Abstract
Objective
Resveratrol is a polyphenolic compound that possesses strong antioxidant and anti-inflammatory activities. This study evaluated the effects of resveratrol on oxidative stress, fibrosis and multiple genes regulation in the kidneys of high fat (HF) diet-fed rats.
Methods
Wistar rats were fed with HF diet for eight weeks. These rats were also treated with resveratrol for eight weeks. Finally, kidney tissue samples were isolated from all sacrificed rats. The histological changes, creatinine and uric acid levels, oxidative stress parameters such as malondialdehyde (MDA), nitric oxide, and advanced oxidation protein product (AOPP) levels were analyzed. The antioxidant enzymes such as catalase, superoxide dismutase (SOD) activities and reduced glutathione (GSH) levels; gene expression of inflammatory and fibrosis-related genes namely, inducible nitric oxide synthase (iNOS), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), transforming growth factor beta1 (TGF-β1), and collagen-1 were assessed. Moreover, gene expression of oxidative stress-related genes such as nuclear factor erythroid 2–related factor 2 (Nrf-2), SOD, catalase, and glutathione reductase, were also assessed.
Results
HF diet-fed rats showed increased creatinine and uric acid levels in plasma which were lowered by resveratrol treatment. The study findings also revealed that resveratrol counterbalanced the oxidative stress and prevented the expression of the inflammatory genes; restored the catalase and SOD activities followed by the up-regulation of antioxidant genes expression in the kidneys of HF diet-fed rats. HF diet caused the Nrf-2 down-regulation followed by the decreased expression of HO-1 and HO-2 genes, which was restored by resveratrol treatment. Moreover, the histological assessment showed lipotoxicity and increased fibrosis in the kidneys of HF diet-fed rats. Resveratrol prevented the kidney fibrosis probably by limiting oxidative stress, inflammation, and down-regulating TGF-β1 mediated signaling pathway.
Conclusion
In conclusion, resveratrol treatment showed beneficial effects in preventing oxidative stress and fibrosis in the kidneys of HF diet-fed rats probably by modulating the gene expression of oxidative stress and inflammation related factors and enzymes.
Keywords: Resveratrol, High fat diet, Oxidative stress, Inflammation, Fibrosis
1. Introduction
Oxidative stress is the abnormality of tissues that failed to manage the free radical-mediated damage to the cells due to a lack of antioxidant defense (Kurutas 2016). Several mechanisms such as mitochondrial electron transport chain, xanthine oxidase system, nicotinamide adenine dinucleotide oxidase system, myeloperoxidase system etc. are involved in reactive oxygen species (ROS) generation in tissues (Di Meo et al., 2016, He et al., 2017). Scavenging enzymes such as superoxide dismutase (SOD), catalase, and glutathione peroxidase activities were reported to be diminished in oxidative stress (He et al., 2017). The SOD can catalyze superoxide into hydrogen peroxide, which is further catalyzed by catalase to water (Di Meo et al., 2016, He et al., 2017). The development of obesity and diabetes are now related to the oxidative stress and, may further deteriorate due to the excessive free radicals mediated tissue damage (Burgos-Morón et al., 2019). Earlier investigations also suggested that obese individual faces chronic kidney dysfunction due to ROS production (Hamza and Dyck, 2014, Ratliff et al., 2016). Our previous investigation showed that the HF diet may also develop kidney dysfunction and fibrosis in rats (Panchal et al., 2011). One of the early signs of tissue damage in kidneys is the increment of the lipid peroxidation product such as malondialdehyde (MDA) and the development of fibrosis (Gyurászová et al., 2020, Mamun et al., 2020). ROS is able to stimulate the collagen secretion from fibroblasts by regulating several growth factors including transforming growth factor-beta (TGF-β) (Isaka 2018). In agreement with this finding, a previous review work showed that the TGF-β expression is increased in the glomerulus and tubular spaces due to increased oxidative stress, leading to fibrosis (Sureshbabu et al., 2016). Transgenic mice having over-expressed TGF-β lead to the development of glomerular immune deposits, mesangial expansion, and matrix proteins deposition, and interstitial fibrosis (Kopp et al., 1996). Therefore, inhibition of ROS production and key gene regulation for antioxidant enzymes in tissues will provide essential protection against oxidative stress-mediated fibrosis in kidneys (Nlandu Khodo et al., 2012, Ruiz et al., 2013).
Activation of a transcription factor known as nuclear factor erythroid-derived 2-related factor 2 (Nrf-2) is responsible for the increased antioxidant enzymes levels and phase-2-detoxifying enzymes such as heme oxygenase-1 (HO-1) (Ruiz et al., 2013, Nabavi et al., 2016). A previous study found that reduced Nrf-2 expression may increase ROS generation and kidney injury (Chen et al., 2019). Moreover, oxidative stress, inflammation, immune cell infiltration, and nuclear factor- kappa B (NF-κB) activation have been found in the remnant kidney of a partial nephrectomy–induced renal damage in rats (Fujihara et al., 2007). Restoration of Nrf-2-HO-1 axis by antioxidants may protect the kidney from injury and fibrogenic process (Selim et al., 2021). A previous study reported the renoprotective activity of resveratrol, a prominent antioxidant compound (Kitada and Koya 2013). Moreover, resveratrol may be able to modulate the function of NF-кB and Nrf-2 in various tissues to prevent oxidative stress and inflammation (Hou et al., 2019). Resveratrol (3,5,4ˈ-tri hydroxyl stilbene) is a natural polyphenolic compound and is well documented for its anticancer, antitumor, and anti-aging properties (Baur and Sinclair 2006). Resveratrol is mainly found in grape skin, red wine, apples, peanuts, soy, and other various fruits and belongs to the stilbene family (Chastang et al., 2018). Previous report suggested that resveratrol could suppress oxidative stress and ameliorate renal injury in HF diet-fed rats (Pan et al., 2014). Resveratrol may also alleviate HF diet-induced kidney injury by decreasing the inflammation, lowering tumor necrosis factor-α, and interleukin-6 concentrations and lipid peroxidation (Cheng et al., 2019). However, resveratrol-mediated prevention of fibrosis in the kidneys of HF diet-fed rats was not appropriately explained before. Thus, this investigation will address the molecular mechanism of resveratrol-mediated preventive effect against oxidative stress and fibrosis in the kidneys of HF diet-fed rats.
2. Material and methods
2.1. Chemicals and reagent kits used in this study
The beef tallow that was used in the HF diet formulation, was purchased from Dhaka New Market, Bangladesh. The reagent kits for assaying creatinine and uric acid were purchased from DCI diagnostics (Budapest, Hungary). Thiobarbituric acid (TBA) was purchased from Sigma (USA), and resveratrol was bought from Xi'an Surnature Biological Technology Co. Ltd, China. The kits for mRNA isolation and RT-PCR were purchased from Thermo-Fisher Scientific (Massachusetts, USA), and Bio-Rad, USA.
2.2. Animals and treatment
In this experiment, Wistar male rats were divided into four groups, where each group had seven rats. All rats were about ten to twelve weeks old, and the range of their body weights were about 185–200 g. All rats were kept in the Animal house unit of the Department of Pharmaceutical Sciences, North South University, where the rats were given free access to water and food. All rats were kept in an individual cage, and the room temperature was (22 ± 3 °C) with 55% humidity. Light and dark circle was maintained for about 12 h each. All experimental protocols were approved by the animal research Ethical Committee of North South University for animal care and experimentation (AEC 006-2017).
The groups are assigned as Control, Control + RSV, HF, and HF + RSV. The Control group only received control food and water for 56 days. Control + RSV group took control food and water with daily oral gavage of resveratrol with a dose of 100 mg/kg body weight. HF group received only a high-fat diet and water for 56 days. Finally, HF + RSV group received a high-fat diet and regular water with daily oral gavage of resveratrol, and the dose was 100 mg/kg body weight. This dose was selected based on the previous reports suggesting that a 3000 mg/kg dose may develop nephrotoxicity in rats (Crowell et al., 2004).
The HF diet was prepared and transformed into a pellet form in the laboratory (Table 1). In control food, calories from fat, carbohydrates and proteins are 13.5%, 57% and 14% respectively. In HF diet, calories percentages were about 48% from fats, 14% from proteins and 37 % from carbohydrates. For all 56 days, body weight, food, and water intake were recorded for all groups. On the 57th day, all rats were sacrificed using ketamine hydrochloride (60 mg/kg), administered intraperitoneally. Blood and kidney samples were collected. The kidney samples were divided into three parts, one for biochemical assay and the cortex was used to isolate the mRNA expression. Another kidney part was used for the histological assessment through various staining processes. Biochemical assay samples were preserved at −20 °C, and the histological samples were kept in neutral buffer formalin of pH 7.4.
Table 1.
Composition of normal and high-fat diet used in this study (approximately for 100 g).
| Control diet | % | HF diet | % |
|---|---|---|---|
| Wheat | 40% | Powdered normal rat feed | 15.5% |
| Wheat Bran | 20% | Sugar | 17.5% |
| Rice Polishing | 0.5 % | Beef tallow (fat) | 20.0% |
| Fish meal | 1.0% | Condensed milk | 39.5% |
| Oil cake | 1.0% | Vitamin mixture | 0.1% |
| Gram | 0.39% | Salt | 0.5% |
| Pulses | 0.39% | Water | 10 mL |
| Milk | 0.38% | ||
| Soybean Oil | 0.15% | ||
| Molasses | 0.095% | ||
| Salt | 0.095% | ||
| Vitamin mixture | 0.1% | ||
| Water | q. s. t. |
In chow food calories were fat, carbohydrates and proteins respectively such as 13.5%, 57% and 14%. In high fat (HF) diet calorie percentage were about 48% fats, 14% proteins and 37% carbohydrate.
2.3. Plasma preparation
The blood was collected from large abdominal veins and taken into heparinized tubes. After the collection of blood samples, they were centrifuged for 15 min at 8000 g and at 4 °C. The plasma was separated, transferred into 1.5 mL microcentrifuge tubes, and stored at −20 °C for biochemical assay.
2.4. Preparation of tissue samples and assays for oxidative stress markers
Kidney tissues were homogenized in phosphate buffer saline (pH 7.4). The homogenates were then centrifuged at 8000 g (at 4 °C) for 15 min. The clear supernatant was collected for the determination of enzymatic activities and protein content.
By following the previously described method, the malondialdehyde (MDA) level was measured for lipid peroxidation in kidney tissue (Rahman et al., 2017). MDA solution was used as the standard curve of malondialdehyde while the unit was expressed as nmol/g tissue.
By measuring nitrate, nitric oxide content was measured in the tissue homogenates (Rahman et al., 2017). Compared to a blank solution, reagent solutions' absorbance with tissue homogenates was measured at 540 nm. The unit of nitric oxide level was expressed as nmol/g tissue using a standard curve.
The advanced oxidation protein product (AOPP) level was also determined by following the previously described method (Rahman et al., 2017). In this assay, chloramine-T was used in different concentrations as standard. The absorbance of chloramine-T was taken at 340 nm, where the range was about 0 to 100 nmol/mg. The concentration unit of AOPP was expressed as nmol/mg chloramine-T equivalents.
2.5. Assay of antioxidant enzyme activities such as catalase, SOD and reduced glutathione (GSH)
The previously published literature described the catalase activity assay in detail (Rahman et al., 2017). An absorbance change of 0.01 units/min is considered as one unit of catalase activity.
SOD activity was also determined by the previously described method (Rahman et al., 2017). In the assay system, auto-oxidation of epinephrine was measured. 50% inhibition of auto-oxidation of epinephrine is defined as one unit enzyme activity.
GSH was also determined using the method previously described by Rahman et al. (Rahman et al., 2017). The yellow color was developed from the reagent mixture, and immediately absorbance was taken at 412 nm. Unit of GSH was measured as ng/mg protein.
2.6. RT-PCR for oxidative stress and inflammation regulatory genes expression
After sacrificing rats of all groups, kidneys were collected immediately, maintaining an RNAse free environment. The total mRNA was isolated from the cortex part of the kidneys, and for the purification of total mRNA; Thermo-Fisher Scientific (Massachusetts, USA) GeneJET RNA purification kit was used. The mRNA concentration was measured in a NanoDrop 2000 spectrophotometer (Thermo-Fisher Scientific, Massachusetts, USA). In T100 Thermal Cycler (Bio-Rad, USA), a cDNA synthesis kit (RevertAid First Strand cDNA Synthesis Kit, Thermo-Fisher Scientific, USA) was used to synthesize cDNA. Two parameters were measured such as inflammation-related proteins and oxidative stress-related proteins where cDNA was quantified in transcript level related qRT-PCR, which was used with SYBR Premix Ex Taq (Tli RNaseH Plus) and it was analyzed with CFX96 C1000 Touch Real-Time PCR Detection System (Bio-Rad, USA) and data were analyzed by CFX Manager TM Software (CFX Manager TM Software) according to the manufacturer’s protocol. In Table 2, oligonucleotides of forwarding and reverse primers in the qRT-PCR were enlisted, in which Primer3 online software was used for primer design. Three processes were involved in a polymerase chain reaction: denaturation, annealing, and extension. Protein denaturation was done at 95 °C for 1 min after that it was amplified by 40 cycles at 95 °C, which stayed for 5 s. The final step was performed for 1 min at 72 °C and after that, the last extension was done for 5 min at 72 °C. β-actin was used as a control for gene expression for normalization and the target gene of transcript level was measured.
Table 2.
The forward and reverse sequence of the primer used in this study.
| Name of gene | Type | Sequence |
|---|---|---|
| iNOS | Forward | 5′-TGGTCCAACCTGCAGGTCTTC-3′ |
| Reverse | 5′-CAGTAATGGCCGACCTGATGTTG-3′ | |
| Catalase | Forward | 5-ATTGCCGTCCGATTCTCC-3 |
| Reverse | 5-CCAGTTACCATCTTCAGTGTAG-3 | |
| MnSOD | Forward | 5-GCTCTAATCACGACCCACT-3 |
| Reverse | 5-CATTCTCCCAGTTGATTACATTC-3 | |
| Glutathione reductase | Forward | 5-GGGCAAAGAAGATTCCAGGTT-3 |
| Reverse | 5-GGACGGCTTCATCTTCAGTGA-3 | |
| Heme oxigenase-1 (HO-1) | Forward | 5-TGCTCGCATGAACACTCTG-3 |
| Reverse | 5-TCCTCTGTCAGCAGTGCCT-3 | |
| Heme oxigenase-2 (HO-2) | Forward | 5-CACCACTGCACTTTACTTCA-3 |
| Reverse | 5-AGTGCTGGGGAGTTTTAGTG-3 | |
| Nrf-2 | Forward | 5-CCC AGCACA TCC AGACAGAC-3 |
| Reverse | 5-TATCCAGGGCAAGCGACT C-3 | |
| IL-6 | Forward | 5-AGCGATGATGCACTGTCAGA-3 |
| Reverse | 5-GGTTTGCCGAGTAGACCTCA-3 | |
| TNF-α | Forward | 5′-ATGTGGAACTGGCAGAGGAG-3′ |
| Reverse | 5′-CCACGAGCAGGAATGAGAAGAG-3′ | |
| TGF-β2 | Forward | 5′-AAGAAGTCACCCGCGTGCTA-3′ |
| Reverse | 5′-TGTGTGATGTCTTTGGTTTTGTC-3′ | |
| β-Actin | Forward | 5′-GCGAGAAGATGACCCAGATC-3′ |
| Reverse | 5′-GGATAGCACAGCCTGGATAG-3′ |
2.7. Histopathological staining
For histological assessment, two staining processes were selected such as hematoxylin/eosin and Sirius red staining. Kidney tissues were kept in neutral buffered formalin for a week to get complete fixation. The tissues were then undergone graded alcohol and xylene treatment and were embedded in paraffin blocks. These blocks were then sectioned carefully at 5 µm thickness, using a microtome. Hematoxylin/eosin staining was used to assess inflammatory cell infiltration, and Sirius red staining was used to see collagen deposition in the kidney section. A light microscope (Zeiss Axioscope, Germany) was used to take the picture at 40X magnification. Image J free software (Version 1.50i) from the National Institute of Health, United States of America was used to semi-quantitatively measure the percentage of fibrosis in the kidney sections.
2.8. Statistical analysis
For statistical calculation, mean ± standard error of mean and mean ± standard deviation were used. All results were evaluated by using One-way ANOVA followed by Tukey post hoc test. Two way ANOVA was also performed to see the effect of diet and treatment on these animals. Graph Pad Prism software (Version 6.2) was used for all the analysis. In all cases, statistical significance was considered at p < 0.05.
3. Results:
3.1. Effect of resveratrol on wet kidney weight, creatinine and uric acid level in plasma of HF diet-fed rats
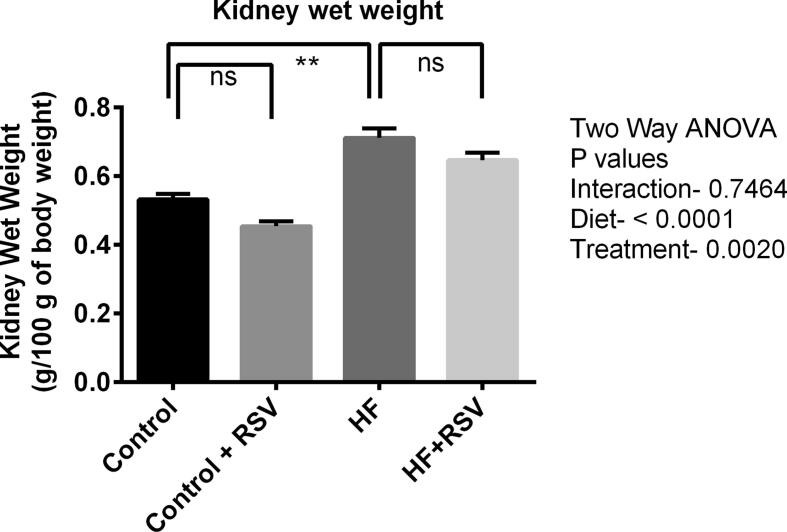
HF diet caused the rise in kidney wet weight in rats compared to the control rats (Fig. 1). The HF diet-fed rats treated with resveratrol showed decreased kidney weight, but the reduction is not significantly different from HF diet-fed rats (Fig. 1). Resveratrol treatment did not change the wet weight of kidneys in control rats.
Fig. 1.
Effect of resveratrol on the kidney wet weight of HF diet-fed rats. Data are presented as mean ± SEM. One-way ANOVA was performed followed by Tukey post hoc test to determine the statistical significance. Asterisk mark (*) is considered significantly different at P < 0.05 and ** is considered significantly different at P < 0.001. ns means not significant. Two-way ANOVA was also performed to see the diet and treatment effect in this study. The p values for Two-way ANOVA are presented in the figure, where P < 0.05 is considered as significant.
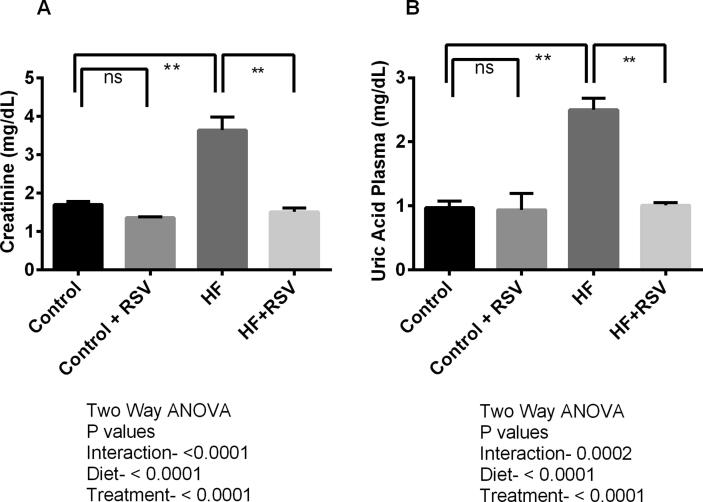
Creatinine level in plasma is increased in HF diet-fed rats compared to the control (P < 0.05). Resveratrol treatment lowered the creatinine level in plasma of HF diet-fed rats (Fig. 2A), however, resveratrol treatment in control rats did not alter the creatinine level in plasma compared to control rats only (Fig. 2A).
Fig. 2.
Effect of resveratrol on creatinine (A) and uric acid (B) level in the kidney of HF diet-fed rats. Data are presented as mean ± SEM. One-way ANOVA was performed followed by Tukey post hoc test to determine the statistical significance. Asterisk mark (*) is considered significantly different at P < 0.05 and ** is considered significantly different at P < 0.001. ns means not significant. Two-way ANOVA was also performed to see the diet and treatment effect in this study. The p values for Two-way ANOVA are presented in the figure, where P < 0.05 is considered as significant.
HF diet fed rats also showed increased uric acid levels (P < 0.05) compared to the control rats (Fig. 2B). Resveratrol treatment prevented the rise of uric acid levels in the plasma of HF diet-fed rats (Fig. 2B). However, no significant changes were observed in uric acid levels in the plasma of control rats treated with resveratrol.
3.2. Effect of resveratrol on oxidative stress parameters in the kidney of HF diet-fed rats
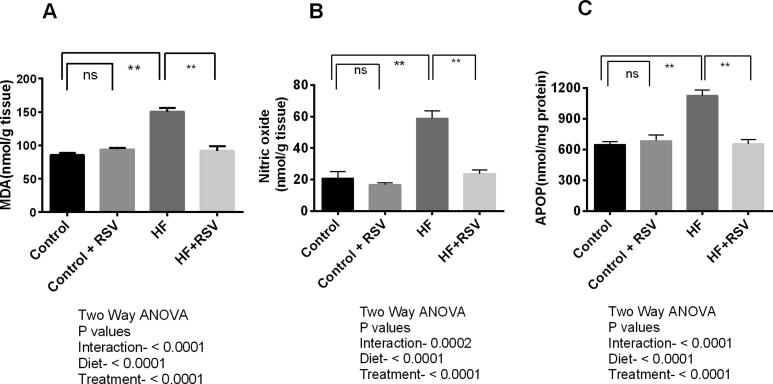
Lipid peroxidation is a crucial parameter for measuring oxidative stress. MDA is known as a byproduct of lipid peroxidation, which was increased significantly (p < 0.05) in the HF diet-fed rats compared to the control rats (Fig. 3A). Resveratrol treatment reduced MDA concentration in the kidneys of HF diet-fed rats significantly (P < 0.05) (Fig. 3A).
Fig. 3.
Effect of resveratrol on oxidative stress parameters (A) MDA (B) NO and (C) AOPP level in the kidney of HF diet-fed rats. Data are presented as mean ± SEM. One-way ANOVA was performed followed by Tukey post hoc test to determine the statistical significance. Asterisk mark (*) is considered significantly different at P < 0.05 and ** is considered significantly different at P < 0.001. ns means not significant. Two-way ANOVA was also performed to see the diet and treatment effect in this study. The p values for Two-way ANOVA are presented in the figure, where P < 0.05 is considered as significant.
In line with this evidence, nitric oxide levels were also increased significantly in the kidney of HF diet-fed rats significantly (P < 0.05) while comparing to the control rats (Fig. 3B). Resveratrol treatment normalized the nitric oxide levels in the kidney homogenates of HF diet-fed rats (Fig. 3B).
HF diet-fed rats also showed increased AOPP levels significantly (p < 0.05) compared to control rats (Fig. 3C). Resveratrol treatment prevented the rise in AOPP levels in the kidney homogenates of HF diet-fed rats compared to HF diet-fed rats (Fig. 3C).
3.3. Effect of resveratrol on antioxidant enzyme activity in the kidney of HF diet-fed rats
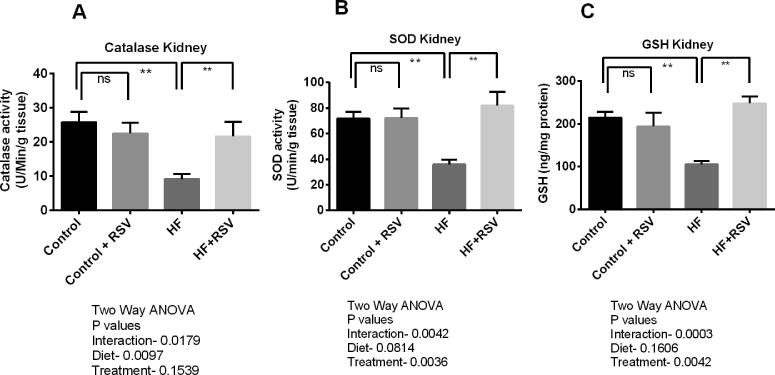
HF diet feeding in rats showed decreased antioxidant enzyme activities in tissues. Compared to control rats, catalase and SOD activities were significantly declined in the kidneys of HF diet-fed rats (p < 0.05). Resveratrol treatment in HF diet-fed rats restored the catalase and SOD activities in the kidneys (Fig. 4A and 4B). Moreover, GSH level was also decreased in the kidneys of HF diet-fed rats significantly (p < 0.05), which was restored by resveratrol treatment (Fig. 4C).
Fig. 4.
Effect of resveratrol on anti-oxidant enzyme activities (A) Catalase (B) SOD and (C) GSH level in the kidney of HF diet-fed rats. Data are presented as mean ± SEM. One-way ANOVA was performed followed by Tukey post hoc test to determine the statistical significance. Asterisk mark (*) is considered significantly different at P < 0.05 and ** is considered significantly different at P < 0.001. ns means not significant. Two-way ANOVA was also performed to see the diet and treatment effect in this study. The p values for Two-way ANOVA are presented in the figure, where P < 0.05 is considered as significant.
3.3.1. Effect of resveratrol on gene expression of inflammation and fibrosis-related proteins in the kidney of HF diet fed rats
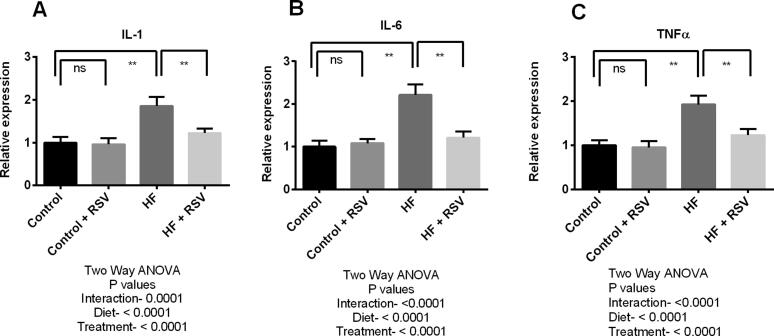
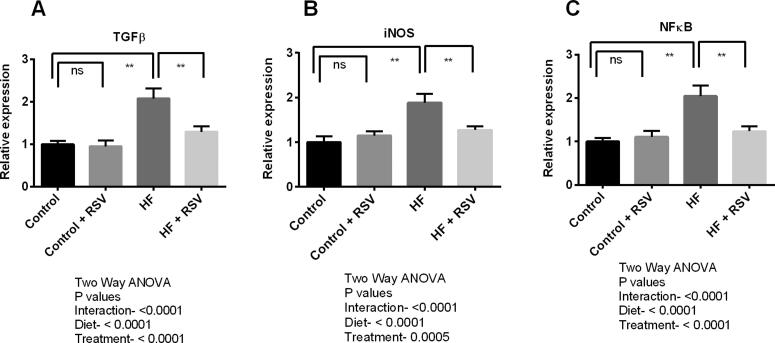
In this experiment, inflammation and fibrosis triggering genes expression were evaluated in the kidneys of HF diet-fed rats. Expression of six genes such as interleukin-1 (IL-1), interleukin-6 (IL-6), transforming growth factor beta-1 (TGF-β1), tumor necrosis factor-alpha (TNF-α), nuclear factor-kappa B (NF-κB), and inducible nitric oxide synthase (iNOS) were analyzed (Fig. 5 and Fig. 6). The study findings revealed that the expression of IL-1, IL-6 and TNF-α genes were significantly (P < 0.05) increased in the kidneys due to HF diet feeding in rats (Fig. 5). TGF-β1, iNOS and NF-кB expressions were also raised significantly in HF diet-fed rats compared to the control rats (Fig. 6). This investigation also revealed that resveratrol treatment successfully suppressed the expression of all these pro-inflammatory and inflammatory genes expression in the kidneys of HF diet-fed rats. It is to be noteworthy that the genes expression of main fibrosis-related proteins such as TGF-β1 and IL-1 were decline significantly (P < 0.05) due to resveratrol treatment in HF diet-fed rats (Fig. 5 and Fig. 6).
Fig. 5.
Effect of resveratrol on inflammatory genes such as IL-1 (A), IL-6 (B) and TNF-α (C) expression in the kidney of HF diet-fed rats. Data are presented as mean ± SD. One-way ANOVA was performed followed by Tukey post hoc test to determine the statistical significance. Asterisk mark (*) is considered significantly different at P < 0.05 and ** is considered significantly different at P < 0.001. ns means not significant. Two-way ANOVA was also performed to see the diet and treatment effect in this study. The p values for Two-way ANOVA are presented in the figure, where P < 0.05 is considered as significant.
Fig. 6.
Effect of resveratrol on inflammatory genes such as TGF-β (A), iNOS (B) and NF-кB (C) expression in the kidney of HF diet-fed rats. Data are presented as mean ± SD. One-way ANOVA was performed followed by Tukey post hoc test to determine the statistical significance. Asterisk mark (*) is considered significantly different at P < 0.05 and ** is considered significantly different at P < 0.001. ns means not significant. Two-way ANOVA was also performed to see the diet and treatment effect in this study. The p values for Two-way ANOVA are presented in the figure, where P < 0.05 is considered as significant.
3.3.2. Effect of resveratrol on gene expression of oxidative stress-related enzymes in the kidney of HF diet-fed rats
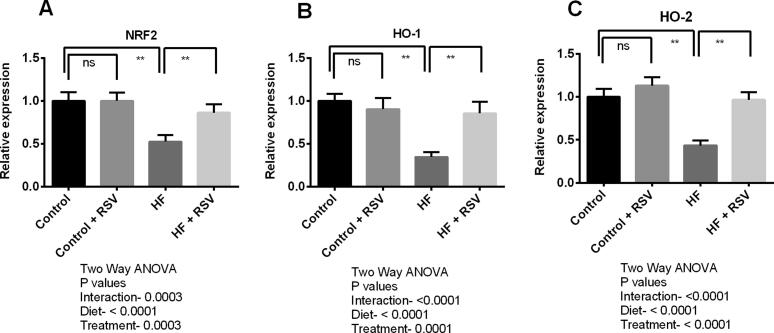
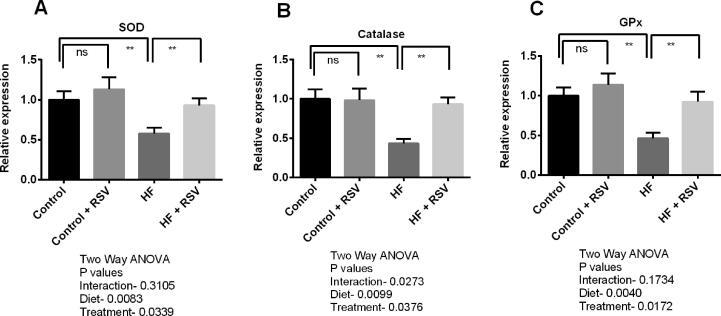
Transcript levels of Nrf2 in the kidneys of HF diet-fed rats were declined compared to the control rats (Fig. 7). Resveratrol treatment restored the Nrf2 expression in the kidneys of HF diet-fed rats (Fig. 7). Significant (p < 0.05) up-regulation of HO-1 and HO-2 transcript levels were also detected in HF diet-fed rats treated with resveratrol (Fig. 7). Further, gene expression of endogenous antioxidant enzymes including SOD, catalase, and GPx was also reduced in the kidneys of HF diet-fed rats (Fig. 8). Further, resveratrol treatment augmented the gene expression of these antioxidant enzymes in the kidneys of HF diet-fed rats significantly (Fig. 8).
Fig. 7.
Effect of resveratrol on antioxidant genes such as NRF-2 (A), HO-1 (B) and HO_2 (C) expression in the kidney of HF diet-fed rats. Data are presented as mean ± SD. One-way ANOVA was performed followed by Tukey post hoc test to determine the statistical significance. Asterisk mark (*) is considered significantly different at P < 0.05 and ** is considered significantly different at P < 0.001. ns means not significant. Two-way ANOVA was also performed to see the diet and treatment effect in this study. The p values for Two-way ANOVA are presented in the figure, where P < 0.05 is considered as significant.
Fig. 8.
Effect of resveratrol on antioxidant genes such as SOD (A), Catalase (B) and GPx (C) expression in the kidney of HF diet-fed rats. Data are presented as mean ± SD. One-way ANOVA was performed followed by Tukey post hoc test to determine the statistical significance. Asterisk mark (*) is considered significantly different at P < 0.05 and ** is considered significantly different at P < 0.001. ns means not significant. Two-way ANOVA was also performed to see the diet and treatment effect in this study. The p values for Two-way ANOVA are presented in the figure, where P < 0.05 is considered as significant.
3.3.3. Histological assessments
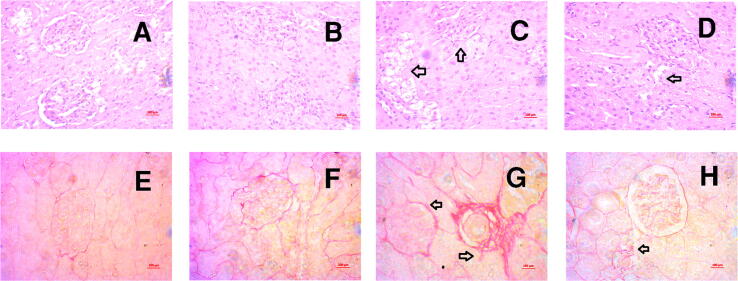
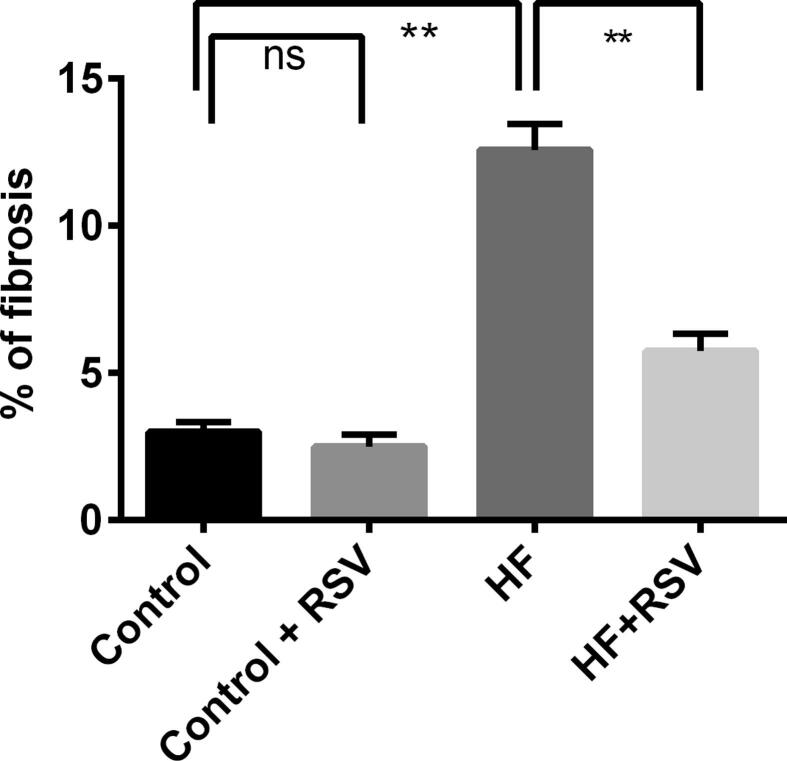
In assessing potential consequences of HF diet induced lipotoxicity on the kidney such as cellular damage, inflammation and fibrosis, hematoxylin and eosin staining, and Sirius red staining revealed that control rats showed no lipid accumulation and necrosis zone in the kidneys (Fig. 9A, Upper panel). Resveratrol-treated control rats also showed similar kidney structures as in the control rats (Fig. 9B). HF diet-fed rats showed lipid accumulation and necrosis in the kidneys (Fig. 9C) which was ameliorated by resveratrol treatment (Fig. 9D). Moreover, control rats and control rats treated with resveratrol showed no fibrosis in the kidney sections (Fig. 9 E and F, Lower panel). HF diet-fed rats showed substantial collagen deposition and fibrosis in the kidneys (Fig. 9 6G, Lower panel) which was prevented by resveratrol treatment (Fig. 9 H, Lower panel). The percentage fibrosis was quantified semi-quantitatively and is presented in Fig. 10. HF diet fed rats showed an increased percentage of fibrosis compared to the control rats, whereas, resveratrol treatment decreased the percentage of fibrosis in the kidneys (Fig. 10).
Fig. 9.
Effect of resveratrol on tissue damage, fibrosis and collagen deposition in the kidney of HF diet-fed rats. A, E-Control rats showed the normal glomerular structure and proximal and distal tubular linings without any necrosis zone, no collagen deposition and fibrosis was also seen. B, F- Control + resveratrol also showed similar structural orientation was found in these kidney sections. C, G- HF showed lipid deposition, shrinkage of glomeruli and thickening of proximal and distal tubules and interstitial fibrosis were observed. and D, H- HF + resveratrol showed reduced or no lipid deposition and fibrosis, and reduced thickening of proximal and distal tubules were observed. Magnification 40X.
Fig. 10.
Effect of resveratrol on % of fibrosis and collagen deposition in the kidney of HF diet-fed rats. Data are presented as mean ± SEM. One-way ANOVA was performed followed by Tukey post hoc test to determine the statistical significance. Asterisk mark (*) is considered significantly different at P < 0.05 and ** is considered significantly different at P < 0.001.
4. Discussion
Chronic kidney dysfunction is increased in metabolic diseases induced by HF diet (Rangel Silvares et al., 2019). This investigation revealed that HF diet feeding in rats developed oxidative stress and declined antioxidant capacities in the kidney. Resveratrol treatment in HF diet-fed rats showed restoration of antioxidant enzymes such as SOD, GPx, and catalase through transcriptional regulation via Nrf-2. This study also revealed that resveratrol treatment prevented fibrosis in the kidneys of HF diet-fed rats.
ROS-induced oxidative stress and lipid peroxidation are significant contributors to HF diet-induced tissue damage in the kidneys (Noeman et al., 2011). In this study, it was evident that HF diet-fed rats showed increased tissue lipid peroxidation. And it is noteworthy to mention that resveratrol treatment may prevent lipid peroxidation in the kidney tissues. This result is supported by previous investigations, which reported that resveratrol might prevent lipid peroxidation in diabetes and kidney dysfunction (Palsamy and Subramanian 2011). Another notable oxidative stress marker is nitric oxide, which may turn into peroxynitrite radicles and cause irreversible cellular damage after reacting with superoxide anions. Nitric oxide may play a dual role in the kidney pathophysiology and may be considered as a harmful marker of chronic kidney disease (Carlström 2021). HF diet-fed rats showed an increased nitric oxide level and iNOS gene expression, which is supported by the previous research reports (Ulla et al., 2017, Martin et al., 2018). It has been reported that iNOS inhibitor, N6-(1-iminoethyl)-l-lysine hydrochloride (l-NIL) administration in HF diet-fed mice prevented the metabolic syndrome and ameliorated proteinuria, decreased N-acetyl-β-d-glucosaminidase excretion and lowered renal triglyceride content (Martin et al., 2018). In the present study, resveratrol normalized the nitric oxide level. Resveratrol also decreased iNOS expression in kidneys of HF diet-fed rats, and our findings are in alignment with the previous study that showed that resveratrol treatment inhibited the iNOS expression (Youn et al., 2009). The benefit could be attributed to the restoration of antioxidant enzyme activities and lowered inflammatory state (Saldanha et al., 2016). However, one of the limitations of this study was the lack of protein expression measurement for iNOS. The mRNA expression sometime may not correlate with the protein synthesis and functional activities. In this study the NO level is significantly increased in the kidneys of HF diet-fed rats which could be attributed to the increased iNOS activity (Cao et al., 2012).
Resveratrol administration stimulates antioxidant function through transcriptional regulation via nuclear factor E2-related factor 2 (Nrf-2) mediated way (Saldanha et al., 2016). This study also revealed that resveratrol augmented Nrf-2 expression in kidneys, leading to increased expression of anti-oxidant genes including HO-1, HO-2, SOD, catalase, and GPx in the kidneys of HF diet-fed rats. In agreement with this, the activities of SOD and catalase were also increased in HF diet-fed rats treated with resveratrol. HO-1 and HO-2 are isoforms of heme-oxygenase enzymes and HO-1 is a more potent antioxidant than the HO-2 (Funes et al., 2020). The previous report suggests that HO-1 provides an antioxidant activity by increasing SOD and catalase activities or decreasing iNOS expression in diabetic rats (Turkseven et al., 2005). Moreover, another study demonstrated that treatment of resveratrol could effectively restore an antioxidant enzyme function which was declined due to kidney dysfunction (Pan et al., 2014). Other study also provided evidence that HF diet may down-regulate MnSOD expression in the kidney of mice which was restored by resveratrol treatment (Zhang et al., 2016).
Lipid accumulation and infiltrating macrophages are the sources of inflammation, leading to the production of inflammatory cytokines in the damaged kidneys. In this study, glomerular disorientation, podocyte loss and lipid accumulation have been noticed in kidney sections of HF diet-fed rats. Inflammatory genes such as IL-1, IL-6, TNF-α, TGF-β, iNOS and NF-κB expression were increased in the kidneys of HF diet-fed rats. Inflammatory cytokines play a crucial role in developing kidney dysfunction in HF diet-induced obesity (Stemmer et al., 2012, van der Heijden et al., 2015). Resveratrol is a potent anti-inflammatory molecule that prevents inflammatory responses (de Sá Coutinho et al., 2018). In this study the transcript levels of inflammatory cytokines such as IL-1, IL-6, TNF-α, and TGF-β and associated factor (NF-кB) and enzyme (iNOS) were increased, in renal tissues, due to the consumption of HF-diet. (Li et al., 2020). Cytokines like IL-6, TNF-α may give signaling for the activation of TGF-β (Kany et al., 2019). Moreover, it has been reported that IL-6 signaling and NF-кB activation lead to the cellular proliferation and thickening of the glomerular basement in diabetic kidney (Navarro-Gonzalez and Mora-Fernandez 2008).
TGF-β is considered the master key regulatory for activating fibroblasts cells to stimulate the production of extracellular matrix (ECM) (Loeffler and Wolf 2013). Moreover, in glomerulosclerosis, TGF-β expression has been increased followed by the increased TGF-β receptors in the glomeruli and the tubulointerstitium region in kidneys (Yamamoto et al., 1996). In this study, HF diet-fed rats showed increased interstitial collagen deposition and TGF-β expression in kidneys. Resveratrol treatment prevented collagen deposition and lowered cytokine expression in the kidneys, including NF-кB and TGF-β expressions. This finding is also supported by a previous study which reported that resveratrol inhibits TGF-β and decreases kidney fibrosis in chronic kidney diseases (Huang et al., 2014).
In this study, a 100 mg/kg dose of resveratrol was used. The daily recommended intake of resveratrol in a human was not found in any literature. The dose used in this study was approximately equal to ∼0.6 g/day based on body surface area comparisons between rats and humans (Reagan-Shaw et al., 2008). However, the total intake of polyphenols is ∼1 g/day (Scalbert and Williamson 2000). Thus, the dose of resveratrol used in this study is realistic in humans.
These findings suggest that resveratrol exerted a remarkable kidney protective effect against HF diet-induced renal dysfunction and fibrosis in rats. This study also provided experimental evidence that resveratrol is a regulator of antioxidant and inflammatory genes and brought down lipid peroxidation levels in kidneys. This effect of resveratrol is a plausible impact and urges further clinical investigation in renal illness.
Funding
This study was supported by the Taif University Researchers Supporting Project (TURSP-2020/197), Taif University, Taif, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The study was carried out at North South University's Department of Pharmaceutical Sciences in Bangladesh. The authors recognize that the North-South University of Bangladesh provides logistical support from the Department of Pharmaceutical Sciences. We appreciate and thank Taif University for the financial support for Taif University Researchers Supporting Project (TURSP-2020/197), Taif University, Taif, Saudi Arabia.
Contributions of Authors
F.I.C., T.Y., Fr.K., and N.S. conducted the investigation. F.I.C., T.Y., Fr.K., N.S., M.N.I., and M.A.A. contributed to analysing and interpreting data and drafting the manuscript. M.M.S., A.A., N.S., M.A.A. and M.A.H. contributed to the conceptualization, visualization and editing of the draft. N.S., M.M.H., M.A.A. and M.A.H. coordinated the research, revised the manuscript and approved the final version for publication. All authors have read and agreed to the published version of the manuscript.
Footnotes
Peer review under responsibility of King Saud University.
References
- Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discovery. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Burgos-Morón E., Abad-Jiménez Z., Marañón A.M.D., et al. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: The battle continues. J. Clin. Med. 2019;8:1385. doi: 10.3390/jcm8091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Inoue K., Sodhi K., Puri N., Peterson S.J., Rezzani R., Abraham N.G. High-fat diet exacerbates renal dysfunction in SHR: reversal by induction of HO-1-adiponectin axis. Obesity. 2012;20(5):945–953. doi: 10.1038/oby.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlström M. Nitric oxide signalling in kidney regulation and cardiometabolic health. Nat. Rev. Nephrol. 2021;17:575–590. doi: 10.1038/s41581-021-00429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastang T., Pozzobon V., Taidi B., Courot E., Clément C., Pareau D. Resveratrol production by grapevine cells in fed-batch bioreactor: Experiments and modelling. Biochem. Eng. J. 2018;131:9–16. [Google Scholar]
- Chen Y., He L., Yang Y., Chen Y., Song Y., Lu X.i., Liang Y. The inhibition of Nrf2 accelerates renal lipid deposition through suppressing the ACSL1 expression in obesity-related nephropathy. Ren. Fail. 2019;41(1):821–831. doi: 10.1080/0886022X.2019.1655450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K., Song Z., Chen Y., Li S., Zhang Y., Zhang H., Zhang L., Wang C., Wang T. Resveratrol protects against renal damage via attenuation of inflammation and oxidative stress in high fat diet-induced obese mice. Inflammation. 2019;42(3):937–945. doi: 10.1007/s10753-018-0948-7. [DOI] [PubMed] [Google Scholar]
- Crowell J.A., Korytko P.J., Morrissey R.L., et al. Resveratrol-associated renal toxicity. Toxicol. Sci. 2004;82:614–619. doi: 10.1093/toxsci/kfh263. [DOI] [PubMed] [Google Scholar]
- de Sá Coutinho D., Pacheco M., Frozza R., Bernardi A. Anti-inflammatory effects of resveratrol: mechanistic insights. Int. J. Mol. Sci. 2018;19(6):1812. doi: 10.3390/ijms19061812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meo S., Reed T.T., Venditti P., Victor V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell. Longevity. 2016;2016:1–44. doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara C.K., Antunes G.R., Mattar A.L., et al. Chronic inhibition of nuclear factor-kappaB attenuates renal injury in the 5/6 renal ablation model. Am. J. Physiol. Renal Physiol. 2007;292:F92–F99. doi: 10.1152/ajprenal.00184.2006. [DOI] [PubMed] [Google Scholar]
- Funes S.C., Rios M., Fernández-Fierro A., Covián C., Bueno S.M., Riedel C.A., Mackern-Oberti J.P., Kalergis A.M. Naturally derived heme-oxygenase 1 inducers and their therapeutic application to immune-mediated diseases. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurászová M., Gurecká R., Bábíčková J., Tóthová Ľ. Oxidative stress in the pathophysiology of kidney disease: implications for noninvasive monitoring and identification of biomarkers. Oxid. Med. Cell. Longevity. 2020;2020:1–11. doi: 10.1155/2020/5478708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza S.M., Dyck J.R.B. Systemic and renal oxidative stress in the pathogenesis of hypertension: modulation of long-term control of arterial blood pressure by resveratrol. Front. Physiol. 2014;5 doi: 10.3389/fphys.2014.00292. 292–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., He T., Farrar S., Ji L., Liu T., Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017;44(2):532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- Hou C.-Y., Tain Y.-L., Yu H.-R., Huang L.-T. The Effects of resveratrol in the treatment of metabolic syndrome. Int. J. Mol. Sci. 2019;20(3):535. doi: 10.3390/ijms20030535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.Z., Wen D., Zhang M., et al. Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF-beta/Smad3 pathway. J. Cell. Biochem. 2014;115:996–1005. doi: 10.1002/jcb.24748. [DOI] [PubMed] [Google Scholar]
- Isaka Y. Targeting TGF-β signaling in kidney fibrosis. Int. J. Mol. Sci. 2018;19:2532. doi: 10.3390/ijms19092532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kany S., Vollrath J.T., Relja B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019;20:6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M., Koya D. Renal protective effects of resveratrol. Oxidative Med. Cellular Longevity. 2013;2013 doi: 10.1155/2013/568093. 568093–568093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp J.B., Factor V.M., Mozes M., et al. Transgenic mice with increased plasma levels of TGF-beta 1 develop progressive renal disease. Lab. Invest. 1996;74:991–1003. [PubMed] [Google Scholar]
- Kurutas E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutrition J. 2016;15 doi: 10.1186/s12937-016-0186-5. 71–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Xiao X., Miao Y., Guo L., Zhen J., Li X., Jiang B., Hu Z. Resveratrol alleviates obesity-associated podocyte injury in ovariectomized obese rats. Exp. Therapeutic Med. 2020 doi: 10.3892/etm.2019.8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler I., Wolf G. Transforming growth factor-β and the progression of renal disease. Nephrol. Dial. Transplant. 2013;29:i37–i45. doi: 10.1093/ndt/gft267. [DOI] [PubMed] [Google Scholar]
- Mamun F., Rahman M.M., Zamila M., Subhan N., Hossain H., Raquibul Hasan S.M., Alam M.A., Haque M.A. Polyphenolic compounds of litchi leaf augment kidney and heart functions in 2K1C rats. J. Funct. Foods. 2020;64:103662. [Google Scholar]
- Martin B., Caron N., Jadot I., Colombaro V., Federici G., Depommier C., Declèves A.-É. Evaluation of inducible nitric oxide synthase inhibition on kidney function and structure in high-fat diet-induced kidney disease. Exp. Physiol. 2018;103(1):125–140. doi: 10.1113/EP086594. [DOI] [PubMed] [Google Scholar]
- Nabavi S.F., Barber A.J., Spagnuolo C., Russo G.L., Daglia M., Nabavi S.M., Sobarzo-Sánchez E. Nrf2 as molecular target for polyphenols: A novel therapeutic strategy in diabetic retinopathy. Crit. Rev. Clin. Lab. Sci. 2016;53(5):293–312. doi: 10.3109/10408363.2015.1129530. [DOI] [PubMed] [Google Scholar]
- Navarro-Gonzalez J.F., Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J. Am. Soc. Nephrol. : JASN. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- Nlandu Khodo S., Dizin E., Sossauer G., Szanto I., Martin P.-Y., Feraille E., Krause K.H., de Seigneux S. NADPH-oxidase 4 protects against kidney fibrosis during chronic renal injury. J. Am. Soc. Nephrol. 2012;23(12):1967–1976. doi: 10.1681/ASN.2012040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noeman S.A., Hamooda H.E., Baalash A.A. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol. Metabolic Syndrome. 2011;3:17. doi: 10.1186/1758-5996-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsamy P., Subramanian S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2–Keap1 signaling. BBA. 2011;1812:719–731. doi: 10.1016/j.bbadis.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Pan Q.-R., Ren Y.-L., Zhu J.-J., Hu Y.-J., Zheng J.-S., Fan H., Xu Y., Wang G., Liu W.-X. Resveratrol increases nephrin and podocin expression and alleviates renal damage in rats fed a high-fat diet. Nutrients. 2014;6(7):2619–2631. doi: 10.3390/nu6072619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal S.K., Poudyal H., Iyer A., Nazer R., Alam A., Diwan V., Kauter K., Sernia C., Campbell F., Ward L., Gobe G., Fenning A., Brown L. High-carbohydrate high fat diet–induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011;57(1):51–64. doi: 10.1097/FJC.0b013e3181feb90a. [DOI] [PubMed] [Google Scholar]
- Rahman M.M., Alam M.N., Ulla A., Sumi F.A., Subhan N., Khan T., Sikder B., Hossain H., Reza H.M., Alam M.A. Cardamom powder supplementation prevents obesity, improves glucose intolerance, inflammation and oxidative stress in liver of high carbohydrate high fat diet induced obese rats. Lipids Health Dis. 2017;16(1) doi: 10.1186/s12944-017-0539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel Silvares R., Nunes Goulart da Silva Pereira E., Eduardo Ilaquita Flores E., et al. High-fat diet-induced kidney alterations in rats with metabolic syndrome: endothelial dysfunction and decreased antioxidant defense. Diabetes Metabolic Syndrome Obesity. 2019;12:1773–1781. doi: 10.2147/DMSO.S211253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff B.B., Abdulmahdi W., Pawar R., Wolin M.S. Oxidant mechanisms in renal injury and disease. Antioxid. Redox Signal. 2016;25(3):119–146. doi: 10.1089/ars.2016.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Ruiz S., Pergola P.E., Zager R.A., et al. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83:1029–1041. doi: 10.1038/ki.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha J.F., Leal V.O., Rizzetto F., et al. Effects of resveratrol supplementation in Nrf2 and NF-kappaB expressions in nondialyzed chronic kidney disease patients: A randomized, double-blind, placebo-controlled, crossover clinical trial. J. Renal Nutrit. 2016;26:401–406. doi: 10.1053/j.jrn.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Scalbert A., Williamson G. Dietary intake and bioavailability of polyphenols. J. Nutrit. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- Selim S., Akter N., Nayan S.I., Chowdhury F.I., Saffoon N., Khan F., Ahmed K.S., Ahmed M.I., Hossain M.M., Alam M.A. Flacourtia indica fruit extract modulated antioxidant gene expression, prevented oxidative stress and ameliorated kidney dysfunction in isoprenaline administered rats. Biochem. Biophys. Rep. 2021;26:101012. doi: 10.1016/j.bbrep.2021.101012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmer K., Perez-Tilve D., Ananthakrishnan G., et al. High fat diet-induced obesity causes an inflammatory and tumor-promoting microenvironment in the rat kidney. Disease Models Mech. 2012;5:627. doi: 10.1242/dmm.009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureshbabu A., Muhsin S.A., Choi M.E. TGF-β signaling in the kidney: profibrotic and protective effects. Am. J. Physiol. Renal Physiol. 2016;310:F596–F606. doi: 10.1152/ajprenal.00365.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkseven S., Kruger A., Mingone C.J., Kaminski P., Inaba M., Rodella L.F., Ikehara S., Wolin M.S., Abraham N.G. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. American Journal of Physiology. Heart Circulatory Physiol. 2005;289(2):H701–H707. doi: 10.1152/ajpheart.00024.2005. [DOI] [PubMed] [Google Scholar]
- Ulla A., Alam M.A., Sikder B., Sumi F.A., Rahman M.M., Habib Z.F., Mohammed M.K., Subhan N., Hossain H., Reza H.M. Supplementation of Syzygium cumini seed powder prevented obesity, glucose intolerance, hyperlipidemia and oxidative stress in high carbohydrate high fat diet induced obese rats. BMC Complem. Alternative Med. 2017;17(1) doi: 10.1186/s12906-017-1799-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden R.A., Bijzet J., Meijers W.C., Yakala G.K., Kleemann R., Nguyen T.Q., de Boer R.A., Schalkwijk C.G., Hazenberg B.P.C., Tietge U.J.F., Heeringa P. Obesity-induced chronic inflammation in high fat diet challenged C57BL/6J mice is associated with acceleration of age-dependent renal amyloidosis. Sci. Rep. 2015;5(1) doi: 10.1038/srep16474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Noble N.A., Cohen A.H., et al. Expression of transforming growth factor-beta isoforms in human glomerular diseases. Kidney Int. 1996;49:461–469. doi: 10.1038/ki.1996.65. [DOI] [PubMed] [Google Scholar]
- Youn J., Lee J.S., Na H.K., et al. Resveratrol and piceatannol inhibit iNOS expression and NF-kappaB activation in dextran sulfate sodium-induced mouse colitis. Nutr. Cancer. 2009;61:847–854. doi: 10.1080/01635580903285072. [DOI] [PubMed] [Google Scholar]
- Zhang N., Li Z., Xu K., Wang Y., Wang Z. Resveratrol protects against high-fat diet induced renal pathological damage and cell senescence by activating SIRT1. Biol. Pharm. Bull. 2016;39(9):1448–1454. doi: 10.1248/bpb.b16-00085. [DOI] [PubMed] [Google Scholar]