Abstract
OBJECTIVE
Although mortality from coronavirus disease 2019 (COVID-19) among youth with type 1 diabetes is rare, severe acute respiratory syndrome coronavirus 2 is associated with increased pediatric hospitalizations for diabetic ketoacidosis (DKA). To clarify whether the relationship between COVID-19 and DKA is coincidental or causal, we compared tissue glucose disposal (TGD) during standardized treatment for DKA between pediatric patients with COVID-19 and those without COVID-19.
RESEARCH DESIGN AND METHODS
We retrospectively compared TGD during standardized therapy for DKA in all children with preexisting type 1 diabetes with or without COVID-19. Cases were assessed beginning with the first case of COVID-19–positive DKA on 19 June 2020 through 2 February 2022.
RESULTS
We identified 93 COVID-19–negative patients and 15 COVID-19–positive patients who were treated for DKA, with similar baseline characteristics between groups. Median TGD was 46% lower among patients who had COVID-19 compared with those who did not (P = 0.013).
CONCLUSIONS
These results suggest that COVID-19 provokes a metabolic derangement over and above factors that typically contribute to pediatric DKA. These findings underscore the significant and direct threat posed by COVID-19 in pediatric type 1 diabetes and emphasize the importance of mitigation and monitoring including through vaccination as a primary prevention.
Introduction
Coronavirus disease 2019 (COVID-19) has arguably afflicted no group more than patients with diabetes. Prior to availability of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine, patients with type 2 diabetes carried a twofold greater risk for mortality (1) and a 3.5-fold greater risk for hospitalization (2) compared with individuals without diabetes. Susceptibility for harm appeared even greater for people with type 1 diabetes. For these patients, the mortality risk was 3.5-fold greater (1) and the risk for hospitalization was 4.5-fold higher (2).
Although diabetes increases the danger of COVID-19 mortality, young age appears to potently mitigate this threat. In a population-wide study of 23,698 COVID-19 deaths in England, only 0.2% of 7,434 deaths in patients with type 2 diabetes occurred in individuals younger than age 40 years (2). None of 364 deaths among patients with type 1 diabetes occurred in individuals younger than age 50 years. When risk of hospitalization with COVID-19 is considered, however, type 1 diabetes remains a considerable hazard for pediatric patients. In an analysis of 43,465 pediatric patients with COVID-19 at 800 U.S. hospitals, type 1 diabetes was the single greatest risk factor for hospitalization, with an adjusted risk ratio of 4.6 for hospitalization compared with children without diabetes (3). In contrast to older patients with type 1 diabetes, registry studies suggest that diabetic ketoacidosis (DKA), not respiratory distress, is strongly associated with this increased hospitalization risk in pediatrics. In the T1D Exchange Registry, 61 of 266 pediatric patients who reported having COVID-19 required hospitalization. Of these, 44 (72%) were hospitalized with DKA. By comparison, 4 patients with COVID-19 were hospitalized for severe hypoglycemia, 3 for respiratory distress, 1 for multisystem inflammatory syndrome in children, and 10 for reasons considered unrelated to COVID-19 (4). In an analysis of the SWEET registry, rates of pediatric DKA hospitalizations during pandemic waves were greatest in countries where COVID-19 mortality was highest (5). In these analyses the investigators infer that while COVID-19 mortality is low in pediatric type 1 diabetes, rates of DKA in conjunction with SARS-CoV-2 are substantial. This association leaves open two possibilities with important clinical implications. On the one hand, because many hospitals universally screen for SARS-CoV-2 on admission, these patients with DKA may have simply had coincidental COVID-19. On the other hand, COVID-19 may have inherent effects contributing to DKA.
Understanding whether the relation between COVID-19 and morbidity in type 1 diabetes is causal or coincidental is critical for developing the policies and approaches that will optimally protect this patient population. If COVID-19 exacerbates metabolic homeostasis more than factors that typically contribute to DKA, this finding would underscore the need for even greater emphasis on immunization and early initiation of diabetes sick day management. To assess these competing models, we investigated the pathophysiologic hypothesis that, among pediatric patients with type 1 diabetes, DKA with COVID-19 is associated with greater insulin resistance than DKA without COVID-19. We reasoned that a difference in insulin resistance during DKA between patients with COVID-19 versus those without COVID-19 would strongly suggest a contributory—rather than coincidental—role for COVID-19 in inducing DKA. To test this hypothesis, we conducted a retrospective cohort study comparing insulin-mediated tissue glucose disposal (TGD) between pediatric patients with and without COVID-19 during standardized treatment for DKA.
Research Design and Methods
Participant Selection
We used an electronic health record (EHR) search query to identify and analyze all hospital admissions for DKA beginning with the first observed case of DKA with a COVID-19–positive test [COVID(+)] at Monroe Carell Jr. Children’s Hospital at Vanderbilt on 19 June 2020 through the end of our review on 2 February 2022. Inclusion criteria included having a diagnosis of type 1 diabetes prior to the hospitalization, which was confirmed on chart review. To ensure the veracity of DKA treatment data (e.g., insulin and glucose [GIR] infusion rates, glucose concentrations, start and end times of infusates, etc.), we only included patients who either initially presented to our hospital or whose insulin infusion was initiated by Vanderbilt’s transport team en route. Thus, patients whose insulin therapy was initiated at an outside hospital were excluded from analysis.
All patients were tested for SARS-CoV-2 on admission with a Centers for Disease Control and Prevention SARS-CoV-2 real-time PCR diagnostic panel. We reviewed charts to identify DKA hospitalizations that were associated with COVID(+) or a negative COVID-19 test [COVID(−)]. In cases where a single patient was hospitalized for DKA both with and without COVID-19 during the retrospective analysis period, we prespecified that we would include only the COVID(+) case in the analysis. If the patient had multiple COVID(−) admissions for DKA during the analysis period, we prespecified that we would analyze only the most recent hospitalization.
The Institutional Review Board of Vanderbilt University approved the study protocol.
DKA Treatment Regimen
Following the diagnosis of DKA, hospital personnel treated all patients using standardized clinical practice guidelines (detailed in Supplementary Material). Patients were treated with use of this protocol if they had pH <7.3, bicarbonate ≤15 mEq/L, and glucose ≥200 mg/dL and ketosis was present by urine or blood testing. Vanderbilt personnel obtained weights on triage for patients who initially presented to Vanderbilt, prior to starting intravenous fluid resuscitation. For patients who were transferred to our hospital by Vanderbilt’s transport team, outside hospital staff provided dosing weights immediately prior to transfer. The treatment regimen included infusing insulin at 0.1 units/kg/h i.v. (1.7 mU/kg/min) after initial intravascular volume resuscitation with an isotonic saline bolus (20 mL/kg or 1 L if weight exceeded 50 kg). Once hyperglycemia abated to <300 mg/dL, a variable dextrose infusion was titrated to avoid hypoglycemia as the metabolic acidosis continued to normalize. The times insulin and glucose infusates were started, stopped, and modified were captured by the medication administration record in the EHR. Criteria for transitioning patients off the intravenous insulin infusion included having an anion gap ≤12 mEq/L and a plasma bicarbonate concentration ≥12 mEq/L.
Data Collection
After identifying hospitalizations for DKA and categorizing the patient cases as COVID(+) or COVID(−), we further reviewed charts to quantify additional covariates that could affect insulin sensitivity. These covariates included age, race, BMI, type 1 diabetes duration, glycosylated hemoglobin (HbA1c), and COVID-19 vaccination status. We collected data related to severity of DKA at presentation, including glucose, pH, and anion gap levels. We then conducted a detailed review to determine the time points when insulin and glucose infusions were started, stopped, and altered for each treatment course.
Calculations
We conceptualized insulin sensitivity as the ability of a unit amount of insulin to effect net glucose removal out of the plasma space while treating DKA. To parameterize TGD, we constructed a mass balance equation for glucose in the plasma space:
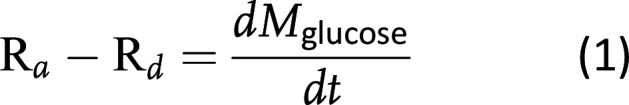 where Ra and Rd are presented as milligrams per kilogram per minute and M is the mass of glucose in plasma. Expanding for each of these components:
where Ra and Rd are presented as milligrams per kilogram per minute and M is the mass of glucose in plasma. Expanding for each of these components:
![]()
![]()
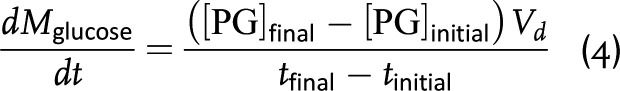 where GIR is the glucose infusion rate into the plasma, EGP is endogenous glucose production (e.g., hepatic glucose production), UGE is urine glucose excretion, [PG]initial and [PG]final are the plasma glucose concentrations at the beginning and ending of insulin infusion, respectively, Vd is the glucose volume of distribution, and tinitial and tfinal are the beginning and ending time points of insulin infusion. Substituting equations 2–4 into equation 1 and rearranging yields an equation to determine the rate of TGD:
where GIR is the glucose infusion rate into the plasma, EGP is endogenous glucose production (e.g., hepatic glucose production), UGE is urine glucose excretion, [PG]initial and [PG]final are the plasma glucose concentrations at the beginning and ending of insulin infusion, respectively, Vd is the glucose volume of distribution, and tinitial and tfinal are the beginning and ending time points of insulin infusion. Substituting equations 2–4 into equation 1 and rearranging yields an equation to determine the rate of TGD:
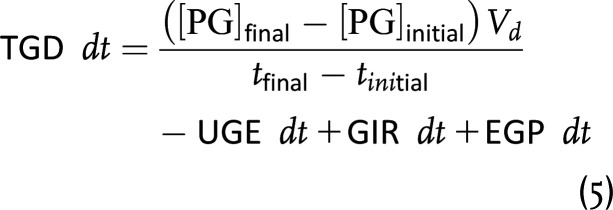
Next, we made three assumptions to facilitate a comparison of TGD between groups:
Based on the investigation by Luzi et al. (6) of insulin’s ability to suppress EGP during DKA (6), the insulin infusion rate used in our DKA treatment protocol (1.7 mU/kg/min) largely suppressed EGP such that differences in EGP between COVID(+) and COVID(−) groups were negligible.
In graded, stepwise hyperglycemic clamp studies, Rave et al. (7) showed that UGE is directly proportional to plasma glucose with a high coefficient of determination (R2 = 0.89). Thus, although UGE was not measured, because plasma glucose concentrations at the beginning and end of insulin infusion were very similar between COVID(+) and COVID(−) groups, differences in UGE between groups were considered negligible.
Differences in insulin clearance kinetics were negligible between COVID(+) and COVID(−) DKA.
With these assumptions, we can simplify and integrate equation 5 to calculate the aggregate TGD for a between-group comparison from the start to the end of the insulin infusion (i.e., tinitial to tfinal):
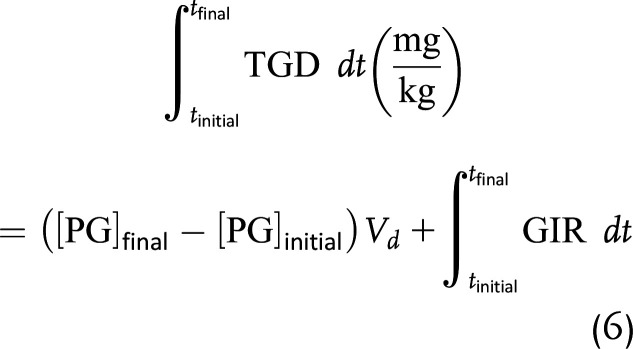 Based on the study of Ishihara et al. of glucose distribution volume in critically ill (8), hemodynamically stable pediatric patients, we used a Vd of 144 mL/kg in our calculations. To quantify TGD as an average rate over the course of the insulin infusion, we divided TGD as calculated in equation 6 by the total time of insulin infusion. Finally, because the total amount of insulin infused differed between COVID(+) and COVID(−) groups, for a between-group comparison we quantified TGD on a per-insulin-unit basis by dividing TGD in milligrams per kilogram per minute by the total amount of insulin infused, yielding TGD in milligrams per kilogram per minute per unit as the primary outcome.
Based on the study of Ishihara et al. of glucose distribution volume in critically ill (8), hemodynamically stable pediatric patients, we used a Vd of 144 mL/kg in our calculations. To quantify TGD as an average rate over the course of the insulin infusion, we divided TGD as calculated in equation 6 by the total time of insulin infusion. Finally, because the total amount of insulin infused differed between COVID(+) and COVID(−) groups, for a between-group comparison we quantified TGD on a per-insulin-unit basis by dividing TGD in milligrams per kilogram per minute by the total amount of insulin infused, yielding TGD in milligrams per kilogram per minute per unit as the primary outcome.
Statistics
The Mann-Whitney U test was used in GraphPad Prism, version 9.3.1 (San Diego, CA), to test for statistically significant differences in TGD between COVID(+) and COVID(−) groups. In sensitivity analyses, to efficiently adjust for confounding with COVID status, we used propensity score matching and inverse probability of treatment weighting (9). Matching was done by age, sex, and weight and resulted in comparisons of 15 COVID(+) patients with 15 COVID(−) patients (1:1 matching). Propensity score weighting creates a synthetic sample in which the distribution of measured baseline covariates is independent of COVID status. Propensity score weighting included all patients and adjustment for age, sex, weight, initial glucose, initial pH, initial anion gap, and duration of type 1 diabetes. Diagnostics were performed to evaluate balance between exposure groups after weighting. Data are summarized as medians and 95% CIs unless otherwise specified.
Results
Patient Characteristics
Among 108 hospital admissions for DKA meeting prespecified inclusion criteria, there were 93 COVID(−) patients and 15 COVID(+) patients. Demographic and clinical characteristics for these patients are summarized in Table 1. Children and adolescents in the COVID(−) and COVID(+) groups had similar ages, diabetes durations, HbA1c levels, weights, and BMIs. Most patients in both groups had a previous hospitalization for DKA. Among the 15 patients admitted with COVID(+) DKA, 13 were unvaccinated, 1 was vaccinated, and the vaccination status for 1 was unknown. Patients in both groups presented with similarly elevated plasma glucose concentrations and similar derangements in acid-base status.
Table 1.
Baseline characteristics of pediatric patients with DKA grouped by COVID-19 status
| Baseline characteristic | COVID(−) DKA (n = 93) | COVID(+) DKA (n = 15) |
|---|---|---|
| Male sex | 57.0 (53) | 53.3 (8) |
| Age, years (interquartile range, total range) | 14.6 (12.6–17.1, 5.4–19.3) | 14.2 (12.4–15.8, 10.9–19.1) |
| Weight, kg | 54.0 (44.6–65.4) | 62.0 (47.3–69.0) |
| Height, m | 1.58 (1.51–1.68) | 1.68 (1.56–1.83) |
| BMI, kg/m2 | 20.9 (18.8–24.6) | 22.3 (20.5–25.2) |
| HbA1c, % | 10.9 (9.4–12.7) | 11.0 (8.6–14.0) |
| HbA1c, mmol/mol | 96 (79–115) | 97 (70–130) |
| Type 1 diabetes duration, years | 5.6 (3.8–8.0) | 4.0 (3.1–7.2) |
| Previous DKA | ||
| No | 10.8 (10) | 13.3 (2) |
| Yes | 89.2 (83) | 86.7 (13) |
| Vaccinated | ||
| No | 83.9 (78) | 86.7 (13) |
| Yes | 8.6 (8) | 6.7 (1) |
| Partial | 2.2 (2) | 0.0 (0) |
| Unknown | 5.4 (5) | 6.7 (1) |
| Mode of arrival | ||
| Presented to VCH ED | 62.4 (58) | 40.0 (6) |
| Transferred from OSH via VCH EMS | 37.6 (35) | 60.0 (9) |
| Race | ||
| White | 73.1 (68) | 80.0 (12) |
| Black | 23.7 (22) | 20.0 (3) |
| Asian | 1.1 (1) | 0.0 (0) |
| Declined to answer | 2.2 (2) | 0.0 (0) |
| Initial plasma glucose, mg/dL | 364 (262–488) | 371 (215–527) |
| Initial BUN, mg/dL | 15 (12–20) | 17 (11–24) |
| Initial creatinine, mg/dL | 1.32 (1.07–1.54) | 1.2 (0.95–1.43) |
| Initial BUN-to-creatinine ratio | 11.7 (9.6–15.1) | 12.6 (9.8–19.2) |
| Initial eGFR (mL/min/m2) | 48.3 (41.8–58.4) | 55.9 (42.7–71.3) |
| Initial pH | 7.17 (7.08–7.25) | 7.11 (7.00–7.25) |
| Anion gap, mEq/L | 22 (18–25) | 21 (15–25) |
| Hypertonic saline administered for suspected cerebral edema | ||
| No | 95.7 (89) | 86.7 (13) |
| Yes | 4.3 (4) | 13.3 (2) |
Data for continuous variables are summarized as medians (interquartile range) unless otherwise indicated and for categorical and ordinal variables as % (n). Estimated glomerular filtration rate (eGFR) was calculated with bedside Schwartz equation. BUN, blood urea nitrogen; ED, emergency department; EMS, emergency medical services; OSH, outside hospital; VCH, Monroe Carell Jr. Children’s Hospital at Vanderbilt.
Treatment Characteristics
Treatment characteristics by COVID-19 group are summarized in Table 2. Despite receiving treatment according to the same clinical practice guidelines—with nearly all children receiving the same insulin infusion rates—patients with COVID(+) DKA required 18% more insulin to resolve their ketoacidosis than COVID(−) patients. This difference likely contributed to 25% longer median hospital lengths of stay for COVID(+) patients than for COVID(−) patients. The mean GIR was 15% higher in the COVID(−) group than in the COVID(+) group. Because the median time required to resolve the ketoacidosis was 2.3 h longer in the COVID(+) group, however, a greater total amount of glucose was infused in COVID(+) patients than in COVID(−) patients. The median final plasma glucose concentration between groups was nearly the same at the end of the insulin infusion, reflecting the standardized treatment regimen.
Table 2.
Treatment characteristics of patients with DKA grouped by COVID-19 status
| Baseline characteristic | COVID(−) DKA (n = 93) | COVID(+) DKA (n = 15) |
|---|---|---|
| Length of stay, days | 0.9 (0.8–1.2) | 1.2 (0.8–1.5) |
| Mean insulin infusion rate, units/kg/min* | 1.7 (1.7–1.7) | 1.7 (1.7–1.7) |
| Total intravenous insulin infused, units/kg | 1.32 (1.00–1.84) | 1.56 (1.07–2.25) |
| Total insulin infusion time, h | 13.3 (10.0–18.7) | 15.6 (10.7–23.0) |
| Mean GIR, mg/kg/min† | 3.8 (3.0–4.5) | 3.3 (2.8–3.4) |
| Total dextrose infused, g/kg | 2.5 (1.8–3.5) | 2.9 (1.3–4.3) |
| Final plasma glucose, mg/dL‡ | 118 (89–146.5) | 112 (88–183) |
| Final BUN, mg/dL‡ | 10 (9–14) | 11 (8–16) |
| Final creatinine, mg/dL‡ | 0.89 (0.77–1.04) | 1.01 (0.85–1.13) |
| Final BUN-to-creatinine ratio‡ | 11.8 (9.5–14.9) | 12.9 (8.3–14.6) |
| Final eGFR (mL/min/m2)‡ | 72.4 (62.9–83.2) | 66.9 (60.2–77.7) |
Data are summarized as median (interquartile range). Estimated glomerular filtration rate (eGFR) calculated with bedside Schwartz equation. BUN, blood urea nitrogen.
From start to completion of intravenous insulin infusion.
From start of glucose infusion until end of insulin infusion.
Immediately preceding discontinuation of the intravenous insulin infusion.
TGD
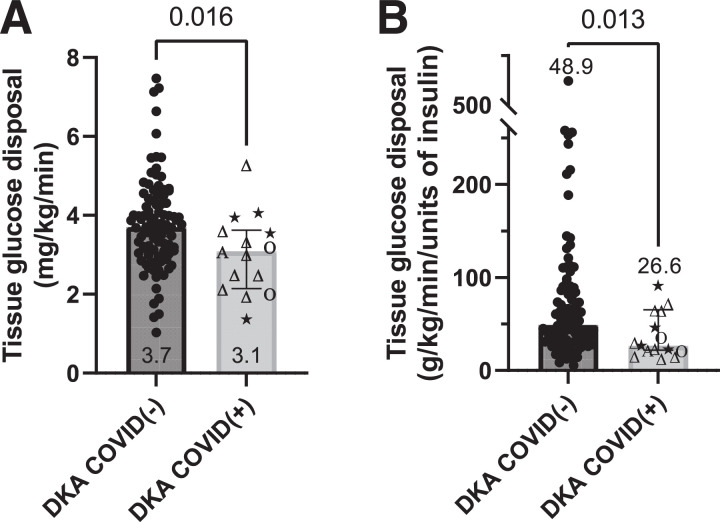
TGD in patients with COVID(+) DKA was 3.09 mg/kg/min vs. 3.70 mg/kg/min in patients with COVID(−) DKA (difference between medians 0.61 mg/kg/min, 95% CI of difference 0.14–1.30, P = 0.016 (Fig. 1A). Because more insulin was infused in COVID(+) patients, we normalized TGD on a per-unit basis as a primary outcome. TGD remained lower in COVID(+) patients than COVID(−) patients: 26.6 vs. 48.9 g/kg/min/unit, respectively (difference in medians 22.3 g/kg/min/unit, 95% CI 3.9–38.3, P = 0.013) (Fig. 1B). There was no apparent relationship between decreased TGD and the predominant SARS-CoV-2 variant at the time of admission.
Figure 1.
TGD during insulin infusion in pediatric patients with DKA with and without COVID-19, expressed as the average glucose Rd in milligrams per kilogram per minute (A) and normalized per unit of insulin infused (in milligrams per kilogram per minute per unit) to account for differing total amounts of insulin infused between groups (B). Greek letters indicate the SARS-CoV-2 variant that predominated at the time of hospital admission (stars indicate “other” variants that were present in 2020). Column scatter plots depict medians and 95% CIs.
Sensitivity Analyses
We conducted two additional sensitivity analyses to address potential confounding covariates present at baseline. First, we propensity score matched the 15 COVID(+) patients with 15 COVID(−) patients COVID on age, sex, and weight. In these individuals, we found the median TGD was 3.09 mg/kg/min in the COVID(+) group and 3.92 mg/kg/min in the COVID(−) group (P = 0.013, Wilcoxon rank sum test, n = 15 per group). These results are similar to what was reported in the full sample (3.70 mg/kg/min vs. 3.09 mg/kg/min, P = 0.016) with a slightly larger difference between groups in the 1:1 matched sample. Second, we considered propensity score weighting to balance subjects on age, sex, weight, initial glucose, initial pH, initial anion gap, and duration of type 1 diabetes. To allow for weights in the software, we used the proportional odds ordinal logistic regression model, which generalizes the Wilcoxon rank sum test to a regression setting. In this analysis, we found that TGD levels were 3.2 times more likely to be lower in COVID(+) patients compared with COVID(−) patients (P < 0.01), which was also consistent with the unweighted analysis (3.3 times more likely, P = 0.015).
Conclusions
These results support the hypothesis that COVID(+) DKA is associated with greater insulin resistance than COVID(−) DKA in children with type 1 diabetes. We found that median TGD during a standardized insulin infusion protocol for DKA was 46% lower when patients had COVID-19 compared with when they did not. This finding provides compelling evidence that COVID-19 induces metabolic derangement over and above factors that typically contribute to pediatric DKA. Thus, although young age in type 1 diabetes protects against severe respiratory illness from SARS-CoV-2, the virus confronts youth with a substantial risk for metabolic morbidity.
These data strongly argue that COVID-19 and DKA are not coincidentally related, which should alert physicians to two critical considerations. First, the diminution in insulin-mediated glucose disposal suggests that COVID-19–induced inflammation lowers the threshold for metabolic decompensation. This propensity for DKA underscores the importance of initiating diabetes sick day management soon after infection. Second, the results highlight the pressing need for vaccination as primary prevention, especially in pediatric patients at increased risk for DKA. We note that 87% of the COVID(+) group had at least one previous DKA admission, yet only 1 of these 15 children was immunized against SARS-CoV-2. While the future of COVID-19 is difficult to predict, it seems likely that vaccinations will continue to ameliorate illness severity and thereby mitigate associated increases in sympathoadrenal tone, insulin resistance, and lipolysis.
The approach taken to test the study’s hypothesis has several strengths. First, because hospital staff treated patients with DKA using a standardized protocol, the confounding effect of differing treatment regimens between groups and individual patients was minimized. Second, we analyzed every hospital admission for DKA in patients with preexisting type 1 diabetes from the first case of COVID(+) DKA until present. This retrospective approach minimized selection bias and produced a robust data set comprising a wide geographic catchment area. For this reason, one can reasonably extrapolate the study’s findings to a broad group of pediatric patients with preexisting type 1 diabetes. At the same time, our approach included only patients whose insulin and glucose infusions were given by our personnel and recorded in our EHR, thereby maximizing the accuracy of the data used to calculate TGD. Third, there was sufficient equipoise between groups for baseline patient characteristics that might have confounded the primary outcome.
Some limitations of the study design and data warrant consideration. First, although our retrospective design generated a robust data set to answer the research question, the approach precluded measuring urinary glucose excretion and endogenous glucose production using isotopic glucose tracer techniques. Thus, we extrapolated from a previous study of endogenous glucose production during DKA in adults with type 1 diabetes (6) and from a hyperglycemic clamp study quantifying urinary glucose excretion in adults with type 2 diabetes (7) to form the assumptions used in quantifying a difference in TGD between groups in this investigation. Second, in a retrospective cohort study we cannot control for or measure all potential unknown confounders (e.g., whether patients with COVID-19 delayed seeking care more than patients without COVID-19). Third, most patients in the analysis were adolescents, rather than younger children, reflecting the most common ages for DKA hospitalization for patients with existing type 1 diabetes at our institution. Thus, one should extrapolate these results to younger children and those with newly diagnosed type 1 diabetes with caution.
We conclude that COVID-19 worsens insulin resistance during DKA over and above that seen during more typical DKA without COVID-19 in pediatric patients with type 1 diabetes. This finding should spur the medical community to undertake a renewed effort to pursue primary prevention in the form of immunization against SARS-CoV-2 in youth with type 1 diabetes. Further, these results should prompt clinicians to teach patients and families to recognize symptoms of impending DKA and promptly initiate sick day management, especially in those individuals at highest risk.
Article Information
Acknowledgments. The authors thank Pradeep Mummidi, Director of Business Analytics at Monroe Carell Jr. Monroe Carell Jr. Children’s Hospital at Vanderbilt, for assistance in using an EHR query to identify patients meeting inclusion criteria for our study.
Funding. Research and personnel for this publication were supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award nos. K23DK123392 (J.M.G.) and R01DK121316 (K.A.D.). The research was also supported by a pilot and feasibility grant from the Vanderbilt Diabetes Research and Training Center (DK020593). J.M.G was supported by a JDRF Career Development Award (5-ECR-2020-950-A-N). K.A.D. was supported by the Katherine Dodd Faculty Scholars Program.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the JDRF.
Duality of Interest. J.M.G. has served as an advisory board member for Eli Lilly, Medtronic, Dompé, vTv Therapeutics, and Mannkind. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. E.S.K. assisted with the study design and conducted chart review. J.C.S. conducted statistical analysis. K.A.D. designed and implemented the standardized clinical practice guidelines used in this study. A.D.C. assisted in calculating key parameters and data analysis. D.J.M. designed the study and analyzed data. J.M.G. designed the study, conducted chart review, calculated key parameters, analyzed data, and wrote the manuscript. All authors critically reviewed and revised the manuscript. J.M.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.20334600.
This article is part of a special article collection available at diabetesjournals.org/journals/collection/52/Diabetes-and-COVID-19.
References
- 1. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol 2020;8:813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gregory JM, Moore DJ. The dual burden of type 1 diabetes and COVID-19. Ann Intern Med 2021;174:703–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kompaniyets L, Agathis NT, Nelson JM, et al. Underlying medical conditions associated with severe COVID-19 illness among children. JAMA Netw Open 2021;4:e2111182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes 2021;13:681–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Danne T, Lanzinger S, de Bock M, et al. A worldwide perspective on COVID-19 and diabetes management in 22,820 children from the SWEET project: diabetic ketoacidosis rates increase and glycemic control is maintained. Diabetes Technol Ther 2021;23:632–641 [DOI] [PubMed] [Google Scholar]
- 6. Luzi L, Barrett EJ, Groop LC, Ferrannini E, DeFronzo RA. Metabolic effects of low-dose insulin therapy on glucose metabolism in diabetic ketoacidosis. Diabetes 1988;37:1470–1477 [DOI] [PubMed] [Google Scholar]
- 7. Rave K, Nosek L, Posner J, Heise T, Roggen K, van Hoogdalem EJ. Renal glucose excretion as a function of blood glucose concentration in subjects with type 2 diabetes--results of a hyperglycaemic glucose clamp study. Nephrol Dial Transplant 2006;21:2166–2171 [DOI] [PubMed] [Google Scholar]
- 8. Ishihara H, Hashiba E, Okawa H, Saito J, Kasai T, Tsubo T. Basic and clinical assessment of initial distribution volume of glucose in hemodynamically stable pediatric intensive care patients. J Intensive Care 2014;2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424 [DOI] [PMC free article] [PubMed] [Google Scholar]