Background:
There are no evidence-based recommendations on the optimal time point to initiate non–vitamin K antagonist oral anticoagulants (NOACs) after acute ischemic stroke in patients with atrial fibrillation. We aimed to investigate the efficacy and safety of early versus delayed initiation of NOAC in these patients.
Methods:
TIMING (Timing of Oral Anticoagulant Therapy in Acute Ischemic Stroke With Atrial Fibrillation) was a registry-based, randomized, noninferiority, open-label, blinded end-point study at 34 stroke units using the Swedish Stroke Register for enrollment and follow-up. Within 72 hours from stroke onset, patients were randomized to early (≤4 days) or delayed (5–10 days) NOAC initiation, with choice of NOAC at the investigators’ discretion. The primary outcome was the composite of recurrent ischemic stroke, symptomatic intracerebral hemorrhage, or all-cause mortality at 90 days. The prespecified noninferiority margin was 3%. Secondary outcomes included the individual components of the primary outcome.
Results:
Between April 2, 2017, and December 30, 2020, 888 patients were randomized to either early (n=450) or delayed (n=438) initiation of NOAC. No patient was lost to 90-day follow-up. Mean age was 78.3 years (SD, 9.9 years); 46.2% were women; 49.1% had previously known atrial fibrillation; and 17.5% prior stroke. The primary outcome occurred in 31 patients (6.89%) assigned to early initiation and in 38 patients (8.68%) assigned to delayed NOAC initiation (absolute risk difference, −1.79% [95% CI, −5.31% to 1.74%]; Pnoninferiority=0.004). Ischemic stroke rates were 3.11% and 4.57% (risk difference, −1.46% [95% CI, −3.98% to 1.07%]) and all-cause mortality rates were 4.67% and 5.71% (risk difference, −1.04% [95% CI, −3.96% to 1.88%]) in the early and delayed groups, respectively. No patient in either group experienced symptomatic intracerebral hemorrhage.
Conclusions:
Early initiation was noninferior to delayed start of NOAC after acute ischemic stroke in patients with atrial fibrillation. Numerically lower rates of ischemic stroke and death and the absence of symptomatic intracerebral hemorrhages implied that the early start of NOAC was safe and should be considered for acute secondary stroke prevention in patients eligible for NOAC treatment.
Registration:
URL: http://www.clinicaltrials.gov; Unique identifier: NCT02961348.
Keywords: anticoagulants, atrial fibrillation, ischemic stroke, secondary prevention
Clinical Perspective.
What Is New?
The TIMING study (Timing of Oral Anticoagulant Therapy in Acute Ischemic Stroke With Atrial Fibrillation) was the first randomized controlled study investigating relevant clinical end points (composite of new ischemic stroke, symptomatic intracerebral hemorrhage, or all-cause death) after initiation of non–vitamin K antagonist oral anticoagulant (NOAC) within the first 10 days after acute ischemic stroke in patients with atrial fibrillation.
Initiating NOAC treatment within 4 days after ischemic stroke was noninferior to initiation of NOAC between days 5 and 10.
No patient experienced symptomatic intracerebral hemorrhage in any study group, and rates of ischemic stroke and death were numerically lower in patients randomized to early initiation of NOAC.
What Are the Clinical Implications?
It seems both safe and reasonable to consider early initiation of NOAC after acute ischemic stroke in patients with atrial fibrillation.
The TIMING study results may facilitate shared decision making between physicians and patients to ensure adequate acute secondary stroke prevention in the early phase of stroke unit care.
Whether early initiation is superior to delayed start remains to be established and requires further investigation in ongoing trials (eg, OPTIMAS [Optimal Timing of Anticoagulation After Acute Ischemic Stroke], NCT03759938; ELAN [Early Versus Late Initiation of Direct Oral Anticoagulants in Post-Ischemic Stroke Patients With Atrial Fibrillation], NCT03148457; and START [Optimal Delay Time to Initiate Anticoagulation After Ischemic Stroke in Atrial Fibrillation], NCT03021928).
Atrial fibrillation is a common cause of ischemic stroke,1–3 and oral anticoagulation treatment, preferably non–vitamin K antagonist oral anticoagulant (NOAC), is highly recommended for stroke prevention.3–7 The optimal time point for initiating anticoagulation after acute ischemic stroke is uncertain8 because the pivotal large-scale studies of NOAC versus warfarin excluded patients with a recent stroke (within 7–30 days).9–12 Because of the sparse evidence, current international guidelines do not provide specific recommendations on the best time point to start anticoagulation in this setting.3–6 The risk of ischemic stroke recurrence seems highest in the days immediately after an ischemic stroke,13,14 but hemorrhagic transformation of the ischemic lesion or intracerebral hemorrhage could offset the advantages of acute secondary prevention.15 Several observational studies indicate possible clinical benefit of early initiation of NOAC therapy to prevent recurrent ischemic stroke.16 Randomized clinical trials on the optimal time point to start NOAC early after acute ischemic stroke in patients with atrial fibrillation are highly warranted,2,8 yet very limited evidence is available,15,17,18 and the lack of consensus among stroke physicians is therefore not surprising.19,20 We aimed to evaluate the efficacy and safety of early versus delayed initiation of NOAC in patients with acute ischemic stroke and atrial fibrillation.
Methods
Design
The TIMING study (Timing of Oral Anticoagulant Therapy in Acute Ischemic Stroke With Atrial Fibrillation) design and protocol have been published.21 In brief, this was an investigator-led, prospective, registry-based, multicenter, open-label, noninferiority, randomized controlled study in 34 of 72 stroke units in Sweden (Table S1). We used the Swedish Stroke Register for enrollment, randomization, and follow-up. The inclusion of a randomization module in the Swedish Stroke Register combines the advantages of a prospective randomized study design with the strengths of a comprehensive clinical quality register and facilitates patient enrollment, data collection, and follow-up.22 The Regional Ethics Committee in Uppsala, Sweden, approved the TIMING study (reference 2016/359).
Patients
The target population was adults (≥18 years of age) with atrial fibrillation and a recent (within 72 hours of symptom onset) ischemic stroke who were eligible for and willing to start NOAC treatment. Atrial fibrillation included paroxysmal, persistent, and permanent atrial fibrillation, which was either previously known or diagnosed during index hospitalization. Patients with ongoing oral anticoagulant therapy at the index stroke were eligible if prior NOAC therapy was interrupted (≥2 days) at the index stroke or the international normalized ratio was ≤1.7 in patients on prior warfarin. After reperfusion therapy, control brain imaging had to be performed before the patient was considered eligible. Patients were ineligible if they had contraindication to NOAC therapy (eg, ongoing bleeding or mechanical heart valve prosthesis) or if they previously participated in the TIMING study. Patients or their nearest relative or representative if the patient did not have the capacity to write provided written informed consent.
Randomization
Eligible patients were‚ within 72 hours from stroke onset and, after written informed consent, randomly allocated to early (≤4 days from stroke onset) or delayed (≥5–10 days from stroke onset) initiation of NOAC by a central computer within the Swedish Stroke Register infrastructure. Participants were randomized in a 1:1 ratio to 1 of the 2 treatment arms. Allocation was stratified by study site.
The choice of NOAC (ie, apixaban, dabigatran, edoxaban, or rivaroxaban) and day to start treatment within the assigned time interval were at the discretion of the treating physician, and all 4 agents were available and reimbursed in Sweden during the entire study period.
Procedures
Study procedures have previously been discussed in detail.21 Web-based case report forms integrated into the Swedish Stroke Register were used to supply information about patients’ demographics, comorbidities, functional status, and drug therapy, including starting time of NOAC, and data were entered by a dedicated study nurse or stroke coordinator at each participating stroke unit. The clinical outcome events were considered within 90 days.
A study-specific event form was used for reporting primary outcome events and major bleeding events within 28 days after index stroke because the regular registry-based surveillance covers only 1 stroke per hospitalization. After 28 days from the index stroke, any new stroke was captured by routine registration in the Swedish Stroke Register. A selection of data, prespecified as of interest for the study, were automatically transferred from the register to the TIMING study database. The regular 90-day follow-up in the Swedish Stroke Register, by postal questionnaire or telephone interviews, was supplemented with 2 additional study questions on persistence with NOAC therapy and any rehospitalization. Patients’ vital status and answers to study-specific questions were verified by local study personnel through medical record reviews to ensure that no study end points were missed or incorrectly reported.
To further minimize incompleteness and to increase the correctness of reported data, logical controls for critical data were programmed into the Swedish Stroke Register. Any changes of data in the Swedish Stroke Register were tracked (audit trail) in the study database. All baseline and outcome data were scrutinized for completeness, range, and consistency within the web-based case report forms by central study personnel.
An independent data and safety monitoring board monitored patient safety and the study conduct.
Outcomes
The primary outcome was a composite of recurrent ischemic stroke, symptomatic intracerebral hemorrhage, or all-cause death within 90 days of index stroke. Recurrent ischemic stroke was defined as a new focal neurological deficit of sudden onset lasting at least 24 hours (or <24 hours if after therapeutic intervention, ie, thrombolysis or thrombectomy, or if the deficit resulted in death within 24 hours), occurring >24 hours after the index ischemic stroke regardless of vascular territory, and not attributable to edema, brain shift, hemorrhagic transformation, intercurrent illness, hypoxia, or drug toxicity.23 Symptomatic intracerebral hemorrhage was defined as a new focal neurological deficit of sudden onset lasting for at least 24 hours and with documented intraparenchymal hematoma (≥10 mm) on imaging (computed tomography or magnetic resonance imaging), including hemorrhagic transformation of the index ischemic stroke, or a new hospitalization for intracerebral hemorrhage registered in the Swedish Stroke Register. Intracerebral hemorrhage was defined as symptomatic if there was an increase of ≥4 points in total National Institutes of Health Stroke Scale (NIHSS) score or ≥2 points in 1 NIHSS category.24
Secondary outcomes included the individual components of the primary outcome and major bleeding events. Bleedings were considered major if they resulted in death or were life threatening, which includes all intracranial hemorrhages regardless of size and symptoms, according to the International Society on Thrombosis and Haemostasis definition,25 or consumed major health-care resources, that is, bleeding events leading to hospitalization.
Observational Cohort in the Swedish Stroke Register
About 5000 patients with ischemic stroke and atrial fibrillation are registered annually in the Swedish Stroke Register by the 72 stroke units in Sweden.1 To investigate whether TIMING study patients had characteristics similar to those of individuals not included in this randomized study, we examined cohorts of nonrandomized patients routinely registered and followed up for 90 days in the Swedish Stroke Register during the same time period as the TIMING study at all Swedish stroke units and separately patients not enrolled in the randomized study at the 34 stroke units participating in TIMING (Figure S1). Patients in these cohorts were, similar to the TIMING study patients, selected by the criteria of acute ischemic stroke and atrial fibrillation and receiving NOAC within 10 days from stroke onset. Patients without a known date of stroke onset or NOAC start were excluded. The Swedish Ethical Review Authority approved this observational study (reference 2021-00430).
Statistical Analyses
The sample size calculation in TIMING was based on findings from the observational Pre-TIMING study.26 The proportion of events of the primary composite variable at 90 days was assumed to be 12% in both treatment regimens. The absolute noninferiority margin was set to 3%. The selection of noninferiority margin was based on magnitudes of commonly observed and clinically meaningful relative and absolute risk reductions in a wide range of secondary stroke preventive trials.27 On the basis of these assumptions, a sample size of 1451 patients per group was considered to be sufficient to test the primary hypothesis (noninferiority) with a power of 80% using a significance level of 5%. Although there is no loss to follow-up in the National Board of Health registers, we conservatively set the sample size to 3000 patients.
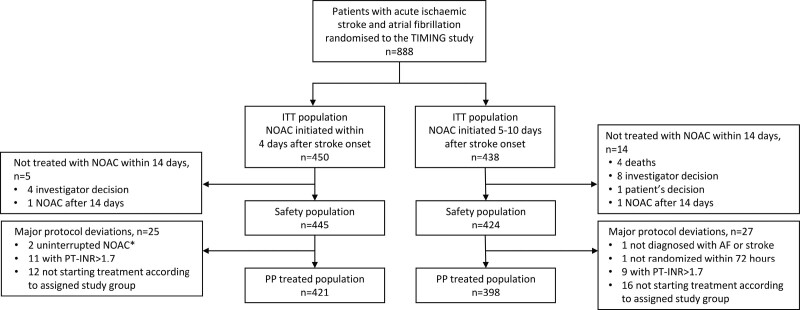
The primary outcome analyses were performed in the intention-to-treat population, consisting of all randomized patients, and in the per-protocol population, which excluded those deviating from inclusion criteria or who did not start NOAC treatment according to the assigned study group (Figure 1). Safety analyses were performed in the safety population, that is, all randomized patients who received at least 1 dose of NOAC during the first 14 days after randomization.
Figure 1.
Study flowchart. INR indicates international normalized ratio; ITT, intention-to-treat; NOAC, non–vitamin K antagonist oral anticoagulant; PP, per-protocol; and TIMING, Timing of Oral Anticoagulant Therapy in Acute Ischemic Stroke With Atrial Fibrillation. *On treatment with NOAC at index event and <2-day (48-hour) treatment interruption.
The prespecified hypotheses to be tested were the noninferiority hypothesis for early start versus delayed start of NOAC treatment and superiority if noninferiority was demonstrated.28 The primary composite outcome of new ischemic stroke, symptomatic intracerebral hemorrhage, or all-cause mortality within 90 days was analyzed with the use of an ordinary z test for proportions, with a Cox proportional hazards model as the sensitivity analysis. Noninferiority was tested by comparing the upper limit of the 2-sided 95% CI for the absolute difference in proportions of events for the 2 treatment regimens with the predefined delta value (ie, the noninferiority margin of 3%). Superiority was tested with a 2-sided test at a significance level of 0.05. Differences between treatment regimens for the individual components of the primary composite variable were displayed in Kaplan-Meier plots and evaluated with Cox proportional hazards models. We performed analyses of subgroups (prespecified before database lock) for the primary end point in the intention-to-treat population using logistic regression models that included treatment group, subgroup factor, and the interaction between treatment group and subgroup. The subgroups were based on age, sex, prior atrial fibrillation, prior diabetes, prior stroke or transient ischemic attack, drugs (antihypertensive, antithrombotic) on admission, stroke severity, and reperfusion therapy.
The study statistician and the coordinating investigators prepared the statistical analysis plan without reference to collected data, and the study steering committee approved the statistical analysis plan before database lock while still blinded to treatment allocation. All statistical analyses were performed with SAS software version 9.4 (SAS Institute Inc, Cary, NC).
Results
From April 2, 2017, to December 30, 2020, 888 patients were randomly assigned to early (n=450) or delayed (n=438) start of NOAC treatment (Figure 1). Patients were followed up and assessed for the primary end point at 90 days, and no patients were lost to follow-up.
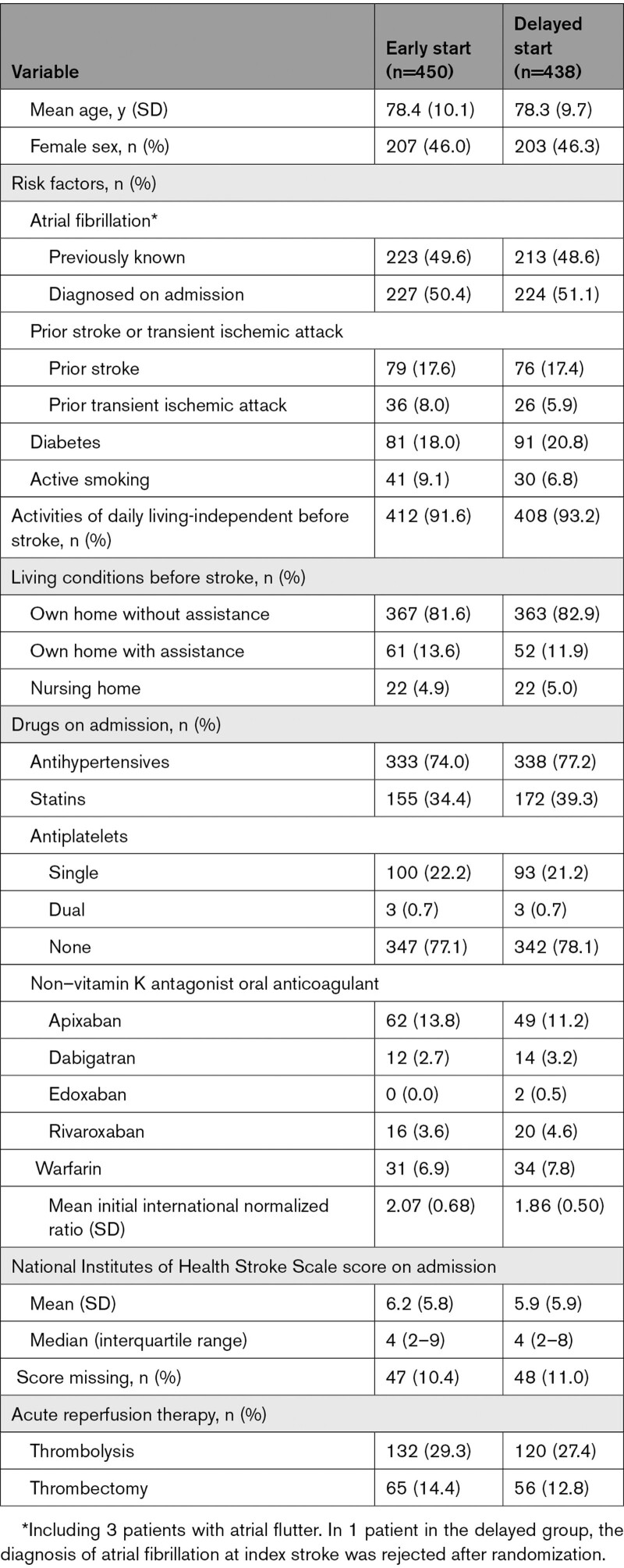
Baseline characteristics are summarized in the Table. Mean age was 78.3 years (SD, 9.9 years); 410 (46.2%) were women; 436 (49.1%) had previously known atrial fibrillation; and 155 (17.5%) had a prior stroke. Before hospitalization, 240 (27.0%) had oral anticoagulation (NOAC or warfarin). Median and mean admission NIHSS scores were 4 (range, 0–26) and 6.1 (SD, 5.9), respectively. Before randomization, 192 patients (21.6%) received thrombolysis only, and 121 patients (13.6%) underwent thrombectomy, of whom 60 (49.6%) received thrombolysis before the thrombectomy. Median NIHSS score at 24 hours after reperfusion therapy was 2 (0–22) in the 313 patients receiving any reperfusion therapy.
Table.
Baseline Characteristics
The 9063 patients in the nonrandomized observational cohort registered in the Swedish Stroke Register during the same time period as the TIMING study was conducted, including 5068 patients not enrolled in the randomized study at stroke units participating in the TIMING study, are presented in Table S2 and Figure S1. The baseline characteristics of the randomized TIMING study patients were similar to those of these observational cohorts in terms of age, sex, prior stroke, and stroke severity, but independence in activities of daily living, newly diagnosed atrial fibrillation, and use of reperfusion therapy were more common in the randomized TIMING study patients.
Among randomized patients, NOAC was on average initiated 66.8 hours (SD, 31.5 hours), that is, on day 3, after stroke onset in the early group and 116.8 hours (SD, 36.5 hours), that is, on day 5, after stroke onset in the delayed group. In total, 17 patients did not start NOAC treatment: 4 in the early and 13 in the delayed group (Figure 1). The choice of NOAC, which was at the discretion of the treating physician, in the early and delayed groups was apixaban in 54.9% and 54.8%, dabigatran in 27.8% and 25.1%, edoxaban in 3.3% and 3.7%, and rivaroxaban in 13.1% and 13.5% of the patients, respectively.
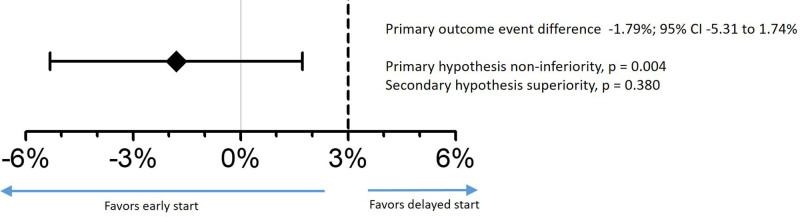
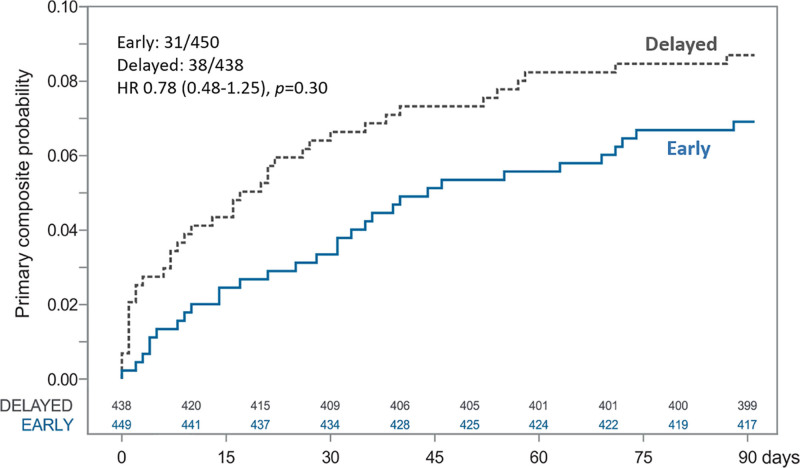
The primary composite outcome of new ischemic stroke, symptomatic intracerebral hemorrhage, or all-cause mortality occurred in 6.89% of the patients assigned to early start and in 8.68% of those assigned to delayed start of NOAC therapy. Early start was noninferior to delayed start of NOAC therapy, with an absolute risk difference of −1.79% (upper limit of the 2-sided 95% CI, 1.74%; Pnoninferiority=0.004). However, early start was not superior to delayed start of NOAC therapy (Figure 2). Time to events and sensitivity analysis for the primary outcome is depicted in Figure 3 and Figure S2A.
Figure 2.
Risk difference in the primary composite outcome for early vs delayed initiation of NOAC at 90 days. Primary outcome was a composite of ischemic stroke, symptomatic intracerebral hemorrhage, or all-cause mortality. Primary hypothesis testing for noninferiority at an absolute 3% margin, and secondary hypothesis testing for superiority. NOAC indicates non–vitamin K antagonist oral anticoagulant.
Figure 3.
Time to the primary composite outcome and Cox proportional hazards analysis for early vs delayed initiation of NOAC until 90 days. Primary outcome was a composite of ischemic stroke, symptomatic intracerebral hemorrhage, or all-cause mortality. HR indicates hazard ratio; and NOAC, non–vitamin K antagonist oral anticoagulant.
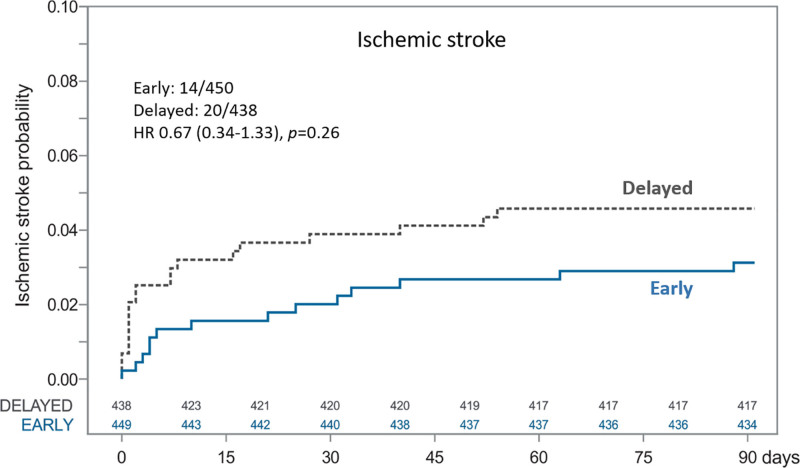
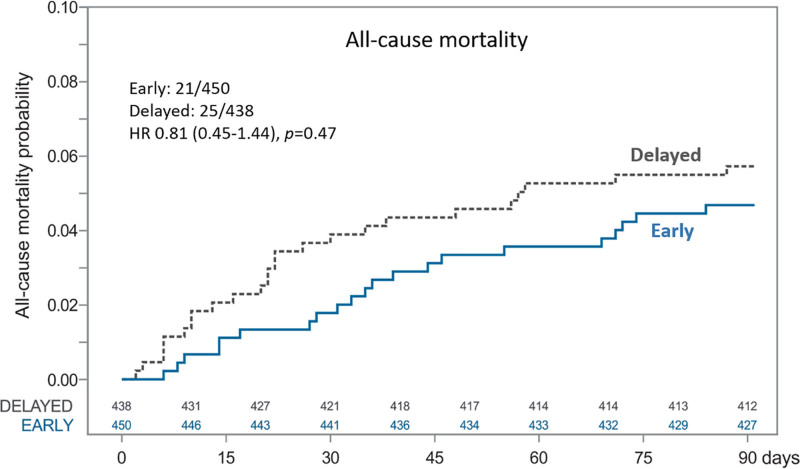
Ischemic stroke rates were 3.11% in the early group and 4.57% in the delayed group, with a risk difference of –1.46% (95% CI, −3.98% to 1.07%) for early versus delayed treatment start. No patient experienced a symptomatic intracerebral hemorrhage in either of the study groups during 90 days of follow-up. All-cause mortality rates were 4.67% in the early group and 5.71% in the delayed group (risk difference, −1.04% [95% CI, −3.96% to 1.88%] for early versus delayed treatment start). Time to ischemic stroke is illustrated in Figure 4 and Figure S2B, and time to death is illustrated in Figure 5 and Figure S2C.
Figure 4.
Time to ischemic stroke and Cox proportional hazards analysis for early vs delayed initiation of NOAC until 90 days. HR indicates hazard ratio; and NOAC, non–vitamin K antagonist oral anticoagulant.
Figure 5.
Time to all-cause mortality and Cox proportional hazards analysis for early vs delayed initiation of NOAC until 90 days. HR indicates hazard ratio; and NOAC, non–vitamin K antagonist oral anticoagulant.
During the first 28 days after randomization, 10 major bleeding events occurred: 7 in the early and 3 in the delayed treatment group. Three of these events were intracerebral hemorrhages not fulfilling the symptom criteria in the outcome definition: 2 cases of hemorrhagic transformation in the early group, which occurred 2 and 17 days after randomization (difference in NIHSS scores before and at event, 1 and 0 points), and 1 case of hemorrhagic transformation in the delayed treatment group, which occurred 13 days after randomization (difference in NIHSS scores, 0 points).
The per-protocol–treated population (Figure 1) consisted of 819 patients (92.2% of the intention-to-treat population). The primary outcome was observed in 28 (6.65%) of the 421 patients receiving early treatment and in 30 (7.54%) of the 398 patients receiving delayed treatment (risk difference, −0.89% [95% CI, −4.41% to 2.63%]; Pnoninferiority=0.0384). Ischemic stroke was observed in 13 patients (3.09%) in the early group and 16 patients (4.02%) in the delayed group (risk difference, −0.93% [95% CI, −3.47% to 1.61%]). All-cause mortality was observed in 19 patients (4.51%) in the early and 19 (4.77%) in the delayed group (risk difference, −0.26% [95% CI, −3.15% to 2.62%}).
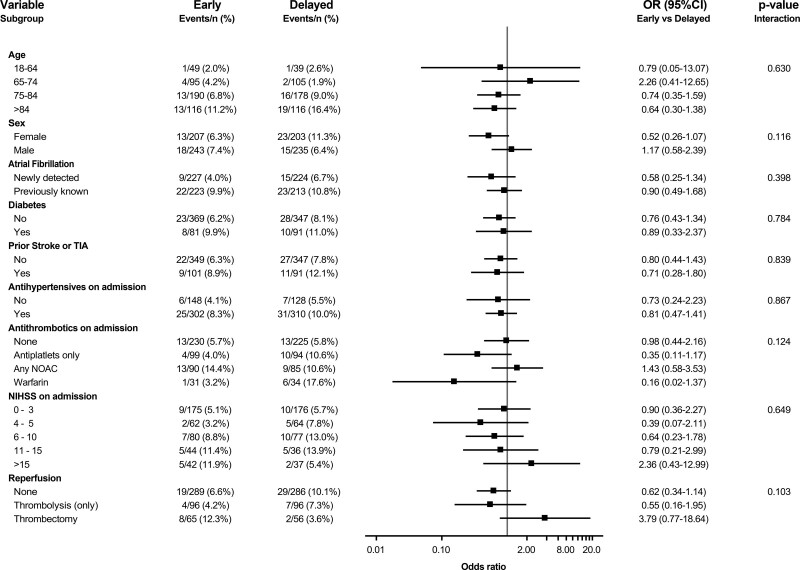
The primary outcome of ischemic stroke, intracranial hemorrhages, or all-cause mortality at 90 days was consistent across all prespecified subgroups (Figure 6).
Figure 6.
Odds ratio for the primary composite outcome in prespecified subgroups. Logistic regression model with treatment group, subgroup factor, and interaction between treatment group and subgroup. NIHSS indicates National Institutes of Health Stroke Scale; NOAC, non–vitamin K antagonist oral anticoagulant; OR, odds ratio; and TIA, transient ischemic attack.
Discussion
TIMING was the first randomized controlled study to evaluate the efficacy and safety of initiation of treatment with NOAC within 10 days of acute ischemic stroke in patients with atrial fibrillation. Early start was noninferior to delayed start of NOAC in terms of the primary composite outcome of ischemic stroke, symptomatic intracerebral hemorrhage, or death. There were numerically lower rates of ischemic stroke and death in patients assigned to early initiation of NOAC, and no patient experienced symptomatic intracerebral hemorrhage in either of the study groups during 90 days of follow-up.
Oral anticoagulation therapy is the cornerstone of primary and secondary ischemic stroke prevention in patients with atrial fibrillation.3,5 During the past decade, NOACs have been established as the preferred alternative to warfarin in this setting on the basis of 4 pivotal randomized controlled trials demonstrating them to be at least as effective for stroke prevention as warfarin with equal or lower risk for major bleeding events, most prominently substantially lower risks for intracranial haemorrhages.7,9–12 The results were also consistent in a meta-analysis of patients with a prior stroke in these 4 pivotal trials, revealing the risk of hemorrhagic stroke to be at least halved.29 It is notable that these trials do not provide any evidence for the optimal time point to start oral anticoagulant treatment in the early phase after acute ischemic stroke because recent stroke (within 7–30 days) was an exclusion criterion. In addition, <30% of the patients in these trials had a prior stroke or transient ischemic attack, except for the rivaroxaban versus warfarin study, in which 52% of patients had a prior stroke, albeit at a mean of 551 days before randomization.30
The high risk of stroke recurrence in the acute phase after ischemic stroke provides a rationale for very early stroke preventive therapy.13,14 The potential advantage of early oral anticoagulation must be weighed against the risk of hemorrhagic transformation of the ischemic lesion or development of intracerebral hemorrhage in this vulnerable phase.15 In a recent systematic review of 28 trials from the Cochrane Library, early anticoagulation was concluded to cause fewer recurrent ischemic strokes, a benefit offset by a similarly sized increase in intracranial hemorrhages.15 Several observational studies indicate possible clinical benefit of early initiation of NOACs in the acute setting after ischemic stroke in patients with atrial fibrillation29; however, data from randomized studies are very sparse. A Korean randomized study of 195 patients evaluating the initiation of treatment with either the NOAC rivaroxaban or warfarin within 5 days after mild stroke in patients with atrial fibrillation reported no difference in the primary outcome of new ischemic or hemorrhagic lesions on brain imaging after 4 weeks.31 The more recent randomized AREST study (Apixaban for Early Prevention of Recurrent Embolic Stroke and Hemorrhagic Transformation) evaluated early anticoagulation with the NOAC apixaban with later initiation of warfarin in patients with atrial fibrillation and stroke or transient ischemic attack. Although this study was based on very few patients, no symptomatic intracerebral hemorrhages were reported in NOAC-treated patients, but in 2.1% symptomatic intracerebral hemorrhages were reported in the warfarin-treated patients.18 So far, no randomized study has evaluated clinical outcomes after early versus delayed initiation of NOACs only after an acute ischemic stroke. This gap in evidence is highlighted in international guidelines3,5 and in the Action Plan for Stroke in Europe.2
It is important to note that none of the patients in the TIMING study experienced a symptomatic intracerebral hemorrhage during the 90-day study period and that overall the rates of major bleeding, including intracranial hemorrhages, were very low during the first 4 weeks. Beyond the clinical importance of safety confirmation, this result partly provides an explanation for the lower-than-anticipated primary composite outcome event rates in the TIMING study. We anticipated a 3% rate of symptomatic intracerebral hemorrhage on the basis of the Pre-TIMING observational study26 and the observational RAF study (Early Recurrence and Cerebral Bleeding in Patients With Acute Ischemic Stroke and Atrial Fibrillation)13 showing a 3.6% rate of cerebral bleedings. However, both the pre-TIMING and RAF studies were conducted in an era when large proportions of patients starting anticoagulant treatment early received vitamin K antagonists, despite their association with substantially higher intracerebral bleeding risk.9–12 Several more recent observational studies, published in parallel with or after the conduct of the TIMING study, have demonstrated substantially lower rates of symptomatic intracranial hemorrhages in patients treated early with NOACs after ischemic stroke in atrial fibrillation.16,32,33
The primary outcome results in TIMING were consistent across prespecified subgroups, including age, sex, previously known atrial fibrillation, and antithrombotic treatment before hospitalization. Signs of potential harm with early initiation of NOAC were implied only in the small groups of patients who underwent thrombectomy or had admission NIHSS scores >15, that is, patients with more severe ischemic strokes. In contrast, there was no sign of interaction with NIHSS score measured at the time of NOAC initiation (data not shown), which reinforces that any subgroup findings should be interpreted as hypothesis generating and need to be confirmed in ongoing randomized controlled trials (OPTIMAS [Optimal Timing of Anticoagulation After Acute Ischemic Stroke], NCT0375993834; ELAN [Early Versus Late Initiation of Direct Oral Anticoagulants in Post-Ischemic Stroke Patients With Atrial Fibrillation], NCT0314845735; and START [Optimal Delay Time to Initiate Anticoagulation After Ischemic Stroke in Atrial Fibrillation]; NCT03021928).
The strengths of the TIMING study include being the first randomized comparison, evaluated by clinically relevant end points, of early versus delayed initiation of NOAC in the setting of acute ischemic stroke and atrial fibrillation, with no patient lost to follow-up. Randomization is fundamental to provide clinical evidence, although randomized trials may be limited by selection bias of specialist trial centers and patients, not necessarily representing real-world practice. TIMING was the first pragmatic study using the comprehensive Swedish Stroke Register, with the addition of a study-specific randomization module, thereby combining the advantages of a prospective randomized study design with the strengths of a comprehensive clinical quality register and facilitating consecutive enrollment and follow-up of less selected patients.22 Another strength was the possibility of assessing the representativeness of the patients voluntarily participating in the TIMING study. These study patients were in many aspects, including age, sex distribution, and NIHSS score on admission, similar to the general Swedish stroke population with atrial fibrillation receiving NOAC treatment except for higher proportions of patients with newly diagnosed atrial fibrillation and reperfusion therapy.
A limitation was the smaller-than-preplanned study population. A number of factors hampering recruitment were identified. For example, stroke physicians wished to start treatment early to protect their patients from recurrent strokes, presumably supported by the transition from vitamin K antagonists to NOACs as the drug of choice during the study period and by extrapolation of safety results from pivotal NOAC versus vitamin K antagonist studies despite the fact that those studies excluded patients with a recent ischemic stroke. In addition, during the conduct of the TIMING study, several observational studies were published underpinning the potential safety of NOACs early after ischemic stroke, which may have created concerns among investigators in the study. More generally, the risk of stroke increases with age, as does the prevalence of cognitive impairment, which could undermine the patients’ capability to comprehend the study and the ability or willingness to provide written informed consent. To enable enrollment of more patients, the steering committee decided to prolong the recruitment period for 1 year, but unfortunately, this coincided with the coronavirus disease 2019 (COVID-19) pandemic during which several study sites were not able and some were not allowed to enroll study patients, an obstacle that the TIMING study shared with many other cardiovascular trials. Thus, the steering committee deemed it impossible to reach the originally planned sample size during the pandemic and, without knowledge of outcome data, terminated the study.
The lack of brain imaging data may also be considered a limitation. Magnetic resonance imaging with diffusion-weighted imaging would be the preferred modality to identify lesion size but is more costly than computed tomography and availability is limited in clinical practice. Moreover, standardizing the timing and performance of imaging and collecting and centrally adjudicating such data were not considered feasible in this pragmatic investigator-initiated and public-funded study.
Conclusions
Early initiation was noninferior to delayed start of NOAC after acute ischemic stroke in patients with atrial fibrillation. The numerically lower rates of ischemic stroke and death, the absence of symptomatic intracerebral hemorrhages, and the overall low rates of major bleedings imply that early initiation of NOAC is safe. Patients with acute ischemic stroke and atrial fibrillation should be considered for acute secondary stroke prevention, although it remains to be established whether early is superior to delayed start.
Article Information
Acknowledgments
The authors thank all patients, site study personnel, the data safety and monitoring board, system developers at the Swedish Stroke Register, and project managers, system developers, data managers, biostatisticians, and the publications manager at UCR. The final cleaned data set will be stored at UCR, the coordinating investigator (J.O., S.Å.) will have access to the data. Deidentified participant data can be made available on reasonable request. However, according to the Public Access to Information and Secrecy Act (2009:400), an interested researcher first must apply to and receive approval from the Swedish Ethical Review Authority. A data-sharing agreement will be put in place before any data are shared. J.O. (coordinating investigator) and S.Å. (co-coordinating investigator) designed and implemented the study, with input from Z.H., P.W., and B.N. (steering committee members). J.O., S.Å., Z.H., and B.N. were involved in protocol development, gaining ethics approval, and obtaining funding. M.B. did the statistical analysis. J.O. and S.Å. accessed and verified the underlying data and wrote the first draft of the manuscript. All authors critically revised the manuscript and approved the final version of the manuscript.
Sources of Funding
The study was funded by the Swedish Research Council (reference 2015-00881).
Disclosures
Dr Oldgren has received honoraria for consultant/advisory boards (including study steering committees and data safety monitoring boards) and lecture fees to his institution (AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daichii Sankyo, Novartis, Pfizer, Portola, Roche Diagnostics, and Sanofi), outside the submitted work. Dr Åsberg has received institutional research grants and lecture fees to her institution (AstraZeneca, Boehringer Ingelheim), outside the submitted work. Dr Hijazi has received lecture and consulting fees (Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, and Roche Diagnostics), outside the submitted work, and receives research support from the Swedish Society for Medical Research (S17-0133), the Swedish Heart-Lung Foundation (20200722), and Uppsala University Hospital, Sweden. Dr Wester has received honoraria for clinical event adjudication in the PORTICO studies (Portopulmonary Hypertension Treatment With Macitentan–A Randomized Clinical Trial; Abbott), outside the submitted work. M. Bertilsson reports no conflicts. Dr Norrving has received honoraria for data monitoring committee work (AstraZeneca), outside the submitted work.
Supplemental Material
Outcome definition
Tables S1 and S2
Figures S1 and S2
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AREST
- Apixaban for Early Prevention of Recurrent Embolic Stroke and Hemorrhagic Transformation
- COVID-19
- coronavirus disease 2019
- NIHSS
- National Institutes of Health Stroke Scale
- NOAC
- non–vitamin K antagonist oral anticoagulant
- RAF
- Early Recurrence and Cerebral Bleeding in Patients With Acute Ischemic Stroke and Atrial Fibrillation
- TIMING
- Timing of Oral Anticoagulant Therapy in Acute Ischemic Stroke With Atrial Fibrillation
J. Oldgren and S. Åsberg contributed equally.
Circulation is available at www.ahajournals.org/journal/circ
Supplemental Material, the podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.122.060666.
For Sources of Funding and Disclosures, see page 1064.
Contributor Information
Signild Åsberg, Email: signild@asberg.se.
Ziad Hijazi, Email: ziad.hijazi@ucr.uu.se.
Per Wester, Email: per.wester@umu.se.
Maria Bertilsson, Email: maria.bertilsson@ucr.uu.se.
Bo Norrving, Email: bo.norrving@med.lu.se.
References
- 1.The Swedish Stroke Register. Accessed September, 8 2021. http://www.riksstroke.org/eng/
- 2.Norrving B, Barrick J, Davalos A, Dichgans M, Cordonnier C, Guekht A, Kutluk K, Mikulik R, Wardlaw J, Richard E, et al. Action plan for stroke in Europe 2018–2030. Eur Stroke J. 2018;3:309–336. doi: 10.1177/2396987318808719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan G-A, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2020;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 4.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 5.Klijn CJ, Paciaroni M, Berge E, Korompoki E, Kõrv J, Lal A, Putaala J, Werring DJ. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: a European Stroke Organisation guideline. Eur Stroke J. 2019;4:198–223. doi: 10.1177/2396987319841187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52:e364–e467. doi: 10.1161/STR.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 7.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 8.Hankey GJ. Unanswered questions and research priorities to optimise stroke prevention in atrial fibrillation with the new oral anticoagulants. Thromb Haemost. 2014;111:808–816. doi: 10.1160/TH13-09-0741 [DOI] [PubMed] [Google Scholar]
- 9.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 10.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 11.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 12.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
- 13.Paciaroni M, Agnelli G, Falocci N, Caso V, Becattini C, Marcheselli S, Rueckert C, Pezzini A, Poli L, Padovani A, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF study. Stroke. 2015;46:2175–2182. doi: 10.1161/STROKEAHA.115.008891 [DOI] [PubMed] [Google Scholar]
- 14.Hart RG, Coull BM, Hart D. Early recurrent embolism associated with nonvalvular atrial fibrillation: a retrospective study. Stroke. 1983;14:688–693. doi: 10.1161/01.str.14.5.688 [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Ouyang M, Yang J, Song L, Yang M, Anderson CS. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2021;10:CD000024. doi: 10.1002/14651858.CD000024.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masotti L, Grifoni E, Dei A, Vannucchi V, Moroni F, Panigada G, Spolveri S, Landini G. Direct oral anticoagulants in the early phase of non valvular atrial fibrillation-related acute ischemic stroke: focus on real life studies. J Thromb Thrombolysis. 2019;47:292–300. doi: 10.1007/s11239-018-1775-2 [DOI] [PubMed] [Google Scholar]
- 17.Seiffge DJ, Werring DJ, Paciaroni M, Dawson J, Warach S, Milling TJ, Engelter ST, Fischer U, Norrving B. Timing of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation. Lancet Neurol. 2019;18:117–126. doi: 10.1016/S1474-4422(18)30356-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labovitz AJ, Rose DZ, Fradley MG, Meriwether JN, Renati S, Martin R, Kasprowicz T, Murtagh R, Kip K, Beckie TM, et al. Early apixaban use following stroke in patients with atrial fibrillation: results of the AREST trial. Stroke. 2021;52:1164–1171. doi: 10.1161/STROKEAHA.120.030042 [DOI] [PubMed] [Google Scholar]
- 19.Rybinnik I, Wong S, Mehta D, Leker RR, Mullen MT, Messé SR, Kasner SE, Cucchiara B. Anticoagulation choice and timing in stroke due to atrial fibrillation: a survey of US Stroke Specialists (ACT-SAFe). J Stroke Cerebrovasc Dis. 2020;29:105169. doi: 10.1016/j.jstrokecerebrovasdis.2020.105169 [DOI] [PubMed] [Google Scholar]
- 20.Munn D, Abdul-Rahim AH, Fischer U, Werring DJ, Robinson TG, Dawson J. A survey of opinion: when to start oral anticoagulants in patients with acute ischaemic stroke and atrial fibrillation? Eur Stroke J. 2018;3:355–360. doi: 10.1177/2396987318787124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Åsberg S, Hijazi Z, Norrving B, Terént A, Öhagen P, Oldgren J. Timing of oral anticoagulant therapy in acute ischemic stroke with atrial fibrillation: study protocol for a registry-based randomised controlled trial. Trials. 2017;18:581. doi: 10.1186/s13063-017-2313-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James S, Rao SV, Granger CB. Registry-based randomized clinical trials: a new clinical trial paradigm. Nat Rev Cardiol. 2015;12:312–316. doi: 10.1038/nrcardio.2015.33 [DOI] [PubMed] [Google Scholar]
- 23.Coull AJ, Rothwell PM. Underestimation of the early risk of recurrent stroke: evidence of the need for a standard definition. Stroke. 2004;35:1925–1929. doi: 10.1161/01.STR.0000133129.58126.67 [DOI] [PubMed] [Google Scholar]
- 24.von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, Treurniet KM, Majoie CB, Marquering HA, Mazya MV, et al. The Heidelberg Bleeding Classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46:2981–2986. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 25.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 26.Åsberg S, Norrving B, Hijazi Z, Oldgren J, Öhagen P, Terént A. Timing of initiation of oral anticoagulants in patients with acute ischemic stroke and atrial fibrillation: the observational Pre-TIMING Study. Eur Stroke J. 2016;1(suppl):672. Abstract. doi: 10.1177/2396987316642910 [Google Scholar]
- 27.Hankey GJ. Secondary stroke prevention. Lancet Neurol. 2014;13:178–194. doi: 10.1016/S1474-4422(13)70255-2 [DOI] [PubMed] [Google Scholar]
- 28.Points to consider when switching between superiority and non-inferiority 2000. Accessed November 3, 2021. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003658.pdf. [DOI] [PMC free article] [PubMed]
- 29.Diener H-C, Hankey GJ, Easton JD, Lip GYH, Hart RG, Caso V. Non-vitamin K oral anticoagulants for secondary stroke prevention in patients with atrial fibrillation. Eur Heart J Suppl. 2020;22(suppl 1):I13–I21. doi: 10.1093/eurheartj/suaa104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hankey GJ, Patel MR, Stevens SR, Becker RC, Breithardt G, Carolei A, Diener HC, Donnan GA, Halperin JL, Mahaffey KW, et al. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol. 2012;11:315–322. doi: 10.1016/S1474-4422(12)70042-X [DOI] [PubMed] [Google Scholar]
- 31.Hong KS, Kwon SU, Lee SH, Lee JS, Kim YJ, Song TJ, Kim YD, Park MS, Kim EG, Cha JK, et al. Rivaroxaban vs warfarin sodium in the ultra-early period after atrial fibrillation-related mild ischemic stroke: a randomized clinical trial. JAMA Neurol. 2017;74:1206–1215. doi: 10.1001/jamaneurol.2017.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seiffge DJ, Paciaroni M, Wilson D, Koga M, Macha K, Cappellari M, Schaedelin S, Shakeshaft C, Takagi M, Tsivgoulis G, et al. Direct oral anticoagulants versus vitamin K antagonists after recent ischemic stroke in patients with atrial fibrillation. Ann Neurol. 2019;85:823–834. doi: 10.1002/ana.25489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Marchis GM, Seiffge DJ, Schaedelin S, Wilson D, Caso V, Acciarresi M, Tsivgoulis G, Koga M, Yoshimura S, Toyoda K, et al. Early versus late start of direct oral anticoagulants after acute ischaemic stroke linked to atrial fibrillation: an observational study and individual patient data pooled analysis. J Neurol Neurosurg Psychiatry. 2022;93:119–125. doi: 10.1136/jnnp-2021-327236 [DOI] [PubMed] [Google Scholar]
- 34.Best JG, Arram L, Ahmed N, Balogun M, Bennett K, Bordea E, Campos MG, Caverly E, Chau M, Cohen H, et al. Optimal timing of anticoagulation after acute ischemic stroke with atrial fibrillation (OPTIMAS): Protocol for a randomized controlled trial. Int J Stroke. 2022;17:583–589. doi: 10.1177/17474930211057722 [DOI] [PubMed] [Google Scholar]
- 35.Fischer U, Trelle S, Branca M, Salanti G, Paciaroni M, Ferrari C, Abend S, Beyeler S, Strbian D, Thomalla G, et al. Early versus Late initiation of direct oral Anticoagulants in post-ischaemic stroke patients with atrial fibrillatioN (ELAN): protocol for an international, multicentre, randomised-controlled, two-arm, open, assessor-blinded trial [published online June 15, 2022]. Eur Stroke J. doi: 10.1177/23969873221106043. https://journals.sagepub.com/doi/full/10.1177/23969873221106043 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.