Abstract
Aim
Since the high cost of reference trastuzumab limits its clinical application, this study aimed to compare the effectiveness and safety of the Zercepac and reference product trastuzumab in neoadjuvant therapy for HER2-positive breast cancer.
Methods
This study retrospectively collected clinical data of patients with early-stageHER2-positive breast cancer, who received trastuzumab, pertuzumab, docetaxel, and platinum as neoadjuvant therapy from November 2020 to July 2021. Patients were divided into the Zercepac and reference trastuzumab groups. Reduction in tumor size, clinical response based on RECIST1.1 criteria, pathological complete response (pCR), and adverse events (AEs) were evaluated. Multivariate logistic regression analyses were used to adjust confounders.
Results
A total of 105 patients were included in the study, among them, 65 were in the Zercepac group and 40 were in the reference trastuzumab group. The percentage of tumor shrinkage from baseline was comparable between the Zercepac and reference trastuzumab group (47.6 ± 18.6% vs. 43.0 ± 19.9%, p = 0.235). Clinical partial response rate was similar between the two groups (81.5% vs. 70.0%, p = 0.172). There were 28 cases of pCR (70.0%) in the reference trastuzumab group and 46 cases of pCR (70.8%) in the Zercepac group (p = 0.933). The choice of Zercepac or reference trastuzumab was not significantly associated with pCR (OR = 0.96, 95%CI: 0.41-2.28, p = 0.933). Adverse events (AEs) were observed in all patients, and the incidence of ≥3 grade AEs was comparable between the two groups (81.5% vs. 70.0%, p = 0.172).
Conclusion
Zercepac has similar effectiveness and safety profile compared with reference trastuzumab in neoadjuvant therapy, which provides treatment options for patients with HER2-positive breast cancer.
1. Introduction
Breast cancer is the most frequently diagnosed cancer and an important cause of premature mortality among women worldwide, comprising up to 25% of all women cancers [1]. Although the incidence of breast cancer is lower in Asia (one out of 35 women, compared to one out of 8 women in the United states), it is predicted to increase in the near future [2, 3]. With breast cancer mortality rate increasing in the world during the past 25 years, prognosis of these patients significantly depends on the availability of treatment [4]. A large number of clinical studies have shown that neoadjuvant therapy significantly improves the chance of achieving high complete pathological response (pCR) rate and disease-free survival (DFS) rate, as well as overall survival (OS), of early breast cancer patients [5–7].
With the introduction of personalized treatment, the molecular biomarkers became important predictors and prognostic indicators of the therapy response in breast cancer patients [8, 9]. Trastuzumab is a human monoclonal antibody targeting HER2, that induces antibody-dependentcell-mediated cytotoxicity and inhibits its signal transduction [10]. Previous studies have proved that neoadjuvant therapy using reference trastuzumab has a significant effect on the prognosis of early-stage [11, 12] and metastatic HER2-positive breast cancer [13–15]. However, it also showed a certain cardiotoxicity in previous trials; thus, the safety and tolerability of Herceptin needs further attention [16, 17].
In many countries, the cost of trastuzumab therapy is not covered by insurance companies. High cost limits its availability for a large group of patients, who may in turn benefit from the usage of HER2 targeting therapy [18]. Zercepac is the first Chinese monoclonal antibody highly similar to reference trastuzumab, with a good application potential in reducing tumor cell proliferation and survival [19, 20]. Recently, Zercepac demonstrated efficacy equivalent to reference trastuzumab for HER2-positive recurrent or metastatic breast cancer in a phase III multicenter clinical trial [21]. However, more studies are needed to evaluate its potential efficacy in neoadjuvant therapy as well as its safety profile. More importantly, the two specification dosage forms are more suitable for Chinese patients, which can reduce the waste of residual fluid. At the same time, Zercepac does not contain preservatives, making it safe to use immediately after dispensing.
This study retrospectively collected the data of patients with HER2-positive breast cancer and evaluated the effectiveness and safety of Zercepac and reference trastuzumab in neoadjuvant therapy. This study will provide evidence for the selection of treatment and intervention strategies in breast cancer research and clinical practice.
2. Methods
2.1. Study Design and Patients
This retrospective cohort study included HER2-positive breast cancer patients who were admitted to the Breast Center of Gulou Hospital, School of Medicine, Nanjing University from November 2020 to July 2021 and received neoadjuvant therapy with trastuzumab (Zercepac or reference trastuzumab). The patients included in the study were all women under 75 years of age, with invasive breast cancer confirmed by histopathology in stages IIA, IIB, or IIIA (TNM staging according to the breast cancer AJCC guidelines [7th edition]). The immunohistology test results of HER2 expression for all patients were classified into HER-2+++, HER-2++ with ISH-positive, and HER-2+. The excluded patients were patients with obvious liver and kidney dysfunction, patients with severe cardiovascular system diseases, and patients with unstable vital signs or cachexia.
This study was approved by the Ethics Committee of Gulou Hospital, School of Medicine, Nanjing University (2021-435-02). The informed consent was waived by the ethic committee due to the retrospective nature of the study.
2.2. Neoadjuvant Therapy
All patients underwent standard 6 cycles of neoadjuvant chemotherapy, and the chemotherapy regimen was TCbHP regimen, which consisted of trastuzumab, pertuzumab, docetaxel, and platinum. Patients were divided into the Zercepac and reference trastuzumab groups, depending on the usage of trastuzumab: Zercepac (Shanghai Fuhong Henlius Biopharmaceutical Co Ltd) or reference trastuzumab (Roche Pharma (Schweiz) Ltd).
2.3. Data Collection
Clinical data such as name, age, gender, body mass index (BMI), menstrual status, molecular and histologic classification, clinical stage, tumor size, location, and metastatic lymph nodes were collected. The images of magnetic resonance imaging (MRI) scanning before and after neoadjuvant therapy were extracted. Laboratory tests included routine blood test and liver function which were obtained from the medical records as well.
2.4. Outcomes
Effectiveness outcomes included the shrinkage of tumor from baseline, clinical response evaluated by MRI scanning according RECIST1.1 criteria, and pathological response (pCR) rate. The Miller–Payne system used in the study has 5 grades: grade 5 is a pCR in breast; grades 1-4 are partial pathological responses according to tumor reduction ratio; from G4 to G1, the degree of tumor reduction gradually decreases [22]. Adverse events were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE 5.0).
2.5. Statistical Analysis
SPSS 26.0 software (IBM, Armonk, NY, USA) was used for data analysis. Continuous variables were presented as mean ± standard deviation (SD), and categorical variables were presented as frequency (percentage). For comparison between two groups, independent Student's t-test and χ2 test were used. The cutoff of continuous variables associated with pCR was determined by receiver operating characteristic (ROC) curve, optimal Youden Index, and area under the curve (AUC). Univariate and multivariate analyses were performed using the logistic regression model to evaluate prognostic factors. p < 0.05 indicated a statistical significance.
3. Results
3.1. Baseline Characteristics
A total of 105 patients with HER2-positive breast cancer received neoadjuvant therapy and were included in the study. Among these patients, 65 patients were in the Zercepac group and 40 patients were in the reference trastuzumab group. The mean age was 48.5 ± 9.3 and 49.2 ± 8.9 years in the Zercepac and reference trastuzumab groups, respectively. The detailed baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics.
| Characteristic | Total (N = 105) | Zercepac (n = 65) | Reference trastuzumab (n = 40) | P |
|---|---|---|---|---|
| Age, years, mean ± SD | 48.73 ± 9.11 | 48.48 ± 9.28 | 49.15 ± 8.93 | 0.715 |
|
| ||||
| Sex, n (%) | ||||
| Female | 105 (100.0) | 65 (100.0) | 40 (100.0) | |
| Male | 0 | 0 | 0 | |
|
| ||||
| BMI, kg/m2, median (range) | 23.20 (19.40, 26.50) | 22.40 (19.40, 26.50) | 23.40 (19.62, 26.47) | 0.877 |
|
| ||||
| Menstruation situation, n (%) | 0.658 | |||
| Premenopausal | 47 (44.8) | 28 (43.1) | 19 (47.5) | |
| Postmenopausal | 58 (55.2) | 37 (56.9) | 21 (52.5) | |
|
| ||||
| Histological typing, n (%) | ||||
| Invasive carcinoma | 105 (100.0) | 65 (100.0) | 40 (100.0) | |
| Other | 0 | 0 | 0 | |
|
| ||||
| Histological grading, n (%) | 0.060∗ | |||
| Grade 1 | 5 (4.8) | 3 (4.6) | 2 (5.0) | |
| Grade 2 | 35 (33.3) | 27 (41.5) | 8 (20.0) | |
| Grade 3 | 65 (61.9) | 35 (53.8) | 30 (75.0) | |
|
| ||||
| Time from diagnosis to trastuzumab treatment, days, media (range) | 6 (4, 9) | 6 (4, 12) | 6 (4, 8) | 0.691 |
|
| ||||
| HER2 status, n (%) | ||||
| - | 0 | 0 | 0 | |
| + | 105 (100.0) | 65 (100.0) | 40 (100.0) | |
|
| ||||
| ER status, n (%) | 0.728 | |||
| - | 45 (42.9) | 27 (41.5) | 18 (45.0) | |
| + | 60 (57.1) | 38 (58.5) | 22 (55.0) | |
|
| ||||
| PR status, n (%) | 0.059 | |||
| - | 67 (63.8) | 46 (70.8) | 21 (52.5) | |
| + | 38 (36.2) | 19 (29.2) | 19 (47.5) | |
|
| ||||
| Molecular typing, n (%) | 0.799 | |||
| HER2+HR- | 41 (39.0) | 26 (40.0) | 15 (37.5) | |
| HER2+HR+ | 64 (61.0) | 39 (60.0) | 25 (62.5) | |
|
| ||||
| Ki67, %, median (range) | 40.00 (40.00, 60.00) | 50.00 (40.00, 60.00) | 40.00 (30.00, 50.00) | 0.026 |
| Maximum tumor diameter, mm, median (range) | 24.00 (18.00, 33.00) | 24.00 (18.00, 34.00) | 22.00 (18.75, 32.00) | 0.815 |
| NLR, median (range) | 2.67 (1.78, 3.56) | 2.57 (1.88, 3.56) | 2.72 (1.64, 3.48) | 0.779 |
| PLR, median (range) | 179.41 (137.73, 219.43) | 174.61 (137.28, 218.39) | 191.96 (139.06, 228.33) | 0.409 |
BMI—Body mass index; HER2—human epidermal growth factor receptor 2; ER—estrogen receptors; PR—progesterone receptors; NLR—neutrophil-to-lymphocyte ratio; PLR—platelet-lymphocyte ratio; SD—standard deviation.
3.2. Effectiveness
Treatment response of individual patients is shown in Figure 1. The percentage of shrinkage of tumor from baseline was comparable between the Zercepac and reference trastuzumab groups (47.6 ± 18.6% vs. 43.0 ± 19.9%, p = 0.235, Table 2). Clinical partial response rate was similar between the two groups (81.5% vs. 70.0%, p = 0.172). Regarding pathological response to neoadjuvant therapy, there were 28 cases of pCR (70.0%) and 12 cases of non-pCR (30.0%) in the reference trastuzumab group, while in the 46 cases achieved pCR in the Zercepac group (70.8%) with no significant difference (p = 0.933, Figure 2). Among the non-pCR patients, in the Zercepac group Grade 2 partial response was reported in 6.2%, Grade 3 was reported in 13.8%, and Grade 4 was reported in 9.2% of patients. In reference trastuzumab group, Grade 2 partial response was reported in 5.0%, Grade 3 in 15.0%, and Grade 4 in 10.0% of patients (Figure 3). There was no statistically significant difference between the two groups (all p > 0.05).
Figure 1.
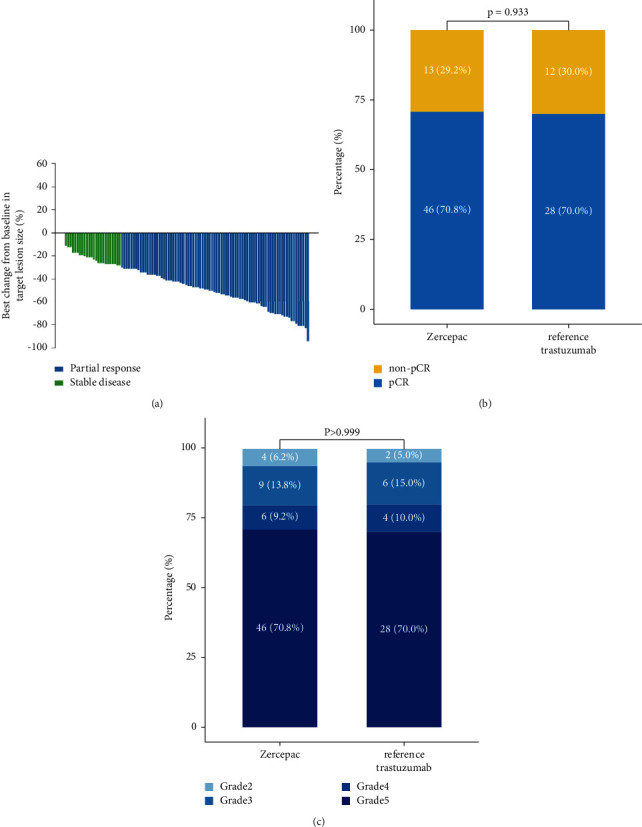
Treatment response.
Table 2.
Effectiveness.
| Total (N = 105) | Zercepac (n = 65) | Reference trastuzumab (n = 40) | p | |
|---|---|---|---|---|
| Percentage of tumor shrinkage from baseline, % | 45.84 ± 19.15 | 47.58 ± 18.60 | 43.00 ± 19.92 | 0.235 |
|
| ||||
| Clinical response, n (%) | 0.172 | |||
| PR | 81 (77.1) | 53 (81.5) | 28 (70.0) | |
| SD | 24 (22.9) | 12 (18.5) | 12 (30.0) | |
|
| ||||
| Pathological response, n (%) | 0.933 | |||
| Non-pCR | 31 (29.5) | 19 (29.2) | 12 (30.0) | |
| pCR | 74 (70.5) | 46 (70.8) | 28 (70.0) | |
|
| ||||
| MP grade, n (%) | >0.999∗ | |||
| Grade 2 | 6 (5.7) | 4 (6.2) | 2 (5.0) | |
| Grade 3 | 15 (14.3) | 9 (13.8) | 6 (15.0) | |
| Grade 4 | 10 (9.5) | 6 (9.2) | 4 (10.0) | |
| Grade 5 | 74 (70.5) | 46 (70.8) | 28 (70.0) | |
PR—partial response; SD—stable disease; pCR—complete pathological response.
Figure 2.
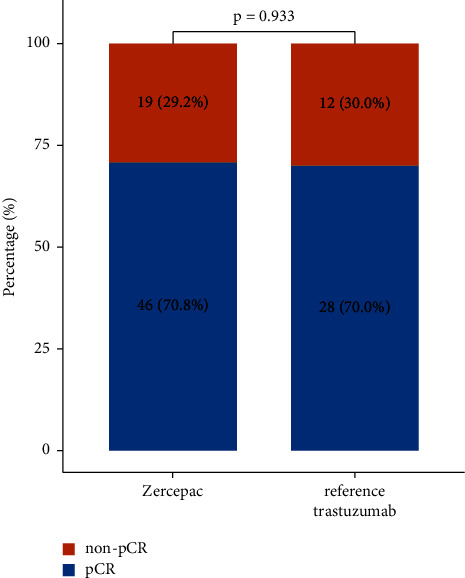
Comparison of complete pathological remission (pCR) rate in patients who received reference trastuzumab and Zercepac.
Figure 3.
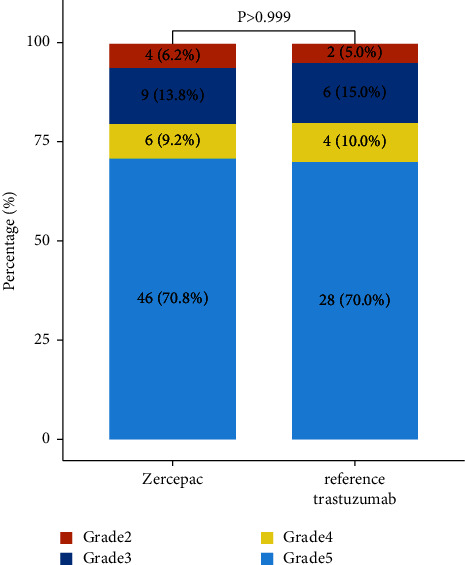
Detailed pathological response to treatment in patients who received reference trastuzumab and Zercepac.
3.3. Predictors of Response
For maximum tumor diameter, neutrophil-to-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and Ki67 expression, ROC curves and optimal Youden Index were used to discriminate the cutoff value (Supplemental Figure 1).
Multivariate analyses entering maximum tumor diameter, NLR, PLR, and Ki67 expression as continuous or categorical variables were carried out. The choice of Zercepac or reference trastuzumab was not significantly associated with pCR (OR = 0.96, 95%CI: 0.41-2.28, p = 0.933). In the meantime, progesterone receptors status (OR = 5.21, 95%CI: 1.64-16.55, p = 0.005), maximum tumor diameter (OR = 0.13, 95%CI: 0.05-0.38, p < 0.001), and NRL (OR = 0.22, 95%CI: 0.12-0.42, p < 0.001) were all independent predictors of response (Table 3).
Table 3.
Univariate and multivariate analyses of factors associated with complete pathological response.
| Characteristics | Univariate analysis | Multivariate analysis 1∗ | Multivariate analysis 2∗∗ | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | |
| Age | 1.02 (0.98-1.07) | 0.327 | ||||
|
| ||||||
| BMI | 0.98 (0.88-1.08) | 0.646 | ||||
|
| ||||||
| Menstruation situation | ||||||
| Premenopausal | 1 | |||||
| Postmenopausal | 0.70 (0.40-1.65) | 0.420 | ||||
|
| ||||||
| Histological grading | ||||||
| Grade 1 | 1 | |||||
| Grade 2 | 6.00 (0.84-43.09) | 0.075 | ||||
| Grade 3 | 3.14 (0.49-20.25) | 0.228 | ||||
|
| ||||||
| Time from diagnosis to trastuzumab treatment | 1.11 (1.00-1.23) | 0.053 | ||||
|
| ||||||
| ER status | ||||||
| - | 1 | |||||
| + | 2.00 (0.85-4.66) | 0.111 | ||||
|
| ||||||
| PR status | ||||||
| - | 1 | 1 | 1 | |||
| + | 3.18 (1.17-8.65) | 0.024 | 4.65 (0.86-25.27) | 0.075 | 5.21 (1.64-16.55) | 0.005 |
|
| ||||||
| Molecular typing | ||||||
| HER2+HR- | 1 | |||||
| HER2+HR- | 2.09 (0.89-4.91) | 0.090 | ||||
|
| ||||||
| Ki67 | 1.02 (0.99-1.05) | 0.171 | ||||
|
| ||||||
| Ki67 (ROC cutoff) | ||||||
| ≤40% | 1 | |||||
| >40% | 2.26 (0.95-5.38) | 0.065 | ||||
|
| ||||||
| Maximum tumor diameter | 0.93 (0.89-0.97) | <0.001 | 0.909 (0.85-0.97) | 0.005 | ||
|
| ||||||
| Maximum tumor diameter (categorical) | ||||||
| ≤30 mm | 1 | 1 | ||||
| >30 mm | 0.20 (0.08-0.49) | <0.001 | 0.14 (0.05-0.38) | <0.001 | ||
|
| ||||||
| NLR | 0.23 (0.13-0.41) | <0.001 | 0.22 (0.12-0.42) | <0.001 | ||
|
| ||||||
| NLR (categorical) | ||||||
| ≤2.68 | 1 | |||||
| >2.68 | 0.00 (0.00-Inf) | 0.989 | ||||
|
| ||||||
| PLR | 1.00 (0.99-1.01) | 0.656 | ||||
|
| ||||||
| PLR (categorical) | ||||||
| ≤189.33 | 1 | |||||
| >189.33 | 1.82 (0.77-4.32) | 0.176 | ||||
|
| ||||||
| Trastuzumab | ||||||
| Zercepac | 1 | |||||
| Reference trastuzumab | 0.96 (0.41-2.28) | 0.933 | ||||
∗ Continuous variable was entered as continuous variable. ∗∗Continuous variable was entered as categorical variables using optimal Youden Index-based cutoff point. OR—odds ratio; BMI—body mass index; HER2—human epidermal growth factor receptor 2; ER—estrogen receptors; PR—progesterone receptors; NLR—neutrophil-to-lymphocyte ratio; PLR—platelet-lymphocyte ratio.
3.4. Safety
The incidence rate of AEs was both 100.0% in the Zercepac and reference trastuzumab groups (Table 4). The most common AEs in the Zercepac group were hair loss (89.2%), elevated AST (aspartate aminotransferase, 69.2%), and fatigue (67.7%), while the most common in the reference trastuzumab group were hair loss (87.5%), decreased appetite (70.0%), and elevated ALT (alanine aminotransferase, 70.0%).
Table 4.
Safety.
| All grades | Grades ≥3 | |||||
|---|---|---|---|---|---|---|
| Zercepac (n = 65) | Reference trastuzumab (n = 40) | p | Zercepac (n = 65) | Reference trastuzumab (n = 40) | p | |
| Any AE | 65 (100.0) | 40 (100.0) | — | 53 (81.5) | 28 (70.0) | 0.172 |
| Nausea | 28 (43.1) | 16 (40.0) | 0.756 | 6 (9.2) | 3 (7.5) | 0.758 |
| Vomit | 33 (50.8) | 17 (42.5) | 0.410 | 9 (13.8) | 4 (10.0) | 0.561 |
| Fatigue | 44 (67.7) | 23 (57.5) | 0.291 | 2 (3.1) | 6 (15.0) | 0.025 |
| Infusion-related reaction | 2 (3.1) | 15 (37.5) | <0.001 | 0 | 1 (2.5) | 0.200 |
| Diarrhea | 36 (55.4) | 17 (42.5) | 0.200 | 5 (7.7) | 5 (12.5) | 0.415 |
| Decreased appetite | 31 (47.7) | 28 (70.0) | 0.025 | 3 (4.6) | 4 (10.0) | 0.283 |
| Elevated AST | 45 (69.2) | 26 (65.0) | 0.653 | 19 (29.2) | 6 (15.0) | 0.096 |
| Elevated ALT | 36 (55.4) | 28 (70.0) | 0.136 | 15 (23.1) | 5 (12.5) | 0.180 |
| Elevated GGT | 12 (18.5) | 11 (27.5) | 0.277 | 1 (1.5) | 0 (0.0) | 0.431 |
| Anemia | 33 (50.8) | 22 (55.0) | 0.673 | 16 (24.6) | 5 (12.5) | 0.132 |
| Thrombocytopenia | 32 (49.2) | 13 (32.5) | 0.093 | 5 (7.7) | 2 (5.0) | 0.591 |
| Hypertriglyceridemia | 14 (21.5) | 17 (42.5) | 0.022 | 3 (4.6) | 5 (12.5) | 0.139 |
| Hypokalemia | 3 (4.6) | 0 | 0.168 | 1 (1.5) | 0 (0.0) | 0.431 |
| Neutropenia | 20 (30.8) | 3 (7.5) | 0.005 | 1 (1.5) | 0 (0.0) | 0.431 |
| Leukopenia | 30 (46.2) | 11 (27.5) | 0.057 | 2 (3.1) | 3 (7.5) | 0.301 |
| Infection | 7 (10.8) | 2 (5.0) | 0.305 | 0 | 0 | — |
| Dizziness | 26 (40.0) | 15 (37.5) | 0.799 | 7 (10.8) | 1 (2.5) | 0.121 |
| Hectic fever | 22 (33.8) | 15 (37.5) | 0.703 | 10 (15.4) | 4 (10.0) | 0.431 |
| Difficulty breathing | 5 (7.7) | 2 (5.0) | 0.591 | 0 | 0 | — |
| Rash | 13 (20.0) | 17 (42.5) | 0.013 | 1 (1.5) | 1 (2.5) | 0.726 |
| Myalgia | 23 (35.4) | 25 (62.5) | 0.007 | 2 (3.1) | 0 (0.0) | 0.263 |
| Cough | 7 (10.8) | 9 (22.5) | 0.104 | 0 | 2 (5.0) | 0.069 |
| Hair loss | 58 (89.2) | 35 (87.5) | 0.787 | 0 | 0 | — |
| Insomnia | 17 (26.2) | 23 (57.5) | 0.001 | 0 | 9 (22.5) | <0.001 |
AST—aspartate aminotransferase; ALT—alanine aminotransferase; GGT—gamma-glutamyl transferase.
Grades ≥3 AEs were reported in 53 (81.5%) and 28 (70.0%) patients in the Zercepac and reference trastuzumab groups (p = 0.172), respectively. The most common grades ≥3 AEs in the Zercepac group were elevated AST (29.2%), anemia (24.6%), and elevated ALT (23.1%), while the most common grades ≥3 AEs in the reference trastuzumab group were insomnia (22.5%), fatigue (15.0%), and evaluated AST (15.0%). The incidence of grade ≥3 insomnia and fatigue was significantly higher in the reference trastuzumab group than those in the Zercepac group (both p < 0.05).
4. Discussion
This retrospective cohort study compared the effectiveness and safety of Zercepac and reference trastuzumab as neoadjuvant therapy in patients with early-stageHER2-positive breast cancer. The findings of this study demonstrated that Zercepac and reference trastuzumab resulted in a comparable pCR rate, which was further confirmed by multivariate analysis. Regarding adverse events, both regimens showed similar safety profile.
In recent years, with the continuous innovation of medical technology, many breast cancer trials are focused on the tumor-promoting gene HER2, which has an impact on cell reproduction, differentiation, and survival [23]. Trastuzumab is a humanized monoclonal antibody derived from recombinant DNA that can selectively suppress the expression of HER2 protein in HER2-positive cells [24, 25]. Previous studies have confirmed the efficacy of reference trastuzumab in patients with HER2-positive metastatic breast cancer combined with chemotherapy [15, 26] and neoadjuvant therapy [11, 27]. Patent expirations for trastuzumab in the European Union (2014) and USA (2019) have led the development of several bioequivalent drugs with low development costs [28]. In the neoadjuvant setting, both biosimilar SB3 and CT-P6 were equivalent to reference trastuzumab with respect to pCR (SB3: 51.7% and 42.0%; CT-P6: 46·8% and 52.4%) in phase 3, randomized trials [29, 30]. In real-world studies, biosimilar CT-P6 demonstrated pCR comparable to reference trastuzumab (CT-P6: 74.4% vs. 69.8%, p = 0.411) in HER2-positiveearly-stage breast cancer [31], while SB3 showed a pCR rate comparable to that seen in previous clinical studies [32]. Consistently, in this retrospective study, the pCR rate was similar in the reference trastuzumab and Zercepac groups (70.8% vs. 70.0%), and further multivariate logistic regression showed the choice of Zercepac or reference trastuzumab was not significantly associated with pCR. Moreover, the percentage of tumor shrinkage and clinical response was comparable between the reference trastuzumab and Zercepac groups. Based on the results of the abovementioned studies, it is suggested that the effectiveness of Zercepac was comparable to that of reference trastuzumab in HER2-positive breast cancer in the neoadjuvant setting.
Reference trastuzumab and its biosimilars were shown to be relatively well tolerated [33]. In the phase 3 clinical trial of trastuzumab SB3, no difference in the incidence of AEs was observed between SB3 and reference trastuzumab (96.6% and 95.2%), with the most common AEs being neutropenia and alopecia [29]. In the randomized clinical trial of trastuzumab CT-P6, the incidence of AEs in patients with HER2-positive breast cancer was comparable between CT-P6 and reference trastuzumab (94% and 95%), while the most commonly reported serious AEs were febrile neutropenia and neutropenia [30]. In this study, though the AEs occurred in all patients in the Zercepac group, grade ≥3 AEs were mostly common AEs of chemotherapy, which were manageable and draw no additional safety concern. Furthermore, the incidence rates of AEs and grade ≥3 AEs were comparable between the Zercepac and reference trastuzumab group. The similar safety profile demonstrated that patients can well tolerate both reference trastuzumab and Zercepac.
This study has the following limitations. Firstly, due to the retrospective design, data loss or ambiguity might occur in the process of data collection. Secondly, this study does not involve follow-up, and the long-term survival rate and quality of life of patients are not included in the results. Whether or not the observed difference in the tumor size reduction would result in different PFS is not known and should be investigated in future studies.
5. Conclusion
In conclusion, the effectiveness and safety of Zercepac were comparable to that of reference trastuzumab in HER2-positive breast cancer when administered in the neoadjuvant setting with pertuzumab, docetaxel, and platinum. This study could provide evidence for the application of domestic trastuzumab Zercepac in the clinical practice, which might contribute to the possibility of anti-HER2 therapy available to a wider range of patients.
Data Availability
The datasets supporting the results of this article are included within the article and supplementary materials. Other datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
This study was approved by the Ethics Committee of Gulou Hospital, School of Medicine, Nanjing University (2021-435-02).
Consent
The informed consent was waived by the ethic committee due to the retrospective nature of the study.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
Supplementary Materials
Supplementary Figure 1. Receiver operating characteristic (ROC) curves used to discriminate the cutoff of Ki67, maximum tumor diameter, neutrophil-to-lymphocyte ratio (NLR), and platelet-lymphocyte ratio (PLR) when predicting complete pathological remission. Cutoff value was determined using Youden Index.
References
- 1.Coughlin S. S. Epidemiology of breast cancer in women. Advances in Experimental Medicine & Biology . 2019;1152:9–29. doi: 10.1007/978-3-030-20301-6_2. [DOI] [PubMed] [Google Scholar]
- 2.Fahad Ullah M. Breast cancer: current perspectives on the disease status. Advances in Experimental Medicine & Biology . 2019;1152:51–64. doi: 10.1007/978-3-030-20301-6_4. [DOI] [PubMed] [Google Scholar]
- 3.Akram M., Iqbal M., Daniyal M., Khan A. U. Awareness and current knowledge of breast cancer. Biological Research . 2017;50(1):p. 33. doi: 10.1186/s40659-017-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azamjah N., Soltan-Zadeh Y., Zayeri F. Global trend of breast cancer mortality rate: a 25-year study. Asian Pacific Journal of Cancer Prevention . 2019;20(7):2015–2020. doi: 10.31557/apjcp.2019.20.7.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willson M. L., Burke L., Ferguson T., Ghersi D., Nowak A. K., Wilcken N. Taxanes for adjuvant treatment of early breast cancer. Cochrane Database of Systematic Reviews . 2019;9(9):p. Cd004421. doi: 10.1002/14651858.CD004421.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaheed M., Wilcken N., Willson M. L., O’Connell D. L., Goodwin A. Sequencing of anthracyclines and taxanes in neoadjuvant and adjuvant therapy for early breast cancer. Cochrane Database of Systematic Reviews . 2019;2(2):p. Cd012873. doi: 10.1002/14651858.CD012873.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montemurro F., Nuzzolese I., Ponzone R. Neoadjuvant or adjuvant chemotherapy in early breast cancer? Expert Opinion on Pharmacotherapy . 2020;21(9):1071–1082. doi: 10.1080/14656566.2020.1746273. [DOI] [PubMed] [Google Scholar]
- 8.Iwamoto T., Kajiwara Y., Zhu Y., Iha S. Biomarkers of neoadjuvant/adjuvant chemotherapy for breast cancer. Chinese Clinical Oncology . 2020;9(3):p. 27. doi: 10.21037/cco.2020.01.06. [DOI] [PubMed] [Google Scholar]
- 9.Jafari S. H., Saadatpour Z., Salmaninejad A., et al. Breast cancer diagnosis: imaging techniques and biochemical markers. Journal of Cellular Physiology . 2018;233(7):5200–5213. doi: 10.1002/jcp.26379. [DOI] [PubMed] [Google Scholar]
- 10.Kreutzfeldt J., Rozeboom B., Dey N., De P. The trastuzumab era: current and upcoming targeted HER2+ breast cancer therapies. Am J Cancer Res . 2020;10(4):1045–1067. [PMC free article] [PubMed] [Google Scholar]
- 11.Swain S. M., Ewer M., Viale G., et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Annals of Oncology . 2018;29(3):646–653. doi: 10.1093/annonc/mdx773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurvitz S. A., Martin M., Jung K. H., et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2-positive breast cancer: three-year outcomes from the phase III KRISTINE study. Journal of Clinical Oncology . 2019;37(25):2206–2216. doi: 10.1200/jco.19.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buiga P., Elson A., Tabernero L., Schwartz J. M. Modelling the role of dual specificity phosphatases in herceptin resistant breast cancer cell lines. Computational Biology and Chemistry . 2019;80:138–146. doi: 10.1016/j.compbiolchem.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Goutsouliak K., Veeraraghavan J., Sethunath V., et al. Towards personalized treatment for early stage HER2-positive breast cancer. Nature Reviews Clinical Oncology . 2020;17(4):233–250. doi: 10.1038/s41571-019-0299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cameron D., Piccart-Gebhart M. J., Gelber R. D., et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. The Lancet . 2017;389(10075):1195–1205. doi: 10.1016/s0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.An J., Sheikh M. S. Toxicology of trastuzumab: an insight into mechanisms of cardiotoxicity. Current Cancer Drug Targets . 2019;19(5):400–407. doi: 10.2174/1568009618666171129222159. [DOI] [PubMed] [Google Scholar]
- 17.Alghafar D. A., Younos I., Baimani K. A., et al. Trastuzumab cardiotoxicity in HER2-positive breast cancer patients in tertiary health care center, sultanate of Oman. Journal of Oncology Pharmacy Practice . 2021;27(2):312–321. doi: 10.1177/1078155220919888. [DOI] [PubMed] [Google Scholar]
- 18.Sarosiek T., Morawski P. Trastuzumab and its biosimilars. Polski Merkuriusz Lekarski . 2018;44(263):253–257. [PubMed] [Google Scholar]
- 19.Zhu X., Wang Y., Zhou M., Wang W. A phase 1 randomized study compare the pharmacokinetics, safety and immunogenicity of HLX02 to reference CN- and EU-sourced trastuzumab in healthy subjects. Cancer Chemotherapy and Pharmacology . 2021;87 doi: 10.1007/s00280-020-04196-9. [DOI] [PubMed] [Google Scholar]
- 20.Kurki P., Pekka B., Sean B., Ingrid T. Safety, immunogenicity and interchangeability of biosimilar monoclonal antibodies and fusion proteins: a regulatory perspective. Drugs . 2021;81 doi: 10.1007/s40265-021-01601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu B., Sun Q., Li T., Teng W., Hu Y. Efficacy, safety, and immunogenicity of hlx02 compared with reference trastuzumab in patients with recurrent or metastatic her2-positive breast cancer: a randomized phase iii equivalence trial. BioDrugs . 2021;35 doi: 10.1007/s40259-021-00475-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W., Liu Y., Zhang H., et al. Prognostic value of residual cancer burden and Miller-Payne system after neoadjuvant chemotherapy for breast cancer. Gland Surgery . 2021;10(12):3211–3221. doi: 10.21037/gs-21-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waks A. G., Winer E. P. Breast cancer treatment: a review. JAMA . 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 24.Bredin P., Walshe J. M., Denduluri N. Systemic therapy for metastatic HER2-positive breast cancer. Seminars in Oncology . 2020;47(5):259–269. doi: 10.1053/j.seminoncol.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Xu Z., Guo D., Jiang Z., et al. Novel HER2-targetingantibody-drug conjugates of trastuzumab beyond T-DM1 in breast cancer: trastuzumab deruxtecan(DS-8201a) and (Vic-)Trastuzumab duocarmazine (SYD985) European Journal of Medicinal Chemistry . 2019;183 doi: 10.1016/j.ejmech.2019.111682.111682 [DOI] [PubMed] [Google Scholar]
- 26.von Minckwitz G., Procter M., de Azambuja E., et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. New England Journal of Medicine . 2017;377(2):122–131. doi: 10.1056/nejmoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneeweiss A., Chia S., Hickish T., et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Annals of Oncology . 2013;24(9):2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 28.Waller C. F., Möbius J., Fuentes-Alburo A. Intravenous and subcutaneous formulations of trastuzumab, and trastuzumab biosimilars: implications for clinical practice. British Journal of Cancer . 2021;124(8):1346–1352. doi: 10.1038/s41416-020-01255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pivot X., Bondarenko I., Nowecki Z., et al. Phase III, randomized, double-blind study comparing the efficacy, safety, and immunogenicity of SB3 (trastuzumab biosimilar) and reference trastuzumab in patients treated with neoadjuvant therapy for human epidermal growth factor receptor 2-positive early breast cancer. Journal of Clinical Oncology . 2018;36(10):968–974. doi: 10.1200/jco.2017.74.0126. [DOI] [PubMed] [Google Scholar]
- 30.Stebbing J., Baranau Y., Baryash V., et al. CT-P6 compared with reference trastuzumab for HER2-positive breast cancer: a randomised, double-blind, active-controlled, phase 3 equivalence trial. The Lancet Oncology . 2017;18(7):917–928. doi: 10.1016/s1470-2045(17)30434-5. [DOI] [PubMed] [Google Scholar]
- 31.Bae S. J., Kim J. H., Ahn S. G., et al. Real-world clinical outcomes of biosimilar trastuzumab (CT-P6) in HER2-positiveearly-stage and metastatic breast cancer. Frontiers Oncology . 2021;11 doi: 10.3389/fonc.2021.689587.689587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berg T., Jensen M. B., Jakobsen E. H., Al-Rawi S., Kenholm J., Andersson M. Neoadjuvant chemotherapy and HER2 dual blockade including biosimilar trastuzumab (SB3) for HER2-positive early breast cancer: population based real world data from the Danish Breast Cancer Group (DBCG) The Breast . 2020;54:242–247. doi: 10.1016/j.breast.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nixon N. A., Hannouf M. B., Verma S. The evolution of biosimilars in oncology, with a focus on trastuzumab. Current Oncology . 2018;25(11):S171–s179. doi: 10.3747/co.25.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Receiver operating characteristic (ROC) curves used to discriminate the cutoff of Ki67, maximum tumor diameter, neutrophil-to-lymphocyte ratio (NLR), and platelet-lymphocyte ratio (PLR) when predicting complete pathological remission. Cutoff value was determined using Youden Index.
Data Availability Statement
The datasets supporting the results of this article are included within the article and supplementary materials. Other datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


