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Ridgway EB, Berry-Candelario J, Grondin RT, Rogers GF, Proctor MR. The management of sagittal synostosis using endoscopic suturectomy and postoperative helmet therapy. J Neurosurg Pediatr. 2011;7:620–6. doi: 10.3171/2011.3.PEDS10418. [DOI] [PubMed] [Google Scholar]
- 18.Berry-Candelario J, Ridgway EB, Grondin RT, Rogers GF, Proctor MR. Endoscope-assisted strip craniectomy and postoperative helmet therapy for treatment of craniosynostosis. Neurosurg Focus. 2011;31:E5. doi: 10.3171/2011.6.FOCUS1198. [DOI] [PubMed] [Google Scholar]
- 19.Isaac KV, Meara JG, Proctor MR. Analysis of clinical outcomes for treatment of sagittal craniosynostosis: a comparison of endoscopic suturectomy and cranial vault remodeling. J Neurosurg Pediatr. 2018;22:467–74. doi: 10.3171/2018.5.PEDS1846. [DOI] [PubMed] [Google Scholar]
- 20.Wes AM, Naran S, Sun J, Mazzaferro D, Xu W, Nguyen P, et al. The Whitaker classification of craniosynostosis outcomes: An assessment of interrater reliability. Plast Reconstr Surg. 2017;140:579e–86e. doi: 10.1097/PRS.0000000000003688. [DOI] [PubMed] [Google Scholar]
- 21.Ghenbot RG, Patel KB, Skolnick GB, Naidoo SD, Smyth MD, Woo AS. Effects of open and endoscopic surgery on skull growth and calvarial vault volumes in sagittal synostosis. J Craniofac Surg. 2015;26:161–4. doi: 10.1097/SCS.0000000000001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honeycutt JH. Endoscopic-assisted craniosynostosis surgery. Semin Plast Surg. 2014;28:144–9. doi: 10.1055/s-0034-1384810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han RH, Nguyen DC, Bruck BS, Skolnick GB, Yarbrough CK, Naidoo SD, et al. Characterization of complications associated with open and endoscopic craniosynostosis surgery at a single institution. J Neurosurg Pediatr. 2016;17:361–70. doi: 10.3171/2015.7.PEDS15187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sood S, Rozzelle A, Shaqiri B, Sood N, Ham SD. Effect of molding helmet on head shape in nonsurgically treated sagittal craniosynostosis. J Neurosurg Pediatr. 2011;7:627–32. doi: 10.3171/2011.4.PEDS116. [DOI] [PubMed] [Google Scholar]
- 25.Marupudi NI, Sood S, Rozzelle A, Ham SD. Effect of molding helmets on intracranial pressure and head shape in nonsurgically treated sagittal craniosynostosis patients. J Neurosurg Pediatr. 2016;18:207–12. doi: 10.3171/2016.1.PEDS15569. [DOI] [PubMed] [Google Scholar]
- 26.Hashmi A, Marupudi NI, Sood S, Rozzelle A. Effect of preoperative molding helmet in patients with sagittal synostosis. J Craniofac Surg. 2017;28:898–903. doi: 10.1097/SCS.0000000000003512. [DOI] [PubMed] [Google Scholar]
- 27.Zubovic E, Lapidus JB, Skolnick GB, Naidoo SD, Smyth MD, et al. Cost comparison of surgical management of nonsagittal synostosis: Traditional open versus endoscope-assisted techniques. J Neurosurg Pediatr. 2020:1–10. doi: 10.3171/2019.11.PEDS19515. [DOI] [PubMed] [Google Scholar]
- 28.Bennett KG, Bickham RS, Robinson AB, Buchman SR, Vercler CJ. Metopic craniosynostosis: a demographic analysis outside an urban environment. J Craniofac Surg. 2016;27:544–7. doi: 10.1097/SCS.0000000000002532. [DOI] [PubMed] [Google Scholar]
- 29.Jimenez DF, McGinity MJ, Barone CM. Endoscopy-assisted early correction of single-suture metopic craniosynostosis: a 19-year experience. J Neurosurg Pediatr. 2018;23:61–74. doi: 10.3171/2018.6.PEDS1749. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen DC, Patel KB, Skolnick GB, Naidoo SD, Huang AH, Smyth MD, et al. Are endoscopic and open treatments of metopic synostosis equivalent in treating trigonocephaly and hypotelorism? J Craniofac Surg. 2015;26:129–34. doi: 10.1097/SCS.0000000000001321. [DOI] [PubMed] [Google Scholar]
- 31.Gociman B, Agko M, Blagg R, Garlick J, Kestle JR, Siddiqi F. Endoscopic-assisted correction of metopic synostosis. J Craniofac Surg. 2013;24:763–8. doi: 10.1097/SCS.0b013e31828696a5. [DOI] [PubMed] [Google Scholar]
- 32.Keshavarzi S, Hayden MG, Ben-Haim S, Meltzer HS, Cohen SR, Levy ML. Variations of endoscopic and open repair of metopic craniosynostosis. J Craniofac Surg. 2009;20:1439–44. doi: 10.1097/SCS.0b013e3181af1555. [DOI] [PubMed] [Google Scholar]
- 33.Jimenez DF, Barone CM. Early treatment of coronal synostosis with endoscopy-assisted craniectomy and postoperative cranial orthosis therapy: 16-year experience. J Neurosurg Pediatr. 2013;12:207–19. doi: 10.3171/2013.4.PEDS11191. [DOI] [PubMed] [Google Scholar]
- 34.Tan SP, Proctor MR, Mulliken JB, Rogers GF. Early frontofacial symmetry after correction of unilateral coronal synostosis: frontoorbital advancement vs endoscopic strip craniectomy and helmet therapy. J Craniofac Surg. 2013;24:1190–4. doi: 10.1097/SCS.0b013e318299742e. [DOI] [PubMed] [Google Scholar]
- 35.Isaac KV, MacKinnon S, Dagi LR, Rogers GF, Meara JG, Proctor MR. Nonsyndromic unilateral coronal synostosis: a comparison of fronto-orbital advancement and endoscopic suturectomy. Plast Reconstr Surg. 2019;143:838–48. doi: 10.1097/PRS.0000000000005383. [DOI] [PubMed] [Google Scholar]
- 36.MacKinnon S, Proctor MR, Rogers GF, Meara JG, Whitecross S, Dagi LR. Improving ophthalmic outcomes in children with unilateral coronal synostosis by treatment with endoscopic strip craniectomy and helmet therapy rather than fronto-orbital advancement. J AAPOS. 2013;17:259–65. doi: 10.1016/j.jaapos.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Hurmerinta K, Kiukkonen A, Hukki J, Saarikko A, Leikola J. Lambdoid synostosis versus positional posterior plagiocephaly, a comparison of skull base and shape of calvarium using computed tomography imaging. J Craniofac Surg. 2015;26:1917–22. doi: 10.1097/SCS.0000000000002098. [DOI] [PubMed] [Google Scholar]
- 38.Zubovic E, Woo AS, Skolnick GB, Naidoo SD, Smyth MD, Patel KB. Cranial base and posterior cranial vault asymmetry after open and endoscopic repair of isolated lambdoid craniosynostosis. J Craniofac Surg. 2015;26:1568–73. doi: 10.1097/SCS.0000000000001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rattani A, Riordan CP, Meara JG, Proctor MR. Comparative analysis of cranial vault remodeling versus endoscopic suturectomy in the treatment of unilateral lambdoid craniosynostosis. J Neurosurg Pediatr. 2020;26:105–12. doi: 10.3171/2020.2.PEDS19522. [DOI] [PubMed] [Google Scholar]
- 40.Elliott RM, Smartt JM, Jr, Taylor JA, Bartlett SP. Does conventional posterior vault remodeling alter endocranial morphology in patients with true lambdoid synostosis? J Craniofac Surg. 2013;24:115–9. doi: 10.1097/SCS.0b013e318270fb4e. [DOI] [PubMed] [Google Scholar]
- 41.Mittermiller PA, Rochlin DH, Menard RM. Endoscopic spring-mediated distraction for unilambdoid craniosynostosis. J Craniofac Surg. 2020;31:2097–100. doi: 10.1097/SCS.0000000000006988. [DOI] [PubMed] [Google Scholar]