Abstract
Psychiatric diseases exact a heavy socioeconomic toll, and it is particularly difficult to identify their risk factors and causative mechanisms due to their multifactorial nature, the limited physiopathological insight, the many confounding factors, and the potential reverse causality between the risk factors and psychiatric diseases. These characteristics make Mendelian randomization (MR) a precious tool for studying these disorders. MR is an analytical method that employs genetic variants linked to a certain risk factor, to assess if an observational association between that risk factor and a health outcome is compatible with a causal relationship. We report the first systematic review of all existing applications and findings of MR in psychiatric disorders, aiming at facilitating the identification of risk factors that may be common to different psychiatric diseases, and paving the way to transdiagnostic MR studies in psychiatry, which are currently lacking. We searched Web of Knowledge, Scopus, and Pubmed databases (until 3 May 2022) for articles on MR in psychiatry. The protocol was preregistered in PROSPERO (CRD42021285647). We included methodological details and results from 50 articles, mainly on schizophrenia, major depression, autism spectrum disorders, and bipolar disorder. While this review shows how MR can offer unique opportunities for unraveling causal links in risk factors and etiological elements of specific psychiatric diseases and transdiagnostically, some methodological flaws in the existing literature limit reliability of results and probably underlie their heterogeneity. We highlight perspectives and recommendations for future works on MR in psychiatry.
Keywords: ADHD, alcohol use disorder, autism spectrum disorder, bipolar disorder, depression, genetics, Mendelian randomization, psychiatry, schizophrenia, systematic review
Introduction
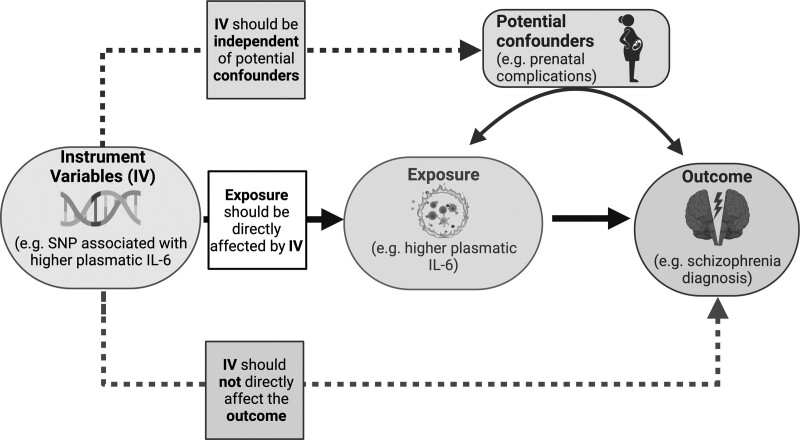
Mental disorders comprehend a wide variety of diseases with different presentations. They are generally characterized by a combination of abnormal thoughts, perceptions, emotions, behavior, and relationships with others. Mental disorders include major depression disorder (MDD), bipolar disorder (BD), schizophrenia (SCZ) and other psychoses, and developmental disorders including autism spectrum disorders (ASD) or attention-deficit and hyperactivity disorder (ADHD). It is estimated that 14.3% of deaths worldwide are caused by mental disorders (for a total of approximately 8 million), which lead to more than 125 million disability-adjusted life-years (Walker et al., 2015; GBD 2019 Mental Disorders Collaborators, 2019). Besides the heavy personal burden on patients and caregivers, psychiatric diseases also exact a heavy socioeconomic toll (Walker et al., 2015). It is particularly difficult to identify risk factors and causative mechanisms for psychiatric diseases due to the limited physiopathological and etiological insight we have, to the many confounding factors, and to the potential reverse causality between the risk factors and these diseases, which are likely multifactorial (i.e. with important genetic and environmental risk factors). This is one of the reasons that make Mendelian randomization (MR) an especially precious tool for studying these diseases. MR is an analytical method that employs genetic variants [or instrumental variables (IVs)] linked to a certain risk factor, to assess if an observational association between that risk factor and a health outcome is compatible with a causal relationship between the risk factor and the chosen outcome. More in detail, every person is inherently assigned a genetic variant that could influence a risk factor in different ways [e.g. a variant that regulates the blood level of LDL could influence the risk of coronary artery disease (CAD)]. In a Mendelian randomized study, one or more of these genetic variants are followed up to verify the development of a specific health outcome. As recapitulated in Fig. 1, MR relies on three main assumptions: a relevance assumption (that the variant is associated with the risk factor, or exposure, in study), an exclusion restriction assumption (that the variants are affecting the outcome only through the risk factor/exposure), and an independence assumption (that these variants have not a shared cause with the outcome) (Emdin et al., 2017).
Fig. 1.
Diagram of a typical Mendelian randomization design, with, as an example, the key assumptions of a possible association between higher plasmatic IL-6 and schizophrenia risk. IL-6, Interleukin 6; IV, instrumental variable; SNP, single-nucleotide polymorphism.
Thus, it is clear the importance of the concept of pleiotropy, which is the production by one single genetic trait [such as a single-nucleotide polymorphism (SNP)] of two or more apparently unrelated outcomes. If pleiotropy arises because an SNP influences one trait (the exposure), which then influences another (i.e. the outcome), then vertical pleiotropy exists and MR can be used to estimate the causal relationship between exposure and outcome. Prominent among the limitations of MR studies, is the nondemonstrable assumption that the exposure mediates the apparent pleiotropic associations (thus reflecting vertical pleiotropy), and that the selected SNP does not influence both the exposure and the outcomes through unrelated pathways (thus violating the exclusion restriction assumption due to ‘horizontal pleiotropy’) (Hemani et al., 2018). With these caveats, MR can use genetic associations obtained with genome-wide association studies (GWAS) to infer causality between exposures and outcomes.
Two main types of MR studies exist: the single sample randomization study and the double sample randomization study. The first one requires the measurement of the gene variant, the risk factor, and health outcome from the same sample of participants. The two-sample MR study requires two different study populations. In this approach, the gene variants and the health outcome are measured from one group, whereas the gene variants and risk factors are measured in another group (Pierce and Burgess, 2013). The main advantage of two-sample MR is that the outcome of interest and the risk factor do not need to be both measured in all participants, which is particularly important if they are expensive or difficult to measure. Thus, two-sample MR allows employing results from GWAS, which are usually precise and large studies (Davies et al., 2018).
To recapitulate, MR is particularly relevant to study risk factors for diseases in which (a) it is difficult to ascertain causality between the risk factor and the disease, (b) reverse causality is possible, and (c) confounding factors are abundant and possibly hidden. Thus, thanks to its potentialities, MR is being used in different studies to evaluate the relationship between possible risk factors and the development of psychiatric diseases. As an example, MR may be employed to assess causality between BD risk and lifetime cannabis use (having ever used cannabis). In fact, if an association between cannabis use and BD were to be found with other study designs, it would be difficult to exclude that it is BD that leads to higher cannabis use, and not vice versa. Similarly, reverse causality is a substantial problem in SCZ psychoneuroimmunology, since associated psychological morbidities can lead to issues with personal hygiene or housing insecurity, which, together with medication side effects, can impact the immune system. Since MR uses genetic information (which generally existed before full-blown psychiatric symptoms) as risk factors, reverse causality is unlikely.
Important insights into MR limitations and strengths can emerge from literature reviews, as well as from the collaboration between clinicians, methodologists, and empirical researchers, from which have benefited also other areas of medical research (Davies et al., 2018). While the interest of MR in psychiatry is increasingly prominent (Wootton et al., 2022), most of the existing reviews focus on specific disorders (Belbasis et al., 2018; Köhler et al., 2018) or risk factors (Treur et al., 2021), or they do not systematically review MR studies in psychiatry (Wootton et al., 2022). In fact, to the best of our knowledge, there is no comprehensive overview of the applications of MR across different psychiatric diseases. Such a transdiagnostic approach would provide a unifying view of the limitations and potential of MR in psychiatry, as well as stimulating insight into potential common risk factors for different psychiatric disorders.
With the present systematic review, we thus aim at providing, for the first time, an unbiased and inclusive view of all existing applications of MR in psychiatric disorders, as defined by Diagnostic and Statistical Manual of Mental Disorders (DSM) or International Classification of Diseases (ICD) diagnoses. Such an approach aims at facilitating the identification of risk factors that may be common to different psychiatric diseases, and at paving the way to transdiagnostic MR studies in psychiatry (which are currently under-investigated), in agreement with current psychiatry research models advising to target aspects common to different pathologies, rather than traditionally defined diagnoses only (Insel, 2014).
Methods
Search strategy and selection criteria
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Supplementary eTable 1, Supplemental Digital Content 1, http://links.lww.com/PG/A287) (Moher et al., 2009), and is registered in the PROSPERO database (registration number CRD42021285647).
We used a two-step approach. First, we searched the Web of Science database by Thomas Reuters, Pubmed, and Scopus. The search strategy included terms related to MR and psychiatry [((Mendelian randomization) OR (Mendelian randomisation)) AND (psychiatry OR bipolar disorder OR borderline personality disorder OR schizophrenia OR depression OR ADHD OR anxiety OR PTSD OR panic))] for articles published until 3 May 2022. We excluded reviews or metanalysis through the search filters. Second, we performed a manual search of the lists of references of retrieved articles. Duplicate references were manually excluded. The remaining articles were screened by title and abstract, and the full texts identified were further inspected for eligibility against a priori-defined exclusion and inclusion criteria. We included original articles in English that met the following Participants, Interventions, Comparators, Outcomes, and Study design criteria. Participants of the study were psychiatric patients of any age, with any psychiatric diagnosis according to DSM or ICD criteria. The Intervention had to include the employment of MR in genetic analysis, applied to the identification of risk factors, to neuroimaging correlations, or to other settings relating to psychiatry (including studies that focused on how psychiatric diseases might be risk factors for other conditions). Comparators were the presence or absence of genetic traits that are determinants of exposure to a certain risk factor (which could be a psychiatric disorder or other risk factors). The Outcome was the risk for a certain condition (both psychiatric and not).
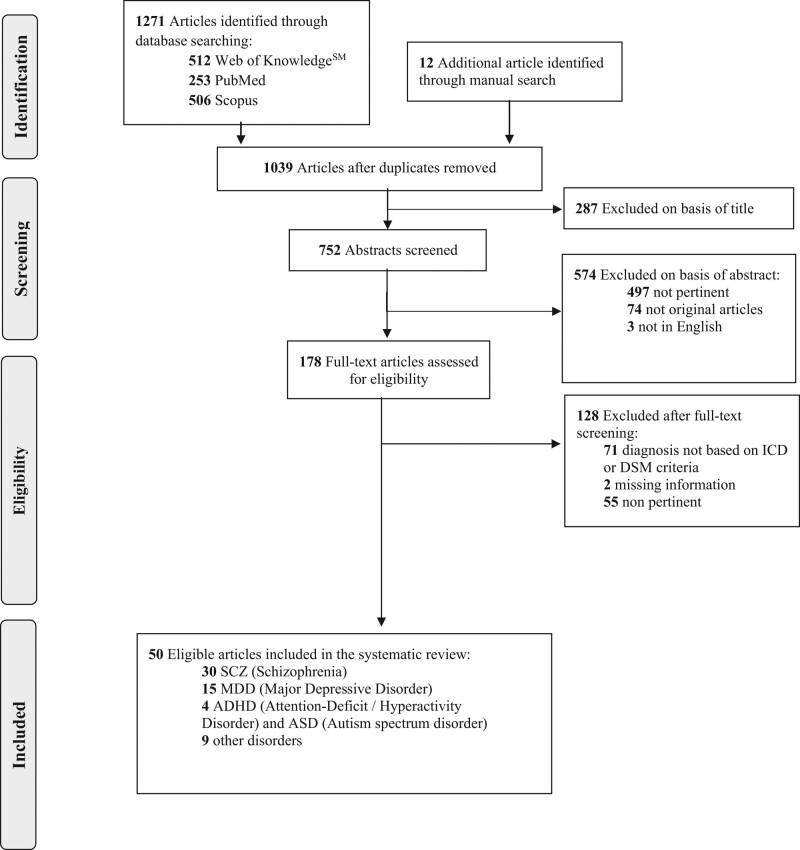
We included all study designs apart from case reports, case series, conference abstracts and presentations, pilot/feasibility studies, reviews, meta-analyses, and systematic reviews. Of note, we did not exclude studies that analyzed (considering different exposures/outcomes, or using different methods from each other) data stored in publicly available databases, or overlapping or partly overlapping populations. The selection process was documented in the PRISMA flow diagram (Fig. 2).
Fig. 2.
PRISMA 2009 flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Data extraction
Data extraction was performed by independent researchers (L.F.S. and S.G.). Any discrepancy was discussed until a consensus was reached. Disagreements were resolved by a third reviewer (G.R.).
The following variables were extracted from each article: authors, year of publication, sample size, genetic information analyzed, main MR method, main findings, presence of pleiotropy analysis, and psychiatric disorder considered as an outcome. If more than one method were employed and results were homogeneous across methods, we reported the first one described. If results obtained through different methods of analysis differed, we reported each method in the corresponding column, and we indicated between parathesis which method was employed to obtain each result in the ‘Outcome and finding’ column. If variables were not available, and no reply was obtained from the corresponding author of the article concerned (at least a 1-month delay for each query), we wrote ‘NG’, not given. If crucial information was missing, we contacted the corresponding author and excluded the study if no answer was received (at least 1-month delay for each query). In the case of articles reporting on more than one psychiatric disease, we included only results on diseases that had been diagnosed according to ICD or DSM criteria, in agreement with our inclusion criteria.
Quality assessment
The quality of the selected studies was assessed independently by two reviewers (S.G. and L.F.S.) with the Newcastle-Ottawa Scale (NOS) (Lo et al., 2014). Any discrepancy was discussed until a consensus was reached. Disagreements were resolved by a third reviewer (G.R.). The NOS is a risk of bias assessment tool that has been used also to evaluate MR studies (Cheng et al., 2020; Spiga et al., 2021). It consists of two sections: one for case-report studies and the other for cohort studies. Studies were evaluated using NOS considering three aspects: patient selection, comparability, and exposure (Supplementary eTable 2, Supplemental Digital Content 1, http://links.lww.com/PG/A287). The risk of bias and concerns regarding applicability were analyzed for each domain.
Results
As described in Fig. 2, 1039 nonduplicates studies were selected through database searching. Two hundred and eighty-seven of these were excluded based on the title, as they were not relevant. A total of 752 articles were screened based on the abstract to exclude articles that were not in English (n = 3), that were not pertinent (i.e. not about MR in psychiatry) (n = 497) or not original articles (e.g. reviews, case reports, metanalyses, and opinion articles) (n = 74). Upon eligibility screening, 128 studies were not eligible as 71 articles included at least some psychiatric patients with diagnoses not based on ICD or DSM criteria, two studies had missing information (and no reply was obtained from the corresponding author of the article concerned after at least 1-month delay for each query), and 55 articles were not pertinent.
The final sample, thus, included 50 articles. Although we searched for studies published from 1966 until 2022, the oldest included study dates back to 2014 (Hung et al., 2014). This confirms the novelty of this research topic. Thirty studies focused on SCZ, 15 on MDD, four on ADHD or ASD, and nine on other disorders. Of note, the sum of the articles included in each group does not equal 50 because studies presenting distinct results concerning multiple diseases are reported in each of the relevant tables for each disease. Details of each study are reported in Tables 1–4.
Table 1.
Mendelian randomization in schizophrenia
| References | Genetic instrument and exposure (P-value) | Main statistic test | Exclusion of pleiotropy | Outcome and findings | Outcome sample size |
|---|---|---|---|---|---|
| Andreu-Bernabeu et al., 2022 | 116 SNPs associated with SCZ (P < 5 × 10−8) | WM | Yes | Bidirectional causal effect between polygenic scores for loneliness/isolation and SCZ risk (WM−β (SE) = 1.37 (0.40); P = 6.14 × 10−4) | 35 476 SCZ cases |
| 19 SNPs associated with loneliness/social isolation (P < 5 × 10−6) | 46 839 controls (PGC) | ||||
| Chen et al., 2022 | 2422 SNPs associated with 41 systemic inflammatory regulators, 2788 SNPs associated with SCZ (P < 1 × 10−6) | IVW | Yes | Genetic predisposition to increased HGF, IL-17, IL-1ra, MCP3, TRAIL causally associated with higher SCZ risk (P < 5 × 10−2) | 36 989 SCZ cases |
| Genetic liability to SCZ causally associated with CTAK and RANTES (P < 3 × 10−2) | 11 3075 controls (PGC) | ||||
| Ni et al., 2022 | >700 SNPs associated with gut microbiota and 28 SNPs associated with SCZ (P < 1 × 10−5) | IVW | Yes | Genetic predisposition to increased class Actinobacteria causally associated with higher SCZ risk (P = 1.33 × 10−3) | 152 805 SCZ cases, 18 473 controls (MiBioGen) |
| No causal association between genetic instruments for SCZ and risk of different gut microbiota composition | |||||
| Perry et al., 2021b | 53 SNP associated with insulin resistance phenotype (P < 5 × 10−3) | IVW | Yes | No causal association between genetic instruments for insulin resistance and SCZ (IVW) | 40 675 SCZ cases |
| 58 SNP associated with CRP (P < 5 × 10−8) | MVMR | No significant association between inflammation-related SNP and SCZ after adjusting for genetic instruments for CRP (MVMR) | 64 643 controls | ||
| Kim et al., 2021 | 4 SNP associated with PD risk (P < 5 × 10−6) | IVW | Yes | Genetic predisposition to PD causally associated with higher SCZ risk (OR, 1.10; 95% CI, 1.05−1.15; P = 3.49 × 10−5) | 35 476 SCZ cases |
| 46 839 controls (PGC) | |||||
| Jin et al., 2021 | 93 SNP associated with PCOS (P < 5 × 10−8) | IVW, | Yes | No causal association between genetic instruments for PCOS predisposition and SCZ risk | 33 640 SCZ cases |
| 43 456 controls | |||||
| Chen et al., 2021 | 31 SNP associated with cigarette smoking (P < 1 × 10−5) | IVW, MR-Egger | Yes | No causal association between genetic instruments for smoking and SCZ | 7711 SCZ cases |
| 18 327 controls | |||||
| Yang et al., 2020 | 530 SNP associated with 2-methoxyacetaminophen sulfate levels (P < 1 × 10−5) | IVW | Yes | Genetic predisposition to higher 2-methoxyacetaminophen sulfate level causally associated with higher SCZ risk (P = 1.73 × 10−5) | 36 989 SCZ cases and 113 075 controls |
| Pasman et al., 2018 | 74 SNP associated with cannabis use (P < 1 × 10−5) | IVW | Yes | Genetic predisposition to cannabis use causally associated with higher SCZ risk (OR, 1.10; 95% CI, 0.99–1.21; P = 0.074) | 130 072 CZ cases |
| 109 with SCZ (P < 5 × 10−8) | Genetic liability to SCZ causally associated with cannabis use (OR, 1.16; 95% CI, 1.06–1.27; P = 0.001) | 180 934 cases of cannabis use | |||
| Zhuang et al., 2020 | 30 SNP associated with Enterobacteriaceae family and order (P = 3.72 × 10−11) | IVW | Yes | Genetic predisposition to increased: | 21 246 SCZ cases |
| Enterobacteriaceae family and order causally associated with higher SCZ risk (OR, 1.09; CI, 1.00–1.18; P = 0.048) | |||||
| Gammaproteobacteria class (OR, 0.90; CI, 0.83–0.98; P = 0.011) causally associated with lower SCZ risk | |||||
| 15 with Gammaproteobacteria class (P < 1.4 × 10−8) | Gut production of serotonin causally associated with higher SCZ risk (OR, 1.07; CI, 1.00–1.15; P = 0.047) | ||||
| 32 with increased gut production of serotonin (P < 8.96 × 10−6) | No causal associations with SCZ risk for other types of gut microbiota | 38 072 controls | |||
| Wootton et al., 2020 | 114 SNP associated with SCZ | IVW | Yes | Genetic predisposition to lifetime smoking causally associated with higher SCZ risk (OR, 2.27; 95% CI, 1.67–3.08; P < 0.001) | 36 989 SCZ cases |
| 126 with lifetime smoking (P < 5 × 10−8) | Genetic liability for SCZ causally associated with higher risk of lifetime smoking (β= 0.022; 95% CI, 0.005–0.038; P = 0.009) | 113 075 controls (PGC) | |||
| 213 372 ever-smokers and 249 318 never-smokers (UK Biobank) | |||||
| Peters et al., 2020 | 319 SNP associated with WHRadjBMI | IVW, MR-Egger | Yes | No causal association between genetic instruments for WHRadjBMI and SCZ risk | 40 675 SCZ cases |
| 64 643 controls (PGC) | |||||
| Kim et al., 2020 | 70 SNP associated with SCZ (P < 5 × 10−8) | IVW | Yes | Genetic liability to SCZ causally associated with higher breast cancer risk (OR, per log odds increase in SCZ risk, 1.069; 95% CI, 1.028–1.112; P < 0.001) | 82 315 SCZ cases (PGC) |
| 117, 146, 204 SNP associated with breast cancer (5 × 10−8, 5 × 10−7, 5 × 10−6respectively) | No causal association between genetic instruments for breast cancer and SCZ | 228 951 breast cancer cases (BCAC) | |||
| Jones et al., 2020 | 5 SNP associated with anxiety | IVW | Yes | Genetic liability to neuroticism (OR, 1.33; 95% CI, 1.12–1.59) and anxiety (OR, 1.10; 95% CI, 1.01–1.19) causally associated with higher SCZ risk | 33 640 SCZ cases |
| 116 with neuroticism (P < 5 × 10−8) | 43 456 controls (PGC) | ||||
| Jang et al., 2020 | 341 SNP associated with substance use phenotypes (smoking, alcohol, or cannabis use) | IVW, WM, MR-Egger; Latent Causal Variable model | Yes | No causal association between genetic instruments for substance use phenotypes and the risk SCZ | 105 318 SCZ cases |
| Song et al., 2021 | 1 SNP associated with pars triangularis volume (P < 5 × 10−8) | IVW | No | Genetic predisposition to lower pars triangularis volume causally associated with SCZ risk (OR, 0.48; 95% CI, 0.34-0.69; P = 5.9 × 10−5) | 40 675 SCZ cases |
| 64 643 controls (PGC) | |||||
| Luo et al., 2020 | 45 SNP associated with SCZ (P < 5 × 10−8) | IVW | Yes | Genetic liability to SCZ causally associated with higher serum uric acid risk (per 10-s% increase in SCZ risk: beta: −0.039, SE: 0.013; P = 0.003) | 35 476 SCZ cases |
| 26 SNP associated with serum uric acid (P < 5 × 10−8) | No causal association between genetic instruments for higher serum uric acid and SCZ | 46 839 controls (PGC) | |||
| Gao et al., 2019 | 110 SNP associated with insomnia (P < 5 × 10−8) | MR-Egger, GSMR | Yes | No causal association between genetic instruments for insomnia and SCZ | 33 426 SCZ cases |
| 32 541 controls | |||||
| Byrne et al., 2019 | 142 SNP associated with SCZ | GSMR | Yes | Genetic liability to SCZ causally associated with higher breast cancer risk (SE = 0.008; P = 2.2 × 10−4) | 40 675 SCZ cases and 64 643 controls (PGC) |
| 191 with breast cancer (P < 5 × 10−8) | No causal association between genetic instruments for breast cancer and SCZ | 122 977 breast cancer cases and 105 974 controls | |||
| Tomioka et al., 2018 | 1 SNP associated with serum pyridoxal levels (P = 0.006) | IVW | No | No causal association between genetic instruments for serum pyridoxal level and SCZ | 365 SCZ cases |
| 911 controls | |||||
| Polimanti et al. 2018 | 10 SNP associated with T2D (P < 5 × 10−8) | Instrumental variable analysis | Yes | No bidirectional causal associations between SCZ and T2D | 34 840 T2D cases and 114 981 controls (DIAGRAM consortium) |
| 108 SNP associated with SCZ (P < 5 × 10−8) | 34 241 SCZ cases and 45 604 controls (PGC) | ||||
| Arafat and Minica, 2018 | 60 SNP associated with birth weight (P < 5 × 10−8) | IVW | Yes | No causal association between genetic instruments for birth weight and SCZ | 34 241 SCZ cases |
| 45 604 controls (PGC) | |||||
| Li et al., 2018 | 13 SNP associated with fasting insulin levels | IVW | Yes | No causal effect of any of the genetic instruments on SCZ risk, nor in Europeans nor in East Asians, in the BMI-adjusted analysis | 84 514 SCZ cases |
| 30 with fasting glucose in Europeans, 14 in East Asians | 126 949 controls (PGC2, BIOX) | ||||
| 36 with HbA1c in Europeans, 27 in East Asians | |||||
| 140 with T2D (for Europeans, East Asians and trans-ancestry groups) (P < 5 × 10−8) | |||||
| Hartwig et al., 2017 | 18 SNP associated with CRP serum levels | IVW | Yes | Genetic predisposition to higher serum CRP causally associated with lower SCZ risk (OR, 0.90; 95% CI, 0.84-0.97; P = 0.005) | 36 989 SCZ cases |
| 1 associated with higher sIL-6R serum levels | Genetic predisposition to higher serum sIL-6R causally associated with higher SCZ risk (OR, 1.06; 95% CI, 1.01-1.12; P = 0.02) | ||||
| 2 associated with higher IL-1Ra serum levels (P < 5 × 10−5) | No causal association between genetic instruments for IL-1Ra and SCZ risk | 113 075 controls (PGC) | |||
| Gage et al., 2017a | 128 SNP associated with SCZ risk (P < 5 × 10−8) | IVW | Yes | Genetic liability to SCZ causally associated with risk for cannabis initiation (OR, 1.10 per doubling of the odds of SCZ; 95% CI, 1.05–1.14; P = 2.64 × 10−5) | 36 989 SCZ cases |
| 21 with cannabis initiation (P < 10−5) | Genetic predisposition to cannabis initiation causally associated with SCZ risk (OR, 1.04 per doubling odds of cannabis initiation; 95% CI, 1.01–1.07; P = 0.019) | 113 075 controls (PGC) | |||
| Gage et al., 2017b | 21 SNP associated with smoking initiation (P < 10−6) | IVW | Yes | Genetic predisposition to smoking initiation causally associated with SCZ risk (OR, 1.73; 95% CI, 1.30–2.25, P < 0.001) | 36 989 SCZ cases |
| 94 with SCZ risk (P < 5 × 10−8) | No causal association between genetic instruments for SCZ and risk for smoking initiation (OR, 1.01; 95% CI, 0.98–1.04; P = 0.32). | 113 075 controls (PGC) | |||
| 35 845 smokers (TAG consortium) | |||||
| Taylor et al., 2016 | 4 SNP associated with serum vitamin D levels (P < 6 × 10−10) | IVW | Yes | No causal association between genetic instruments for higher serum vitamin D and SCZ risk. | 36 989 SCZ cases |
| 113 075 controls (PGC) | |||||
| Prins et al., 2016 | 18 SNP associated with CRP levels (P < 1 × 10−4) | GRS IV | Yes | Genetic predisposition to higher serum CRP causally associated with reduced SCZ risk (per 10-s% increase in CRP level; OR, 0.86; 95% CI, 0.79–0.94; P < 0.0010) | 34 241 SCZ cases |
| 45 604 controls (PGC) | |||||
| Inoshita et al., 2016 | 15 SNP associated with CRP serum levels (P < 5 × 10−8) | IVW | Yes | Genetic predisposition to higher serum CRP causally associated with SCZ risk (OR, 1.10; 95% CI, 1.02–1.19; P = 0.015) | 418 SCZ cases |
| 1365 controls | |||||
| Wium-Andersen et al., 2015a | 1 SNP associated with smoking intensity | IVW | No | Genetic predisposition to higher smoking intensity causally associated with SCZ risk (OR, 1.06; 95% CI, 1.04–1.08) in ever- and never-smokers combined, but not in each group alone. | 67 SCZ cases |
| 40 014 ever-smokers and 23 282 never-smokers |
BCAC, Breast Cancer Association Consortium; BIOX, Bio-X Institutes; CI, confidence interval; CRP, C-reactive protein; CUD, cannabis use disorder; HGF, hepatocyte growth factor; CTACK, cutaneous T-cell attracting chemokine; GRS IV, Genetic risk score instrumental variable; (GS)MR, (Generalized Summary-based) Mendelian randomization; (s)IL-R, (soluble) interleukin receptor; IVW, inverse variance–weighted; MCP3, monocyte-specific chemokine 3; MVMR, multivariable Mendelian randomization; OR, odds ration; PGC, psychiatric genomic consortium; RANTES, regulated on Activation; SCZ, schizophrenia; SE, standard error; SNP, single-nucleotide polymorphisms; s%, symmetric percentage; TAG, Tobacco and Genetics Consortium; TRAIL, TNF-related apoptosis-inducing ligand; T2D, type 2 diabetes; WHRadjBMI, waist-to-hip ratio adjusted for BMI; WM, weighted median.
Table 4.
Mendelian randomization in other psychiatric disorders
| Reference | Genetic instrument and exposure (P-value) | Main statistic test | Exclusion of pleiotropy | Outcome and findings | Outcome sample size |
|---|---|---|---|---|---|
| Chen et al., 2022 | 2422 SNPs associated with increased 41 systemic inflammatory regulators (P < 1 × 10−6) | IVW | Yes | Genetic predisposition to increased CTAK (P = 1.17 × 10−2) and IL-18 (P = 1 × 10−2) causally associated with higher OCD risk (P < 5 × 10−2) | 2688 OCD cases, |
| 7037 controls | |||||
| Causal association between the genetic predisposition to OCD and the levels of inflammatory regulators not investigated | |||||
| Ni et al., 2022 | >700 SNPs associated with gut microbiota and >3 SNPs associated with each psychiatric disorder (P < 1 × 10−5) | IVW | Yes | Genetic predisposition to increased | 46 351 ASD cases, 51 710 BD, 14 307 TS, 9725 OCD cases, 23 809 AD, 18,473 controls (MiBioGen) |
| Family Prevotellaceae causally associated with higher ASD risk (P = 5.31 × 10−4) | |||||
| Class Betaproteobacteria causally associated with higher BD risk (P = 1.53 × 10−3) | |||||
| Class Bacteroidia and order Bacteroidales with causally associated with higher TS risk (P = 2.51 × 10−3 and 2.51 × 10−3) | |||||
| No causal association between genetic instruments for gut microbiota composition and the risk for OCD or AD | |||||
| No causal association between genetic instruments for psychiatric disorders and risk of different gut microbiota composition | |||||
| Jin et al., 2021 | 93 SNP associated with PCOS (P < 5 × 10−8) | IVW, WM | Yes | Genetic predisposition to PCOS causally associated with higher OCD risk based on IVW (OR, 1.339; 95% CI, = 1.083–1.657; P = 0.007) and WM analysis (OR, 1.493; 95% CI, 1.145–1.946; P = 0.003) | 2688 OCD cases and 7037 controls (PGC) |
| 7016 AD cases and 14 745 controls | |||||
| No causal association between genetic instruments for PCOS predisposition and AD risk | |||||
| Zhao et al., 2021 | 30 SNP associated with serum urate concentration (P < 1 × 10−700) | IVW | Yes | No causal association between genetic instruments for increased urate levels and the risk for the psychiatric disorders analyzed | 2688 OCD cases and 7037 controls |
| 16 992 AN cases and 55 525 controls | |||||
| 23 212 PTSD cases and 151 447 controls | |||||
| Rosoff et al., 2021 | 74 SNP associated with educational attainment (P < 5 × 10−8) | IVW | Yes | Genetically determined increased educational attainment causally associated with reduced AUD risk (ORIVW = 0.508; 95% CI, 0.315–0.819; P = 5.52 × 10−3) | 8485 AUD cases |
| 20 657 controls | |||||
| Wium-Andersen et al., 2015a | 2 SNP associated with alcohol consumption (P < 1 × 10−5) | IVW | No | Genetic predisposition to alcohol consumption causally associated with higher risk for AUD (OR, 28.6; 95% CI, 6.47–126 for an increase of 1 drink/day estimated from the genetic instrument; P = NG) | 4736 cases of AUD |
| 78 154 total participants | |||||
| Jefsen et al., 2021 | 6 SNP associated with lifetime cannabis use | IVW regression | Yes | Genetic liability to BD causally associated with lifetime cannabis use (P = 7 × 10−6) | 20 352 BD cases and 31 358 controls |
| 17 with bipolar disorder (P < 5 × 10−8) | |||||
| No causal effect of the genetic risk for lifetime cannabis use on the risk for BD. | |||||
| Vermeulen et al., 2021 | 126 SNP associated with smoking cigarettes (P < 5 × 10−8) | IVW | Yes | Genetic predisposition to smoking cigarettes causally associated with BD risk (smoking initiation ORIVW = 1.46; 95% CI, 1.28–1.66; P = 1.44 × 10−8, lifetime smoking ORIVW = 1.72; 95% CI, 1.29–2.28; P = 1.8 × 10−4) | 20 352 BD cases and 31 358 controls |
| Wium-Andersen et al., 2016 | 4 SNP associated with elevated CRP (P < 5 × 10−8) | Instrumental variable analysis | Yes | Doubling in genetically determined CRP causally associated with late-onset BD risk (OR, 4.66; 95% CI, 0.89–24.3; P = 0.15) | 93 late-onset BD cases and 78 809 controls |
(GS)MR, (generalized summary-based) Mendelian randomization; AD, anxiety disorder; AN, anorexia nervosa; ASD, autism spectrum disorder; AUD, alcohol use disorder; BD, bipolar disorder; CI, confidence interval; CRP, c-reactive protein; CTACK, cutaneous T-cell attracting chemokine; IVW, inverse variance–weighted; OCD, obsessive-compulsive disorder; OR, odds ratio; PCOS, polycystic ovary syndrome; SNP, single-nucleotide polymorphism; TS, Tourette syndrome; WM, weighted median.
The NOS scores were fair and homogenous among the studies. More in detail, all studies scored high in the ‘selections of patients’ and ‘comparability’. Further details are provided in Supplementary eTable 2, Supplemental Digital Content 1, http://links.lww.com/PG/A287.
Mendelian randomization in schizophrenia
The most numerous group of articles that we included is on SCZ, comprising 30 articles (Table 1) (Wium-Andersen et al., 2015; Prins et al., 2016; Taylor et al., 2016; Inoshita et al., 2016; Gage et al., 2017a; Gage et al., 2017b; Hartwig et al., 2017; Arafat and Minica, 2018; Li et al., 2018; Pasman et al., 2018; Polimanti et al., 2018; Tomioka et al., 2018; Byrne et al., 2019; Gao et al., 2019; Jang et al., 2020; Jones et al., 2020; Kim et al., 2020, 2021; Luo et al., 2020; Peters et al., 2020; Wootton et al., 2020; Yang et al., 2020; Zhuang et al., 2020; Jin et al., 2021; Johnson et al., 2021; Perry et al., 2021b; Song et al., 2021; Chen et al., 2021, 2022; Andreu-Bernabeu et al., 2022; Ni et al., 2022). Most studies focused on inflammation, metabolic traits, or substance use and their associations with SCZ.
Contradictory findings exist on the association between smoking and risk for SCZ: while Chen et al. (2021) did not find any causal association between their genetic instrument for smoking and SCZ risk, two other studies with more numerous sample sizes did show that genetic predisposition to smoking was causally associated with higher SCZ risk, but not vice versa (Gage et al., 2017b; Wootton et al., 2020). On the other hand, there may be a bidirectional causal association between cannabis use and SCZ that is genetic risk for SCZ is causally associated with cannabis use and vice versa (Gage et al., 2017a; Pasman et al., 2018).
Metabolic syndrome is a well-known comorbidity of patients with SCZ, but this is likely due to antipsychotic use. In fact, no causal relationship has been highlighted between genetic instruments for metabolic traits [e.g. insulin resistance (Li et al., 2018; Perry et al., 2021b), BMI (Peters et al., 2020), or diabetes (Polimanti et al., 2018)] and SCZ risk.
Findings on the link between the immune system and SCZ risk are contrasting (Prins et al., 2016; Hartwig et al., 2017; Chen et al., 2022). Two papers studying partially overlapping samples from the Psychiatric Genomics Consortium (PGC) highlighted that a genetic predisposition for a higher serum C-reactive protein (CRP) was causally associated with reduced SCZ risk (Prins et al., 2016; Hartwig et al., 2017), whereas a study on a smaller sample size reported opposite findings (Inoshita et al., 2016). Studying systemic inflammation in SCZ is particularly relevant considering that two recent studies showed causal links between genetic predisposition to altered gut microbiota composition and SCZ risk (Zhuang et al., 2020; Ni et al., 2022), and it is known that microbiota composition might influence systemic immunity (Lo et al., 2021). However, the classes of gut bacteria identified as risk factors for SCZ differ between these two studies [i.e. actinobacteria by Ni et al. (2022) and gammaproteobacteria by Zhuang et al. (2020)].
Interestingly, genetic liability to other psychiatric disorders (i.e. anxiety and neuroticism) was causally associated with higher SCZ risk (Jones et al., 2020), whereas this was not true for insomnia (Gao et al., 2019).
Finally, two works (Byrne et al., 2019; Kim et al., 2020) found that genetic liability to SCZ was causally associated with an increased risk for developing breast cancer (but not vice versa), in partially overlapping populations.
Mendelian randomization in major depressive disorder
We included 15 studies on MR in MDD (Table 2) including data from populations ranging between 500 and 143 265 MDD patients (Wium-Andersen et al., 2014; Hung et al., 2014; Wium-Andersen et al., 2015a; Wium-Andersen et al., 2015b; Kwok et al., 2016; Sequeira et al., 2017; Clarke et al., 2017; Michalek et al., 2017; Cai et al., 2019; Choi et al., 2019; Milaneschi et al., 2019; Gill et al., 2019; Huang et al., 2020; Lu et al., 2021; Maharjan et al., 2021). Studies employed most often inverse variance–weighted analysis, and they all accounted for pleiotropic effects, apart from (Michalek et al., 2017). These mostly evaluated causal associations between exposure to metabolic traits, or to other diseases (such as CAD or stroke) and MDD. MDD was, thus, used as exposure and/or outcome. For instance, genetic variables for diabetes (Clarke et al., 2017), higher plasmatic CRP (Wium-Andersen et al., 2014), vitamin D or n3-PUFA (Milaneschi et al., 2019) or BMI (Hung et al., 2014) did not lead to an increased risk for MDD. While no bidirectional causal association between genetic instruments for Alzheimer’s disease and MDD was identified (Huang et al., 2020), genetic liability to MDD appears to impair functional outcome after a stroke (Gill et al., 2019), and to be causally associated with cardiovascular disease (Lu et al., 2021), as well as with higher risk of small vessel stroke, although this effect is not present for large artery nor cardioembolic strokes (Cai et al., 2019).
Table 2.
Mendelian randomization in major depressive disorder
| Reference | Genetic instrument and exposure (P-value) | Main statistic test | Exclusion of pleiotropy | Outcome and findings | Outcome sample size |
|---|---|---|---|---|---|
| Maharjan et al., 2021 | 121 SNP associated with total testosterone | IVW | Yes | No causal association between genetic instruments for increased female testosterone or SHBG levels and MDD | 521 MDD cases |
| 91 with bioavailable testosterone | |||||
| 173 with serum SHBG protein | |||||
| 368 controls | |||||
| Lu et al., 2021 | 102 SNP associated with MDD (P < 5 × 10−8) | IVW | Yes | Genetic liability to MDD causally associated with higher CAD (OR, 1.14; 95% CI, 1.06–1.24; P = 1.0 × 10−3) and myocardial infarction (OR, 1.21; 95% CI, 1.11–1.33; P = 4.8 × 10−5) risks | 60 801 CAD cases, including 43 676 with myocardial infarction, |
| 123 504 controls | |||||
| Huang et al., 2020 | 32 SNP associated with Alzheimer’s Disease, and 65 SNP with MDD (P < 0.002) | IVW | Yes | No bidirectional causal association between Alzheimer’s Disease and MDD genetic instruments | 9240 MDD cases |
| 9519 controls | |||||
| Milaneschi et al., 2019 | 6 SNP associated with vitamin D | IVW | Yes | No bidirectional causal association between vitamin D or n3-PUFA and MDD genetic instruments | 1700 MDD cases |
| 347controls | |||||
| 7 with n3-PUFA | |||||
| 37 with MDD (P < 0.05) | |||||
| Gill et al., 2019 | 56 SNP associated with MDD (P < 5 × 10-8) | IVW | Yes | Genetic liability to MDD causally associated with functional outcome after ischemic stroke (OR of poor outcome per 1-SD increase in genetically determined risk of MDD, 1.81; 95% CI, 0.98–3.35; P = 0.06). | 60 341 ischemic stroke cases |
| 454 450 control subjects (MEGASTROKE consortium) | |||||
| Cai et al., 2019 | 72 SNP associated with MDD (P < 1 × 10−6) | IVW | Yes | Genetic liability to MDD: | 34 217 ischemic stroke cases |
| causally associated with higher risk of small vessel stroke, (OR, 1.33; 95% CI, 1.08–1.65; P = 0.009) | |||||
| 406 111 controls | |||||
| but not with large artery stroke, cardioembolic stroke, or all ischemic stroke | |||||
| Choi et al., 2019 | SNP associated with: | IVW | Yes | Genetic predisposition to higher accelerometer-based physical activity causally associated with reduced risk for MDD (IVW OR, 0.74 for MDD per 1-SD unit increase in mean acceleration; 95% CI, 0.59-0.92; P = 0.006) | 143 265 MDD |
| MDD (n = 17) (P < 1 × 10−6) | 91 084 accelerometer-based activity | ||||
| Accelerometer-based (n = 12) and self-reported (n = 25) physical activity (P < 1 × 10−7) | |||||
| 377 234 self-reported activity | |||||
| No causal association between genetic instruments for accelerometer-based activity and MDD | |||||
| No causal association between genetic instruments for self-reported activity and MDD | |||||
| Michalek et al., 2017 | 1 SNP associated with shorter leukocyte telomere lengths | Generalized linear model | NO | Genetic predisposition to shorter telomere lengths causally associated with: | 1628 MDD cases |
| 1140 controls | |||||
| Increased risk for childhood-onset MDD (P ≤ 0.05) | |||||
| Increased risk for childhood-onset MDD relative to adult-onset MDD cases (P ≤ 0.001) | |||||
| No association with adult-onset MDD | |||||
| Wium-Andersen et al., 2014 | 4 SNP associated with increased plasma CRP levels (P < 5.1 × 10−59) | Regression model | Yes | No causal association between genetic instruments for increased plasma CRP levels and MDD | 1183 MDD |
| 77 626 controls | |||||
| Sequeira et al., 2017 | 120 SNP associated with age at menarche (P < 0.0001) | Structural mean model | Yes | Genetic liability to early menarche: | 3920 girls (ALSPAC cohort) |
| Causally associated with higher levels of depressive symptoms at 14 years (OR, per risk allele 1.02; 95% CI, 1.005–1.04, n = 2404). | |||||
| No association with MDD after 14 years | |||||
| Kwok et al., 2016 | 3 SNP associated with coffee consumption (P < 5 × 10−8) | IVW | Yes | No causal association between genetic instruments for coffee consumption and MDD | 9240 MDD cases |
| 9519 controls (PGC) | |||||
| Clarke et al., 2017 | 10 SNP associated with T2D (P ≤ 5 × 10−8) | Mixed linear models | Yes | No causal association between genetic instruments for T2D and MDD | 2697 MDD cases |
| 20 800 controls (GS: SFHS) | |||||
| Hung et al., 2014 | 32 SNP associated with higher BMI (P < 5 × 10−8) | Maximum likelihood estimation model | Yes | No causal association between genetic instruments for higher BMI and MDD | 2430 MDD cases |
| 792 controls | |||||
| Wium-Andersen et al., 2015b | 1 SNP associated with smoking intensity | IVW | No | No causal association between genetic instruments for higher smoking intensity and MDD risk in any group | 1067 MDD cases |
| 40 014 ever-smokers and 23 282 never-smokers | |||||
| Wium-Andersen et al., 2015a | 2 SNP associated with alcohol consumption (P < 1 × 10−5) | IVW | No | Genetic predisposition to alcohol consumption causally associated with higher risk for hospitalization/death with MDD (OR, 4.52; 95% CI, 0.99–20.5; P = NG) | 4777 cases of hospitalization/death with MDD |
| 78 154 total participants |
Longitudinal Study of Parents and Children; CAD, coronary artery disease; CI, confidence interval; GS: SFHS, Generation Scotland: the Scottish Family Health Study; IVW, inverse variance–weighted; MDD, major depressive disorder; n3-PUFA, omega-3 polyunsaturated fatty acid; NG, not given; OR, odds ration; PGC, psychiatric genomic consortium; SHBG, sex-hormone-binding globulin; SNP, single-nucleotide polymorphisms; T2D, type 2 diabetes.
Interestingly, two articles focused on causal risk factors for early-onset MDD (Michalek et al., 2017; Sequeira et al., 2017). The latter (Sequeira et al., 2017) showed that genetic liability to early menarche was causally associated with higher levels of depressive symptoms at 14 years but not after 14 years. The first one (Michalek et al., 2017) found that genetic predisposition to shorter telomere lengths was causally associated with increased risk for childhood-onset MDD.
Mendelian randomization in attention-deficit and hyperactivity disorder and autism spectrum disorders
Four articles on ADHD or ASD satisfied the inclusion criteria (Jang et al., 2020; Peyre et al., 2021; Vilar-Ribó et al., 2021; Zhao et al., 2021) (Table 3). One studied the interaction between these two disorders, using a genetic instrument for ADHD and ASD (in 18 381 cases) as an outcome to show that genetic predisposition to ADHD was causally associated with an increased risk of ASD (Peyre et al., 2021).
Table 3.
Mendelian randomization in attention-deficit and hyperactivity disorder and autism spectrum disorders
| Reference | Genetic instrument and exposure (P-value) | Main statistic test | Exclusion of pleiotropy | Outcome and findings | Outcome sample size |
|---|---|---|---|---|---|
| Zhao et al., 2021 | 30 SNP associated with serum urate concentration (P < 1 × 10−700) | IVW | Yes | No causal association between genetic instruments for increased urate levels and ADHD risk | 19 099 ADHD cases |
| 34 194 controls (PGC) | |||||
| Peyre et al., 2021 | 40 SNP associated with ADHD | IVW | Yes | Genetic predisposition to ADHD causally associated with an increased risk of ASD (P < 0.001) | 18 381 ASD cases |
| 27 969 controls | |||||
| Vilar-Ribó et al., 2021 | 164 SNP associated with smoking initiation, alcohol or cocaine dependence, lifetime cannabis use and ever addicted to illicit drugs | IVW | No | Genetic liability to ADHD causally associated with smoking initiation (IVW OR, 1.20; P = 2.24 × 10−21), age of smoking initiation (IVW OR, 0.94; P = 4.27 × 10−4), cigarettes per day (IVW OR, 1.08; P = 8.23 × 10−4) and lifetime cannabis use (IVW OR, 1.15; P = 2.01 × 10−3) | 989 ADHD cases among whom (overlapping samples): |
| 409 cases smoking initiation, 511 controls | |||||
| 177 alcohol dependence cases, 679 controls | |||||
| MR-PRESSO | |||||
| 172 cocaine dependence cases, 777 controls | |||||
| Genetic predisposition to smoking initiation causally associated with higher ADHD risk (MR-PRESSO OR, 2.46; P = 3.66 × 10−17) | |||||
| 298 cases of lifetime cannabis use, 655 controls | |||||
| Genetic predisposition to lifetime cannabis use causally associated with higher ADHD risk (IVW OR, 1.46; P = 8 × 10−4) | 345 cases of ever addicted to illicit drugs, 609 controls | ||||
| 12 SNP associated with ADHD (P < 5 × 10−6) | |||||
| No causal effect of genetic liability to ADHD on smoking cessation, alcohol dependence, cocaine dependence or ever addicted to illicit drugs and vice versa | |||||
| Jang et al., 2020 | 341 SNP associated with substance use phenotypes (smoking, alcohol, or cannabis use) | IVW, WM, MR-Egger; Latent Causal Variable model | Yes | No causal association between genetic instruments for substance use phenotypes and the risk for ADHD | 53 293 ADHD cases |
ADHD, attention-deficit and hyperactivity disorder; ASD, autism spectrum disorders; IVW, inverse variance–weighted; MR-PRESSO, Mendelian Randomization Pleiotropy RESidual Sum and Outlier; OR, odds ratio; SNP, single-nucleotide polymorphism.
The second study focused on ADHD and its relationship with the use of substances (Vilar-Ribó et al., 2021). It showed that being genetically exposed to ADHD causally increased the risk of smoking tobacco or cannabis and vice versa, that is genetic predisposition to smoking initiation and to lifetime cannabis was causally linked with a higher risk of suffering from ADHD. On the other side, the study did not show a causal effect of genetic liability to ADHD on smoking cessation, alcohol dependence, cocaine dependence, or addiction to illicit drugs and vice versa (Vilar-Ribó et al., 2021).
Mendelian randomization in other psychiatric disorders
We included in this section and heterogeneous group of nine articles that focused on psychiatric diseases not included in previous tables (Table 4) (Wium-Andersen et al., 2015a; Jang et al., 2020; Jefsen et al., 2021; Jin et al., 2021; Rosoff et al., 2021; Vermeulen et al., 2021; Zhao et al., 2021; Chen et al., 2022; Ni et al., 2022). Among these, we included three studies on BD (Wium-Andersen et al., 2016; Jefsen et al., 2021; Vermeulen et al., 2021). The two most recent ones focused on the relationship between cannabis or tobacco smoking and BD, finding that genetic liability to suffering from BD was causally linked with lifetime cannabis use, whereas there was no causal effect of genetic risk for lifetime cannabis use on BD risk (Jefsen et al., 2021). However, genetic predisposition to smoking cigarettes appeared to be causally associated with BD risk (Vermeulen et al., 2021). These studies analyzed data from the same GWAS study, including 20 352 BD cases and 31 358 controls (Stahl et al., 2019). The third study explored the link between systemic inflammation and BD, finding that increased plasmatic CRP was causally associated with the development of late-onset BD, although the number of patients included in the study was limited (93 late-onset BD cases) (Wium-Andersen et al., 2016).
Only one study (Rosoff et al., 2021) satisfied our inclusion criteria among those that employed MR in alcohol use disorder (AUD). In this work, investigators used a genetic instrument for higher educational attainment and found that it was causally associated with lower AUD risk in 8485 AUD cases (Rosoff et al., 2021). Intriguingly, a recent study found that genetic predisposition to polycystic ovary syndrome was causally associated with higher obsessive-compulsive disorder (OCD) risk but not with some other psychiatric disorders (i.e. anxiety disorder and SCZ) (Jin et al., 2021). Considering the inflammatory component in PCOS pathophysiology, it is very interesting to report that a recent study showed that genetic predisposition to increased inflammatory markers (CTAK and IL-18) was causally associated with higher OCD risk.
Discussion and limitations
We systematically reviewed the evidence of the employ of MR in psychiatric diseases. We included 50 articles, divided into four groups on the basis of the psychiatric disorder assessed: SCZ (the most numerous group), MDD, ADHD/ASD, or other psychiatric diseases.
Overall, the present findings confirm that MR offers a unique opportunity of unraveling causal links in risk factors and etiological elements of psychiatric diseases, both in specific disorders and transdiagnostically.
This is especially true considering the important challenges encountered when employing more traditional research methods to ascertain causality between a certain risk factor and psychiatric disorders, which have often a multifactorial cause, and a behavioral impact that may expose the patients to further risk factors. As mentioned in the introduction, MR can control for reverse causality when investigating disease causes and can use existing GWAS studies with no need for long, time-consuming longitudinal cohort recruiting. Furthermore, the ever-increasing development of (epi)genome sequencing technologies is providing additional data for further MR studies. No study included here studied epigenomic data with MR, but this is an expanding field (Grau-Perez et al., 2019) and might be an interesting perspective for psychiatric diseases.
Most commonly, studies included in this review evaluated the impact of metabolic traits, inflammation, or substance abuse on psychiatric risk. Few significant, causal links between psychiatric diseases and risk factors are confirmed by different groups in different populations, at the same time. However, some promising findings emerge. Overall, the causal relationships between depression and cardiovascular risk (of stroke in particular) that have been identified (Cai et al., 2019; Gill et al., 2019; Lu et al., 2021) are particularly interesting considering the increasing evidence on depression and serotoninergic axis dysregulation as important players in stroke physiopathology (Colpo et al., 2019; Damsbo et al., 2019; Saccaro et al., 2022a). While the present review did not identify a definitive causal link between systemic inflammation and psychiatric disorders (the results on SCZ, MDD, or BD patients, for example, are not conclusive), the link between inflammation and psychiatric disorders is another topic of major interest in recent years (Wium-Andersen et al., 2016; Han and Ham, 2021; Murphy et al., 2021; Saccaro et al., 2021a, 2021b; Saccaro et al., 2022b), and further studies, employing MR as well, could unravel the pathophysiological role of inflammation in psychiatric disorders, with diagnostic, prognostic or therapeutic potential. To do so, however, it is important to focus on the right targets and to correctly interpret results. As a paradigmatic example, Perry et al. (2021a) and Hartwig et al. (2017) found that genetic variants in the IL-6 pathway purportedly linked with higher serum IL-6 or sIL-6R levels are causally associated with SCZ. While the interpretation offered by the authors is that higher levels of such plasmatic markers are causally implied in the SCZ development, a mechanistic insight into the IL-6 pathway in humans is lacking, and, thus, it has been argued that these conclusions need to be tempered (Pearce, 2022). In fact, other interpretations of these results are possible. For instance, there is evidence in animal models that maternal immune activation causes lasting proinflammatory changes, including higher IL-6 plasmatic levels, in the fetus (Mandal et al., 2013). Thus, variants associated with higher serum IL-6 levels might, in fact, be associated with pregnancy complications or maternal-fetal interactions, which are well-known risk factors for SCZ (Pearce, 2022). Should this be the case, the exclusion restriction assumption (which, as discussed in the background, is crucial for MR soundness), would not be respected. Although the aforementioned findings that we have taken as an example, in this case, remain relevant and interesting, it is crucial to critically evaluate the results of MR and consider potential alternative interpretations or confounders, as recapitulated in Fig. 1.
Similarly, two independent studies (Table 4) identified causal roles of genetic predisposition to PCOS (Jin et al., 2021) and higher IL-18, a proinflammatory cytokine (Chen et al., 2022), in the risk of developing OCD. However, IL-18 itself has been associated with PCOS (Yang et al., 2011; Zhang et al., 2020), although the mechanisms underlying these associations are yet to be clarified. While the authors appropriately excluded pleiotropy in the MR analyses (Jin et al., 2021; Chen et al., 2022), further research is needed to ascertain the role of inflammation, PCOS, or other uninvestigated variables [such as metabolic syndrome, linking the previous two risk factors (March et al., 2010)], in the pathogenesis of OCD.
Another example of cases in which key MR assumptions may not be met is the utilization of fat mass and obesity-associated protein (FTO) alleles as IV for BMI. Although FTO alleles have been used as a genetic instrument for BMI in MR studies (Tan et al., 2019), evidence suggests that this might not be a reliable BMI indicator (Walter et al., 2015). Should this be the case, the relevance assumption (that the variant is associated with the risk factor, or exposure, in study), would not be met.
It is, thus, clear how MR studies at the same time guide and are guided by mechanistic, preclinical ones. In fact, the choice of genetic targets is clearly of the utmost importance for performing MR studies, as the whole analysis relies on this step. In some cases, articles employed genetic instruments, whose phenotypes are more difficultly identifiable, for instance, physical activity as measured by accelerometer in MDD, which seems to be protective for MDD (Choi et al., 2019). Although a relaxed threshold has been used in this study to select SNP for the genetic instrument, the findings are in agreement with existing evidence on the association between reduced physical activity and unipolar (Burton et al., 2013) or bipolar depression (Saccaro et al., 2021c) and actigraphy is an emerging technique, among others, for monitoring psychiatric diseases.
As mentioned above, thanks to the inclusion of results from different psychiatric disorders, this systematic review unveils transdiagnostic findings across several distinct psychiatric diseases that deserve further investigation. Besides the causal relationships between psychiatric disorders (ADHD and SCZ in particular) and smoking, which had already been identified by previous works focused on substance abuse (Treur et al., 2021), we identify some preliminary transdiagnostic evidence of the causal involvement of CRP levels in the pathophysiology of late-onset BD and SCZ, that warrants additional research, as mentioned above. In fact, this link is particularly relevant in light of the increasing evidence showing that peripheral inflammation is associated with neuroinflammation and can have an impact on central nervous system development and function (Felger, 2018), besides being associated with psychiatric symptoms and disorders (Saccaro et al., 2021a, 2021b). Our transdiagnostic approach revealed also interesting causal relationships between psychiatric disorders. Genetic liability to anxiety was causally associated with higher SCZ risk (Jones et al., 2020), and a DSM/ICD diagnosis of ADHD was causally associated with an increased risk of ASD (Peyre et al., 2021). While observational studies had highlighted some of these associations, MR allows to speculate on the direction of causality, which may lead to new avenues of pathophysiological research and prevention.
However, the aforementioned findings are in part undermined by minor methodological issues. In fact, some caveats must be reminded, since important assumptions need to be met before considering findings from MR trustworthy, as detailed in the introduction. Some of these are inherent in MR designs, and, thus, accounted for in the included articles. These include, for example, the statistical problems derived from the fact that, in MR, the randomization process is confided to the natural distribution in the population of genetic variants, which may sometimes be, in fact, nonrandom (e.g. due to linkage disequilibrium, or ‘cryptic relatedness’), which have been discussed in detail in the MR literature (Voight and Pritchard, 2005; Nitsch et al., 2006; Boef et al., 2015). Furthermore, the heterogeneity of the populations included in the MR studies may represent a confounder in the analyses performed by the papers that we reviewed. Population heterogeneity can impact both the definition of the genetic IV and of the outcome population. However, our stringent inclusion criteria (exclusively DSM/ICD-based diagnoses) reduce the risk of unwanted population heterogeneity in the samples of psychiatry patients.
Other important points include taking account of potential pleiotropy, choosing solid genetic instruments, and precise inclusion/exclusion criteria for patients and control. In fact, during the full-text screening of studies for inclusion in the present systematic review, we found that a considerable number of articles enrolled some or all psychiatric patients based on self-assessment questionnaires or clinical scores other than DSM or ICD criteria. Accurate and homogenous diagnostic criteria are essential in genetic studies in particular to avoid biases (such as selection bias) and to obtain results that are meaningful to the clinical population studied. For these reasons, we included fewer studies compared to other existing reviews of MR in specific psychiatric disorders (Belbasis et al., 2018; Köhler et al., 2018; Treur et al., 2021). For instance, the majority of studies on MDD did not use DSM nor ICD to diagnose MDD as an outcome and were therefore excluded. Similarly, many works on PTSD were based on data from the UK Biobank [which used self-reported data (Davis et al., 2020), or from the PGC-Posttraumatic Stress Disorder group (PGC-PTSD) (Duncan et al., 2018)], which included studies that did not diagnose patients based on DSM nor ICD (but, for example, based on a Structured Interview Scale derived from the PTSD Checklist or the modified PTSD Symptom Scale). These studies were, thus, not included. On the contrary, other disorders, for example, SCZ, were accurately diagnosed according to the DSM or the ICD in most of the papers we examined. Our stringent criteria on patients’ diagnoses allowed us to select a sample of studies that have very good scores in the NOS criteria ‘selections of patients’, in agreement with the overall fair scores in the other domains of the NOS evaluation (Supplementary eTable 2, Supplemental Digital Content 1, http://links.lww.com/PG/A287).
On the other side, the vast majority of the studies we included did accurately assess and account for possible pleiotropy in their analyses.
One last limitation common to some papers that we included (e.g. Choi et al., 2019; Milaneschi et al., 2019) may be the fact that researchers employed relaxed P-values thresholds for SNP selection to build the genetic instrument. While this method of relaxing the statistical threshold is often used in MR research when few significant SNPs are available, it exposes to the risk of basing the research on false genetic instruments or of not meeting the relevance assumption.
This review shows how MR can offer unique opportunities for unraveling causal links in risk factors and etiological elements of specific psychiatric diseases and across disorders. These relationships are otherwise difficult to uncover, but some methodological flaws in the existing literature limit the reliability of results of MR studies in psychiatry and probably underlie result heterogeneity. While most existing MR studies in psychiatry accurately employ and report analyses to exclude pleiotropy, as well as sources of genetic IVs, a few points are pivotal for future MR studies. These should first of all be informed by results from other study designs and use them to validate their findings and look for potential confounders; second, they should attempt to falsify their key assumptions, or at least clearly state them; and, finally, MR studies in psychiatry should carefully assess and report whether the patients included both for the exposure and the outcome analysis are indeed psychiatric patients with a certain DSM- or ICD-based psychiatric diagnosis.
Acknowledgements
Figure 1 was created with Biorender.com.
PROSPERO registration: the protocol is registered in PROSPERO(CRD42021285647).
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.psychgenetics.com.
References
- Andreu-Bernabeu Á, Díaz-Caneja CM, Costas J, De Hoyos L, Stella C, Gurriarán X, et al. (2022). Polygenic contribution to the relationship of loneliness and social isolation with schizophrenia. Nat Commun 13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arafat S, Minica CC. (2018). Fetal origins of mental disorders? an answer based on mendelian randomization. Twin Res Hum Genet 21:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belbasis L, Köhler CA, Stefanis N, Stubbs B, van Os J, Vieta E, et al. (2018). Risk factors and peripheral biomarkers for schizophrenia spectrum disorders: an umbrella review of meta-analyses. Acta Psychiatr Scand 137:88–97. [DOI] [PubMed] [Google Scholar]
- Boef AG, Dekkers OM, le Cessie S. (2015). Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int J Epidemiol 44:496–511. [DOI] [PubMed] [Google Scholar]
- Burton C, McKinstry B, Szentagotai Tătar A, Serrano-Blanco A, Pagliari C, Wolters M. (2013). Activity monitoring in patients with depression: a systematic review. J Affect Disord 145:21–28. [DOI] [PubMed] [Google Scholar]
- Byrne EM, Ferreira MAR, Xue A, Lindström S, Jiang X, Yang J, et al. (2019). Is schizophrenia a risk factor for breast cancer?-evidence from genetic data. Schizophr Bull 45:1251–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Cai B, Zhang H, Sun W, Wang Y, Zhou S, et al. (2019). Major depression and small vessel stroke: a Mendelian randomization analysis. J Neurol 266:2859–2866. [DOI] [PubMed] [Google Scholar]
- Chen J., et al. (2021). Cigarette smoking and schizophrenia: Mendelian randomisation study. Br J Psychiatry 218: 98–103. [DOI] [PubMed] [Google Scholar]
- Chen X., et al. (2022). Systemic inflammatory regulators and 7 major psychiatric disorders: a two-sample Mendelian randomization study. Prog Neuropsychopharmacol Biol Psychiatry 116:110534. [DOI] [PubMed] [Google Scholar]
- Cheng TS, Day FR, Lakshman R, Ong KK. (2020). Association of puberty timing with type 2 diabetes: a systematic review and meta-analysis. PLoS Med 17:e1003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KW, Chen CY, Stein MB, Klimentidis YC, Wang MJ, Koenen KC, Smoller JW; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium (2019). Assessment of bidirectional relationships between physical activity and depression among adults: a 2-sample Mendelian randomization study. JAMA Psychiatry 76:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TK, Obsteter J, Hall LS, Hayward C, Thomson PA, Smith BH, et al. (2017). Investigating shared aetiology between type 2 diabetes and major depressive disorder in a population based cohort. Am J Med Genet B Neuropsychiatr Genet 174: 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2019 Mental Disorders Collaborators (2019). Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990 – 2019: a systematic analysis from the Global Burden of Disease Study 2019 Accepted for publication at The Lancet Psychiatry. Lancet Psychiatry 0366:1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpo GD, Venna VR, McCullough LD, Teixeira AL. (2019). Systematic review on the involvement of the kynurenine pathway in stroke: pre-clinical and clinical evidence. Front Neurol 10:778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsbo AG, Kraglund KL, Buttenschøn HN, Johnsen SP, Andersen G, Mortensen JK. (2019). Serotonergic regulation and cognition after stroke: the role of antidepressant treatment and genetic variation. Cerebrovasc Dis 47:72–79. [DOI] [PubMed] [Google Scholar]
- Davies NM, Holmes MV, Davey Smith G. (2018). Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362:k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KAS, Coleman JRI, Adams M, Allen N, Breen G, Cullen B, et al. (2020). Mental health in UK Biobank - development, implementation and results from an online questionnaire completed by 157 366 participants: a reanalysis. BJPsych Open 6:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Ratanatharathorn A, Aiello AE, Almli LM, Amstadter AB, Ashley-Koch AE, et al. (2018). Largest GWAS of PTSD (N=20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol Psychiatry 23:666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdin CA, Khera AV, Kathiresan S. (2017). Mendelian randomization. JAMA 318:1925–1926. [DOI] [PubMed] [Google Scholar]
- Felger JC. (2018). Imaging the role of inflammation in mood and anxiety-related disorders. Curr Neuropharmacol 16:533–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage SH, Jones HJ, Burgess S, Bowden J, Davey Smith G, Zammit S, Munafò MR. (2017a). Assessing causality in associations between cannabis use and schizophrenia risk: a two-sample Mendelian randomization study. Psychol Med 47:971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage SH, Jones HJ, Taylor AE, Burgess S, Zammit S, Munafò MR. (2017b). Investigating causality in associations between smoking initiation and schizophrenia using Mendelian randomization. Sci Rep 7:40653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Meng LX, Ma KL, Liang J, Wang H, Gao Q, Wang T. (2019). The bidirectional causal relationships of insomnia with five major psychiatric disorders: a Mendelian randomization study. European Psychiatry 60:79–85. [DOI] [PubMed] [Google Scholar]
- Gill D, James NE, Monori G, Lorentzen E, Fernandez-Cadenas I, Lemmens R, et al.; International Stroke Genetics Consortium and the GISCOME Network (2019). Genetically determined risk of depression and functional outcome after ischemic stroke. Stroke 50:2219–2222. [DOI] [PubMed] [Google Scholar]
- Grau-Perez M, Agha G, Pang Y, Bermudez JD, Tellez-Plaza M. (2019). Mendelian Randomization and the environmental epigenetics of health: a systematic review. Curr Environ Health Rep 6:38–51. [DOI] [PubMed] [Google Scholar]
- Han KM, Ham BJ. (2021). How inflammation affects the brain in depression: a review of functional and structural MRI studies. J Clin Neurol (Korea) 17:503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig FP, Borges MC, Horta BL, Bowden J, Davey Smith G. (2017). Inflammatory biomarkers and risk of schizophrenia: a 2-sample Mendelian randomization study. JAMA Psychiatry 74:1226–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemani G, Bowden J, Davey Smith G. (2018). Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet 27:R195–R208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zuber V, Matthews PM, Elliott P, Tzoulaki J, Dehghan A. (2020). Sleep, major depressive disorder, and Alzheimer disease: a Mendelian randomization study. Neurology 95:e1963–e1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CF, Rivera M, Craddock N, Owen MJ, Gill M, Korszun A, et al. (2014). Relationship between obesity and the risk of clinically significant depression: Mendelian randomisation study. Br J Psychiatry 205: 24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoshita M, Numata S, Tajima A, Kinoshita M, Umehara H, Nakataki M, et al. (2016). A significant causal association between C-reactive protein levels and schizophrenia. Sci Rep 6:26105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Insel TR. (2014). The nimh research domain criteria (rdoc) project: precision medicine for psychiatry. Am J Psychiatry 395–397. [DOI] [PubMed] [Google Scholar]
- Jang SK, Saunders G, Liu M, Jiang Y, Liu DJ, Vrieze S; 23andMe Research Team (2022). Genetic correlation, pleiotropy, and causal associations between substance use and psychiatric disorder. Psychol Med 52:968–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefsen OH, Speed M, Speed D, Østergaard SD. (2021). Bipolar disorder and cannabis use: a bidirectional two-sample Mendelian randomization study. Addict Biol 26:e13030. [DOI] [PubMed] [Google Scholar]
- Jin L, Yu JE, Chen Y, Pang H, Sheng J, Huang H. (2021) ‘Polycystic ovary syndrome and risk of five common psychiatric disorders among European women: a two-sample Mendelian randomization study’, Front Genet 12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Hatoum AS, Deak JD, Polimanti R, Murray RM, Edenberg HJ, et al. (2021) ‘The relationship between cannabis and schizophrenia: a genetically informed perspective’, Addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HJ, Martin D, Lewis SJ, Davey Smith G, O'Donovan MC, Owen MJ, et al. (2020) ‘A Mendelian randomization study of the causal association between anxiety phenotypes and schizophrenia’, Am J Med Genet B Neuropsychiatr Genet 183: 360–369. [DOI] [PubMed] [Google Scholar]
- Kim K, Kim S, Myung W, Shim I, Lee H, Kim B, et al. (2021) ‘Shared genetic background between parkinson’s disease and schizophrenia: a two-sample mendelian randomization study’, Brain Sci 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim K, Myung W, Lee H, Kim H, Kim DK, Won HH. (2020) ‘Two-sample Mendelian randomization study for schizophrenia and breast cancer’, Precis Future Med 4:21–30. [Google Scholar]
- Köhler CA, Evangelou E, Stubbs B, Solmi M, Veronese N, Belbasis L, et al. (2018). Mapping risk factors for depression across the lifespan: an umbrella review of evidence from meta-analyses and Mendelian randomization studies. J Psychiatr Res 103:189–207. [DOI] [PubMed] [Google Scholar]
- Kwok MK, Leung GM, Schooling CM. (2016). Habitual coffee consumption and risk of type 2 diabetes, ischemic heart disease, depression and Alzheimer’s disease: a Mendelian randomization study. Sci Rep 6:36500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chen P, Chen J, Xu Y, Wang Q, Li X, et al. (2018). Glucose and insulin-related traits, type 2 diabetes and risk of schizophrenia: a Mendelian randomization study. EBioMedicine 34:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo BC, Chen GY, Núñez G, Caruso R. (2021). Gut microbiota and systemic immunity in health and disease. Int Immunol 33:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CK, Mertz D, Loeb M. (2014). Newcastle-Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Wang Z, Georgakis MK, Lin H, Zheng L. (2021). Genetic liability to depression and risk of coronary artery disease, myocardial infarction, and other cardiovascular outcomes. Journal of the American Heart Association 10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Wen Z, Li Y, Chen Z, Long X, Bai Y, et al. (2020). Assessment causality in associations between serum uric acid and risk of schizophrenia: a two-sample bidirectional Mendelian randomization study. Clin Epidemiol 12:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharjan DT, Syed AAS, Lin GN, Ying W. (2021). Testosterone in female depression: a meta-analysis and Mendelian randomization study. Biomolecules 11:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal M, Donnelly R, Elkabes S, Zhang P, Davini D, David BT, Ponzio NM. (2013). Maternal immune stimulation during pregnancy shapes the immunological phenotype of offspring. Brain Behav Immun 33:33–45. [DOI] [PubMed] [Google Scholar]
- March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. (2010). The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 25:544–551. [DOI] [PubMed] [Google Scholar]
- Michalek JE, Kepa A, Vincent J, Frissa S, Goodwin L, Hotopf M, et al. (2017). Genetic predisposition to advanced biological ageing increases risk for childhood-onset recurrent major depressive disorder in a large UK sample. J Affect Disord 213:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaneschi Y, Peyrot WJ, Nivard MG, Mbarek H, Boomsma DI, WJH Penninx B. (2019). A role for vitamin D and omega-3 fatty acids in major depression? An exploration using genomics. Transl Psychiatry 9:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CE, Walker AK, Weickert CS. (2021). Neuroinflammation in schizophrenia: the role of nuclear factor kappa B. Transl Psychiatry 11:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni JJ, Xu Q, Yan SS, Han BX, Zhang H, Wei XT, et al. (2021). Gut microbiota and psychiatric disorders: a two-sample Mendelian randomization study. Front Microbiol 12:737197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch D, Molokhia M, Smeeth L, DeStavola BL, Whittaker JC, Leon DA. (2006). Limits to causal inference based on Mendelian randomization: a comparison with randomized controlled trials. Am J Epidemiol 163:397–403. [DOI] [PubMed] [Google Scholar]
- Pasman JA, Verweij KJH, Gerring Z, Stringer S, Sanchez-Roige S, Treur JL, et al.; 23andMe Research Team; Substance Use Disorders Working Group of the Psychiatric Genomics Consortium; International Cannabis Consortium (2018). GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal influence of schizophrenia. Nat Neurosci 21:1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce BD. (2022). Mendelian randomization of cytokines in schizophrenia and depression: what does this tell us about causal chains in these illnesses? Brain Behav Immun 99:130–131. [DOI] [PubMed] [Google Scholar]
- Perry BI, Upthegrove R, Kappelmann N, Jones PB, Burgess S, Khandaker GM. (2021a). Associations of immunological proteins/traits with schizophrenia, major depression and bipolar disorder: a bi-directional two-sample mendelian randomization study. Brain Behav Immun 97:176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry BI, Burgess S, Jones HJ, Zammit S, Upthegrove R, Mason AM, et al. (2021b). The potential shared role of inflammation in insulin resistance and schizophrenia: a bidirectional two-sample mendelian randomization study. PLoS Med 18:e1003455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T, Nüllig L, Antel J, Naaresh R, Laabs BH, Tegeler L, et al. (2020). The role of genetic variation of BMI, body composition, and fat distribution for mental traits and disorders: a look-up and Mendelian randomization study. Front Genet 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyre H, Schoeler T, Liu C, Williams CM, Hoertel N, Havdahl A, Pingault JB. (2021). Combining multivariate genomic approaches to elucidate the comorbidity between autism spectrum disorder and attention deficit hyperactivity disorder. J Child Psychol Psychiatry Allied Discip. [DOI] [PubMed] [Google Scholar]
- Pierce BL, Burgess S. (2013). Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol 178:1177–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimanti R, Gelernter J, Stein DJ. (2018). Genetically determined schizophrenia is not associated with impaired glucose homeostasis. Schizophr Res 195:286–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins BP, Abbasi A, Wong A, Vaez A, Nolte I, Franceschini N, et al. (2016). Investigating the causal relationship of C-reactive protein with 32 complex somatic and psychiatric outcomes: a large-scale cross-consortium Mendelian randomization study. PLoS Med 13:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoff DB, Clarke TK, Adams MJ, McIntosh AM, Davey Smith G, Jung J, Lohoff FW. (2021). Educational attainment impacts drinking behaviors and risk for alcohol dependence: results from a two-sample Mendelian randomization study with ~780,000 participants. Mol Psychiatry 26:1119–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccaro LF, Schilliger Z, Perroud N, Piguet C. (2021a). Inflammation, anxiety, and stress in attention-deficit/hyperactivity disorder. Biomedicines 9:1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccaro LF, Schilliger Z, Dayer A, Perroud N, Piguet C. (2021b). Inflammation, anxiety, and stress in bipolar disorder and borderline personality disorder: a narrative review. Neurosci Biobehav Rev 127:184–192. [DOI] [PubMed] [Google Scholar]
- Saccaro LF, Amatori G, Cappelli A, Mazziotti R, Dell’Osso L, Rutigliano G. (2021c). Portable technologies for digital phenotyping of bipolar disorder: a systematic review. J Affect Disord 295:323–338. [DOI] [PubMed] [Google Scholar]
- Saccaro LF, Pico F, Chadenat ML, Richard O, Launay JM, Bastenaire B, et al. (2022a). Platelet, plasma, urinary axis markers in hyperacute brain ischemia patients: a prospective study. Front Neurol 12:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccaro LF, Crokaert J, Piguet C. (2022b). Structural and functional MRI correlates of inflammation in bipolar disorder: a systematic review. PsyArXiv. [DOI] [PubMed] [Google Scholar]
- Sequeira ME, Lewis SJ, Bonilla C, Smith GD, Joinson C. (2017). Association of timing of menarche with depressive symptoms and depression in adolescence: Mendelian randomisation study. Br J Psychiatry 210:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Qian W, Wang W, Yu S, Lin GN. (2021). Mendelian randomization studies of brain MRI yield insights into the pathogenesis of neuropsychiatric disorders. BMC Genomics 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga F, Gibson M, Dawson S, Smith GD, Munafò MR, Higgins JP. (2021). Tools for the assessment of quality and risk of bias in Mendelian randomization studies: a systematic review 1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, et al.; eQTLGen Consortium; BIOS Consortium; Bipolar Disorder Working Group of the Psychiatric Genomics Consortium (2019). Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet 51:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AG, Kifley A, Flood VM, Holliday EG, Scott RJ, Cumming RG, et al. (2019). Evaluating the associations between obesity and age-related cataract: a Mendelian randomization study. Am J Clin Nutr 110:969–976. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Burgess S, Ware JJ, Gage SH, Richards JB, Davey Smith G, Munafò MR. (2016). Investigating causality in the association between 25(OH)D and schizophrenia. Sci Rep 6:26496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka Y, Numata S, Kinoshita M, Umehara H, Watanabe SY, Nakataki M, et al. (2018). Decreased serum pyridoxal levels in schizophrenia: meta-analysis and Mendelian randomization analysis. J Psychiatry Neurosci 43:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treur JL, Munafò MR, Logtenberg E, Wiers RW, Verweij KJH. (2021). Using Mendelian randomization analysis to better understand the relationship between mental health and substance use: a systematic review. Psychol Med 51:1593–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen JM, Wootton RE, Treur JL, Sallis HM, Jones HJ, Zammit S, et al. (2021). Smoking and the risk for bipolar disorder: evidence from a bidirectional Mendelian randomisation study. Br J Psychiatry 218:88–94. [DOI] [PubMed] [Google Scholar]
- Vilar-Ribó L, Sánchez-Mora C, Rovira P, Richarte V, Corrales M, Fadeuilhe C, et al. (2021). Genetic overlap and causality between substance use disorder and attention-deficit and hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 186:140–150. [DOI] [PubMed] [Google Scholar]
- Voight BF, Pritchard JK. (2005). Confounding from cryptic relatedness in case-control association studies. PLoS Genet 1:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ER, McGee RE, Druss BG. (2015). Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 72:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S, Kubzansky LD, Koenen KC, Liang L, Tchetgen Tchetgen EJ, Cornelis MC, et al. (2015). Revisiting mendelian randomization studies of the effect of body mass index on depression. Am J Med Genet B Neuropsychiatr Genet 168:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wium-Andersen MK, Ørsted DD, Tolstrup JS, Nordestgaard BG. (2015a). Increased alcohol consumption as a cause of alcoholism, without similar evidence for depression: a Mendelian randomization study. Int J Epidemiol 44:526–539. [DOI] [PubMed] [Google Scholar]
- Wium-Andersen MK, Orsted DD, Nordestgaard BG. (2014). Elevated C-reactive protein, depression, somatic diseases, and all-cause mortality: a mendelian randomization study. Biol Psychiatry 76:249–257. [DOI] [PubMed] [Google Scholar]
- Wium-Andersen MK, Ørsted DD, Nordestgaard BG. (2015b). Tobacco smoking is causally associated with antipsychotic medication use and schizophrenia, but not with antidepressant medication use or depression. Int J Epidemiol 44:566–577. [DOI] [PubMed] [Google Scholar]
- Wium-Andersen MK, Ørsted DD, Nordestgaard BG. (2016). Elevated C-reactive protein and late-onset bipolar disorder in 78 809 individuals from the general population. Br J Psychiatry 208:138–145. [DOI] [PubMed] [Google Scholar]
- Wootton RE, Richmond RC, Stuijfzand BG, Lawn RB, Sallis HM, Taylor GMJ, et al. (2020). Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. Psychol Med 50:2435–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton RE, Jones HJ, Sallis HM. (2022). Mendelian randomisation for psychiatry: how does it work, and what can it tell us? Mol Psychiatry 27:53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yan B, Zhao B, Fan Y, He X, Yang L, et al. (2020). Assessing the causal effects of human serum metabolites on 5 major psychiatric disorders. Schizophr Bull 46:804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Qiao J, Li R, Li MZ. (2011). Is interleukin-18 associated with polycystic ovary syndrome? Reprod Biol Endocrinol 9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang X, Xu J, Zhu Y, Chen X, Hu Y. (2020). IL-18 and IL-18 binding protein concentration in ovarian follicular fluid of women with unexplained infertility to PCOS during in vitro fertilization. J Reprod Immunol 138:103083. [DOI] [PubMed] [Google Scholar]
- Zhao SS, Qian Y, Mackie SL, Wen C, Mao Y. (2021). Genetically predicted serum urate levels have no causal role on depression or other psychiatric disorders. Clin Rheumatol 40:3729–3733. [DOI] [PubMed] [Google Scholar]
- Zhuang Z, Yang R, Wang W, Qi L, Huang T. (2020). Associations between gut microbiota and Alzheimer’s disease, major depressive disorder, and schizophrenia. J Neuroinflammation 17:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.