Abstract
Purpose of the Review:
Sarcopenia is the loss of muscle quantity and strength. It is highly prevalent in patients with inflammatory bowel disease (IBD) and is associated with periods of ongoing inflammation. This review will summarize the prior work in the field and highlight areas for future research.
Recent Findings:
The presence of sarcopenia has been associated with adverse outcomes in different populations. Most recently, sarcopenia has been associated with adverse postoperative outcomes and an increased likelihood of surgery in IBD. Despite this, significant heterogeneity among these studies limits the ability to draw definitive conclusions.
Summary:
The importance of sarcopenia in inflammatory bowel disease (IBD) is only beginning to be recognized. Future studies assessing it utility both as a risk stratification tool and a modifiable factor in IBD are needed.
Keywords: aging, Crohn’s disease, ulcerative colitis, geriatrics, inflammation, muscle loss, outcomes
Introduction
Sarcopenia is a term that was first coined in 1988 to describe age-associated muscle loss. The word stems from the Greek sarx, meaning flesh, and penia, meaning loss.1 Sarcopenia is defined by a progressive and widespread loss of skeletal muscle mass, strength, and function, with skeletal muscle comprising 40-50% of human body weight.2 While sarcopenia was first described as an age-associated process, it is increasingly recognized to be a sequela of conditions associated with weight loss and cachexia. A modern definition of sarcopenia describes it as a “syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with a risk of adverse outcomes such as physical disability, poor quality of life and death.”3
The pathophysiology of sarcopenia is complex. Sarcopenia is a multifactorial process with contributions from apoptosis, mitochondrial dysfunction, endogenous and/or exogenous hormonal effects, motor neuron loss, immobility, age-related muscle dysfunction, malnutrition and cachexia. The development of sarcopenia implicates changes in stellate cell recruitment, protein oxidation, inflammation, and changes in anabolic signaling.3
Although often overlapping, sarcopenia is a distinct entity from frailty and cachexia, as it can be present in their absence. In recognition of the growing number of older adults seen in the clinical practice of IBD, aging-related syndromes are being studied as they pertain to these patients. The applications of frailty in inflammatory bowel diseases (IBD) have been described, but data assessing the role of sarcopenia in IBD are much more limited.4-7 In this review, we aim to provide a broad overview of the sparse, but existent literature regarding the role of sarcopenia in IBD. We conclude by delineating future directions to advance the study of sarcopenia in IBD and highlight avenues for intervention.
Sarcopenia and Aging
As evidenced by the origins of the term itself, sarcopenia is most often associated with aging. In fact, the progressive loss of muscle mass and strength is often considered a hallmark of aging. Additionally, numerous studies of sarcopenia in older adults found that it is associated with a number of adverse outcomes including disability, frailty, hospitalization and even mortality.3, 8 Despite a number of interventions to attenuate and even reverse muscle mass in older adults, none are proven to fully reverse sarcopenia.9 The etiopathogenesis of sarcopenia in aging has been linked to chronic, low-grade, systemic inflammation characterized by the presence of pro-inflammatory cytokines, which may contribute to the development of sarcopenia.10 9
Sarcopenia and Inflammation
Animal models suggest that inflammation is broadly associated with a dampened anabolic response and increased catabolism of muscle. We see evidence of this in conditions such as heart failure, cancer, chronic obstructive pulmonary disease (COPD) and AIDS, which all ultimately lead to skeletal muscle wasting.11, 12,13 A study of 441 adults ≥60 years of age demonstrated that sarcopenic older adults had significantly higher levels of circulating interleukin (IL)-6 and tumor necrosis factor (TNF)-α, coupling inflammation with aging.14 Analogously, one study demonstrated that anti-inflammatory treatment was associated with a decrease in serum IL-6 levels as well as an improvement in muscle performance and mobility in hospitalized older adults.15 While direct causality between inflammation and sarcopenia has yet to be established, there is evidence of an association within the context of IBD.9
Sarcopenia and IBD
IBD is a chronic inflammatory condition of the gastrointestinal system comprised of two main subtypes: Crohn’s disease (CD) and ulcerative colitis (UC).16 Patients with IBD often have periods of ongoing inflammation and are therefore at risk for weight loss, malnutrition, and the prolonged use of glucocorticoids, which all impact muscle strength and mass. IBD, like other chronic inflammatory disease states, disrupts the growth hormone (GH)/insulin-like growth factor (IGF)-1 axis, which plays a crucial role in regulating linear skeletal and muscle growth in children, which is maintained throughout adulthood. In a study of 344 individuals with IBD, 41% of patients met inclusion criteria for sarcopenia or probable sarcopenia, even while in remission.17,18
The study of sarcopenia in patients with IBD remains limited to largely retrospective reports of small groups of heterogeneous patients with varying definitions of sarcopenia (Table 1). One study of 101 pediatric IBD patients with sarcopenia, as defined by being in the lowest quartile for area of psoas muscle divided by total body surface area, had an increased risk for disease flares and need for biologics as compared to patients in the highest quartile.18 In a Korean cohort of patients with CD, patients with muscle loss as defined by cross-sectional imaging, were found to have elevated inflammatory markers; ie: C-reactive protein (CRP). In this cohort however, sarcopenia was not associated with need for hospitalization, surgery, use of corticosteroids or escalation of immunosuppression.19 This is in contrast to other studies which have shown sarcopenia to be an independent predictor of major adverse events. For example, in a study of 89 patients hospitalized for the management of acute severe ulcerative colitis, sarcopenia was significantly associated with the need for medical and/or surgical rescue therapy.20
Table 1:
Diagnostic criteria to define sarcopenia used in studies of patients with inflammatory bowel diseases (IBD)
| Modality of Diagnosis |
Criteria | Study | Study Population |
|---|---|---|---|
| Functional | Continuous measures of fixed-velocity resistive movement at both knees | Subramaniam et al AP&T 2015 | CD Mean age: 33±11 |
| Computed Tomography (CT) | Skeletal muscle mass <52.4cm2/m2 for males <38.5cm2/m2 for females | Adams et al Inflamm Bowel Dis, 2017 | All IBD Median age: 35 (IQR: 26-50) |
| CT | Skeletal Muscle Index (SMI: skeletal muscle area/height2) at 3rd lumbar vertebra <42cm2/m2 for males <38cm2/m2 for females | Bamba et al, PLoS One, 2017 | All IBD CD median age: 29 (IQR: 25-37) UC median age: 39 (IQR: 28-55) |
| CT | Total Psoas muscles Area (TPA) at 4th lumbar vertebra <567.4 mm2/m2 for males <355.8 mm2/m2 for females | Fujikawa et al, Surg Today, 2017 | UC Mean age: 40±14 |
| CT | Total Psoas Index (TPI) or mean Hounsfield Unit Average Calculations (HUAC) at 3rd lumbar vertebra TPI <5.2cm2/m2 / HUAC <18.8 for males TPI <4cm2/m2 / HUAC <20.3 for females | Pedersen et al, Inflamm Bowel Dis, 2017 | All IBD & non-IBD controls Mean age: 43 |
| CT | SMI at 3rd lumbar vertebra <55cm2/m2 for males <39cm2/m2 for females | Zhang et al, J Parenter Enteral Nutr, 2017 | CD Mean age: 32±11 |
| CT | SMI at 3rd lumbar vertebra <55cm2/m2 for males <39cm2/m2 for females | Zhang et al, Clin Nutr, 2017 | All IBD & non-IBD controls CD: 33±11 UC: 40±14 |
| CT | SMI at 3rd lumbar vertebra <55cm2/m2 for males <39cm2/m2 for females | Cushing et al, J Crohns Colitis, 2018 | UC Mean age: 43 (range 9-86) |
| CT | SMI at 3rd lumbar vertebra If BMI <25 kg/m2: <43cm2/m2 for males <41cm2/m2 for females If BMI ≥25 kg/m2: <53cm2/m2 for males | O’Brien et al, Eur Radiol Exp, 2018 | All IBD Mean age: 42 (range 20-80) |
| CT | SMI at 3rd lumbar vertebra <52.4 cm2/m2 for males <38.5 cm2/m2 for females | Carvalho et al, Gastrointest Disord, 2019 | CD Mean age: 33 (range 11-80) |
| CT | SMI at 3rd lumbar vertebra <49cm2/m2 for males <31cm2/m2 for women | Lee et al, Intest Res, 2020 | CD Mean age: 30±11 |
| Magnetic Resonance Imaging (MRI) | Psoas Area Index (PAI) <4th quartile | Atlan et al, J Pediatr Gastroenterol Nutr, 2021 | All IBD & non-IBD controls Median age: 15 (IQR: 13-17) |
| CT or MRI | PMTH (Psoas Muscle Thickness normalized to Height) at the umbilicus <17.8 mm/m in males <14.8 mm/m in females | Alipour et al, Scand J Gastroenterol, 2021 | All IBD Mean age: 43±15 |
| CT | SMI at 3rd lumbar vertebra <42cm2/m2 for males <38cm2/m2 for females | Bamba et al, Inflamm Bowel Dis, 2021 | All IBD CD median age 31 (IQR: 25-41) UC median age 36 (IQR: 25-49) |
| CT | SMI at 3rd lumbar vertebra <42.44 cm2/m2 for males <33.48 cm2/m2 for females | Ge et al, Eur J Clin Nutr, 2021 | UC Mean age: 44±1 |
| Functional & Physical | Functional: Hand grip <32kg for males Hand grip <22 kg for females Gait speed ≤ 0.8 m/s Physical: Calf circumference <33cm Fat Free Mass Index (FFMI) <17kg/m2 for males <15kg/m2 for females Skeletal Muscle Mass Index (SMMI) <9.2kg/m2 for males <7.4kg/m2 for females | Unal et al, Eur J Gastroenterol Hepatol, 2021 | All IBD Mean age: 49 (range: 22-87) |
Age is in years CD: Crohn’s disease UC: ulcerative colitis IQR: Inter-quartile range
At the time of this review, a prospective multi-modal assessment of sarcopenia is underway in patients with chronic inflammatory disorders (liver disease, IBD and rheumatoid arthritis), and is focused on exploring the mechanisms driving sarcopenia development. To date, no results have been published.21
Sarcopenia and IBD Surgery
Sarcopenia is a predictor of adverse outcomes in patients undergoing abdominopelvic surgery, but there is limited data assessing this in IBD.22 A retrospective study of 178 patients with IBD who had sarcopenia assessed on preoperative imaging, concluded that it conferred a significantly higher risk for blood transfusions, longer length of stay, ICU admission, deep vein thromboses (DVT) and postoperative infections.23 This has been supported by additional studies with an increased risk of postoperative morbidity and readmission after intestinal resection in sarcopenic patients with both CD and UC.24-27 However, these findings have not been duplicated in all studies. Eighty five IBD patients with sarcopenia defined by psoas muscle index, were not found to have an increased risk of postoperative complications.28 A systematic review as well as a separate meta-analysis of 885 patients with IBD found sarcopenia to be independently predictive of surgical outcomes when adjusting for relevant clinical variables.29, 30 Although data suggest an association between operative risk and sarcopenia in IBD, conclusions are difficult given the retrospective nature, varied methodology, and omission of measures of muscle strength in these reports.
Sarcopenia has also been investigated as a marker for the need of intestinal surgery in IBD. In a study of 72 patients with IBD, sarcopenia was significantly associated with intestinal resection.31 Similarly, in a study of patients with acute severe ulcerative colitis, patients who were sarcopenic had an increased risk for colectomy.32 This finding, however, has not been consistently replicated, but may be due to the lack of power in these smaller studies (n=58).24
Sarcopenic Obesity
A review of sarcopenia, in IBD or otherwise, is not complete without a discussion of sarcopenic obesity, defined as a relative reduction of muscle mass or strength due to an increase in fat mass.33 As weight increases in healthy adults, mechanoreceptors in bone and muscle respond by producing growth factors, balancing this increase in weight with an increase in bone and muscle mass. As dietary intake, absorption, and metabolism change, this homeostasis can be disrupted and lead to a disproportionate increase in fat and the development of sarcopenic obesity.34, 35 As a result, sarcopenic obesity is present in IBD as well. In a retrospective study of 90 patients with IBD, 20% of patients who were sarcopenic also had a body mass index indicating obesity.36 Thus, BMI is often not reflective of an individual’s muscle mass, strength or fitness state, and has limited utility as a stand-alone prediction tool.37
Interventions to Ameliorate Sarcopenia in Patients with IBD
Improvement in sarcopenia among older adults may result from nutrition and strength training.38-40 Although data are limited in the setting of immune-mediated diseases, there is a mounting body of evidence that sarcopenia is modifiable and that targeted interventions can be beneficial.41, 42 In one study, patients with UC were noted to have improvement in muscle mass after colectomy.43 In the only prospective study assessing this, 19 patients with Crohn’s disease who were treated with infliximab (an anti-tumor necrosis factor [TNF]-α agent) noted a gain of muscle volume and strength as a result of treatment (diet and exercise levels were held constant). This indicates that sarcopenia is at least in part influenced by inflammation and may be modifiable.44
Defining and Diagnosing Sarcopenia
A significant barrier to advancing the study of sarcopenia is the lack of established definition and diagnostic criteria.29 As defined by the European Working Group on Sarcopenia in Older People and by the Foundation for the National Institutes of Health Sarcopenia Project, sarcopenia is defined as a loss of both muscle mass and strength.45 However, in current studies in IBD, sarcopenia has been assessed only by muscle quantity, which limits their clinical utility.46 Furthermore, these studies have used varying measures, cutoff values, and timelines to assess muscle mass on cross-sectional imaging (Table 1).47
Future Directions
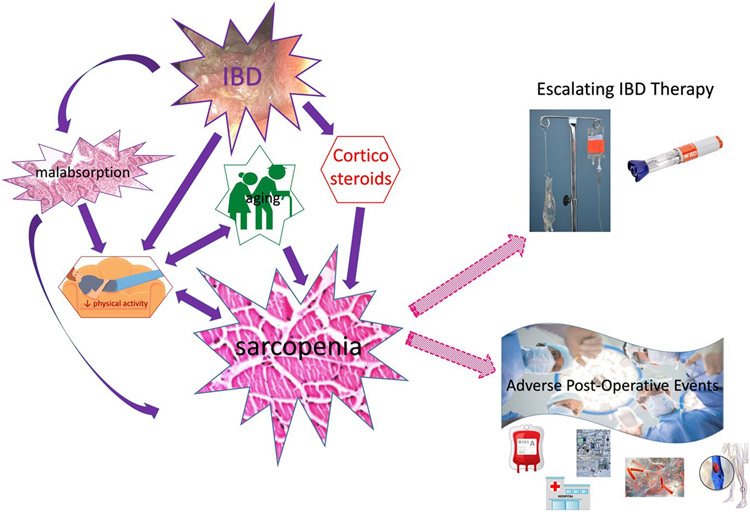
As patients with IBD age, sarcopenia will become increasingly prevalent.48, 49 Therefore, understanding the complex interplay between inflammation, aging, and the factors that influence sarcopenia will be critical to advancing the care of our patients with IBD. Figure 1 summarizes current knowledge about the relationship between IBD, sarcopenia, and adverse outcomes, whereas Table 2 summarizes knowledge gaps and research priorities that address the gaps to advance the study of sarcopenia in patients with IBD.
Figure 1: Conceptual model for the relationships between inflammatory bowel disease (IBD) and sarcopenia with a summary of reported adverse events associated with sarcopenia in patients with IBD.
Retrospective studies until 2021 demonstrate that sarcopenia in patients with IBD are associated with a greater need for immunosuppressive therapy, including infliximab or cyclosporine rescue for patients hospitalized with acute severe ulcerative colitis, as well as a number of adverse post-operative events such as increased hospital length of stay, re-admission, ICU stays, deep vein thrombosis, infections and blood transfusions.
Table 2:
Knowledge gaps and research priorities for the study of sarcopenia in inflammatory bowel diseases (IBD)
| Knowledge Gap | Research Priorities |
|---|---|
| Lack of standardized and tailored measures of sarcopenia in patients with IBD | Determine the cross-sectional imaging assessment of muscle quantity, as well as IBD-specific cutoff values |
| Include prospective measures of muscle strength as part of operational definition of sarcopenia | |
| Describing trajectories of sarcopenia in patients with IBD | Characterize how modifiable risk factors for sarcopenia in patients with IBD |
| Determine time course of reversibility of sarcopenia in patients with IBD | |
| Determining associations with sarcopenia in patients with IBD | Characterize how sarcopenia is an independent predictor of:
|
Any study of sarcopenia will require a uniform definition and measure. The lack of a universally accepted definition tailored to patients with IBD likely explains the wide-ranging heterogeneity of results. Future studies should focus on evaluating the different cross-sectional measurements of muscle mass and incorporating functional measurements of muscle strength. Once optimal measures and values of sarcopenia have been established and accepted, future studies can prospectively validate these findings and assess the relationship between sarcopenia and its outcomes.
Existing studies suggest that sarcopenia may be a valuable and readily available risk stratification tool for patients with IBD. In the preoperative state, sarcopenia can be readily assessed through routine preoperative imaging and measures of muscle strength and can predict risk for adverse postoperative outcomes. This can aid current risk stratification tools which notably omit physiologic markers of functional status and target preoperative interventions focused on strength training and nutrition. Additionally, this can be studied and applied as a risk stratification tool for patients newly starting biologics, and is already being implemented in oncologic care.50, 51
Conclusion
The potential for reversing sarcopenia in patients with IBD is critical. Interventions need to be specifically assessed in a population of IBD patients both young and old. The contribution that inflammation and IBD-specific treatments have on the development and improvement of sarcopenia may enhance our ‘treat-to-target’ paradigm. This is an area in need of active investigation.
Funding:
R03AG074059 [BK]
Footnotes
Conflict of Interest Statement
All the authors have no relevant conflicts of interest to declare
ASF reports the following disclosures: consulting for GuidePoint, Janssen, LEK, M3 and Schlesinger
BK reports the following disclosures: advisory board for Pfizer, Inc
TK and SC have no disclosures
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References:
- 1.Rosenberg IH. Sarcopenia: Origins and Clinical Relevance. The Journal of Nutrition 1997;127:990S–991S. [DOI] [PubMed] [Google Scholar]
- 2.Marcell TJ. Review Article: Sarcopenia: Causes, Consequences, and Preventions. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 2003;58:M911–M916. [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kochar B, Jylhava J, Soderling J, et al. Prevalence and Implications of Frailty in Older Adults with Incident Inflammatory Bowel Diseases: a Nationwide Cohort Study. Clin Gastroenterol Hepatol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faye AS, Wen T, Soroush A, et al. Increasing Prevalence of Frailty and Its Association with Readmission and Mortality Among Hospitalized Patients with IBD. Digestive Diseases and Sciences 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faye AS, Colombel J-F. Aging and IBD: A New Challenge for Clinicians and Researchers. Inflammatory Bowel Diseases 2021. [DOI] [PubMed] [Google Scholar]
- 7.Kochar B, Orkaby AR, Ananthakrishnan AN, et al. Frailty in inflammatory bowel diseases: an emerging concept. Therapeutic Advances in Gastroenterology 2021;14:175628482110254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Visser M, Schaap LA. Consequences of sarcopenia. Clin Geriatr Med 2011;27:387–99. [DOI] [PubMed] [Google Scholar]
- 9.Dalle S, Rossmeislova L, Koppo K. The Role of Inflammation in Age-Related Sarcopenia. Front Physiol 2017;8:1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018;15:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balage M, Averous J, Remond D, et al. Presence of low-grade inflammation impaired postprandial stimulation of muscle protein synthesis in old rats. J Nutr Biochem 2010;21:325–31. [DOI] [PubMed] [Google Scholar]
- 12.Schakman O, Dehoux M, Bouchuari S, et al. Role of IGF-I and the TNFalpha/NF-kappaB pathway in the induction of muscle atrogenes by acute inflammation. Am J Physiol Endocrinol Metab 2012;303:E729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakuma K, Aoi W, Yamaguchi A. Current understanding of sarcopenia: possible candidates modulating muscle mass. Pflugers Arch 2015;467:213–29. [DOI] [PubMed] [Google Scholar]
- 14.Bian A-L, Hu H-Y, Rong Y-D, et al. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. European Journal of Medical Research 2017;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beyer I, Bautmans I, Njemini R, et al. Effects on muscle performance of NSAID treatment with Piroxicam versus placebo in geriatric patients with acute infection-induced inflammation. a double blind randomized controlled trial. BMC Musculoskeletal Disorders 2011;12:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abraham C, Cho JH. Inflammatory Bowel Disease. New England Journal of Medicine 2009;361:2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unal NG, Oruc N, Tomey O, et al. Malnutrition and sarcopenia are prevalent among inflammatory bowel disease patients with clinical remission. Eur J Gastroenterol Hepatol 2021. [DOI] [PubMed] [Google Scholar]
- 18.Atlan L, Cohen S, Shiran S, et al. Sarcopenia is a Predictor for Adverse Clinical Outcome in Pediatric Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr 2021;72:883–888. [DOI] [PubMed] [Google Scholar]
- 19.Lee CH, Yoon H, Oh DJ, et al. The prevalence of sarcopenia and its effect on prognosis in patients with Crohn’s disease. Intestinal Research 2020;18:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cushing KC, Kordbacheh H, Gee MS, et al. Sarcopenia is a Novel Predictor of the Need for Rescue Therapy in Hospitalized Ulcerative Colitis Patients. Journal of Crohn's and Colitis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhaliwal A, Williams FR, Quinlan JI, et al. Evaluation of the mechanisms of sarcopenia in chronic inflammatory disease: protocol for a prospective cohort study. Skeletal Muscle 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simonsen C, de Heer P, Bjerre ED, et al. Sarcopenia and Postoperative Complication Risk in Gastrointestinal Surgical Oncology: A Meta-analysis. Annals of Surgery 2018;268:58–69. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen M, Cromwell J, Nau P. Sarcopenia is a Predictor of Surgical Morbidity in Inflammatory Bowel Disease. Inflammatory Bowel Diseases 2017;23:1867–1872. [DOI] [PubMed] [Google Scholar]
- 24.Carvalho D, Viana C, Marques I, et al. Sarcopenia is associated with Postoperative Outcome in Patients with Crohn’s Disease Undergoing Bowel Resection. Gastrointestinal Disorders 2019;1:201–209. [Google Scholar]
- 25.Fujikawa H, Araki T, Okita Y, et al. Impact of sarcopenia on surgical site infection after restorative proctocolectomy for ulcerative colitis. Surgery Today 2017;47:92–98. [DOI] [PubMed] [Google Scholar]
- 26.Zhang T, Cao L, Cao T, et al. Prevalence of Sarcopenia and Its Impact on Postoperative Outcome in Patients With Crohn's Disease Undergoing Bowel Resection. Journal of Parenteral and Enteral Nutrition 2017;41:592–600. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien S, Kavanagh RG, Carey BW, et al. The impact of sarcopenia and myosteatosis on postoperative outcomes in patients with inflammatory bowel disease. European Radiology Experimental 2018;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alipour O, Lee V, Tejura TK, et al. The assessment of sarcopenia using psoas muscle thickness per height is not predictive of post-operative complications in IBD. Scand J Gastroenterol 2021;56:1175–1181. [DOI] [PubMed] [Google Scholar]
- 29.Ryan E, McNicholas D, Creavin B, et al. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflammatory Bowel Diseases 2019;25:67–73. [DOI] [PubMed] [Google Scholar]
- 30.Erős A, Soós A, Hegyi P, et al. Sarcopenia as an independent predictor of the surgical outcomes of patients with inflammatory bowel disease: a meta-analysis. Surgery Today 2020;50:1138–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bamba S, Sasaki M, Takaoka A, et al. Sarcopenia is a predictive factor for intestinal resection in admitted patients with Crohn’s disease. PLOS ONE 2017;12:e0180036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ge X, Xia J, Wu Y, et al. Sarcopenia assessed by computed tomography is associated with colectomy in patients with acute severe ulcerative colitis. European Journal of Clinical Nutrition 2021. [DOI] [PubMed] [Google Scholar]
- 33.Stenholm S, Harris TB, Rantanen T, et al. Sarcopenic obesity: definition, cause and consequences. Current Opinion in Clinical Nutrition and Metabolic Care 2008;11:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morley JE. The aging gut: physiology. Clin Geriatr Med 2007;23:757–67, v-vi. [DOI] [PubMed] [Google Scholar]
- 35.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci 2000;904:437–48. [DOI] [PubMed] [Google Scholar]
- 36.Adams DW, Gurwara S, Silver HJ, et al. Sarcopenia Is Common in Overweight Patients with Inflammatory Bowel Disease and May Predict Need for Surgery. Inflammatory Bowel Diseases 2017;23:1182–1186. [DOI] [PubMed] [Google Scholar]
- 37.Dogan SC, Hizmetli S, Hayta E, et al. Sarcopenia in women with rheumatoid arthritis. Eur J Rheumatol 2015;2:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beckwee D, Delaere A, Aelbrecht S, et al. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J Nutr Health Aging 2019;23:494–502. [DOI] [PubMed] [Google Scholar]
- 39.Beaudart C, Dawson A, Shaw SC, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporosis International 2017;28:1817–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cruz-Jentoft AJ, Romero-Yuste S, Chamizo Carmona E, et al. Sarcopenia, immune-mediated rheumatic diseases, and nutritional interventions. Aging Clinical and Experimental Research 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhaliwal A, Quinlan JI, Overthrow K, et al. Sarcopenia in Inflammatory Bowel Disease: A Narrative Overview. Nutrients 2021;13:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang T, Ding C, Xie T, et al. Skeletal muscle depletion correlates with disease activity in ulcerative colitis and is reversed after colectomy. Clinical Nutrition 2017;36:1586–1592. [DOI] [PubMed] [Google Scholar]
- 44.Subramaniam K, Fallon K, Ruut T, et al. Infliximab reverses inflammatory muscle wasting (sarcopenia) in Crohn's disease. Alimentary Pharmacology & Therapeutics 2015;41:419–428. [DOI] [PubMed] [Google Scholar]
- 45.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age and Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Studenski SA, Peters KW, Alley DE, et al. The FNIH Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates. The Journals of Gerontology: Series A 2014;69:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bamba S, Inatomi O, Takahashi K, et al. Assessment of Body Composition From CT Images at the Level of the Third Lumbar Vertebra in Inflammatory Bowel Disease. Inflamm Bowel Dis 2021;27:1435–1442. [DOI] [PubMed] [Google Scholar]
- 48.Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2021;18:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prado CMM, Baracos VE, McCargar LJ, et al. Sarcopenia as a Determinant of Chemotherapy Toxicity and Time to Tumor Progression in Metastatic Breast Cancer Patients Receiving Capecitabine Treatment. Clinical Cancer Research 2009;15:2920–2926. [DOI] [PubMed] [Google Scholar]
- 51.Ganju RG, Morse R, Hoover A, et al. The impact of sarcopenia on tolerance of radiation and outcome in patients with head and neck cancer receiving chemoradiation. Radiother Oncol 2019;137:117–124. [DOI] [PubMed] [Google Scholar]