Abstract
Whole cells and lipopolysaccharides (LPSs) extracted from Burkholderia cepacia, Pseudomonas aeruginosa, Stenotrophomonas maltophilia, and Escherichia coli were compared in their ability to stimulate tumor necrosis factor alpha (TNF-α) from the human monocyte cell line MonoMac-6. B. cepacia LPS, on a weight-for-weight basis, was found to have TNF-α-inducing activity similar to that of LPS from E. coli, which was approximately four- and eightfold greater than the activity of LPSs from P. aeruginosa and S. maltophilia, respectively. The LPS-stimulated TNF-α production from monocytes was found to be CD14 dependent. These results suggest that B. cepacia LPS might play a role in the pathogenesis of inflammatory lung disease in cystic fibrosis, and in some patients it might be responsible, at least in part, for the sepsis-like cepacia syndrome.
In recent years, Burkholderia cepacia infection in cystic fibrosis (CF) patients has become a clinical issue of increasing concern. B. cepacia is highly transmissible from person to person (4, 9) and intrinsically resistant to many antibiotics (3, 10). Once acquired, B. cepacia infection is rarely eradicated from the lungs of CF patients. In addition, a proportion of patients who acquire B. cepacia infection develop a rapidly fatal pneumonia and sepsis with a high mortality rate, the so-called cepacia syndrome (6, 8). However, the pathogenic mechanisms involved in this syndrome remain unclear. Indeed, the nature of the virulence factors that contribute to the pathogenicity of B. cepacia in CF remains largely unknown. Due to its potent inflammatory activity and association with sepsis, lipopolysaccharide (LPS) might be an important virulence factor in B. cepacia infections. Elevated tumor necrosis factor alpha (TNF-α) levels have been implicated in both systemic and local inflammations (7) and may play a role in cepacia syndrome (5). In this report, we studied the activity of B. cepacia whole cells as well as purified LPS in stimulating human monocytes to release TNF-α and compared it with the activities of other gram-negative bacteria that also colonize the lungs of CF patients, Pseudomonas aeruginosa and Stenotrophomonas maltophilia. Patients carrying these organisms may, over time, suffer a chronic deterioration of lung function, but there are no reports that acquisition of these organisms is followed by the acute pulmonary deterioration sometimes seen in B. cepacia-infected patients. Escherichia coli was included in this study since the LPS from this organism is often reported to represent the most biologically active form of LPS (11), and it is used as a reference standard for the measurement of LPS activity by the Limulus assay.
The human monocytic cell line MonoMac-6 (MM6) was obtained from the German collection of microorganisms and cell cultures (Deutsche Sammlung von Mikroorganismen), Braunschweig, Germany. In this study, we used 13 B. cepacia clinical CF isolates (from 10 of which LPS was purified), 7 B. cepacia environmental isolates (from 4 of which LPS was purified), 8 S. maltophilia isolates (from all 8 of which LPS was purified), and 7 P. aeruginosa isolates (from all 7 of which LPS was purified). E. coli O111:B4 LPS was purchased from Sigma Chemical Co., Poole, United Kingdom, and LPS was also purified from E. coli NCTC 10418. LPSs were purified by the phenol-water extraction method (2) and characterized by silver staining on a sodium dodecyl sulfate–14% polyacrylamide gel. Coomassie blue staining of the gels did not reveal any visible protein bands in the purified LPSs. The activity of different doses of LPS to produce TNF-α was established by stimulating MM6 cells with LPS concentrations ranging from 0.1 to 1000 ng/ml. However, at an LPS concentration above 500 ng/ml, the linearity of the TNF-α response decreased, and thus 500 ng of LPS per ml was used as the optimum concentration for this study. MM6 cells suspended at 106/ml of RPMI 1640 medium containing 10% fetal calf serum (Gibco) and 5 ng of phorbol myristate acetate per ml (13) were stimulated with 500 ng of LPS or 106 CFU of whole bacteria (all bacterial isolates were grown under identical conditions). MM6 incubated with phorbol myristate acetate only (without LPS) did not produce any significant amounts of TNF-α. The cells were incubated overnight, and the supernatants were collected after centrifugation at 3,000 × g for 3 min followed by filtration through a 0.2-μm-pore-size syringe filter (Acrodisc; Gelman Sciences, Ann Arbor, Mich.). Supernatants were stored at −80°C until assayed. The TNF-α enzyme-linked immunosorbent assay (ELISA) was performed with monoclonal anti-human TNF-α antibody and biotinylated anti-human TNF-α antibody (R & D, Minneapolis, Minn.) in accordance with the manufacturer’s instructions.
Statistical significance was calculated by the nonparametric Mann-Whitney test by using Minitab release 10 software. The role of CD14 in the LPS-stimulated TNF-α production was investigated by using monoclonal anti-human CD14 antibodies MY4 (Coulter Corporation, Miami, Fla.) and UCHM-1 (Sigma). The monoclonal anti-human CD7 antibody 3A1 (Sigma) was used as an isotype control antibody for MY4 (immunoglobulin G2b). Five microliters (2.5 μg) of monoclonal antibody was incubated with 106 MM6 cells at room temperature for 30 min, and the cells were then washed with RPMI 1640 medium to remove unbound antibody. The MM6 cells were then stimulated with 500 ng of LPS. The TNF-α produced was measured by an ELISA as described above.
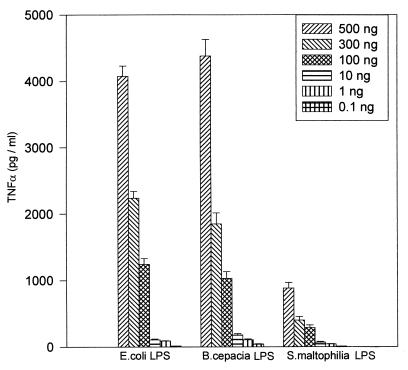
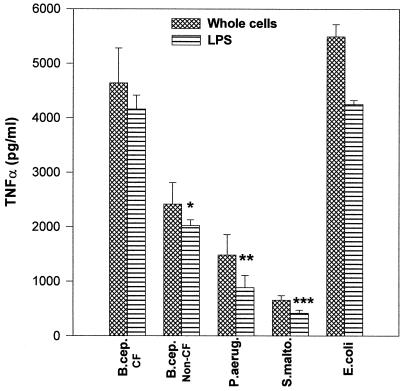
Figure 1 shows the TNF-α production in MM6 cells stimulated with different doses of LPS. All of the LPS preparations used gave a dose response for TNF-α production, although there was variation in the total TNF-α stimulated by each type of LPS. A 500-ng/ml dose of LPS was found to be optimal for each LPS type for TNF-α production. Therefore, the different LPS types were compared at this dose. The ELISA results (Fig. 2) showed that B. cepacia LPS from CF patients induced a concentration of TNF-α similar to that induced by E. coli LPS (mean TNF-α concentration of 4,000 pg/ml). On the other hand, B. cepacia LPS from environmental isolates had half the activity (2,000 pg/ml) of that from CF isolates (Fig. 2). Moreover, LPS from B. cepacia CF isolates had fourfold the activity of P. aeruginosa LPS (1,000 pg/ml) and eightfold the activity of S. maltophilia LPS (500 pg/ml). TNF-α stimulated by the corresponding whole bacterial cells showed a similar pattern to that produced by the extracted LPS (Fig. 2). If LPS-mediated macrophage stimulation plays a role in the development of inflammatory lung pathology, the differences in the TNF-α stimulatory activity between LPSs from B. cepacia, P. aeruginosa, and S. maltophilia may in part explain the different pathogenic sequelae observed with lung infections due to these organisms in CF patients. S. maltophilia, in particular, is similar to B. cepacia in many aspects, such as high patient-to-patient infection rate, resistance to most antibiotics, and no significant exotoxin production. However, unlike the case with B. cepacia, despite numerous investigations, there have yet to be reports of acute pulmonary inflammation occurring following S. maltophilia acquisition (1).
FIG. 1.
Dose response of LPS-induced TNF-α production from stimulated MM6 cells. MM6 cells (106/ml) were incubated with 0.1 to 500 ng of LPS per ml, and TNF-α production was measured by an ELISA. The TNF-α produced was proportional to the LPS concentration up to an LPS dose of 500 ng/ml. Bars represent the mean (+ standard error) of four separate experiments.
FIG. 2.
TNF-α production by MM6 cells stimulated by whole bacteria and the extracted LPS. The bars show the mean (+ standard error) of TNF-α production in response to the different LPS types (number of different isolates = 4 to 10 in each group) from four separate experiments. ∗, P = 0.021; ∗∗, P = 0.0054; ∗∗∗, P = 0.00012 (all versus results for B. cepacia [B.cep.] CF isolates). P.aerug, P. aeruginosa; S.malto, S. maltophila.
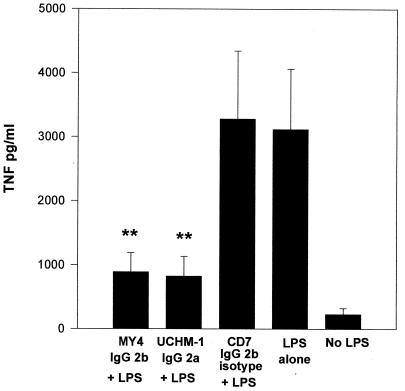
To study the role of CD14 in B. cepacia LPS-mediated TNF-α stimulation, monoclonal anti-human CD14 antibodies were used to block CD14 binding sites and consequently inhibit TNF-α production. When monoclonal antibodies were added, TNF-α production was significantly inhibited following B. cepacia LPS stimulation in comparison with TNF-α production in control cells or those cells incubated with the isotype antibody (Fig. 3). We found similar results with S. maltophilia and P. aeruginosa. Contrary to a previous report by Shaw et al. (12), our investigations suggest that B. cepacia LPS-mediated TNF-α stimulation is almost completely mediated by CD14.
FIG. 3.
Production of TNF-α from MM6 cells stimulated with B. cepacia LPS (500 ng/ml) and in the presence of the anti-CD14 monoclonal antibodies MY4 and UCHM-1 and the MY4 isotype control anti-CD7. ∗∗, P = 0.0004 versus results for LPS alone. IgG, immunoglobulin G.
We conclude that B. cepacia LPS is a potent immunostimulator and that chronic colonization may lead to LPS shedding and TNF-α production, resulting in tissue damage and lung inflammation.
REFERENCES
- 1.Denton M. Stenotrophomonas maltophilia: an emerging problem in cystic fibrosis patients. Rev Med Microbiol. 1997;8:15–19. [Google Scholar]
- 2.Galanos C, Luderitz O, Westphal O. A new method for the extraction of R-lipopolysaccharide. Eur J Biochem. 1969;9:245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 3.Goldman D A, Klinger J D. Pseudomonas cepacia: biology, mechanisms of virulence, epidemiology. J Pediatr. 1986;108:806–812. doi: 10.1016/s0022-3476(86)80749-1. [DOI] [PubMed] [Google Scholar]
- 4.Govan J R W, Brown P H, Maddison J, et al. Evidence of transmission of Pseudomonas cepacia by social contact in cystic fibrosis. Lancet. 1993;342:15–19. doi: 10.1016/0140-6736(93)91881-l. [DOI] [PubMed] [Google Scholar]
- 5.Govan J R W, Hughes J E, Vandamme P. Burkholderia cepacia: medical, taxonomic and ecological issues. J Med Microbiol. 1996;45:395–407. doi: 10.1099/00222615-45-6-395. [DOI] [PubMed] [Google Scholar]
- 6.Govan J R W, Nelson J W. Microbiology of lung infection in cystic fibrosis. Br Med Bull. 1992;48:912–930. doi: 10.1093/oxfordjournals.bmb.a072585. [DOI] [PubMed] [Google Scholar]
- 7.Greally P, Hussein M J, Cook A J, Sampson A P, Piper P J, Price J F. Sputum tumor necrosis factor-α and leukotriene concentrations in cystic fibrosis. Arch Dis Child. 1993;68:389–392. doi: 10.1136/adc.68.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lancet. Pseudomonas cepacia—more than a harmless commensal? Lancet. June 6, 1992;339:1385–1386. . (Editorial.) [PubMed] [Google Scholar]
- 9.Lipuma J J, Dasen S E, Neilson D W, Stern R C, Stull T L. Person-to-person transmission of Pseudomonas cepacia between patients with cystic fibrosis. Lancet. 1990;336:527–532. doi: 10.1016/0140-6736(90)92571-x. [DOI] [PubMed] [Google Scholar]
- 10.Prince A. Antibiotic resistance of Pseudomonas species. J Pediatr. 1986;108:830–834. doi: 10.1016/s0022-3476(86)80753-3. [DOI] [PubMed] [Google Scholar]
- 11.Riestschel E-T, Brade L, Lindner B, Zahringer U. Biochemistry of lipopolysaccharides. In: Morrison D C, Ryan J L, editors. Bacterial endotoxic lipopolysaccharides. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 3–41. [Google Scholar]
- 12.Shaw D, Poxton I R, Govan J R W. Biological activity of Burkholderia (Pseudomonas) cepacia lipopolysaccharide. FEMS Immunol Med Microbiol. 1995;11:99–106. doi: 10.1111/j.1574-695X.1995.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe N, Nakada K, Kobayashi Y. Processing and release of tumor necrosis factor α. Eur J Biochem. 1998;253:576–582. doi: 10.1046/j.1432-1327.1998.2530576.x. [DOI] [PubMed] [Google Scholar]