Abstract
Kinetic analysis of two mutations within Pseudomonas aeruginosa exoenzyme S (ExoS) showed that a E379D mutation inhibited expression of ADP-ribosyltransferase activity but had little effect on the expression of NAD glycohydrolase activity while a E381D mutation inhibited expression of both activities. These data identify ExoS as a biglutamic acid ADP-ribosyltransferase, where E381 is the catalytic residue and E379 contributes to the transfer of ADP-ribose to the target protein.
Pseudomonas aeruginosa is a gram-negative opportunistic pathogen in patients with neutropenia, cystic fibrosis, and burn wounds (1, 15, 19, 21). The prevalence of multidrug-resistant strains complicates the control of P. aeruginosa (3), which has prompted studies to define the molecular basis for its pathogenesis. P. aeruginosa possesses an array of virulence factors, which makes it a successful opportunistic pathogen (6), including the ADP-ribosyltransferases, exotoxin A, and exoenzyme S.
Exoenzyme S was identified by Iglewski and coworkers as an ADP-ribosyltransferase of P. aeruginosa (8). Cloning the two forms of exoenzyme S showed that the 53-kDa form of exoenzyme S (now termed exoenzyme T [ExoT]) and the 49-kDa form of exoenzyme S (now termed exoenzyme S [ExoS]) were encoded by separate genes that were located on the P. aeruginosa chromosome (10, 22). While alignment of their primary amino acid sequences showed that ExoS and ExoT possess 76% homology (22), the specific activity of ExoT in catalyzing the ADP-ribosyltransferase reaction is only 0.2% of that of ExoS (14, 22). Kinetic analysis of the catalytic domains of ExoS and ExoT showed that the primary defect of ExoT was a lower Vmax relative to that of ExoS (14).
Recent studies have shown ExoS to be a bifunctional toxin. The amino-terminal 216 amino acids of ExoS, which possesses limited homology to YopE of Yersinia and SptP of Salmonella (4, 5, 22), catalyzes rho-dependent actin depolymerization (17), while the carboxyl-terminal 222 amino acids comprises the ADP-ribosyltransferase domain (designated ΔN222) (9). Recent studies have shown that Glu381 is a candidate active-site glutamic acid of ExoS (13). The goal of this study was to determine whether ExoS was a mono- or biglutamic acid ADP-ribosyltransferase by measuring the kinetic properties of the mutant ΔN222-E379D.
Two site-directed mutations were introduced into the nucleotide sequence coding for ΔN222, 5′-GAATCTCTTTATCATTCTTGT-3′ (resulting in mutant ΔN222-E379D) and 5′-TATAGAGAATATCTTTATCAT-3′ (resulting in mutant ΔN222-E381D), by using the Sculptor system (Amersham) (the nucleotide in bold represents base change introduced). Residues within the ΔN222 sequence were designated with respect to the amino acid sequence of ExoS (10). Mutated DNA was sequenced to confirm the presence of the desired mutation and that secondary mutations had not been introduced and then subcloned into pET15b (Novagen). Recombinant proteins were expressed in Escherichia coli BL21 (DE3) and purified to 75 to 80% homogeneity, as His-tagged fusion proteins as previously described (22). These proteins contain six-histidine amino acids at the amino terminus of ΔN222, but the presence of the His tag does not influence expression of enzymatic activities (11). ADP-ribosyltransferase and NAD glycohydrolase activities were measured as previously described (22). Intracellular expression of various forms of ΔN222 in CHO cells was performed as previously described (16).
The ADP-ribosylation of soybean trypsin inhibitor (SBTI) by ΔN222-E379D and ΔN222-E381D was FAS dependent. The specific activity of ΔN222-E379D in the ADP-ribosyltransferase reaction was 700-fold slower than that of the wild type, ΔN222, while the specific activity of ΔN222-E381D was 300-fold slower than that of the wild type (Table 1). Kinetic analysis (average of three independent experiments) showed that, at variable NAD, ΔN222-E379D possessed a Km(app) for NAD of 169 ± 39 μM with a kcat of 1.49 ± 0.17 mol/min/mol, while, at variable SBTI, the Km(app) for SBTI was 371 ± 90 μM, with a kcat of 3.91 ± 1.14 mol/min/mol. Relative to those of the wild type, ΔN222, the Km(app)s of ΔN222-E379D for NAD and SBTI had increased 3- and 10-fold, respectively, while the kcat/Km(app) ratio had decreased about 300-fold. The determined kinetic defect of the E379 mutation was similar to that which we had previously determined for the E381 mutation (13), which indicated that both glutamic acids were required for efficient catalytic expression of ADP-ribosyltransferase activity.
TABLE 1.
Catalytic activity of ΔN222, ΔN222-E379D, and ΔN222-E381Da
| Reaction | Enzyme | Sp actb | Relative activity (%)c |
|---|---|---|---|
| ADP-ribosyltransferase | |||
| ΔN222 | 85.5 ± 21.2 | 100 | |
| ΔN222-E379D | 0.11 ± 0.01 | 0.13 | |
| ΔN222-E381D | 0.30 ± 0.02 | 0.35 | |
| NAD glycohydrolase | |||
| ΔN222 | 2.68 ± 0.35 | 100 | |
| ΔN222-E379D | 0.71 ± 0.12 | 26.5 | |
| ΔN222-E381D | 0.001 ± 0.0004 | 0.05 |
Enzyme assays were performed as described in Materials and Methods.
Specific activity is expressed as moles per minute per mole of enzyme (mean ± standard deviation).
Activities are expressed relative to that of ΔN222, which was normalized to 100%. Data are presented as the average of experiments performed in triplicate. Statistical analysis was performed by using Sigmaplot 4.01.
To enhance the resolution of the role of E379 in catalysis, an analysis of the role of E379 in the NAD glycohydrolase reaction was determined. ΔN222-E379D catalyzed the NAD glycohydrolase reaction at 0.71 mol of nicotinamide released/min/mol, which was only fourfold slower than that of ΔN222 (Table 1). In contrast, ΔN222-E381D catalyzed the NAD glycohydrolase reaction at a specific activity of 0.001 mol of nicotinamide released/min/mol of ΔN222-E381D, which was about 2,000-fold slower than that of ΔN222.
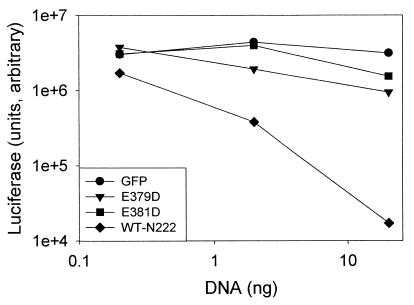
CHO cells were cotransfected with plasmids encoding either ΔN222, ΔN222-E381D, or ΔN222-E379D and reporter plasmid pEGFPN1 (Clonetech). Intracellular expression of ΔN222 caused a dose-dependent decrease in enhanced green fluorescent protein (EGFP) expression (data not shown), as previously observed (16). In contrast, expression of ΔN222-E381D did not modify EGFP expression, while expression of ΔN222-E379D caused only a slight reduction in EGFP expression. This indicated that neither ΔN222-E379D nor ΔN222-E381D had a substantial effect on reporter protein expression in CHO cells. Quantitation of a second reporter protein, luciferase, showed that while transfection with pΔN222 inhibited luciferase expression in a dose-dependent process, transfection with pΔN222-E379D or pΔN222-E381D did not have a substantial effect on luciferase expression in CHO cells (Fig. 1). In addition, while transfection with 300 ng of ΔN222 was cytotoxic to CHO cells, which was measured as the uptake of trypan blue, CHO cells transfected with 300 ng of either ΔN222-E379D or ΔN222-E381D excluded trypan blue (data not shown). This indicated that intracellular expression of neither ΔN222-E379D nor ΔN222-E381D was cytotoxic to CHO cells and that intracellular expression of ADP-ribosyltransferase, but not NAD glycohydrolase activity, correlated with the cytotoxic phenotype of ΔN222 (16).
FIG. 1.
Reporter protein expression in CHO cells cotransfected with the ADP-ribosyltransferase domain of ExoS (ΔN222) and noncatalytic mutants. CHO cells were cotransfected with 20.0 ng, 2.0 ng, or 0.2 ng of effector DNA and 180 ng of reporter plasmid pCMV10luc. Total DNA was normalized with the addition of pEGFPN1. Effector DNA expressed the following proteins: ΔN222 (WT-N222), ΔN222-E379D (E379D), ΔN222-E381D (E381D), or EGFP (GFP). Twenty four hours posttransfection, the cells were lysed and luciferase expression was assayed as previously described (16). The data presented are the average of duplicate assays. The experiment was repeated twice, and a representative experiment is shown.
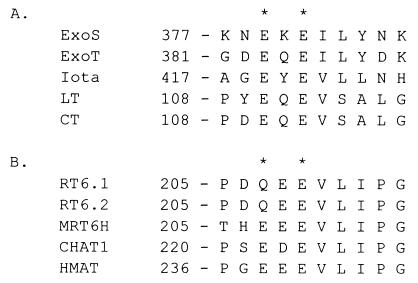
Previous studies in our laboratory identified Glu381 as a potential active-site glutamic acid residue of ExoS (13), which allowed the sequence alignment of ExoS with other ADP-ribosyltransferases. The primary amino acid sequence adjacent to the active-site glutamic acid is conserved among ExoS and cholera toxin, heat-labile enterotoxin (LT) iota toxin of Clostridium perfringens, and several eukaryotic ADP-ribosyltransferases (Fig. 2), which are biglutamic acids, since mutagenesis studies have shown that both glutamic acids are required for expression of wild-type levels of enzymatic activity (2, 7, 12, 18, 20). These ADP-ribosyltransferases have been termed biglutamic acid transferases.
FIG. 2.
Alignment of the active-site glutamic acids of several prokaryotic and eukaryotic ADP-ribosyltransferases. (A) The prokaryotic enzymes P. aeruginosa ExoS (ExoS; accession no. L27629), P. aeruginosa ExoT (ExoT; accession no. L46800), iota toxin of C. perfringens (Iota; accession no. AF037328), LT from E. coli (accession no. P43530), and cholera toxin (CT; accession no. P01555) are aligned. (B) The eukaryotic enzymes rat T-cell antigen (RT6.1 and RT6.2) (accession no. P17982 and P20974), mouse homologue of RT6 (MRT6H; accession no. P17981), chicken bone marrow cell ADP-ribosyltransferase 1 (CHAT1; accession no. D31864), and human skeletal muscle ADP-ribosyltransferase (HMAT; accession no. P52961) are aligned. Asterisks indicate active-site glutamic acids.
This study shows that ExoS is also a biglutamic acid transferase, since both Glu379 and Glu381 are required for efficient expression of ADP-ribosyltransferase activity. A catalytic role for two glutamic acids has been reported for other biglutamic acid ADP-ribosyltransferases, such as LT, rodent T-cell antigen, and iota toxin (7, 12, 18). As observed for ExoS, it was noted that the determined sequence of the eukaryotic RT6 antigen included a Gln at position 207 and the antigen expressed only NAD glycohydrolase activity, while mutagenesis to a Glu at position 207 yielded a protein that now expressed ADP-ribosyltransferase activity (7). Studies with LT suggested that Glu112 contributed more to catalysis than Glu110 (2), since substitutions at Glu112 resulted in a reduction in kcat of over 100-fold while the kcat of the Glu110 mutant was reduced 40-fold. In the case of ExoS, both Glu379 and Glu381 appear to play a role in catalysis, since the aspartic acid mutation at either residue reduced activity by >100-fold and the principal defect appears to be in enzyme turnover, not substrate or target protein binding affinity. In addition, the E379D mutant possessed considerable NAD glycohydrolase activity, while the E381D mutant was deficient in catalyzing the NAD glycohydrolase reaction. Thus, it appears that the E379D and E381D mutations play unique roles in the catalytic process, where Glu381 contributes to an early step in the catalytic reaction mechanism involving NAD hydrolysis while Glu379 contributes to a later step in the transferase reaction, possible the transfer of ADP-ribose to arginine within the target protein. The lower NAD glycohydrolase activity catalyzed by ΔN222-E379D relative to that of the wild type, ΔN222, is probably due to its threefold-higher Km for NAD observed in the kinetic analysis of ADP-ribosyltransferase activity. Identification of both E379 and E381 as components of the ADP-ribosyltransferase reaction should allow the engineering of a double mutant of ExoS which is essentially void of ADP-ribosyltransferase activity, which will assist in the in vivo characterization of the modulation of host cell physiology by ExoS.
Acknowledgments
The study was supported by the National Institutes of Health grants F37-AI10017 to K.J.P. and AI-30162 to J.T.B.
We acknowledge Suyan Liu, who engineered the ΔN222-E381D mutant, and Gradin Gonsalvez, who assisted in the construction of the eukaryotic expression vectors for ΔN222-E379D and ΔN222-E381D.
REFERENCES
- 1.Bodey G P, Bolivar R, Fainstein V, Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983;5:279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- 2.Cieplak W, Jr, Mead D J, Messer R J, Grant C C. Site-directed mutagenic alteration of potential active-site residues of the A subunit of Escherichia coli heat-labile enterotoxin. Evidence for a catalytic role for glutamic acid 112. J Biol Chem. 1995;270:30545–30550. doi: 10.1074/jbc.270.51.30545. [DOI] [PubMed] [Google Scholar]
- 3.Finland M. Changing patterns of susceptibility of common bacterial pathogens to antimicrobial agents. Ann Intern Med. 1972;76:1009–1036. doi: 10.7326/0003-4819-76-6-1009. [DOI] [PubMed] [Google Scholar]
- 4.Frithz-Lindsten E, Du Y, Rosqvist R, Forsberg A. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol Microbiol. 1997;25:1125–1139. doi: 10.1046/j.1365-2958.1997.5411905.x. [DOI] [PubMed] [Google Scholar]
- 5.Fu Y, Galan J E. The Salmonella typhimurium tyrosine phosphatase SptP is translocated into host cells and disrupts the actin cytoskeleton. Mol Microbiol. 1998;27:359–368. doi: 10.1046/j.1365-2958.1998.00684.x. [DOI] [PubMed] [Google Scholar]
- 6.Goranson J, Frank D W. Genetic analysis of exoenzyme S expression by Pseudomonas aeruginosa. FEMS Microbiol Lett. 1996;135:149–255. doi: 10.1111/j.1574-6968.1996.tb07981.x. [DOI] [PubMed] [Google Scholar]
- 7.Hara N, Tsuchiya M, Shimoyama M. Glutamic acid 207 in rodent T-cell RT6 antigens is essential for arginine-specific ADP-ribosylation. J Biol Chem. 1996;271:29552–29555. doi: 10.1074/jbc.271.47.29552. [DOI] [PubMed] [Google Scholar]
- 8.Iglewski B H, Sadoff J, Bjorn M J, Maxwell E S. Pseudomonas aeruginosa exoenzyme S: an adenosine diphosphate ribosyltransferase distinct from toxin A. Proc Natl Acad Sci USA. 1978;75:3211–3215. doi: 10.1073/pnas.75.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight D A, Finck-Barbancon V, Kulich S M, Barbieri J T. Functional domains of Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1995;63:3182–3186. doi: 10.1128/iai.63.8.3182-3186.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulich S M, Yahr T L, Mende-Mueller L M, Barbieri J T, Frank D W. Cloning the structural gene for the 49-kDa form of exoenzyme S (exoS) from Pseudomonas aeruginosa strain 388. J Biol Chem. 1994;269:10431–10437. [PubMed] [Google Scholar]
- 11.Kulich S M, Frank D W, Barbieri J T. Expression of recombinant exoenzyme S of Pseudomonas aeruginosa. Infect Immun. 1995;63:1–8. doi: 10.1128/iai.63.1.1-8.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lobet Y, Cluff C W, Cieplak W., Jr Effect of site-directed mutagenic alterations on ADP-ribosyltransferase activity of the A subunit of Escherichia coli heat-labile enterotoxin. Infect Immun. 1991;59:2870–2879. doi: 10.1128/iai.59.9.2870-2879.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Kulich S M, Barbieri J T. Identification of glutamic acid 381 as a candidate active site residue of exoenzyme S. Biochemistry. 1996;35:2754–2758. doi: 10.1021/bi952340g. [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Yahr T, Frank D W, Barbieri J T. Biochemical relationships between the 53-kilodalton (Exo53) and 49-kilodalton (ExoS) forms of exoenzyme. J Bacteriol. 1997;179:1609–1613. doi: 10.1128/jb.179.5.1609-1613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendelson M, Gurtman A, Szabo S, Neibart E, Meyers B, Policar M, Cheung T, Lillienfeld D, Hammer G, Reddy S, et al. Pseudomonas aeruginosa bacteremia in patients with AIDS. Clin Infect Dis. 1994;18:886–895. doi: 10.1093/clinids/18.6.886. [DOI] [PubMed] [Google Scholar]
- 16.Pederson K J, Barbieri J T. Intracellular expression of the ADP-ribosyltransferase domain of Pseudomonas aeruginosa is cytotoxic to eukaryotic cells. Mol Microbiol. 1998;30:751–760. doi: 10.1046/j.1365-2958.1998.01106.x. [DOI] [PubMed] [Google Scholar]
- 17.Pederson, K. J., A. Vallis, K. Aktories, D. W. Frank, and J. T. Barbieri. The amino terminal domain of Pseudomonas aeruginosa ExoS disrupts actin filaments via small-molecular-weight GTP binding proteins. Submitted for publication. [DOI] [PubMed]
- 18.Perelle S, Domenighini M, Popoff M R. Evidence that Arg-295, Glu-378, and Glu-380 are active-site residues of the ADP-ribosyltransferase activity of iota toxin. FEBS Lett. 1996;395:191–194. doi: 10.1016/0014-5793(96)01035-6. [DOI] [PubMed] [Google Scholar]
- 19.Roilides E, Butler K N, Husson R N, Mueller B V, Lewis L L, Pizzo P A. Pseudomonas infections in children with human immunodeficiency virus infection. Pediatr Infect Dis J. 1992;11:547–553. doi: 10.1097/00006454-199207000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Takada T, Iida K, Moss J. Conservation of a common motif in enzymes catalyzing ADP-ribose transfer. Identification of domains in mammalian transferases. J Biol Chem. 1995;270:541–544. doi: 10.1074/jbc.270.2.541. [DOI] [PubMed] [Google Scholar]
- 21.Tummler B, Bosshammer J, Breitenstein S, Brockhausen I, Gudowius P, Herrmann C, Herrmann S, Heuer T, Kubesch P, Mekus F, Romling U, Schmidt K, Spangenberg C, Walter S. Infections with Pseudomonas aeruginosa in patients with cystic fibrosis. Behring Inst Mitt. 1997;98:249–355. [PubMed] [Google Scholar]
- 22.Yahr T L, Barbieri J T, Frank D W. Genetic relationship between the 53- and 49-kilodalton forms of exoenzyme S from Pseudomonas aeruginosa. J Bacteriol. 1996;178:1412–1419. doi: 10.1128/jb.178.5.1412-1419.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]