Abstract
Originally intended for life-saving salvage therapy, the use of temporary mechanical circulatory support (MCS) devices has become increasingly widespread in a variety of clinical settings in the contemporary era. Their use as a short-term, prophylactic support vehicle has expanded to include procedures in the catheterization laboratory, electrophysiology suite, operating room and intensive care unit. Accordingly, MCS device design and technology continue to develop at a rapid pace. In this Review, we describe the functionality, indications, management and complications associated with temporary MCS, together with scenario-specific utilization, goal-directed development and bioengineering of future devices. We address various considerations for the use of temporary MCS devices in both prophylactic and rescue scenarios, with input from stakeholders from various cardiovascular specialties, including interventional and heart failure cardiology, electrophysiology, cardiothoracic anaesthesiology, critical care and cardiac surgery.
Subject terms: Heart failure, Cardiology
Originally intended for life-saving salvage therapy, the use of temporary mechanical circulatory support (MCS) devices has become increasingly widespread. In this Review, Salter and colleagues discuss the functionality, indications, management and complications associated with temporary MCS in specific clinical scenarios.
Key points
The use of mechanical circulatory support (MCS) devices involves several different stakeholders and requires a multidisciplinary approach to consideration and management.
Choosing the appropriate MCS device involves a thorough evaluation of the patient’s phenotype, history, physical condition, laboratory data, haemodynamic deficit (univentricular or biventricular compromise) and echocardiographic findings and the objectives of care.
Optimal patient outcome is continually reassessed and is based on a balanced intersection between the objectives and level of support, the risk of complications, timing and the available resources.
Important opportunities for MCS innovation include challenges related to pump size, vascular access, biocompatibility and use in the ambulatory setting.
Introduction
The use of temporary mechanical circulatory support (MCS) devices has evolved from exclusive use as a rescue strategy for cardiogenic shock to functioning as an integral tool in various aspects of cardiovascular care. Indeed, temporary MCS is now routinely relied on for procedures in the catheterization laboratory, electrophysiology suite, operating room and intensive care unit. Expanded indications have led to rapid innovation in MCS device design and technology, with several devices and variations from which to choose, depending on the clinical scenario. The use of MCS devices involves several different stakeholders and requires a multidisciplinary approach to consideration and management. Various aspects of temporary MCS care must be considered holistically for an integrated effort from specialists in intensive care, cardiothoracic surgery, anaesthesiology, heart failure, electrophysiology and interventional cardiology, and from nursing and other support staff. Although a comprehensive review of the indications, design, technology, management and pitfalls of temporary MCS devices is a moving target, the incremental value of this Review lies in its multidisciplinary authorship and perspectives on how temporary MCS devices can be considered in a variety of prophylactic and rescue scenarios.
Current devices
Temporary MCS devices differ according to the level of support provided, haemodynamic effects, contraindications for use, site of vascular access and placement, sheath size required for delivery and associated complications, some of which are summarized in Table 1. Device selection involves a thorough evaluation of the patient’s phenotype, history, physical examination, laboratory data, imaging findings and goals of care. Although operator experience and institutional resources must also be considered, specific device selection is ultimately contingent on the degree of univentricular or biventricular haemodynamic support required. Optimal patient outcome is based on a balance between the goals and level of haemodynamic support as well as the risk of complications, timing and available resources, all of which are reassessed on a continual basis (Box 1). A brief summary of available temporary MCS devices is given below and in Figs. 1 and 2, followed by their practical applications according to indication and subspecialty.
Table 1.
Comparison of temporary mechanical circulatory support devices
| Device (examples) | Circuit | Level of cardiac output support | Contraindications | Device-specific complications |
|---|---|---|---|---|
| Intra-aortic balloon pump | Aorta | +/− | Moderate-to-severe aortic valve insufficiency, severe peripheral vascular disease, aortic dissection, aortic aneurysm | Renal or gut ischaemia, balloon rupture, aortic plaque embolism, limb ischaemia |
| Microaxial flow pump (Impella) | Left ventricle to aorta | ++ | Moderate-to-severe aortic valve insufficiency, left ventricular thrombus, mechanical aortic valve, severe peripheral vascular disease, aortic dissection | Malignant ventricular arrhythmias, cardiac or vascular injury, haemolysis, limb ischaemia (left) |
| Right atrium to pulmonary artery | Mechanical tricuspid or pulmonary valve, severe tricuspid valve stenosis, severe pulmonary valve stenosis or insufficiency, thrombosis in the vena cava or right atrium or ventricle | |||
| Percutaneous centrifugal (TandemHeart; right percutaneous CentriMag, dual-lumen devices) | Left atrium to femoral artery (TandemHeart) | +++ | Moderate-to-severe aortic valve insufficiency, severe peripheral vascular disease | Air embolism, limb ischaemia, stroke |
| Right atrium or ventricle to pulmonary artery | Mechanical tricuspid or pulmonary valve, severe tricuspid valve stenosis, severe pulmonary valve stenosis or insufficiency, thrombosis in the vena cava or right atrium or ventricle, superior vena cava or internal jugular vein stenosis or occlusion | Cardiac injury, tamponade | ||
| Peripheral venoarterial extracorporeal membrane oxygenation | Right atrium to femoral (or axillary) artery | ++++ | Severe aortic valve insufficiency, severe peripheral vascular disease | Limb ischaemia, pulmonary oedema, intracardiac thrombus, stroke |
| Surgical centrifugal | Left atrium or ventricle to aorta; right atrium or ventricle to pulmonary artery | ++++ | Patient not a surgical candidate | Complications of sternotomy or thoracotomy, bleeding, stroke |
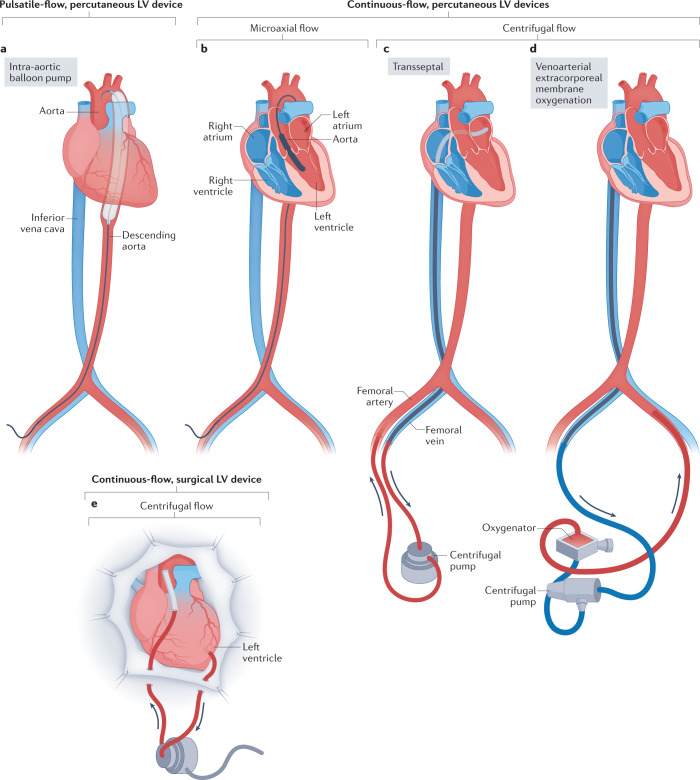
Fig. 1. Left ventricular circulatory support devices.
a, The intra-aortic balloon pump uses counterpulsation to provide circulatory support and is commonly inserted via the femoral or axillary artery. b, Microaxial flow devices traverse the aortic valve via the femoral or axillary artery and continuously remove blood from the left ventricular (LV) cavity to achieve unloading. c, Transseptal percutaneous assist devices are powered by a centrifugal pump and are inserted via the femoral vein, across the intra-atrial septum and into the left atrium, with an outflow cannula in the femoral artery. Devices using centrifugal pumps require a separate control and monitoring module. d, Venoarterial extracorporeal membrane oxygenation uses a centrifugal pump to pull venous blood from the right atrium, through an oxygenator and into the arterial circulation via the outflow cannula in the aorta. Common cannulation sites include the femoral or axillary vessels or via central cannulation by thoracotomy or sternotomy. Extracorporeal membrane oxygenation can be used for left, right or biventricular support. e, Surgical extracorporeal centrifugal devices provide LV support via sternotomy or minimally invasive thoracotomy. Inflow cannulation is from the left ventricle or left atrium, and outflow cannulation is into the aorta.
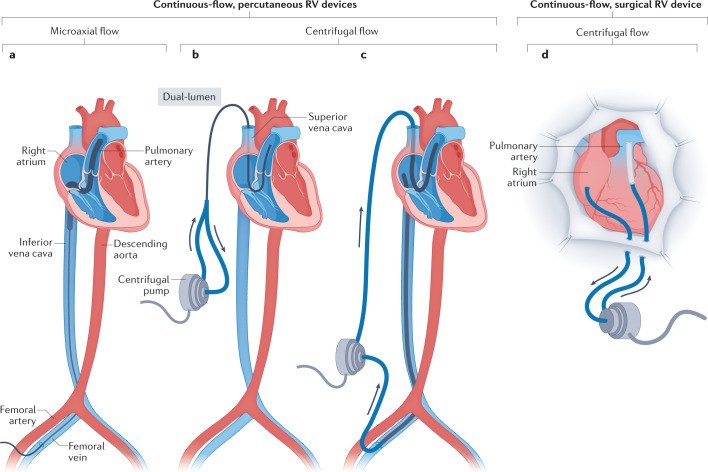
Fig. 2. Right ventricular circulatory support devices.
a, Microaxial flow devices for right ventricular (RV) support are inserted percutaneously through the femoral vein and into the pulmonary artery. b, Dual-lumen percutaneous assist devices draw blood from the right atrium or right ventricle to an external continuous centrifugal pump and out to the pulmonary artery. c, Percutaneous cannulas draw blood from the right atrium to a continuous centrifugal pump and into the pulmonary artery via the femoral, internal jugular or subclavian vein. d, Surgical extracorporeal centrifugal devices provide RV support via sternotomy or minimally invasive thoracotomy. Inflow cannulation is from the right atrium or right ventricle and outflow cannulation is into the pulmonary artery.
Box 1 Maximizing the benefit of temporary MCS.
Optimal patient outcome with temporary mechanical circulatory support (MCS) is contingent upon the dynamic balance between the objectives, level of support required, risk of complications, timing and available resources:
What are the objectives of support (recovery, bridge to transplantation, durable device)?
What level of support (including univentricular or biventricular) is required to deliver the best haemodynamic benefit?
What are the relevant complications for the patient?
When is the optimal timing of insertion and removal of temporary MCS?
Are the necessary resources available to support the patient through temporary MCS?
Intra-aortic balloon pump
The intra-aortic balloon pump (IABP), commonly inserted via the axillary or femoral artery, uses counterpulsation to increase mean arterial blood pressure, coronary perfusion pressure and cardiac output (by approximately 1 l/min), resulting in a decrease in left ventricular (LV) end-diastolic pressure (LVEDP), LV wall stress and myocardial oxygen demand1–4. The quality of support provided by an IABP is dependent on several factors, including heart rate, heart rhythm and systemic vascular resistance. IABP counterpulsation is synchronized with the cardiac cycle and, therefore, disturbances in heart rate and rhythm can compromise device function. Tachycardia reduces the time spent in diastole, limiting IABP inflation and, consequently, the intended increases in coronary perfusion pressure, whereas arrhythmias lead to dyssynchrony between balloon inflation and deflation5. Contraindications to IABP use include severe peripheral vascular disease and aortic dissection, tortuosity and aneurysm. In addition, counterpulsation in the setting of clinically significant aortic valve regurgitation is counterproductive, leading to increased LV wall stress, rather than producing the desired cardioprotective effects2.
Percutaneous left ventricular devices
The two major classes of percutaneous LV support devices are microaxial flow pumps (such as the Impella (Abiomed)) and extracorporeal centrifugal devices. Both devices allow increases in cardiac output, cardiac power and mean arterial blood pressure, with corresponding increases in coronary and systemic perfusion pressures. Effective LV ‘unloading’ reduces LVEDP, leading to decreased wall tension and, therefore, myocardial oxygen demand4,6–9. Microaxial flow devices traverse the aortic valve and continuously remove blood from the LV cavity to achieve unloading. The left-sided Impella comes in various sizes and can deliver 2.5–5.5 l/min of flow, depending on the device. The Impella 2.5 and Impella CP can be inserted percutaneously via the femoral artery. The Impella 5 and Impella 5.5 use a larger introducer sheath, requiring a surgical cut down for insertion, and are most often placed via the axillary artery. Pump flow is determined by the pressure difference between the aorta and left ventricle and the pump support level (P-level). The Impella CP with SmartAssist additionally provides measurements of LVEDP, mean arterial blood pressure and cardiac power output in real-time to support optimization and weaning6. Several important contraindications exist to the placement of left-sided microaxial flow pumps, including severe aortic valve stenosis (≤0.6 cm2), moderate-to-severe aortic valve regurgitation, clinically significant peripheral arterial disease, and the presence of a mechanical aortic valve or LV thrombus6,7. Lastly, ideal positioning of the Impella device is approximately 3.5 cm below the aortic valve, into the middle of the left ventricle, as seen on echocardiography7. Device migration and malpositioning can lead to ineffective circulatory support and unloading, as well as haemolysis and arrhythmias. Algorithms incorporated into controllers can detect suction and automatically adjust pump speed to compensate, if these events occur.
The TandemHeart (LivaNova) percutaneous assist device is a continuous flow, centrifugal pump, left atrium to femoral artery system. A 21F transseptal inflow cannula is inserted via the femoral vein into the left atrium, while an outflow cannula (15F–19F) is placed into the descending aorta, thereby bypassing the left ventricle. The device is powered by an extracorporeal electromagnetic motor that drives a plastic impeller at a speed of 3,000–7,500 rpm and can deliver flows of up to 5 l/min8,9. Despite the haemodynamic benefits observed with the use of TandemHeart, more widespread implementation is limited by the challenges of device insertion (that is, the need for transseptal puncture), especially in emergency situations. Improper placement or device dislodgement into the right atrium can precipitate substantial right-to-left shunting of deoxygenated blood. Device fixation is therefore paramount, as minor changes in position can compromise the intended circulatory support.
Percutaneous right ventricular devices
Like the temporary LV support options discussed above, options for percutaneous right ventricular (RV) support similarly include microaxial and extracorporeal centrifugal devices. These devices directly reduce right atrial and RV pressures and increase blood flow across the pulmonary artery, leading to increases in mean pulmonary arterial pressure, LV preload and, in turn, cardiac output in the setting of preserved or assisted LV function10,11.
The Impella RP system is a percutaneous microaxial RV assist device that operates on the same principles as the other Impella devices. A 22F catheter sits in the inferior vena cava and delivers blood from the inlet area to the outlet opening in the pulmonary artery12. Although data to support the ubiquitous or routine use of the Impella RP in the setting of RV failure are lacking, emergency use authorization was granted specifically to treat RV failure related to coronavirus disease 2019 (COVID-19), including patients with pulmonary emboli13. The ProtekDuo (LivaNova) differs from the Impella RP in design and is a dual-lumen, percutaneous RV assist device that directs blood from the right atrium into the pulmonary artery and is connected to an external continuous centrifugal pump. The system supports up to 5 l/min of blood flow, and an oxygenator can be inserted for extracorporeal membrane oxygenation (ECMO). The ProtekDuo is manufactured in two sizes (29F and 31F) and is typically inserted via the right internal jugular vein14.
The Dual Lumen Cannula (Spectrum Medical) is another advanced, dual-lumen, percutaneous RV assist device that is inserted via the right internal jugular vein and connects to a centrifugal pump and oxygenator (if required). In contrast to the ProtekDuo, the Dual Lumen Cannula assist device drains blood from the right ventricle and returns blood to the pulmonary artery15. The system comes in 31F, 27F and 24F sizes, supporting up to 5 l/min of blood flow. Insertion via the internal jugular vein for both the ProtekDuo and Dual Lumen Cannula devices can allow greater patient mobility than with a device inserted via the femoral vein.
Percutaneous RV assist device support can also be provided by using two percutaneously placed cannulas, one from a femoral vein for the inflow arm and another from the internal jugular or subclavian vein into the pulmonary artery for the outflow arm. This circuit can be connected to an extracorporeal centrifugal pump, such as the CentriMag Acute Circulatory Support System (Abbott). The CentriMag consists of a magnetically levitated rotor and can be used to provide support with or without an oxygenator for ECMO16,17.
Contraindications to right-sided device placement include mechanical tricuspid or pulmonary valves, severe tricuspid or pulmonary stenosis or regurgitation, disorders of the pulmonary artery wall that prohibit device placement, and mural thrombus of the right atrium or vena cava18.
Venoarterial extracorporeal membrane oxygenation
Venoarterial (VA) ECMO uses a centrifugal pump to pull venous blood from the right atrium, through a membrane oxygenator and into the arterial circulation via the outflow cannula. Commonly, the venous inflow cannula (18F–28F) is inserted into a femoral vein and advanced to the right atrium, whereas the arterial outflow cannula (15F–19F) is placed in a femoral artery. Other sites of cannulation include the axillary vessels or via central cannulation by thoracotomy or sternotomy.
The haemodynamic effects of VA ECMO are dictated by the cannula size, volume status and pump speed (up to 8 l/min). The objectives of VA ECMO are to improve systemic circulation and reduce myocardial demand by decreasing RV preload, pulmonary blood flow, LVEDP and LV end-diastolic volume. Additionally, by decreasing central venous pressure, blood flow to organs in the portal circulation improves19. However, as ECMO flow is increased, LV afterload rises and can result in increased wall stress, LVEDP, pulmonary capillary wedge pressure and myocardial oxygen demand, potentially impeding LV recovery4,19. Increased afterload can be attenuated by reductions in systemic vascular resistance and LV decompression or ‘venting’, usually achieved by early institution of inotropic support, IABP placement, percutaneous LV assist devices, atrial septostomy, percutaneous transseptal venting of the left atrium, percutaneous pulmonary artery venting, or surgical venting of the left atrium or left ventricle4,20,21. Although no guideline recommendations exist for how to establish LV decompression, common indications include persistently elevated pulmonary capillary wedge pressure (>15 mmHg) or pulmonary artery diastolic blood pressure (>25 mmHg) when receiving support, aortic valve closure throughout the cardiac cycle, persistent pulmonary oedema, refractory ventricular arrhythmia, or LV distension seen on echocardiography20,22. In patients with cardiogenic shock, microaxial flow pump-assisted LV unloading in the setting of ECMO (so-called ECPella), has been shown to decrease 30-day mortality, but its widespread use remains controversial owing to varying rates of complications when compared with ECMO support alone23,24.
Increasingly, VA ECMO is being deployed to complement high-quality cardiopulmonary resuscitation after cardiac arrest25. Compared with conventional cardiopulmonary resuscitation alone, extracorporeal cardiopulmonary resuscitation has been shown to allow increases in coronary perfusion pressure and higher rates of successful defibrillation and return of spontaneous circulation, potentially underlying associations with improved survival rates and neurological outcomes26,27.
Surgical extracorporeal centrifugal devices
Temporary haemodynamic support (univentricular or biventricular) is also provided using surgically implanted cannulas and extracorporeal centrifugal pumps. Implantation is commonly performed via sternotomy or minimally invasive thoracotomy. The CentriMag, which can provide up to 10 l/min of flow, is the most frequently used pump and is approved for prolonged use because of its favourable haemocompatibility profile, with low rates of haemolysis.
MCS device complications
Temporary MCS devices are associated with several complications that can span the cardiovascular, haematological, immune and neurological systems, or can be intrinsic to the mechanical pump itself. Complications can vary and must be weighed against the potential clinical benefit for individual patients28 (Box 2; Table 1).
Box 2 Shared complications of temporary MCS.
Mechanical
Device migration
Device malfunction
Cardiovascular
Valvular injury
Chamber perforation
Vascular injury
Limb ischaemia
Access-site haemorrhage
Haematological
Haemolysis
Thrombocytopenia
Thromboembolism
Haemorrhage
Neurological
Cerebrovascular accident
Peripheral nerve injury
Infectious
Sepsis
Access-site infection
MCS, mechanical circulatory support.
Cardiovascular complications
Cardiovascular complications from MCS devices can be related to direct cardiac injury or vascular access. Direct cardiac complications include valvular injury (with aortic valve injury being more common with the use of trans-aortic devices, resulting in early or late development of aortic valve insufficiency) as well as chamber perforation (from cannulas placed in the atrium) potentially resulting in shunting, pericardial effusion and cardiac tamponade. Vascular complications include distal limb ischaemia and dissection of cannulated vessels28. The risk of distal limb ischaemia relates to cannula size, patient vessel size, urgency of deployment and concomitant vasopressor use. In extreme cases, limb ischaemia can necessitate amputation. The large femoral arterial cannulas used for ECMO render patients especially vulnerable, necessitating strategies such as distal limb perfusion and near-infrared spectroscopy monitoring to help to reduce the incidence of this devastating complication29,30.
Haematological complications
Haematological complications from MCS devices include (but are not limited to) haemorrhage, anaemia, platelet dysfunction, thrombocytopenia and thrombosis. Despite routine anticoagulation, the risk of pump thrombosis persists. With some forms of temporary MCS (in particular ECMO), intracardiac stasis can contribute to this risk and result in pump thrombosis and subsequent thromboembolic events. MCS-related bleeding is multifactorial and secondary to acquired coagulation deficits, haemolysis from high shear forces, thrombocytopenia, access-related issues and requisite anticoagulation.
Infectious complications
Infectious complications are commonly encountered in the setting of MCS, given the multiple sites of external cannulation access combined with critical illness and prolonged hospital stays28. Infectious complications can range from local access-site infections to systemic illness, including bacteraemia and sepsis. Although some institutions use prophylactic antibiotic therapy for patients receiving MCS, no evidence is available to support this generalized approach28,31.
Neurological complications
Neurological injury can be multifactorial in critically ill patients, but is commonly attributed to the migration of microemboli in an MCS device. Haemodynamically unstable patients requiring MCS often have associated periods of cerebral hypoperfusion, hypoxia or metabolic derangement that contribute to cerebrovascular accidents. The rate of stroke is similar in patients with an IABP, TandemHeart or Impella devices, but is notably higher among those supported with ECMO28.
Practical application by indication
Temporary MCS devices are relied on for procedures in the catheterization laboratory, electrophysiology suite, operating room and intensive care unit. Commonly used devices, corresponding indications and the rationale for implementation are reviewed below and summarized in Table 2.
Table 2.
Temporary MCS: indications, rationale and commonly used devices
| Indication | Rationale | MCS devices |
|---|---|---|
| Cardiogenic shock |
Haemodynamics refractory to pharmacotherapy Acute myocardial infarction or heart failure related |
IABP, pLVAD, VA ECMO |
| High-risk PCI |
Procedural coronary or systemic hypotension Haemodynamic support for complex revascularization |
IABP, pLVAD |
| Cardiac surgery |
Haemodynamic compromise before surgery Pre-emptive in high-risk patients undergoing surgery Perioperative circulatory collapse Post-cardiotomy shock |
IABP, pLVAD, pRVAD, VA ECMO, extracorporeal centrifugal MCS |
| Advanced heart failure |
Preoperative optimization Bridge to recovery, decision or durable therapy |
IABP, pLVAD, pRVAD, VA ECMO, extracorporeal centrifugal MCS |
| Electrophysiology laboratory |
High risk of heart failure or decompensation during procedure High PAINESD score |
pLVAD, VA ECMO |
IABP, intra-aortic balloon pump; MCS, mechanical circulatory support; PCI, percutaneous coronary intervention; pLVAD, percutaneous left ventricular assist device; pRVAD, percutaneous right ventricular assist device; VA ECMO, venoarterial extracorporeal membrane oxygenation.
Cardiogenic shock
Despite advances in medical management, mortality in patients with cardiogenic shock, especially in the setting of acute myocardial infarction (AMI-CS), remains remarkably high at 40–60%32. The objectives of management in cardiogenic shock are to increase cardiac output, maintain systemic blood pressure and preserve end-organ perfusion. Therefore, the most common indication for temporary MCS is cardiogenic shock refractory to medical management. The selection of patients with cardiogenic shock who might derive benefit from temporary MCS is based largely on the Society for Cardiovascular Angiography and Interventions (SCAI) stages, which grade the severity of cardiogenic shock by haemodynamic parameters and markers of end-organ dysfunction33. Various scoring systems that incorporate major prognostic variables, including haemodynamic, biochemical and other relevant patient factors, have been proposed to identify predictors of adverse outcome and treatment strategies in the setting of cardiogenic shock34 (Table 3). Although the potential exists for these scoring systems to guide the use of temporary MCS, varied methodologies and populations of derivation and validation limit their widespread clinical applicability.
Table 3.
Comparison of scoring systems to evaluate the indication for MCS
| Score | Derivation population | Predictors (points) | Outcomes by score |
|---|---|---|---|
| CardShock104 | Cardiogenic shock (AMI and non-AMI) |
Acute coronary syndrome aetiology (1) Age >75 years (1) Previous MI or CABG surgery (1) Confusion at presentation (1) LVEF <40% (1) Blood lactate level (mmol/l): <2 (0), 2–4 (1), >4 (2) eGFR (ml/min/1.73 m2): >60 (0), 30–60 (1), <30 (2) |
Total score: 0–9 In-hospital mortality: Score 1–3 (low risk), 8.7% Score 4–6 (intermediate risk), 36% Score 7–9 (high risk), 77% |
| SAVE105 | Cardiogenic shock receiving ECMO |
Diagnosis: myocarditis (3), refractory ventricular tachycardia or fibrillation (2), previous heart or lung transplantation (3), congenital heart disease (−3), other (0) Age (years): 18–38 (7), 39–52 (4), 53–62 (3), ≥63 (0) Body weight (kg): ≤65 (1), 65–89 (2), ≥90 (0) Cardiac: pre-ECMO cardiac arrest (−2), pre-ECMO diastolic blood pressure ≥40 mmHg (3), pre-ECMO pulse pressure ≤20 mmHg (−2) Respiratory: pre-ECMO duration of intubation (h): ≤10 (0), 11–29 (−2), ≥30 (−4); peak inspiratory pressure ≤20 cmH2O (3) Renal failure: acute (−3), chronic (−6), pre-ECMO HCO3 level ≤15 mmol/l (−3) Liver failure (−3) Central nervous system dysfunction (−3) |
Total score: −35 to 17 In-hospital survival: Score >5 (class I), 75% Score 1–5 (class II), 58% Score −4 to 0 (class III), 42% Score −9 to −5 (class IV), 30% Score ≤−10 (class V), 18% |
| IABP Shock II106 | AMI with cardiogenic shock undergoing PCI |
Age >73 years (1) History of cerebrovascular attack (2) Glucose level >191 mg/dl (1) Creatinine level >1.5 mg/dl (1) Lactate level >5 mmol/l (2) TIMI flow grade <3 after PCI (2) |
Total score: 0–9 30-day mortality: Score 0–2 (low risk), 23.8% Score 3–4 (intermediate risk), 49.2% Score 5–9 (high risk), 76.6% |
AMI, acute myocardial infarction; CABG, coronary artery bypass graft; ECMO, extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
All forms of MCS improve cardiac output and blood pressure to varying degrees; however, each interacts with the heart and vasculature uniquely to exert device-specific haemodynamic effects. Unique changes in pressure–volume relationships encountered with the various temporary MCS devices in the setting of cardiogenic shock are depicted in Fig. 3. Consideration of specific temporary MCS device use must be based on the clinical scenario, which can occur de novo, as in the setting of AMI-CS or myocarditis, or as a presentation of worsening disease, as in patients with pre-existing heart failure whose condition deteriorates35.
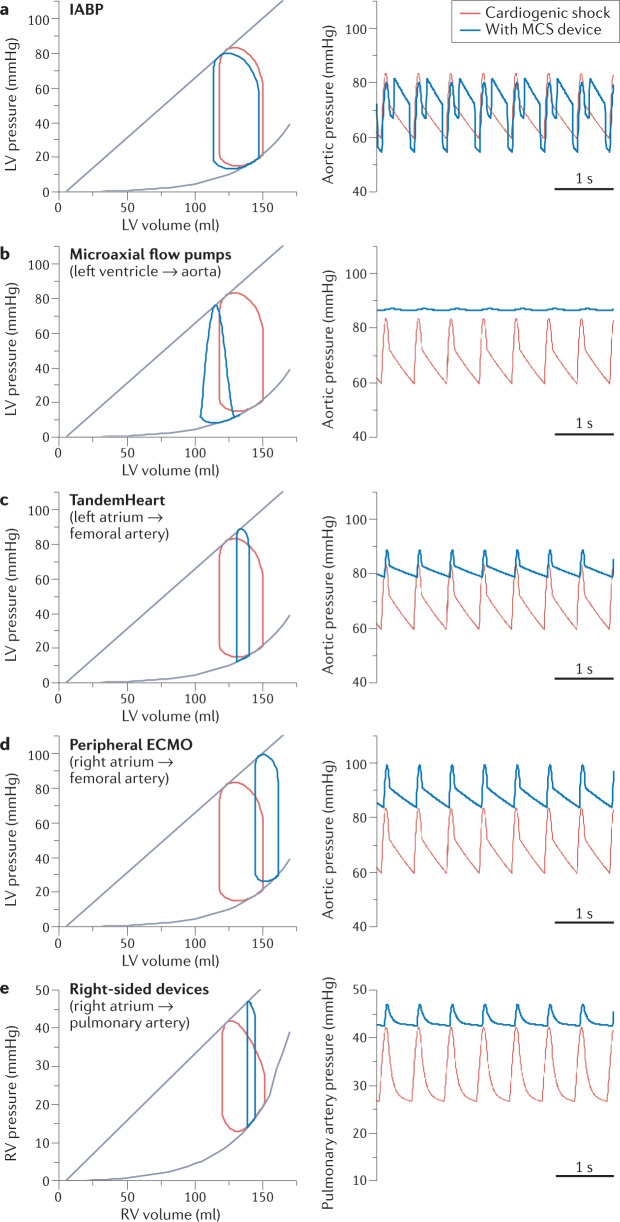
Fig. 3. Effects of MCS devices on pressure–volume loops and blood pressure.
All temporary mechanical circulatory support (MCS) devices improve cardiac output (left) and blood pressure (right) to varying degrees. In this figure, the primary haemodynamic effects of temporary MCS devices are compared to illustrate the fundamental differences in how the devices interact with the heart and vasculature. Red lines indicate tracings under baseline cardiogenic shock conditions; blue lines show tracings after the introduction of the specified MCS device. a, Intra-aortic balloon pump (IABP). The tracings show the effects of counterpulsation, with a small reduction in left ventricular (LV) preload and a small increase in stroke volume. b, Microaxial flow pumps (connecting the left ventricle to the aorta). The tracings show substantial unloading (decreased LV end-diastolic pressure and pulmonary capillary wedge pressure), triangulation of the pressure–volume loop (indicating loss of isovolumic periods), decreased pressure–volume area (correlating with decreased myocardial oxygen consumption) and LV–aortic pressure uncoupling with a closed aortic valve. c, TandemHeart (connecting the left atrium to the femoral artery). The tracings show LV unloading (decreased LV end-diastolic pressure and pulmonary capillary wedge pressure), decreased LV stroke volume, increased aortic pressure (due to overall increased systemic blood flow), decreased pressure–volume area and preserved aortic valve opening (crucial for preventing LV and aortic root stasis and thrombosis). d, Peripheral extracorporeal membrane oxygenation (ECMO; connecting the right atrium to the femoral artery). The tracings show increasing LV preload (increased LV end-diastolic pressure and pulmonary capillary wedge pressure), decreased LV stroke volume, increased aortic pressure (due to overall increased systemic blood flow), increased pressure–volume area (correlating with increased myocardial oxygen consumption) and preserved aortic valve opening (crucial for preventing LV and aortic root stasis and thrombosis). e, Right-sided microaxial and extracorporeal centrifugal devices (connecting the right atrium to the pulmonary artery). The tracings show right ventricular (RV) unloading (decreased RV end-diastolic pressure and central venous pressure), decreased RV stroke volume, increased pulmonary artery pressure (due to overall increased pulmonary blood flow), decreased RV pressure–volume area and preserved pulmonary valve opening (crucial for preventing RV and pulmonary artery root stasis and thrombosis). All tracings were obtained using the Harvi cardiovascular simulation108 and are used with permission from PVLoops.
Although use of the IABP has decreased over the past 10 years, it remains the most common temporary MCS device used in the setting of AMI-CS (approximately 70% of patients)36,37. However, despite its frequent use, randomized controlled trials have not shown an associated increase in survival. The IABP SHOCK II trial38–40 randomly assigned 600 patients with AMI-CS undergoing planned revascularization to IABP or no circulatory support and reported no significant difference in mortality at 30 days, 6 months, 1 year and 6 years. Several limitations might account for this lack of demonstrated benefit: a patient population with mild-to-moderate cardiogenic shock, low rates of device insertion before percutaneous coronary intervention (PCI) and a high rate of patient crossover to the IABP group. Importantly, patients with mechanical complications from myocardial infarction (such as ventricular septal defect or severe mitral valve regurgitation) were not included. Nevertheless, the American Heart Association (AHA)/American College of Cardiology (ACC) guidelines41 changed the class of recommendation for the use of an IABP in the setting of AMI-CS from class I to class IIa, with acknowledgement of potential utility among patients with mechanical complications42. The European Society of Cardiology (ESC) guidelines43 take a firmer stance, advocating against the routine use of an IABP in patients with AMI-CS (class III, level of evidence B), but give a class IIa, level of evidence C recommendation for use in patients with AMI-CS and mechanical complications.
Other temporary MCS devices that provide greater levels of haemodynamic support than an IABP have also been investigated in the setting of cardiogenic shock. Of these, percutaneous microaxial flow pumps, such as the Impella device, have gained the most popularity44. The ISAR-SHOCK trial45 randomly assigned 25 patients with AMI-CS to receive an Impella LP 2.5 or an IABP and found that the microaxial flow device produced a greater increase in cardiac index than the IABP at 30 min after device insertion. Similarly, patients with AMI-CS supported with the microaxial flow pump were reported to have lower requirements for inotropes and lower lactate levels than patients supported with an IABP46. However, despite these haemodynamic benefits, percutaneous microaxial flow pumps have not been shown to increase survival45,47. Specifically, patients with AMI-CS supported with Impella were retrospectively matched to patients included in the IABP-SHOCK trial, and no significant difference in all-cause mortality was reported (48.5% versus 46.4%; P = 0.64)48. Additionally, a retrospective, propensity-matched analysis of 3,000 patients undergoing PCI for AMI-CS demonstrated an increase in cost and risk of bleeding and death with a microaxial flow pump compared with an IABP49. Given these disparate and limited data, multiple professional cardiovascular societies have emphasized that insufficient evidence is available to support uniform use of percutaneous LV assist devices in the clinical setting of cardiogenic shock (class IIb, level of evidence C)41,50. The results from the ongoing, randomized, prospective DanGer Shock trial51 might elucidate whether microaxial flow pumps do confer a survival benefit compared with standard of care in patients with AMI-CS. Furthermore, the National Cardiogenic Shock Initiative52 is a prospective registry in the USA that will determine whether the use of a prespecified algorithm to identify cardiogenic shock together with early implantation of the Impella CP device leads to improved outcomes in patients with AMI-CS.
In studies from 2005 and 2006 involving patients with AMI-CS, use of the TandemHeart compared with an IABP was associated with improved haemodynamic profiles, but increased rates of complications and no significant difference in 30-day mortality53,54.
VA ECMO is commonly used in patients with cardiogenic shock55,56. For patients with profound shock, VA ECMO can serve as a bridge to myocardial recovery, surgery or durable MCS. A retrospective analysis of 105 patients who underwent VA ECMO for cardiogenic shock showed a 1-year survival of 42%, with most survivors receiving heart transplantation or durable MCS57. A similar rate of survival to hospital discharge was demonstrated in a retrospective analysis of 756 patients in the Extracorporeal Life Support Organization registry58.
Overall, although cardiogenic shock remains the most common indication for temporary MCS in a variety of forms, randomized trials to support the benefit of this approach are lacking. Despite the use of MCS devices in this setting, registry data indicate a persistently high mortality. The Cardiogenic Shock Working Group is establishing a national, multicentre, retrospective registry of real-world experience of patients with cardiogenic shock, including >1,500 patients supported with various forms of temporary MCS, which will hopefully inform best practice and future clinical trials in this field59.
High-risk, assisted PCI
MCS during PCI is most commonly used to support patients with AMI-CS and those undergoing high-risk coronary procedures. Patients who are older, with complex coronary artery disease with or without haemodynamic compromise, low ejection fraction and other relevant comorbidities are considered to be at high risk (Box 3). In these patients, procedures are often longer, with periods of coronary and systemic hypoperfusion, and MCS-assisted PCI can allow time for complete revascularization while providing sufficient cardiac output, LV unloading, and myocardial and end-organ perfusion.
The BCIS-1 trial60 randomly assigned 301 patients with low ejection fraction undergoing high-risk PCI to elective IABP support or no support. Although no significant difference was reported in major adverse cardiovascular events or mortality at 28 days (the primary end point), the risk of procedural complications, including prolonged procedural hypotension necessitating escalation of support, was lower in the IABP group, but the risk of access-site complications and bleeding was higher60. The subsequent observation of a reduction in mortality in the IABP group at 2 years61 has led to varied interpretations of the overall results.
Numerous retrospective, observational and prospective studies have sought to evaluate the safety and efficacy of using the Impella device in the setting of high-risk PCI62. One large observational analysis of 48,000 patients raised concern, because use of the microaxial pump was associated with increased rates of bleeding, stroke and death as well as costs62. However, the series of prospective PROTECT studies63,64 have yielded more promising data. The PROTECT II study64 found no significant difference in the composite of major adverse events at discharge or 30 days with the Impella 2.5 device compared with an IABP. The FDA granted premarket approval for use of the Impella 2.5 device in this clinical setting on the basis of secondary and per-protocol analyses, which included improved haemodynamic profiles64, ability to accomplish longer rotational atherectomy for plaque modification65, reduction in acute kidney injury66 and fewer adverse events at 90 days. Subsequently, the post-approval, single-group PROTECT III study67 in 1,143 patients undergoing Impella-supported, non-emergent PCI demonstrated a reduction in the primary composite end point of death, stroke, myocardial infarction and repeat procedures at 90 days when compared with patients supported with the Impella device in the PROTECT II study. In the prospective PROTECT IV trial68, high-risk patients with complex coronary artery disease and reduced LV function undergoing revascularization will be randomly assigned to receive Impella circulatory support or no planned haemodynamic support during PCI.
The optimal timing of MCS implementation before revascularization is also a topic of ongoing research. The STEMI Pilot Trial69 randomly assigned 50 patients to PCI or delayed reperfusion after a period of unloading with the Impella device and demonstrated the safety and feasibility of LV unloading using Impella to allow time for cardioprotection and reduced reperfusion injury.
Current AHA/ACC guidelines include a recommendation that elective insertion of an appropriate haemodynamic support device as an adjunct to PCI might be reasonable in carefully selected high-risk patients (class IIb, level of evidence B)70. The ESC guidelines recommend that temporary MCS (without device specification) should be considered in non-emergent, high-risk PCI procedures, such as left main coronary artery disease, single remaining patent coronary artery and complex chronic total occlusions, performed by adequately experienced operators at centres that have access to circulatory support and onsite cardiovascular surgery71.
Box 3 High-risk PCI criteria.
Although no universal definition exists of the criteria for high-risk percutaneous coronary intervention (PCI), the following factors are used to identify patients who might benefit from mechanical circulatory support.
Clinical
Acute coronary syndrome
Severe left ventricular dysfunction (ejection fraction <35%)
Decompensated heart failure
Arrhythmias
Anatomical
Unprotected left main coronary artery disease or equivalent
Three-vessel disease
High SYNTAX score
Multivessel disease
Last patent conduit
Complex chronic total occlusion
Heavy calcification
Complex bifurcation
Large area of myocardium at risk
Comorbidities
Advanced age
Severe valvular disease
Severe lung disease
Chronic kidney disease
The electrophysiology laboratory
The use of temporary MCS in the electrophysiology laboratory has been implemented to facilitate substrate mapping and ablation of haemodynamically unstable ventricular tachycardia (VT) as well as to mitigate post-procedural heart failure and haemodynamic compromise.
Data on whether temporary MCS leads to better outcomes among patients undergoing VT ablation are mixed. Preliminary studies demonstrated the benefit of safely leaving patients supported with percutaneous LV assist devices in VT for longer periods of time to allow improved mapping and ablation during ongoing VT72,73. Safe maintenance in VT led to more VT terminations with radiofrequency ablation (P = 0.03), but did not result in increased freedom from VT during follow-up72. In a larger, non-randomized study in which use of the Impella device (n = 109) was compared with no percutaneous LV assist device (n = 85), no significant difference was found in the rate of successful VT termination during ablation or freedom from VT recurrence during follow-up74. Although no significant differences were observed, patients who received Impella support were sicker and had lower LV ejection fractions (26 ± 10% versus 39 ± 16%; P < 0.001), with more New York Heart Association (NYHA) class ≥III heart failure (51% versus 25%; P < 0.001) and more frequent electrical storm (49% versus 34%; P = 0.04), leading the researchers to conclude that temporary MCS allowed sicker patients to achieve equivalent outcomes to those in less-sick patients74. However, other studies have disproved this theory75, rendering the evidence to support the benefit of temporary MCS for the end points of reducing VT recurrence and mortality after catheter ablation uncertain.
Temporary MCS is also used in the electrophysiology laboratory in high-risk patient populations to reduce acute haemodynamic decompensation and worsening heart failure after ablation, which has been reported in up to 11% of patients76. Long-term mortality in these patients is driven by heart failure, which was the most common cause of death in the VANISH trial (n = 36/71 deaths)77, with registries showing higher mortality in patients with lower ejection fraction and higher NYHA class78.
Microaxial flow devices are the most common form of MCS used during VT ablation owing to their relative ease of use; however, the required transaortic position can limit access for endocardial–epicardial mapping and they are also susceptible to electromagnetic interference79. ECMO provides a higher degree of circulatory support and oxygenation for sicker, haemodynamically unstable patients. In a report of 64 patients over 5 years, ECMO support for VT ablation was associated with a low procedure-related mortality, and the researchers propose the potential added benefit of being able to bridge decompensated patients to permanent LV assist device implantation or heart transplantation if needed80.
High-risk patients presenting for VT ablation can be identified using the PAINESD score, which gives points for the presence of the following high-risk variables76,81: pulmonary disease (five points), age >60 years (three points), ischaemic cardiomyopathy (six points), NYHA class III/IV heart failure (six points), LV ejection fraction <25% (three points), VT storm (five points) and diabetes mellitus (three points). A high PAINESD score (typically ≥15 points) has been associated with significantly higher rates of acute haemodynamic decompensation and early mortality after VT ablation76,81. In a study of 75 patients undergoing VT ablation, pre-emptive use of MCS in patients with a high PAINESD score was associated with a reduced rate of death or heart transplantation (33% versus 66%; P < 0.01) compared with when it was not used82. Therefore, the primary benefit of haemodynamic support during VT ablation is from mitigation of periprocedural acute haemodynamic decompensation and heart failure rather than VT ablation success.
For high-risk patients (with a high PAINESD score) being considered for VT ablation, consultation with heart failure specialists and cardiac surgeons can be prudent, for optimization of symptoms and volume status, for consideration of pre-emptive temporary MCS, and for potential evaluation for advanced therapies (such as heart transplantation or durable MCS) as appropriate.
Cardiac surgery
MCS devices have become an integral component of the repertoire used in most general cardiac surgical programmes. Although most frequently used as a rescue for haemodynamic collapse after surgery, their use as an adjunct in selected patients undergoing complex or high-risk surgery is also increasing. There are three broad applications of temporary MCS in a non-transplantation cardiac surgical practice: management of cardiogenic shock before surgical intervention, pre-emptive implantation for high-risk patients undergoing surgery, and management of perioperative and post-cardiotomy circulatory compromise.
Haemodynamic compromise before surgical intervention
Cardiogenic shock can be a presentation of various valvular, myocardial or coronary pathologies requiring surgical intervention. In addition to the common aetiologies and manifestations discussed above, other pre-surgical conditions that can present with cardiogenic shock include massive pulmonary embolism and iatrogenic complications of percutaneous interventions.
Patients in cardiogenic shock are at high operative risk when undergoing cardiac surgery. For example, coronary artery bypass graft surgery in the setting of cardiogenic shock is associated with an approximately 20% operative mortality, rising to 33% when patients have unknown neurological status at the time of surgery83 and as high as 50% if there are mechanical complications (ventricular septal or papillary muscle rupture)84. Although some conditions require emergent resolution beyond institution of MCS (such as aortic valve insufficiency or ischaemia and coronary artery disease), temporary circulatory support can be used as a bridge to optimize organ function and avoid the risk of death associated with a salvage procedure in preparation for a more controlled, urgent surgical operation. For example, in a study of 28 patients with post-infarction ventricular septal rupture, surgery was deferred in those at high surgical risk. These patients were initially managed with VA-ECMO to improve haemodynamic profiles and metabolic status (n = 11). Despite their higher risk profile (assessed by INTERMACS status and the presence of multiorgan failure), the use of temporary MCS in these patients was associated with a perioperative mortality that was similar to that in less-sick patients who underwent surgery without temporary MCS optimization85. Temporary MCS in this setting can also allow care teams to weigh the benefits of surgery in moribund patients who would otherwise be at prohibitively high surgical risk.
The effects on preload, afterload and (when applicable) shunt flow must be considered when selecting a bridging device86. The adequacy of MCS during resuscitation and patient stability in the ensuing hours and days often determine the timing of surgery.
Pre-emptive application in high-risk patients undergoing surgery
Post-cardiotomy cardiogenic shock and resultant multiorgan dysfunction remain the leading causes of early mortality after cardiac surgery87. Therefore, efforts are focused on its prevention, ensuring preoperative optimization and modern myocardial protection, as needed. Risk factors for post-cardiotomy cardiogenic shock include pre-existing ventricular dysfunction, long ischaemic periods and low baseline cardiac output. In patients with these high-risk characteristics who are undergoing surgery, pre-emptive, temporary MCS can help to avoid high doses of pharmacological support and promote cardiac recovery in the early postoperative period. Pre-emptive MCS, placed before weaning from cardiopulmonary bypass or if weaning fails, carries a relatively low incremental risk compared with when deployed for cardiogenic shock88. Given that the requirement for biventricular support is less common, percutaneous LV assist devices (as opposed to ECMO) are ideal. The microaxial flow pump Impella 5.5 device is becoming increasingly popular for this application owing to its relative ease of implantation via the axillary artery or aorta, offering the potential for full LV support as well as the option of removal without re-sternotomy89.
Post-cardiotomy shock and perioperative circulatory collapse
In addition to pre-existing cardiac dysfunction or injury, inadequate myocardial protection, ischaemia–reperfusion injury and/or surgical complications all contribute to the risk of post-cardiotomy cardiogenic shock. Post-cardiotomy shock can manifest after unsuccessful weaning from cardiopulmonary bypass as a progressive haemodynamic decline after cardiopulmonary bypass separation or in the postoperative intensive care unit setting, and is associated with up to 60% mortality during or shortly after the index hospitalization90,91. In these scenarios of extreme haemodynamic compromise, ECMO is the most commonly used form of temporary MCS. However, if cardiac recovery or repair is unlikely, temporary MCS (ECMO) is not generally recommended unless durable support or heart transplantation are options. Further, advanced therapeutic options should be considered if a patient cannot be weaned after 3–4 days of MCS, because the likelihood of cardiac recovery after ≥7 days of post-cardiotomy MCS are low91.
Although circulatory collapse after cardiac surgery is infrequent, it is associated with a 30-day postoperative mortality of >50%92. When conventional resuscitation efforts fail in the setting of postoperative cardiac arrest, extracorporeal cardiopulmonary resuscitation (ECMO or cardiopulmonary bypass) can be performed centrally or peripherally. Although the likelihood of patient survival is based on several factors, such as the underlying cause of cardiac arrest, comorbidities, effectiveness and duration of initial resuscitation, and the promptness of the institution of ECMO, survival rates in the range of 30–40% have been reported93.
Advanced heart failure
Although AMI-CS has traditionally been the focus of clinical and research inquiry for the use of temporary MCS, heart failure-related cardiogenic shock is currently more prevalent35. Patients with heart failure often also constitute the ‘high-risk’ populations undergoing the various interventions (including PCI, cardiac ablation procedures and cardiac surgery), in whom the use of temporary MCS has already been discussed. The following section uniquely focuses on the role of temporary MCS in patients with advanced heart failure under consideration for durable MCS and heart transplantation.
The use of temporary MCS can be considered before durable MCS surgery in selected patients at high surgical risk with pre-existing heart failure for the purposes of achieving clinical stability and maintaining end-organ perfusion. Similar considerations to those discussed in the previous section on pre-emptive MCS in cardiac surgery are relevant.
In patients with advanced heart failure who are on the waiting list for heart transplantation in the USA94, the use of temporary MCS has particularly increased over the past 4 years, tied to important implications for transplantation prioritization. In 2018, the United Network for Organ Sharing (UNOS) allocation system was modified in an effort to ensure appropriate prioritization of patients in most urgent need of transplantation (Table 4). Patients receiving temporary MCS are accordingly designated status 1–3, with shorter waiting list times than patients designated status 4–6. Patients receiving ECMO or biventricular support devices are designated status 1, followed by patients supported with an IABP or a percutaneous ventricular assist device, who are designated status 2–3. Patients are maintained on these forms of temporary MCS for longer periods of time than originally intended, via alternative access (often axillary rather than femoral artery) to allow mobility. Although shown to be safe and effective in some studies94, the increased use of temporary MCS in this setting has sparked debate about appropriateness of use as well as effects on longer-term outcomes after heart transplantation95.
Table 4.
Comparison of old and new systems for adult heart allocation in the USA
| Old system for adult heart allocation | New system for adult heart allocation | Criteria |
|---|---|---|
| Status 1A | Status 1 |
Venoarterial extracorporeal membrane oxygenation Non-dischargeable surgical biventricular assist device Mechanical circulatory support with life-threatening ventricular tachycardia |
| Status 2 |
Non-dischargeable, surgically implanted, non-endovascular left ventricular assist device Intra-aortic balloon pump Ventricular tachycardia or ventricular fibrillation, mechanical support not required Mechanical circulatory support device with device malfunction or mechanical failure Total artificial heart, biventricular assist device, right ventricular assist device, or ventricular assist device for patients with single ventricle Percutaneous endovascular mechanical circulatory support device |
|
| Status 3 |
Dischargeable left ventricular assist device for discretionary 30 days Multiple inotropes or single high-dose inotrope with continuous haemodynamic monitoring Venoarterial extracorporeal membrane oxygenation after 7 days; percutaneous endovascular circulatory support device or intra-aortic balloon pump after 14 days Non-dischargeable, surgically implanted, non-endovascular left ventricular assist device after 14 days Mechanical circulatory support device with one of the following: device infection, haemolysis, pump thrombosis, right-sided heart failure, mucosal bleeding or aortic valve insufficiency |
|
| Status 1B, status 2 | Status 4 |
Dischargeable left ventricular assist device without discretionary 30 days Inotropes without haemodynamic monitoring Re-transplantation Diagnosis of one of the following: congenital heart disease, ischaemic heart disease with intractable angina, hypertrophic cardiomyopathy, restrictive cardiomyopathy or amyloidosis |
| – | Status 5 | On the waiting list for at least one other organ at the same hospital |
| – | Status 6 | All others |
The updated United Network for Organ Sharing (UNOS) allocation system prioritizes patients in urgent need of transplantation. Those patients receiving temporary mechanical circulatory support are designated as UNOS status 1 and 2, with the shortest waiting list times. Table modified with permission from ref.107, Elsevier.
If allograft function is suboptimal (primary graft dysfunction) at the time of heart transplantation, ECMO, IABP or another form of temporary MCS can be used to rest the allograft and allow adequate end-organ perfusion. Although its causes remain elusive, primary graft dysfunction is encountered in approximately 10–30% of all heart transplantations and is a challenging clinical scenario96. Observational reports in which the prompt use of VA ECMO allowed myocardial recovery and even a possibility of decreased mortality have been encouraging, but further studies are needed97. Advances to increase access to heart transplantation are progressing at a rapid pace, demanding dedicated studies to improve our understanding of the role and effects of temporary MCS in the management of patients with advanced heart failure.
Future development
Despite the major advances and varied technologies for providing MCS, important opportunities exist for additional innovation, including improvements to pump flow capacity, pump size, haemocompatibility and vascular access.
Expandable pumps and sheaths allow device insertion and withdrawal at smaller French sizes, while facilitating increases in the flow rate that is typically possible given the constraints of native vessel size. One novel expandable microaxial flow pump can be advanced through a 9F sheath and expanded to 18F to achieve peak flows of approximately 3.5 l/min, a rate similar to that of flow pumps requiring substantially larger access98.
Temporary MCS in the ambulatory setting is an area of investigation intended to facilitate physical rehabilitation and allow outpatient support while the patient awaits cardiac recovery or more definitive therapy. The Intravascular Ventricular Assist System (iVAS; NuPulse)99 is an extended use, minimally invasive, ambulatory IABP intended to be used as a bridge to transplantation. The initial feasibility trial demonstrated early positive experiences with the device100, and larger investigations are underway. For patients with decompensated heart failure, the current UNOS allocation system limits opportunities for assessment of recovery, given the shorter waiting list times for urgent transplantation candidates receiving temporary MCS. Higher flow devices (>5 l/min) providing nearly full support and greater levels of LV unloading for longer durations (>14 days) might allow cardiac recovery without proceeding directly to durable LV assist device or transplantation and are currently being studied for this purpose101. Furthermore, support systems are being developed for longer-term outpatient applications (up to 1 year) in which the purge line will be eliminated but, much like durable ventricular assist devices, there will be a percutaneous drive line and an external controller and battery. These devices will be implantable without a thoracotomy or large-bore outflow graft requiring surgical anastomosis to the proximal aorta.
Novel cannulas and cannulation strategies for MCS systems are being investigated and developed. Transcaval implantation of microaxial flow devices is a novel approach introduced for patients with small, tortuous or diseased femoral or iliac arteries. An electrified needle with guidewire crosses the inferior vena cava into the aorta, and the microaxial device can then be advanced to its final transvalvular position102. Another strategy known as left atrial venoarterial (LAVA) ECMO103 uses a fenestrated cannula (24F VFEM024; Edwards Lifesciences) inserted via the femoral vein and advanced through the interatrial septum. Blood is simultaneously withdrawn from the right and left atria, which offers the potential advantage of LV unloading without additional access for venting.
Finally, a major area of innovation is the development of smart pumps that monitor haemodynamics and can predict instability for pre-emptive intervention. Additionally, artificial intelligence-based software algorithms are being used to aid in choosing which support strategy (drug-based and/or device-based) is optimal for an individual patient.
Conclusions
Although historically reserved for rescue therapy, temporary MCS devices are now being routinely used in a variety of clinical scenarios. Expanded indications and applications demand multidisciplinary team education and collaborative efforts to establish best practices. Continued innovation in bioengineering coupled with clinical trial assessment is required to elucidate the specific scenarios, patient populations and timings in which temporary MCS confers clinical benefit that outweighs potential complications.
Author contributions
B.S.S., C.R.G., S.R.D., G.W.S., N.M., A.C.A., D.B. and A.L. researched data for the article. B.S.S., C.R.G., A.C.A., D.B. and A.L. discussed the content. All the authors wrote the manuscript. B.S.S, C.R.G., M.M.W., A.C.A., D.B. and A.L. reviewed and/or edited the article before submission.
Peer review
Peer review information
Nature Reviews Cardiology thanks José González-Costello and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
S.R.D. declares receiving research grant support from Biosense Webster and owning equity in Farapulse (acquired by Boston Scientific) and Manual Surgical Sciences. D.B. declares receiving an unrestricted educational grant from Abiomed, acting as a consultant to PVLoops and receiving consulting fees from CardioDyme. A.L. declares receiving speaker honoraria from Zoll, being on a data safety and monitoring board for Sequana, being on the advisory board of Bioventrix and being a speaker for Novartis. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Parissis H, et al. IABP: history-evolution-pathophysiology-indications: what we need to know. J. Cardiothorac. Surg. 2016;11:122. doi: 10.1186/s13019-016-0513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishna M, Zacharowski K. Principles of intra-aortic balloon pump counterpulsation. Contin. Educ. Anaesth. Crit. Care Pain. 2009;9:24–28. doi: 10.1093/bjaceaccp/mkn051. [DOI] [Google Scholar]
- 3.Getinge. Intra-aortic balloon counterpulsation. Getingehttps://www.getinge.com/me/products/hospital/counterpulsation/ (2022).
- 4.Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of mechanical circulatory support. J. Am. Coll. Cardiol. 2015;66:2663–2674. doi: 10.1016/j.jacc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Schreuder JJ, et al. Beat-to-beat effects of intraaortic balloon pump timing on left ventricular performance in patients with low ejection fraction. Ann. Thorac. Surg. 2005;79:872–880. doi: 10.1016/j.athoracsur.2004.07.073. [DOI] [PubMed] [Google Scholar]
- 6.Abiomed. Impella: the world’s smallest heart pump. Abiomedhttps://www.abiomed.com/products-and-services/impella (2022).
- 7.Gottula AL, et al. Impella in transport: physiology, mechanics, complications, and transport considerations. Air Med. J. 2021;41:114–127. doi: 10.1016/j.amj.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Telukuntla KS, Estep JD. Acute mechanical circulatory support for cardiogenic shock. Methodist. Debakey Cardiovasc. J. 2020;16:27–35. doi: 10.14797/mdcj-16-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saffarzadeh A, Bonde P. Options for temporary mechanical circulatory support. J. Thorac. Dis. 2015;7:2102–2111. doi: 10.3978/j.issn.2072-1439.2015.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapur NK, et al. Mechanical circulatory support devices for acute right ventricular failure. Circulation. 2017;136:314–326. doi: 10.1161/CIRCULATIONAHA.116.025290. [DOI] [PubMed] [Google Scholar]
- 11.Anderson M, et al. Outcomes of patients with right ventricular failure requiring short-term hemodynamic support with the Impella RP device. J. Heart Lung Transpl. 2018;37:1448–1458. doi: 10.1016/j.healun.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Abiomed. Impella RP with SmartAssist. Abiomedhttps://www.heartrecovery.com/products-and-services/impella/impella-rp (2022).
- 13.FDA. Fact Sheet for Patients: emergency use of Impella RP system during the COVID-19 outbreak. FDAhttps://www.fda.gov/media/138462/download (2020).
- 14.Upadhyay R, Alrayes H, Arno S, Kaushik M, Basir MB. Current landscape of temporary percutaneous mechanical circulatory support technology. US Cardiol. Rev. 2021;15:e21. doi: 10.15420/usc.2021.15. [DOI] [Google Scholar]
- 15.Spectrum Medical. Dual Lumen Cannula. Spectrum Medicalhttps://www.spectrummedical.com/quantum-perfusion-for-the-icu-cath-lab-and-transport/quantum-sterile-technologies-icu/cannulas/dual-lumen-rv-to-pa-cannula (2020).
- 16.Takayama H, et al. A novel approach to percutaneous right-ventricular mechanical support. Eur. J. Cardiothorac. Surg. 2012;41:423–426. doi: 10.1016/j.ejcts.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 17.Abbott. About the CentriMag circulatory support system. Abbotthttps://www.cardiovascular.abbott/us/en/hcp/products/heart-failure/mechanical-circulatory-support/centrimag-acute-circulatory-support-system/about.html (2022).
- 18.Abiomed. Impella RP system with the automated impella controller. FDAhttps://www.fda.gov/media/138463/download (2020).
- 19.Tsangaris A, et al. Overview of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) support for the management of cardiogenic shock. Front. Cardiovasc. Med. 2021;8:686558. doi: 10.3389/fcvm.2021.686558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cevasco M, et al. Left ventricular distension and venting strategies for patients on venoarterial extracorporeal membrane oxygenation. J. Thorac. Dis. 2019;11:1676–1683. doi: 10.21037/jtd.2019.03.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Fares AA, et al. Optimal strategy and timing of left ventricular venting during veno-arterial extracorporeal life support for adults in cardiogenic shock. Circ. Heart Fail. 2019;12:e006486. doi: 10.1161/CIRCHEARTFAILURE.119.006486. [DOI] [PubMed] [Google Scholar]
- 22.Truby LK, et al. Incidence and implications of left ventricular distention during venoarterial extracorporeal membrane oxygenation support. ASAIO J. 2017;63:257–265. doi: 10.1097/MAT.0000000000000553. [DOI] [PubMed] [Google Scholar]
- 23.Schrage B, et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation. 2020;142:2095–2106. doi: 10.1161/CIRCULATIONAHA.120.048792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo JJ, et al. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J. Am. Coll. Cardiol. 2019;73:654–662. doi: 10.1016/j.jacc.2018.10.085. [DOI] [PubMed] [Google Scholar]
- 25.Extracorporeal Life Support Organization. ECLS international summary of statistics. Extracorporeal Life Support Organizationhttps://www.elso.org/Registry/InternationalSummaryandReports/InternationalSummary.aspx (2022).
- 26.Sakamoto T, et al. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: a prospective observational study. Resuscitation. 2014;85:762–768. doi: 10.1016/j.resuscitation.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 27.Stub D, Byrne M, Pellegrino V, Kaye DM. Extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in a sheep model of refractory ischaemic cardiac arrest. Heart Lung Circ. 2013;22:421–427. doi: 10.1016/j.hlc.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Subramaniam AV, Barsness GW, Vallabhajosyula S, Vallabhajosyula S. Complications of temporary percutaneous mechanical circulatory support for cardiogenic shock: an appraisal of contemporary literature. Cardiol. Ther. 2019;8:211–228. doi: 10.1007/s40119-019-00152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patton-Rivera K, et al. Using near-infrared reflectance spectroscopy (NIRS) to assess distal-limb perfusion on venoarterial (V-A) extracorporeal membrane oxygenation (ECMO) patients with femoral cannulation. Perfusion. 2018;33:618–623. doi: 10.1177/0267659118777670. [DOI] [PubMed] [Google Scholar]
- 30.Guglin M, et al. Venoarterial ECMO for adults: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019;73:698–716. doi: 10.1016/j.jacc.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 31.Extracorporeal Life Support Organization. General guidelines for all ECLS cases. Extracorporeal Life Support Organizationhttps://www.elso.org/Portals/0/ELSO%20Guidelines%20General%20All%20ECLS%20Version%201_4.pdf (2017).
- 32.Elgendy IY, Van Spall HGC, Mamas MA. Cardiogenic shock in the setting of acute myocardial infarction. Circ. Cardiovasc. Interv. 2020;13:e009034. doi: 10.1161/CIRCINTERVENTIONS.120.009034. [DOI] [PubMed] [Google Scholar]
- 33.Baran DA, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter. Cardiovasc. Interv. 2019;94:29–37. doi: 10.1002/ccd.28329. [DOI] [PubMed] [Google Scholar]
- 34.Kalra S, et al. Risk prediction in cardiogenic shock: current state of knowledge, challenges and opportunities. J. Card. Fail. 2021;27:1099–1110. doi: 10.1016/j.cardfail.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Abraham J, et al. Heart failure-related cardiogenic shock: pathophysiology, evaluation and management considerations: review of heart failure-related cardiogenic shock. J. Card. Fail. 2021;27:1126–1140. doi: 10.1016/j.cardfail.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Akodad M, Delmas C, Bonello L, Duflos C, Roubille F. Intra-aortic balloon pump: is the technique really outdated? Esc. Heart Fail. 2020;7:1025–1030. doi: 10.1002/ehf2.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhruva SS, et al. Use of mechanical circulatory support devices among patients with acute myocardial infarction complicated by cardiogenic shock. JAMA Netw. Open. 2021;4:e2037748. doi: 10.1001/jamanetworkopen.2020.37748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiele H, et al. Intraaortic balloon pump in cardiogenic shock complicating acute myocardial infarction: long-term 6-year outcome of the randomized IABP-SHOCK II trial. Circulation. 2019;139:395–403. doi: 10.1161/CIRCULATIONAHA.118.038201. [DOI] [PubMed] [Google Scholar]
- 39.Thiele H, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382:1638–1645. doi: 10.1016/S0140-6736(13)61783-3. [DOI] [PubMed] [Google Scholar]
- 40.Thiele H, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N. Engl. J. Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 41.O’Gara PT, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 42.van Diepen S, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 43.Ibanez B, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 44.Khera R, et al. Trends in the use of percutaneous ventricular assist devices: analysis of national inpatient sample data, 2007 through 2012. JAMA Intern. Med. 2015;175:941–950. doi: 10.1001/jamainternmed.2014.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seyfarth M, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J. Am. Coll. Cardiol. 2008;52:1584–1588. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 46.Alushi B, et al. Impella versus IABP in acute myocardial infarction complicated by cardiogenic shock. Open Heart. 2019;6:e000987. doi: 10.1136/openhrt-2018-000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouweneel DM, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J. Am. Coll. Cardiol. 2017;69:278–287. doi: 10.1016/j.jacc.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 48.Schrage B, et al. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation. 2019;139:1249–1258. doi: 10.1161/CIRCULATIONAHA.118.036614. [DOI] [PubMed] [Google Scholar]
- 49.Elliott Miller P, et al. Clinical outcomes and cost associated with an intravascular microaxial left ventricular assist device vs intra-aortic balloon pump in patients presenting with acute myocardial infarction complicated by cardiogenic shock. JAMA Intern. Med. 2022 doi: 10.1001/jamainternmed.2022.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neumann F-J, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur. Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 51.Udesen NJ, et al. Rationale and design of DanGer shock: Danish-German cardiogenic shock trial. Am. Heart J. 2019;214:60–68. doi: 10.1016/j.ahj.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 52.US National Library of Medicine. Clinicaltrials.govhttps://clinicaltrials.gov/ct2/show/NCT03677180 (2022).
- 53.Burkhoff D, Cohen H, Brunckhorst C, O’Neill WW. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am. Heart J. 2006;152:469.e1–469.e8. doi: 10.1016/j.ahj.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 54.Thiele H, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur. Heart J. 2005;26:1276–1283. doi: 10.1093/eurheartj/ehi161. [DOI] [PubMed] [Google Scholar]
- 55.Thiagarajan RR, et al. Extracorporeal Life Support Organization registry international report 2016. ASAIO J. 2017;63:60–67. doi: 10.1097/MAT.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 56.Ouweneel DM, et al. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med. 2016;42:1922–1934. doi: 10.1007/s00134-016-4536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dangers L, et al. Extracorporeal membrane oxygenation for acute decompensated heart failure. Crit. Care Med. 2017;45:1359–1366. doi: 10.1097/CCM.0000000000002485. [DOI] [PubMed] [Google Scholar]
- 58.Acharya D, et al. Extracorporeal membrane oxygenation in myocardial infarction complicated by cardiogenic shock: analysis of the ELSO registry. J. Am. Coll. Cardiol. 2020;76:1001–1002. doi: 10.1016/j.jacc.2020.06.062. [DOI] [PubMed] [Google Scholar]
- 59.US National Library of Medicine. Clinicaltrials.govhttps://www.clinicaltrials.gov/ct2/show/NCT04682483 (2022).
- 60.Perera D, et al. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: a randomized controlled trial. JAMA. 2010;304:867–874. doi: 10.1001/jama.2010.1190. [DOI] [PubMed] [Google Scholar]
- 61.Perera D, et al. Long-term mortality data from the balloon pump-assisted coronary intervention study (BCIS-1): a randomized, controlled trial of elective balloon counterpulsation during high-risk percutaneous coronary intervention. Circulation. 2013;127:207–212. doi: 10.1161/CIRCULATIONAHA.112.132209. [DOI] [PubMed] [Google Scholar]
- 62.Amin AP, et al. The evolving landscape of Impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. 2020;141:273–284. doi: 10.1161/CIRCULATIONAHA.119.044007. [DOI] [PubMed] [Google Scholar]
- 63.Dixon SR, et al. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (the PROTECT I trial): initial U.S. experience. JACC Cardiovasc. Interv. 2009;2:91–96. doi: 10.1016/j.jcin.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 64.O’Neill WW, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012;126:1717–1727. doi: 10.1161/CIRCULATIONAHA.112.098194. [DOI] [PubMed] [Google Scholar]
- 65.Cohen MG, et al. Optimizing rotational atherectomy in high-risk percutaneous coronary interventions: insights from the PROTECT ΙΙ study. Catheter. Cardiovasc. Interv. 2014;83:1057–1064. doi: 10.1002/ccd.25277. [DOI] [PubMed] [Google Scholar]
- 66.Flaherty MP, et al. Hemodynamic support with a microaxial percutaneous left ventricular assist device (Impella) protects against acute kidney injury in patients undergoing high-risk percutaneous coronary intervention. Circ. Res. 2017;120:692–700. doi: 10.1161/CIRCRESAHA.116.309738. [DOI] [PubMed] [Google Scholar]
- 67.Popma, J. PROTECT III first look: high-risk PCI outcomes in 800 Impella-supported patients. TCTMDhttps://www.tctmd.com/slide/protect-iii-first-look-high-risk-pci-outcomes-800-impella-supported-patients (2019).
- 68.US National Library of Medicine. Clinicaltrials.govhttps://clinicaltrials.gov/ct2/show/NCT04763200 (2022).
- 69.Kapur NK, et al. Unloading the left ventricle before reperfusion in patients with anterior ST-segment-elevation myocardial infarction. Circulation. 2019;139:337–346. doi: 10.1161/CIRCULATIONAHA.118.038269. [DOI] [PubMed] [Google Scholar]
- 70.Lawton JS, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e18–e114. doi: 10.1161/CIR.0000000000001038. [DOI] [PubMed] [Google Scholar]
- 71.Windecker S, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur. Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 72.Miller MA, et al. Activation and entrainment mapping of hemodynamically unstable ventricular tachycardia using a percutaneous left ventricular assist device. J. Am. Coll. Cardiol. 2011;58:1363–1371. doi: 10.1016/j.jacc.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 73.Miller MA, et al. Percutaneous hemodynamic support with Impella 2.5 during scar-related ventricular tachycardia ablation (PERMIT 1) Circ. Arrhythm. Electrophysiol. 2013;6:151–159. doi: 10.1161/CIRCEP.112.975888. [DOI] [PubMed] [Google Scholar]
- 74.Kusa S, et al. Outcomes of ventricular tachycardia ablation using percutaneous left ventricular assist devices. Circ. Arrhythm. Electrophysiol. 2017;10:e004717. doi: 10.1161/CIRCEP.116.004717. [DOI] [PubMed] [Google Scholar]
- 75.Turagam MK, et al. Hemodynamic support in ventricular tachycardia ablation: an International VT Ablation Center Collaborative Group study. JACC Clin. Electrophysiol. 2017;3:1534–1543. doi: 10.1016/j.jacep.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 76.Santangeli P, et al. Early mortality after catheter ablation of ventricular tachycardia in patients with structural heart disease. J. Am. Coll. Cardiol. 2017;69:2105–2115. doi: 10.1016/j.jacc.2017.02.044. [DOI] [PubMed] [Google Scholar]
- 77.Sapp JL, et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N. Engl. J. Med. 2016;375:111–121. doi: 10.1056/NEJMoa1513614. [DOI] [PubMed] [Google Scholar]
- 78.Tung R, et al. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: an International VT Ablation Center Collaborative Group study. Heart Rhythm. 2015;12:1997–2007. doi: 10.1016/j.hrthm.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vallabhajosyula S, et al. Venoarterial extracorporeal membrane oxygenation support for ventricular tachycardia ablation: a systematic review. ASAIO J. 2020;66:980–985. doi: 10.1097/MAT.0000000000001125. [DOI] [PubMed] [Google Scholar]
- 80.Baratto F, et al. Extracorporeal membrane oxygenation for hemodynamic support of ventricular tachycardia ablation. Circ. Arrhythm. Electrophysiol. 2016;9:e004492. doi: 10.1161/CIRCEP.116.004492. [DOI] [PubMed] [Google Scholar]
- 81.Santangeli P, et al. Acute hemodynamic decompensation during catheter ablation of scar-related ventricular tachycardia: incidence, predictors, and impact on mortality. Circ. Arrhythm. Electrophysiol. 2015;8:68–75. doi: 10.1161/CIRCEP.114.002155. [DOI] [PubMed] [Google Scholar]
- 82.Muser D, et al. Outcomes with prophylactic use of percutaneous left ventricular assist devices in high-risk patients undergoing catheter ablation of scar-related ventricular tachycardia: a propensity-score matched analysis. Heart Rhythm. 2018;15:1500–1506. doi: 10.1016/j.hrthm.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 83.Cox ML, et al. Outcomes after coronary artery bypass grafting in patients with myocardial infarction, cardiogenic shock and unresponsive neurological state: analysis of the Society of Thoracic Surgeons database. Eur. J. Cardiothorac. Surg. 2018;54:710–716. doi: 10.1093/ejcts/ezy114. [DOI] [PubMed] [Google Scholar]
- 84.Elbadawi A, et al. emporal trends and outcomes of mechanical complications in patients with acute myocardial infarction. JACC Cardiovasc. Interv. 2019;12:1825–1836. doi: 10.1016/j.jcin.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 85.Ariza-Solé A, et al. The role of perioperative cardiorespiratory support in post infarction ventricular septal rupture-related cardiogenic shock. Eur. Heart J. Acute Cardiovasc. Care. 2020;9:128–137. doi: 10.1177/2048872618817485. [DOI] [PubMed] [Google Scholar]
- 86.Ronco D, et al. Mechanical circulatory support as a bridge to definitive treatment in post-infarction ventricular septal rupture. JACC Cardiovasc. Interv. 2021;14:1053–1066. doi: 10.1016/j.jcin.2021.02.046. [DOI] [PubMed] [Google Scholar]
- 87.Hansen LS, Sloth E, Hjortdal VE, Jakobsen C-J. Follow-up after cardiac surgery should be extended to at least 120 days when benchmarking cardiac surgery centers. J. Cardiothorac. Vasc. Anesth. 2015;29:984–989. doi: 10.1053/j.jvca.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 88.Tong MZY, Weiss AJ, Bakaeen F, Soltesz EG. CABG in failing hearts: a how-to-guide to using modern mechanical support as backup. Innovations. 2021;16:227–230. doi: 10.1177/15569845211016455. [DOI] [PubMed] [Google Scholar]
- 89.Anderson M, et al. Impella 5.5 direct aortic implant and explant techniques. Ann. Thorac. Surg. 2021;111:e373–e375. doi: 10.1016/j.athoracsur.2020.09.069. [DOI] [PubMed] [Google Scholar]
- 90.Kowalewski M, et al. Venoarterial extracorporeal membrane oxygenation for postcardiotomy shock-analysis of the Extracorporeal Life Support Organization registry. Crit. Care Med. 2021;49:1107–1117. doi: 10.1097/CCM.0000000000004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lorusso R, et al. Structured review of post-cardiotomy extracorporeal membrane oxygenation: part 1 – adult patients. J. Heart Lung Transpl. 2019;38:1125–1143. doi: 10.1016/j.healun.2019.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vakil K, et al. Long-term outcomes of patients who had cardiac arrest after cardiac operations. Ann. Thorac. Surg. 2016;102:512–517. doi: 10.1016/j.athoracsur.2016.01.092. [DOI] [PubMed] [Google Scholar]
- 93.Mazzeffi MA, et al. Outcomes of extracorporeal cardiopulmonary resuscitation for refractory cardiac arrest in adult cardiac surgery patients. J. Thorac. Cardiovasc. Surg. 2016;152:1133–1139. doi: 10.1016/j.jtcvs.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 94.Clerkin KJ, et al. Impact of temporary percutaneous mechanical circulatory support before transplantation in the 2018 heart allocation system. JACC Heart Fail. 2022;10:12–23. doi: 10.1016/j.jchf.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Silvestry SC, Rogers JG. Rinse, wash, repeat: the evolution of the UNOS heart transplant allocation system. JACC Heart Fail. 2022;10:24–26. doi: 10.1016/j.jchf.2021.10.010. [DOI] [PubMed] [Google Scholar]
- 96.Kobashigawa J, et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J. Heart Lung Transpl. 2014;33:327–340. doi: 10.1016/j.healun.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 97.DeRoo SC, et al. Extracorporeal membrane oxygenation for primary graft dysfunction after heart transplant. J. Thorac. Cardiovasc. Surg. 2019;158:1576–1584. doi: 10.1016/j.jtcvs.2019.02.065. [DOI] [PubMed] [Google Scholar]
- 98.Abiomed. First patients treated with the world’s smallest heart pump, the 9Fr impella ECP. Abiomedhttps://www.abiomed.com/about-us/news-and-media/press-releases/first-patients-treated-worlds-smallest-heart-pump-9fr-impella-ecp (2020).
- 99.Uriel N, et al. Clinical outcomes and quality of life with an ambulatory counterpulsation pump in advanced heart failure patients. Circ. Heart Fail. 2020;13:e006666. doi: 10.1161/CIRCHEARTFAILURE.119.006666. [DOI] [PubMed] [Google Scholar]
- 100.Jeevanandam V, et al. The first-in-human experience with a minimally invasive, ambulatory, counterpulsation heart assist system for advanced congestive heart failure. J. Heart Lung Transpl. 2018;37:1–6. doi: 10.1016/j.healun.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 101.Topkara VK, et al. Recovery with temporary mechanical circulatory support while waitlisted for heart transplantation. J. Am. Coll. Cardiol. 2022;79:900–913. doi: 10.1016/j.jacc.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Afana M, et al. Transcaval access for the emergency delivery of 5.0 liters per minute mechanical circulatory support in cardiogenic shock. Catheter. Cardiovasc. Interv. 2021;97:555–564. doi: 10.1002/ccd.29235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Singh-Kucukarslan G, et al. Hemodynamic effects of left-atrial venous arterial extra-corporeal membrane oxygenation (LAVA-ECMO) ASAIO J. 2021 doi: 10.1097/MAT.0000000000001628. [DOI] [PubMed] [Google Scholar]
- 104.Harjola V-P, et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur. J. Heart Fail. 2015;17:501–509. doi: 10.1002/ejhf.260. [DOI] [PubMed] [Google Scholar]
- 105.Schmidt M, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur. Heart J. 2015;36:2246–2256. doi: 10.1093/eurheartj/ehv194. [DOI] [PubMed] [Google Scholar]
- 106.Pöss J, et al. Risk stratification for patients in cardiogenic shock after acute myocardial infarction. J. Am. Coll. Cardiol. 2017;69:1913–1920. doi: 10.1016/j.jacc.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 107.Estep JD, Soltesz E, Cogswell R. The new heart transplant allocation system: early observations and mechanical circulatory support considerations. J. Thorac. Cardiovasc. Surg. 2020 doi: 10.1016/j.jtcvs.2020.08.113. [DOI] [PubMed] [Google Scholar]
- 108.Burkhoff, D., Dickstein, M. L. & Schleicher, T. Harvi - online (accessed 21 November 2019, PVLoops); https://harvi.online.