PURPOSE
Representativeness in acute leukemia clinical research is essential for achieving health equity. The National Cancer Institute's mandate for Comprehensive Cancer Centers (CCCs) to define and assume responsibility for cancer control and treatment across a geographic catchment area provides an enforceable mechanism to target and potentially remediate participatory inequities.
METHODS
We examined enrollee characteristics across 15 Cancer and Leukemia Group B/Alliance cooperative group adult acute leukemia clinical trials (N = 3,734) from 1998 to 2013, including participation in optional companion biobanks. We determined enrollment odds by race-ethnicity for all participants adjusted for national incidence, and for those enrolled at CCCs adjusted for catchment area incidence. We modeled biobank participation by sociodemographics using logistic regression.
RESULTS
Non-Hispanic (NH)-White patients were more likely to be enrolled than NH-Black, NH-Asian, or Hispanic patients (odds ratio [OR], 0.75, 0.48, and 0.44, respectively; all P < .001), but less likely than NH-Native American patients (OR, 1.91; P < .001), adjusted for national incidence. Enrollment odds were lower for NH-Black, NH-Asian, and Hispanic patients at CCCs adjusted for catchment area incidence (OR, 0.57, 0.26, and 0.32, respectively; P < .001); differences were driven by overenrollment of NH-White patients from outside self-defined catchment areas (18.1% v 12.3%; χ2 P = .01) and by CCCs with less absolute enrollee diversity (rank sum P = .03). Among all enrollees, NH-White race-ethnicity and lower neighborhood deprivation correlated with biobank participation (OR, 1.81 and 1.45, respectively; P = .01 and .03). For CCC enrollees, the correlation of race-ethnicity with biobank participation was attenuated by a measure accounting for their site's degree of enrollment disparity but not neighborhood deprivation.
CONCLUSION
Acute leukemia clinical research disparities are substantial and driven by structural trial enrollment barriers at CCCs. Real-time CCC access and enrollment monitoring is needed to better align research participation with local populations.
INTRODUCTION
Representativeness in clinical cancer research is essential for achieving health equity. Because of correlations between social determinants of health, mutational profiles, pharmacokinetics, and outcomes, over-representation or under-representation of demographic groups in clinical research can significantly limit the generalizability of research findings.1-5 If clinical trials are not representative, real-world efficacy, safety, and care delivery needs remain unknown.5,6 Inequitable representation in biobanking has the potential to misdirect drug and biomarker development toward overenrolled groups and impair pharmacogenomic advancements relevant to those under-represented.7,8 Moreover, trials offer high-quality care and access to novel treatments,9,10 and participatory equity is an ethically obligatory research practice.11,12
CONTEXT
Key Objective
Despite considerable race-ethnic disparities in adult acute leukemia clinical trial enrollment, there are knowledge gaps for targeting enrollment disparities at a scale amenable to intervention. We aimed to understand whether site catchment area diversity drives the representativeness of multicenter trials, and what additive participatory disparities exist for companion biobanking studies.
Knowledge Generated
Enrollment disparities were largest for non-Hispanic Black and Asian and Hispanic patients as measured against the self-defined catchment areas of participating National Cancer Institute–designated Comprehensive Cancer Centers; disparities were exacerbated by overenrollment of non-Hispanic White patients from outside these areas. Additive disparities in biobank participation were seen and appeared driven by trial enrollment disparities at these sites.
Relevance (J.W. Friedberg)
-
This study identifies the National Cancer Institute-Comprehensive Cancer Center catchment area as a target for improving overall trial and biobank representativeness for acute leukemia. Prospective monitoring of catchment area enrollment relative to its leukemia incidence is an opportunity to ensure participatory equity.*
*Relevance section written by JCO Editor-in-Chief Jonathan W. Friedberg, MD.
The most prominently documented adult trial enrollment disparities are by race and/or ethnicity, where individuals identifying as Black/African American, Asian American/Pacific Islander, Native American, and Hispanic/Latinx are frequently under-represented relative to incident disease burden.1,13 The presence, degree, and reasons for under-representation vary by cancer and trial type.13,14 Adult acute myeloid (AML) and lymphoblastic leukemia (ALL) are aggressive but curable blood cancers with distinctive care needs that make trial enrollment processes unique: patients require therapy within days of diagnosis, treatment is frequently inpatient, substantial proportions are treated and enrolled at National Cancer Institute (NCI) Comprehensive Cancer Centers (CCCs), and reliance on multicenter studies is higher because of lower prevalence.4,15,16 Our ClinicalTrials.gov national trial analysis found that Black, Native American, Asian, and Hispanic adults with acute leukemia were underenrolled by 51%, 56%, 36%, and 48%, respectively,13 but data sources limited patient- or site-level assessment.
Research elucidating the differences between local populations diagnosed with acute leukemia and those who participate in multicenter research is both limited and crucial for targeting interventions. Over the past 2 decades, the NCI has introduced increasing requirements for CCCs to define, describe, and be responsible for cancer control and treatment across geographic catchment areas.17 These areas represent an enforceable mechanism for generating participatory equity. Thus far, they remain an underutilized method for measuring performance at a geographic level small enough to be the target of equity-focused interventions.
In this context, important questions must be addressed so that remediating interventions can be developed. These include if and how catchment area enrollment diversity drives the representativeness of multicenter trials, and what additive participatory inequities exist for companion biobanking studies. Such questions are also central to the recent US Food and Drug Administration initiative to enforce trial enrollment equity.18 To address them, we analyzed the characteristics of participants enrolling on multicenter acute leukemia clinical trials run by the Cancer and Leukemia Group B (CALGB; now part of the Alliance for Clinical Trials in Oncology [Alliance]) cooperative group, and their optional biobanks, against national and CCC catchment area demographic distributions of acute leukemia.
METHODS
Protocol Selection
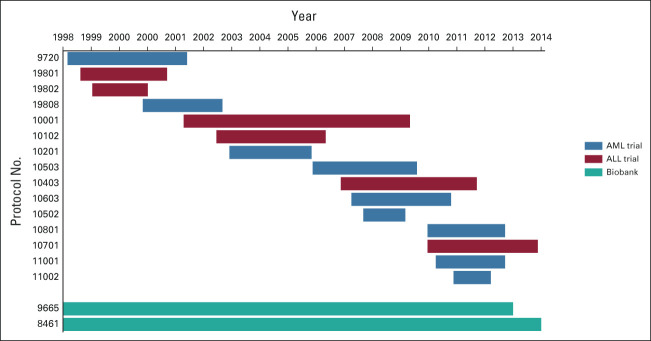
This analysis includes data from patients enrolled on the 15 CALGB/Alliance primary AML or ALL clinical trials that enrolled patients from 1998 to 2013. Enrollees' consent to participate in the two optional tissue biobanking protocols was recorded. An enrollment timeline is shown in Figure 1. Protocol names, NCT numbers, and enrollment periods are shown in the Data Supplement (online only). Institutional review board approval was obtained at each site before patient registration to any of the studies.
FIG 1.
Timeline of clinical trial and biobank study enrollment periods. ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia.
Patient Selection
All patients registered to the clinical trials were included in the initial data set. Patients registered to the biobanks outside these trials were not included to allow for accurate comparisons between incidence, trial enrollment, and biobank participation. Patients registered to the clinical trials were excluded if they were enrolled at a site outside the United States (n = 497; 11.0%) or did not have data on place of residence (n = 272; 6.0%) and/or race-ethnicity (n = 102; 2.3%) available.
Data Collection
Patient data collection was performed by the CALGB/Alliance Statistics and Data Management Center using a secure database. Each participant signed an institutional review board–approved, protocol-specific informed consent document in accordance with federal and institutional guidelines. The following demographic, clinical, and institutional information from the time of registration was abstracted: age, sex, race, ethnicity, date of registration, home zip code, enrollment site, and clinical trial and biobanking protocol consents. The data cutoff date was August 26, 2021. Data quality was ensured by review of data by the CALGB/Alliance Statistics and Data Center and by the study chairperson following Alliance policies.
Incidence Estimates
National adult incidence estimates from the SEER data (SEER*Stat version 8.3.8)19 and the 2010 US Census data20 were used to determine the number of adults diagnosed with AML and ALL in each demographic category. We identified adult incidence via the SEER-13 data set by International Classification of Diseases-0-3/WHO-2008 site and pathology recode categories. Census populations by age, sex, race, and ethnicity for the total US adult population and each SEER registry area were identified to estimate age- and sex-standardized national incidence. Race and ethnicity data were reconciled into five mutually exclusive race-ethnic categories of non-Hispanic (NH)-White (White), NH-Black/African American (Black), NH-Asian/Pacific Islander (Asian), NH-Native American/Alaska Native (Native American), and Hispanic; persons reporting as more than one race were excluded from incidence estimates.
NCI CCCs have been federally mandated to measure and designate their geographic catchment areas, within which they assume responsibility for cancer control and treatment.17,21 Catchment area incidence was determined for CCCs recruiting ≥ 10 patients to the clinical trials; catchment area geographies (Data Supplement) were obtained from public reports and/or CCC representatives. Population coverage estimates by age, sex, race, and ethnicity for these catchment areas were determined using census data. Age- and sex-standardized catchment area incidence was estimated by transforming national incidence by the catchment areas' adult population coverage and demographic proportions.
Sociodemographic Variables
Patient home zip code was used to assign the following sociodemographic variables: distance from site, rural-urban status, and neighborhood deprivation. Straight-line distance from site was calculated using great circles assuming a spherical earth.22 Rural-urban status was assigned and dichotomized according to Rural-Urban Continuum Codes (Data Supplement).23 Neighborhood deprivation was assigned using the national Area Deprivation Index (ADI), a composite measure of socioeconomic disadvantage that ranges from 1 to 100 on the basis of place of residence, with lower scores indicating less disadvantage.24
Statistical Methods
Descriptive analysis was performed using demographic, clinical, and institutional data collected in the CALGB/Alliance database. Comparisons between participants enrolled at CCCs versus other sites were assessed using the χ2, Fisher's exact, or Wilcoxon rank-sum tests, as appropriate.25,26 To assess incidence-adjusted enrollment diversity, we determined enrollment fractions (EFs) for each race-ethnic category, defined as the number of trial enrollees within a subgroup over a given period divided by the estimated incidence in that subgroup during the period.27 We compared EFs by race-ethnic category using the χ2 test with White enrollees as the comparator group and reporting odds ratios (ORs) with 95% CIs. Comparisons were performed for trial enrollees and biobank participants against national incidence. To estimate enrollment at CCCs, EF comparisons were performed for enrollees from CCCs that recruited ≥ 10 patients to the clinical trials against national incidence and the incidence of those CCCs' catchment areas in aggregate and individually.
We modeled the probability of consent to participate in a biobank by age, sex (male or female), acute leukemia subtype (AML or ALL), race-ethnicity (dichotomized as Black, Asian, Native American, Hispanic, and Other v White, and individually), year of enrollment, neighborhood deprivation, rural-urban status, and distance from site using univariable logistic regression models clustered by trial. Separate models were run for CCC sites recruiting ≥ 10 patients to include a measure of site disparity (EF odds for diverse enrollees compared with White enrollees within individual catchment areas), as site enrollment diversity characteristics have been shown to correlate with companion study participation.28 Multivariable models were built with variables significant in univariable analysis; interactions were assessed between race-ethnicity and other variables. All statistical tests were two-sided, and P < .05 was considered statistically significant. The study was approved by the Dana-Farber Office for Human Research Studies.
RESULTS
Patient Demographics and Clinical Trial Characteristics
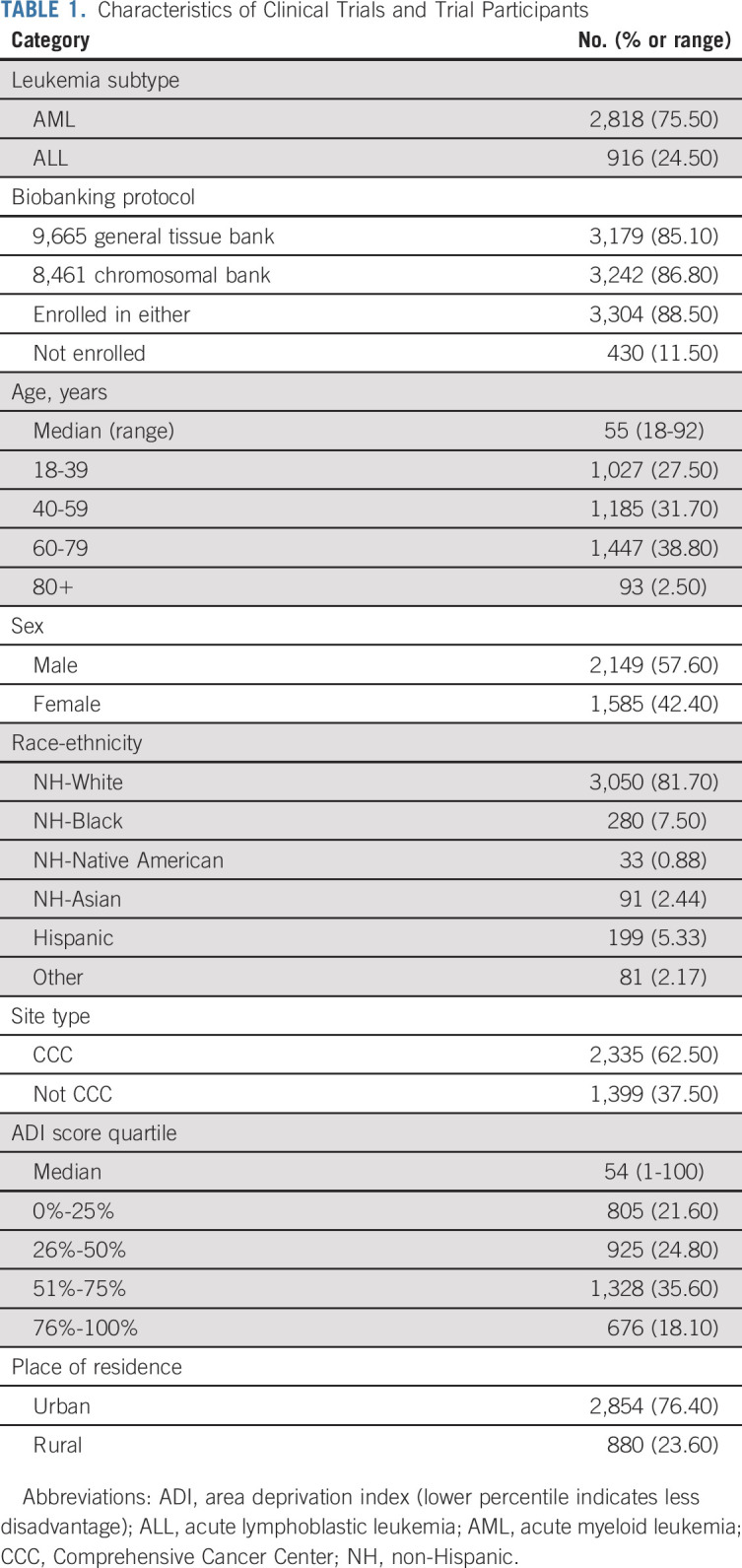
A total of 4,503 patients were enrolled onto the clinical trials; 3,734 (83.0%) were included in the analysis. Among US participants, fewer persons with ALL (3.0% v 7.0%) and from CCC sites (4.9% v 9.5%) were excluded; there were no differences by age, sex, or race-ethnicity (Data Supplement). Demographics are summarized in Table 1, with counts by race-ethnic category in the Data Supplement. Demographic comparisons between enrollees at CCCs and non-CCC sites are shown in the Data Supplement. At CCCs, there were more participants with AML, who identified as White, and who lived in neighborhoods with higher deprivation and more urbanity.
TABLE 1.
Characteristics of Clinical Trials and Trial Participants
Clinical Trial Enrollment
Clinical trial EF odds (N = 3,734) for Black (OR 0.75; 95% CI, 0.66 to 0.84), Asian (OR 0.48; 95% CI, 0.39 to 0.60), and Hispanic patients (OR 0.44; 95% CI, 0.38 to 0.51) were significantly lower than White patients, adjusted for national incidence, whereas Native American EF odds were significantly higher (OR 1.91; 95% CI, 1.35 to 2.71; Fig 2A). EF odds were lower for Asian and Hispanic patients at CCC sites recruiting ≥ 10 patients, unchanged for Black patients, and higher for Native American patients, adjusted for national incidence (n = 2,225; Fig 2B); opposite trends were seen for patients recruited at other sites (n = 1,509; Data Supplement). When adjusted for catchment area incidence, EF odds at CCC sites recruiting ≥ 10 patients decreased for Black and Asian patients, increased for Native American patients, and remained stable for Hispanic patients (Fig 2C). Five sites with indigenous health partnerships were responsible for 66% of Native American enrollment; a sensitivity analysis of Native American enrollment without these sites is shown in the Data Supplement.
FIG 2.
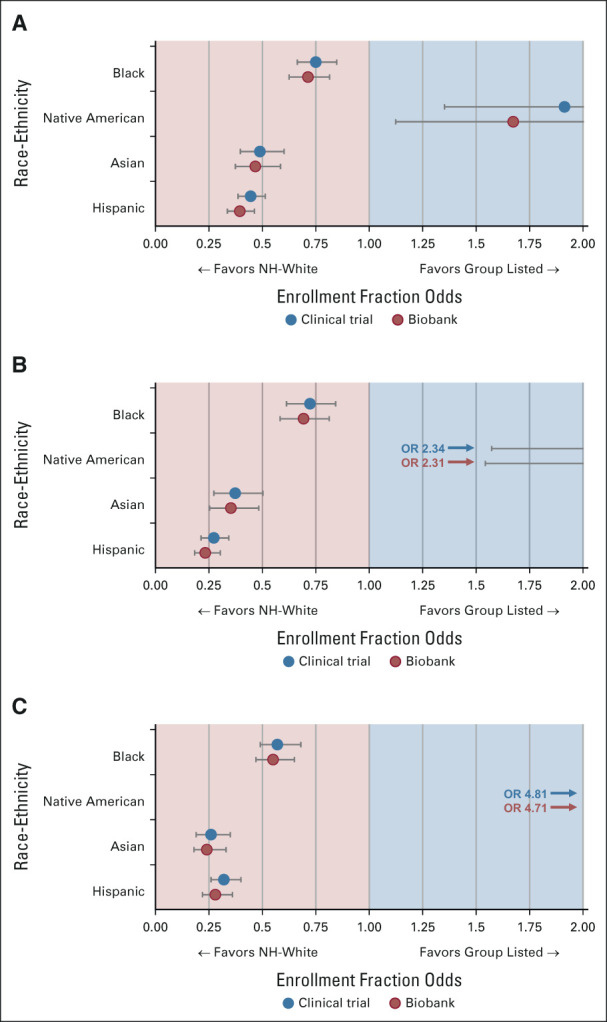
Enrollment fraction odds for trial enrollees and biobank participants by race-ethnicity. (A) Odds of enrollment compared with NH-Whites for all trial enrollees and biobank participants adjusted for national incidence (N = 3,734). (B) Odds of enrollment for patients enrolled at CCC sites recruiting 10 or more participants, adjusted for national incidence (n = 2,225). (C) Odds of enrollment for patients enrolled at CCC sites recruiting 10 or more participants, adjusted for these sites' catchment area incidence (n = 2,225). 95% CIs are shown as error bars. CCC, Comprehensive Cancer Center; NH, non-Hispanic; OR, odds ratio.
Among the 17 CCCs recruiting ≥ 10 patients (n = 2,225), site-specific enrollment diversity varied relative to catchment area incidence (Fig 3 and Data Supplement). Twelve sites (n = 1,671) recruited White patients at a higher proportion, whereas five (n = 554) did not. Five sites' overenrollment reached statistical significance (site disparity OR, 0.15-0.59; all P < .05; bold in Fig 3); one site significantly overenrolled diverse patients (OR, 1.67; 95% CI, 1.06 to 2.63). Sites with higher absolute proportions of White trial enrollees were more likely to overenroll White persons relative to catchment area incidence (Wilcoxon rank-sum P = .03). Three hundred ninety (17.5%) participants were from outside self-defined catchment areas, and as incident race-ethnic diversity increased, higher proportions of enrollees came from outside the catchment area (β = .45; 95% CI, 0.11 to 0.79). Comparisons between enrollees within (n = 1,835) and outside catchment areas (n = 390) are shown in the Data Supplement. There were no differences by age, sex, or leukemia subtype; White enrollees were more likely to come from outside catchment areas (18.1% v 12.3%; χ2 P = .01).
FIG 3.
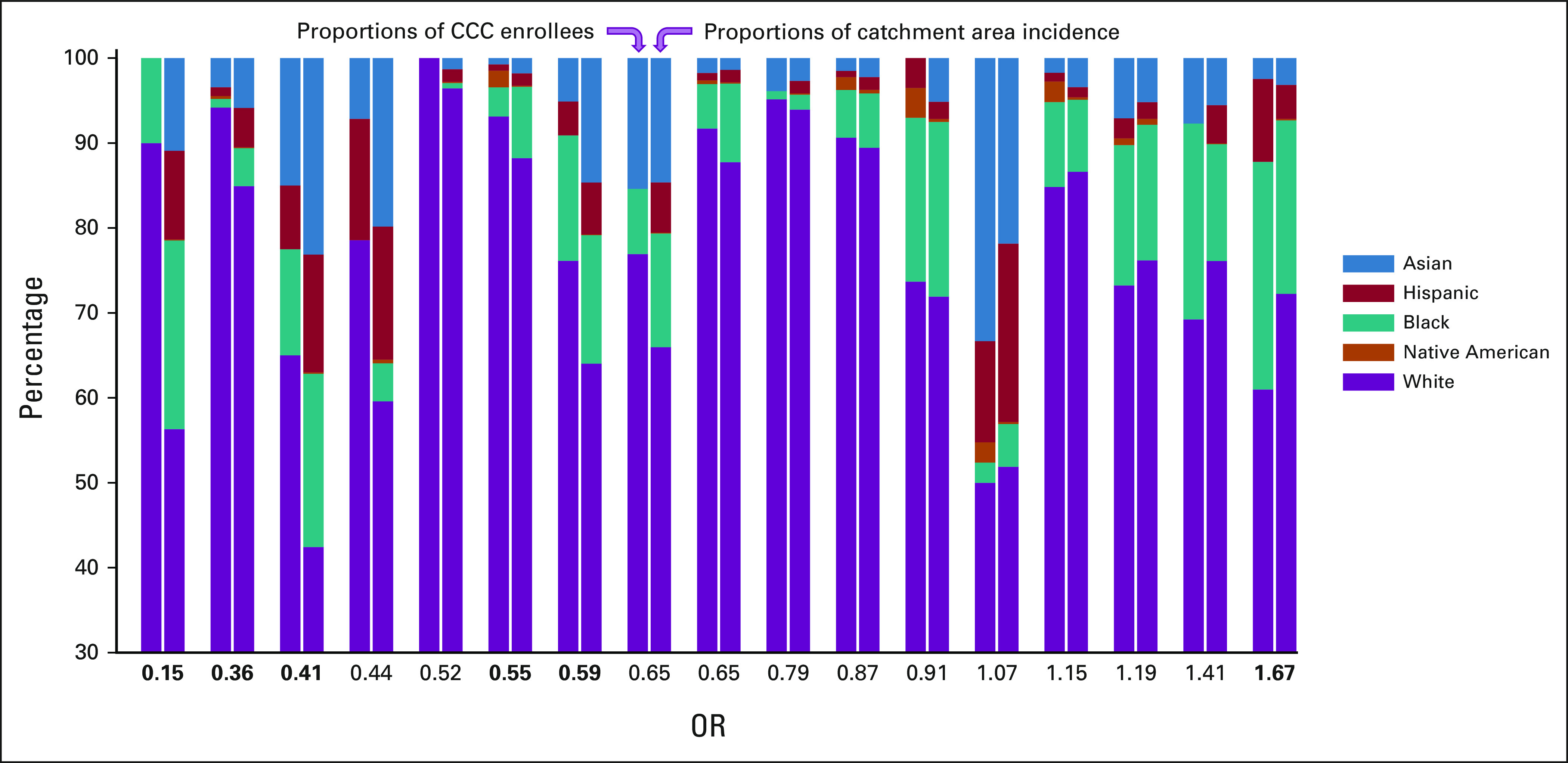
Enrollment proportions by race-ethnic group for the 17 CCC sites recruiting ≥ 10 patients to clinical trials relative to catchment area incidence. Each sites' proportions are displayed as stacked bars, with the proportion of all enrollees at the site in the left bar and the proportion of the catchment areas incident cases in the right bar. Site disparity ORs are shown below the corresponding sites (χ2 odds of diverse enrollment relative to non-Hispanic White enrollment, using estimated site catchment area incidence). Bold values indicate significant differences (P < .05). Sites are positioned left to right in order of increasing odds of enrolling diverse patients. Note that the vertical axis scale starts at 30%. CCC, Comprehensive Cancer Center; OR, odds ratio.
Biobank Participation
Biobank participation periods are shown in Figure 1. Biobank EF odds (N = 3,734) were significantly lower for Black (OR 0.71; 95% CI, 0.62 to 0.81), Asian (OR 0.46; 95% CI, 0.37 to 0.58), and Hispanic patients (OR 0.39; 95% CI, 0.33 to 0.46) compared with White patients, adjusted for national incidence, and Native American EF odds were significantly higher (OR 1.67; 95% CI, 1.12 to 2.47; Fig 2A); biobank EF odds were lower than clinical trial EF odds for each diverse race-ethnic group, but there were no statistically significant declinations. Analogous findings were seen when biobank enrollees were stratified by CCC sites recruiting ≥ 10 patients (n = 2,225) and other sites (n = 1,509) compared with national incidence, and for the former group compared with catchment area incidence (Fig 2 and Data Supplement). The exception to this was for Native American enrollees at other sites, where participation was not significantly different. A higher proportion of enrollees from inside CCC catchment areas participated in biobanking (97.0%) than did those from outside (93.3%; χ2 P < .001; Data Supplement).
Biobank Participation Among Trial Enrollees
Biobank participation proportions for each trial by race-ethnicity and annualized proportions are shown in the Data Supplement. Univariable models of biobank participation among all enrollees (N = 3,734) are shown in the Data Supplement. In these models, White race-ethnicity (OR 1.80) and lower neighborhood deprivation (OR 1.45) were correlated with biobank participation (both P < .05). Univariable models restricted to CCC sites recruiting ≥ 10 patients (n = 2,225) are shown in the Data Supplement. In these models, older age, AML subtype, White race-ethnicity, lower neighborhood deprivation, rural place of residence, and enrolling at a site with higher trial enrollment disparities correlated with biobank participation.
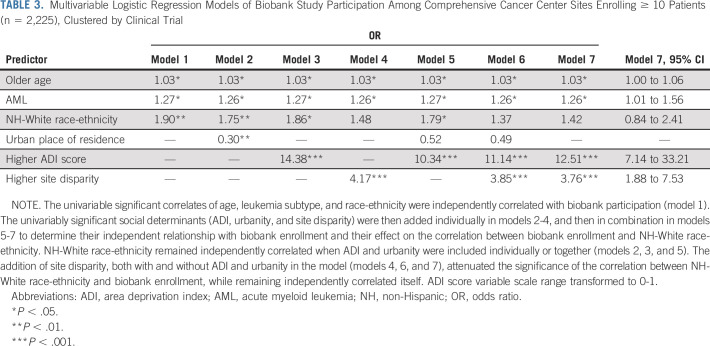
In a multivariable model of all enrollees and including significant univariable correlates (N = 3,734; Table 2), both White race-ethnicity and being from an area with less neighborhood deprivation independently correlated with biobank enrollment (OR 1.81; 95% CI, 1.17 to 2.79 and 1.45; 95% CI, 1.03 to 2.04). In multivariable modeling of CCC sites recruiting ≥ 10 patients (n = 2,225), the univariable significant correlates of age, leukemia subtype, and race-ethnicity were significantly correlated in an initial model (Table 3, model 1). The other univariably significant social determinants (ADI, urbanity, and site disparity) were then added individually (models 2-4) and in combination (models 5-7) to determine their relationship with biobank enrollment and their effect on the correlation between biobank enrollment and White race-ethnicity. White race-ethnicity remained independently correlated when ADI and urbanity were included individually or together (models 2, 3, and 5). The addition of site disparity, both with and without ADI and urbanity in the model (models 4, 6, and 7), attenuated the significance of the correlation between White race-ethnicity and biobank enrollment, while remaining independently correlated itself.
TABLE 2.
Final Multivariable Logistic Regression Model of Biobank Study Participation of All Trial Enrollees (N = 3,734), Clustered by Clinical Trial
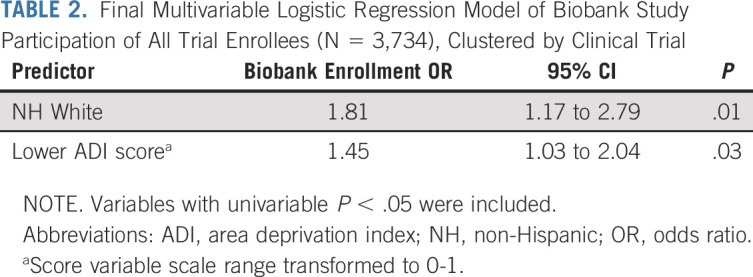
TABLE 3.
Multivariable Logistic Regression Models of Biobank Study Participation Among Comprehensive Cancer Center Sites Enrolling ≥ 10 Patients (n = 2,225), Clustered by Clinical Trial
Models of the other sites (n = 1,509; Data Supplement) found that ADI score and urbanity were univariable predictors of enrollment. Only lower ADI remained significantly correlated in a multivariable model (OR 7.14; 95% CI, 4.17 to 14.29). As there were unadjusted differences in AML and ALL biobank participation among all enrollees (AML: 93.4%; ALL, 73.3%; χ2 P < .001), exploratory models stratified by leukemia subtype are shown in the Data Supplement. Models with individual race-ethnicities are shown in the Data Supplement.
DISCUSSION
In this analysis of participatory disparities among a cooperative group's acute leukemia clinical trials and biobanking studies, we found trial enrollment to be significantly lower for Black, Asian, and Hispanic patients compared with White patients, with additive disparities in biobank participation even among those enrolled on clinical trials. Enrollment relative to national incidence was higher for Native American patients, reflecting the impact that indigenous health partnerships may have on enrollment equity. Trial enrollment disparities were largest for NCI CCCs relative to their self-defined catchment areas, which appear to have been amplified by recruitment of White patients from outside these areas. Biobank participation disparities by race-ethnicity were independent of neighborhood deprivation, but not of CCCs' trial enrollment disparity. Together, these data suggest structural advantages for White patients with acute leukemia in accessing clinical research, especially through CCC trial enrollment, and that dedicated health partnerships may remediate some aspects of structural disadvantage.
Access to CCCs can vary by race-ethnicity,29 distance, insurance type, educational level,30 and additional social determinants.16 The few data specific to adult acute leukemia are from the California Cancer Registry,4 which found lower rates of CCC treatment for Hispanic and Black persons (OR 0.79 and 0.66, respectively). Those differences reflect the benefit of involving community sites in multicenter trials; they are also smaller than the disparities seen herein, suggesting additive disparities in access and enrollment. Our data also show that enrollment disparities between CCCs are not uniform, vary by demographic mix, and have downstream effects on biobank participation. CCCs' enrollment disparities were almost uniformly increased by over-recruitment of White patients from outside self-defined catchment areas. Many CCCs are recognized for innovative research and recruit patients with lower-prevalence cancers from far away; nonetheless, their studies must be available equitably to the population for whom they are responsible.
A notable exception was for Native American enrollees, who were enrolled at higher rates compared with White patients. Five sites in Ohio, upstate New York, and North Carolina with indigenous health partnerships31,32 were responsible for 66% of these enrollees. Given the long history of marginalization and barriers to care for this group, these data suggest that such partnerships—which focus on overcoming population-specific structural, cultural, and interpersonal barriers—may provide useful strategies for achieving enrollment equity.31 At the same time, this positive result should neither overshadow the overall participatory disparities seen nor the evidence that indigenous communities remain vastly underserved.33,34
Numerous sociodemographic disparities in cancer clinical trial enrollment are known,27,35 but there are relatively few data assessing how trial enrollment inequities relate to companion biobank participation. A study of CALGB solid tumor trials found that among the 81% of enrollees who consented to an optional companion study, non-White patients were half as likely to consent.28 Our data show a similar rate of disparity in biobank participation among acute leukemia trial enrollees, with some differences on the basis of leukemia subtype. They also demonstrate that much of the disparity between biobank participation and incidence is attributable to trial enrollment rates.
This disparity between incidence and trial enrollment appears to have a downstream effect on biobank participation at high-enrolling CCCs. At these sites, White patients remained more likely to participate in a biobank independent of age, leukemia subtype, neighborhood deprivation, and urbanity. However, inclusion of a site-level trial disparity measure attenuated the significant association between race-ethnicity and biobank participation, suggesting that those barriers impeding representative trial enrollment also compromise equitable biobank participation. Although previous studies concluded that privacy concerns and desire for control of samples were responsible for biobank participation disparities,36,37 our findings support emerging literature that implicates structural barriers, including differences in access to biobanking, being asked to participate, and the availability of culturally competent recruitment methods.38-40 Given disease prevalence and the growing complexity of performing mutation-specific acute leukemia trials, efforts should focus on characterizing and reducing disparate participatory barriers between catchment areas, NCI CCCs, and their clinical research enterprises.
Our analysis has limitations. First, groupings by race-ethnicity are incomplete reflections of social and environmental determinants of health, culture, and structural racism.41,42 For example, while incorporating some potential sociodemographic mediators, the retrospective nature of these data limited us to geospatially derived surrogate measures, and analogous data for incident cases were not accessible. Second, geographic estimates of catchment areas for non-CCC sites were not available, limiting direct comparison. Third, CCCs are not required to use a uniform methodology for defining catchment areas, although most use health records data to generate geographic areas that encompass ≥ 80% of patients seen.21,37 As these designations denote areas for which CCCs have assumed cancer control and treatment responsibilities; however, they remain important for policy and population health equity. Fourth, cooperative group trials have characteristics that differ from other studies; findings are not generalizable beyond this trial mechanism. Finally, to ensure model stability, primary analyses included only significant univariable predictors in multivariable models; this may have omitted some variables with clinical relevance.
The importance of NCI CCCs in advancing research for the acute leukemias4,15 gives them an outsized responsibility to recruit equitably. When assessed with respect to their self-defined catchment areas, our data highlight targets for intervention by site, geography, and structural level, as well as a potential avenue for improvement through community partnerships. Reasonable next steps for improving participatory equity include integrating institutional and registry data sets to understand barriers between the catchment area, CCC, and trial; trial-embedded collection of social determinant data; and further developing community-engaged interventions for historically marginalized groups. Through such a multilevel approach, the effect of programmatic efforts and specific interventions could be measured, and participatory equity potentially realized.
Ann-Kathrin Eisfeld
Employment: Karyopharm Therapeutics (I)
Wendy Stock
Honoraria: AbbVie, Pfizer
Consulting or Advisory Role: Jazz Pharmaceuticals, Kite, a Gilead Company, Kura Oncology, GlaxoSmithKline, Morphosys, Pfizer, Servier, Deciphira, BEAm, Newave Pharmaceutical, AstraZeneca, Kronos Bio, Pluristem Therapeutics
Patents, Royalties, Other Intellectual Property: Royalties for a chapter in Up to Date
Travel, Accommodations, Expenses: Pfizer
Sumithra Mandrekar
Honoraria: BeiGene
Consulting or Advisory Role: Flatiron Health, Harbinger Oncology Inc
Other Relationship: Beigene
Richard A. Larson
Consulting or Advisory Role: Novartis, Celgene, ARIAD, CVS Caremark, Epizyme, Actinium Pharmaceuticals, Servier, Immunogen
Research Funding: Daiichi Sankyo (Inst), Celgene (Inst), Astellas Pharma (Inst), Novartis (Inst), Rafael Pharmaceuticals (Inst), Cellectis (Inst), Gilead/Forty Seven
Patents, Royalties, Other Intellectual Property: UpToDate
Richard M. Stone
Honoraria: Prime Oncology, Medscape, Research to Practice, DAVA Pharmaceuticals
Consulting or Advisory Role: Amgen, AbbVie, Agios, Celgene, Novartis, Actinium Pharmaceuticals, Arog, Astellas Pharma, Macrogenics, Takeda, Biolinerx, Daiichi-Sankyo, Trovagene, Gemoab, Syntrix Biosystems, ElevateBio, Syndax, Syros Pharmaceuticals, BerGenBio, Janssen, Innate Pharma, Foghorn Therapeutics, Aprea Therapeutics, GlaxoSmithKline, CTI BioPharma Corp, Bristol Myers Squibb, Boston Pharmaceuticals, Onconova Therapeutics, Jazz Pharmaceuticals
Research Funding: Novartis (Inst), Agios (Inst), AbbVie/Genentech (Inst)
Christopher S. Lathan
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Lilly, Bristol Myers Squibb Foundation, Pfizer, Grail, Johnson and Johnson
Daniel J. DeAngelo
Consulting or Advisory Role: Pfizer, Amgen, Novartis, Takeda, Blueprint Medicines, Incyte, Gilead Sciences, Jazz Pharmaceuticals
Research Funding: Novartis (Inst), AbbVie (Inst), Glycomimetics (Inst), Blueprint Medicines (Inst)
John C. Byrd
Stock and Other Ownership Interests: Vincerx Pharma
Honoraria: AstraZeneca, Novartis, Syndax, Trillium Therapeutics
Consulting or Advisory Role: Janssen, Kura Oncology, Novartis, Syndax, AstraZeneca, Newave Pharmaceutical
Research Funding: Acerta Pharma (Inst), Pharmacyclics (Inst), Zencor
Patents, Royalties, Other Intellectual Property: OSU Patents (Inst)
Travel, Accommodations, Expenses: Gilead Sciences, Janssen, Novartis, Pharmacyclics, TG Therapeutics
No other potential conflicts of interest were reported.
DISCLAIMER
The sponsors had no role in gathering, analyzing, or interpreting the data. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
PRIOR PRESENTATION
Presented in part at the 63rd Annual American Society of Hematology Annual Meeting and Exposition, Atlanta, GA, December 11, 2021 (abstr 111).
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821, U10CA180882, and U24CA196171 (to the Alliance for Clinical Trials in Oncology); UG1CA233180 (to Dana-Farber/Partners CancerCare); UG1CA233327 (to the University of Chicago); UG1CA233331 (to the Ohio State University); T32 CA092203 and P50 CA206963 (to A.H.); R35 CA197734 and UG1CA233338 (to J.C.B.); and by the Evelyn and Sidney Rieder Family Trust Fellowship in Acute Lymphocytic Leukemia Research (to A.H.); additional support for the Alliance can be found here: https://acknowledgments.alliancefound.org.
AUTHOR CONTRIBUTIONS
Conception and design: Andrew Hantel, Sumithra Mandrekar, John C. Byrd, Gregory A. Abel
Provision of study materials or patients: Wendy Stock, Sumithra Mandrekar, Richard A. Larson
Collection and assembly of data: Andrew Hantel, Wendy Stock, Sawyer Jacobson, Richard A. Larson, Daniel J. DeAngelo, John C. Byrd, Gregory A. Abel
Data analysis and interpretation: Andrew Hantel, Jessica Kohlschmidt, Ann-Kathrin Eisfeld, Wendy Stock, Sumithra Mandrekar, Richard M. Stone, Christopher S. Lathan, Daniel J. DeAngelo, John C. Byrd, Gregory A. Abel
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Inequities in Alliance Acute Leukemia Clinical Trial and Biobank Participation: Defining Targets for Intervention
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ann-Kathrin Eisfeld
Employment: Karyopharm Therapeutics (I)
Wendy Stock
Honoraria: AbbVie, Pfizer
Consulting or Advisory Role: Jazz Pharmaceuticals, Kite, a Gilead Company, Kura Oncology, GlaxoSmithKline, Morphosys, Pfizer, Servier, Deciphira, BEAm, Newave Pharmaceutical, AstraZeneca, Kronos Bio, Pluristem Therapeutics
Patents, Royalties, Other Intellectual Property: Royalties for a chapter in Up to Date
Travel, Accommodations, Expenses: Pfizer
Sumithra Mandrekar
Honoraria: BeiGene
Consulting or Advisory Role: Flatiron Health, Harbinger Oncology Inc
Other Relationship: Beigene
Richard A. Larson
Consulting or Advisory Role: Novartis, Celgene, ARIAD, CVS Caremark, Epizyme, Actinium Pharmaceuticals, Servier, Immunogen
Research Funding: Daiichi Sankyo (Inst), Celgene (Inst), Astellas Pharma (Inst), Novartis (Inst), Rafael Pharmaceuticals (Inst), Cellectis (Inst), Gilead/Forty Seven
Patents, Royalties, Other Intellectual Property: UpToDate
Richard M. Stone
Honoraria: Prime Oncology, Medscape, Research to Practice, DAVA Pharmaceuticals
Consulting or Advisory Role: Amgen, AbbVie, Agios, Celgene, Novartis, Actinium Pharmaceuticals, Arog, Astellas Pharma, Macrogenics, Takeda, Biolinerx, Daiichi-Sankyo, Trovagene, Gemoab, Syntrix Biosystems, ElevateBio, Syndax, Syros Pharmaceuticals, BerGenBio, Janssen, Innate Pharma, Foghorn Therapeutics, Aprea Therapeutics, GlaxoSmithKline, CTI BioPharma Corp, Bristol Myers Squibb, Boston Pharmaceuticals, Onconova Therapeutics, Jazz Pharmaceuticals
Research Funding: Novartis (Inst), Agios (Inst), AbbVie/Genentech (Inst)
Christopher S. Lathan
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Lilly, Bristol Myers Squibb Foundation, Pfizer, Grail, Johnson and Johnson
Daniel J. DeAngelo
Consulting or Advisory Role: Pfizer, Amgen, Novartis, Takeda, Blueprint Medicines, Incyte, Gilead Sciences, Jazz Pharmaceuticals
Research Funding: Novartis (Inst), AbbVie (Inst), Glycomimetics (Inst), Blueprint Medicines (Inst)
John C. Byrd
Stock and Other Ownership Interests: Vincerx Pharma
Honoraria: AstraZeneca, Novartis, Syndax, Trillium Therapeutics
Consulting or Advisory Role: Janssen, Kura Oncology, Novartis, Syndax, AstraZeneca, Newave Pharmaceutical
Research Funding: Acerta Pharma (Inst), Pharmacyclics (Inst), Zencor
Patents, Royalties, Other Intellectual Property: OSU Patents (Inst)
Travel, Accommodations, Expenses: Gilead Sciences, Janssen, Novartis, Pharmacyclics, TG Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Nipp RD, Hong K, Paskett ED: Overcoming barriers to clinical trial enrollment. Am Soc Clin Oncol Ed Book 39:105-114, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Bhatnagar B, Kohlschmidt J, Mrozek K, et al. : Poor survival and differential impact of genetic features of Black patients with acute myeloid leukemia. Cancer Discov 11:626-637, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shigematsu H, Lin L, Takahashi T, et al. : Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 97:339-346, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Ho G, Wun T, Muffly L, et al. : Decreased early mortality associated with the treatment of acute myeloid leukemia at National Cancer Institute-designated cancer centers in California. Cancer 124:1938-1945, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aristizabal P, Winestone LE, Umaretiya P, et al. : Disparities in pediatric Oncology: The 21st century opportunity to improve outcomes for children and adolescents with cancer. Am Soc Clin Oncol Ed Book 41:e315-e326, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warnecke RB, Oh A, Breen N, et al. : Approaching health disparities from a population perspective: The National Institutes of Health Centers for population health and health disparities. Am J Public Health 98:1608-1615, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kehl KL, Lathan CS, Johnson BE, et al. : Race, poverty, and initial implementation of precision medicine for lung cancer. J Natl Cancer Inst 111:431-434, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wadhwa L: Landscape of pediatric biobanking: Challenges and current efforts. Biopreserv Biobank 19:119-123, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Koo KC, Lee JS, Kim JW, et al. : Impact of clinical trial participation on survival in patients with castration-resistant prostate cancer: A multi-center analysis. BMC Cancer 18:468, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheurer ME, Lupo PJ, Schuz J, et al. : An overview of disparities in childhood cancer: Report on the Inaugural Symposium on Childhood Cancer Health Disparities, Houston, Texas, 2016. Pediatr Hematol Oncol 35:95-110, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen MS Jr, Lara PN, Dang JH, et al. : Twenty years post-NIH revitalization act: Enhancing minority participation in clinical trials (EMPaCT): Laying the groundwork for improving minority clinical trial accrual: Renewing the case for enhancing minority participation in cancer clinical trials. Cancer 120:1091-1096, 2014. (suppl 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Declaration of Helsinki, 59th WMA General Assembly. Seoul, South Korea, World Medical Association, 2008 [Google Scholar]
- 13.Hantel A, Luskin MR, Garcia JS, et al. : Racial and ethnic enrollment disparities and demographic reporting requirements in acute leukemia clinical trials. Blood Adv 5:4352-4360, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loree JM, Anand S, Dasari A, et al. : Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol 5:e191870, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfson J, Sun CL, Wyatt L, et al. : Adolescents and young adults with acute lymphoblastic leukemia and acute myeloid leukemia: Impact of care at specialized cancer centers on survival outcome. Cancer Epidemiol Biomarkers Prev 26:312-320, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfson JA, Sun CL, Wyatt LP, et al. : Impact of care at comprehensive cancer centers on outcome: Results from a population-based study. Cancer 121:3885-3893, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paskett ED, Hiatt RA: Catchment areas and community outreach and engagement: The new mandate for NCI-designated cancer centers. Cancer Epidemiol Biomarkers Prev 27:517-519, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Ong MBH: Project equity: FDA considers options to increase diverse enrollment in clinical trials. Cancer Lett 47:4, 2021 [Google Scholar]
- 19.Surveillance, Epidemiology, and End Results (SEER) Program; SEER*Stat Database (ed Version 8.3.8). Rockville, MD, National Cancer Institute, DCCPS, Surveillance Research Program, 2020 [Google Scholar]
- 20.Overview of Race and Hispanic Origin: 2010. Washington, DC, US Department of Commerce, Census Bureau, 2011 [Google Scholar]
- 21.Blake KD, Ciolino HP, Croyle RT: Population health assessment in NCI-designated cancer center catchment areas. Cancer Epidemiol Biomarkers Prev 28:428-430, 2019 [DOI] [PubMed] [Google Scholar]
- 22.Boscoe FP, Henry KA, Zdeb MS: A nationwide comparison of driving distance versus straight-line distance to hospitals. Prof Geogr 64, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rural-Urban Continuum Codes. Washington, DC, USDA Economic Research Service, 2013 [Google Scholar]
- 24.Kind AJH, Buckingham WR: Making neighborhood-disadvantage metrics accessible—The neighborhood atlas. N Engl J Med 378:2456-2458, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altman DG: Practical Statistics for Medical Research. Boca Raton, FL, Chapman & Hall/CRC, 1999 [Google Scholar]
- 26.Fleiss JL, Levin B, Paik MC: Statistical Methods for Rates and Proportions (ed 3). Hoboken, NJ, J. Wiley, 2003 [Google Scholar]
- 27.Murthy VH, Krumholz HM, Gross CP: Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA 291:2720-2726, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Dressler LG, Deal AM, Owzar K, et al. : Participation in cancer pharmacogenomic studies: A study of 8456 patients registered to clinical trials in the cancer and leukemia group B (Alliance). J Natl Cancer Inst 107:djv188, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sultan DH, Gishe J, Hanciles A, et al. : Minority use of a National Cancer Institute-designated comprehensive cancer center and non-specialty hospitals in two Florida regions. J Racial Ethn Health Disparities 2:373-384, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Huang LC, Ma Y, Ngo JV, et al. : What factors influence minority use of National Cancer Institute-designated cancer centers? Cancer 120:399-407, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roswell Park Cancer Institute: Center for indigenous cancer research. https://www.roswellpark.org/screening-prevention/indigenous-communities
- 32.Southeastern American Indian Cancer Health Equity Partnership. Chapel Hill, NC, UNC Lineberger Comprehensive Cancer Center, 2020 [Google Scholar]
- 33.Zavala VA, Bracci PM, Carethers JM, et al. : Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer 124:315-332, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haozous EA: American Indians and Alaska Natives: Resolving disparate cancer outcomes. Clin J Oncol Nurs 24:107-110, 2020 [DOI] [PubMed] [Google Scholar]
- 35.Unger JM, Vaidya R, Hershman DL, et al. : Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst 111:245-255, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong SJ, Drake B, Goodman M, et al. : Race, trust in doctors, privacy concerns, and consent preferences for biobanks. Health Commun 35:1219-1228, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrett NJ, Rodriguez EM, Iachan R, et al. : Factors associated with biomedical research participation within community-based samples across 3 National Cancer Institute-designated cancer centers. Cancer 126:1077-1089, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagiwara N, Berry-Bobovski L, Francis C, et al. : Unexpected findings in the exploration of African American underrepresentation in biospecimen collection and biobanks. J Cancer Educ 29:580-587, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.John EM, Sangaramoorthy M, Koo J, et al. : Enrollment and biospecimen collection in a multiethnic family cohort: The Northern California site of the Breast Cancer Family Registry. Cancer Causes Control 30:395-408, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ewing AT, Erby LA, Bollinger J, et al. : Demographic differences in willingness to provide broad and narrow consent for biobank research. Biopreserv Biobank 13:98-106, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vince RA Jr, Eyrich NW, Mahal BA, et al. : Reporting of racial health disparities research: Are we making progress? J Clin Oncol 40:8-11, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unger JM, Moseley AB, Cheung CK, et al. : Persistent disparity: Socioeconomic deprivation and cancer outcomes in patients treated in clinical trials. J Clin Oncol 39:1339-1348, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]