Abstract
OBJECTIVE
Continuous glucose monitoring (CGM) is associated with improved outcomes in type 1 diabetes, but racial-ethnic disparities exist in use. We were interested in examining whether addressing structural health care barriers would change provider prescribing behaviors to make CGM access more equitable.
RESEARCH DESIGN AND METHODS
From January 2019 to December 2021, we used multilevel stakeholder input to develop and implement several non-grant-funded practice transformations targeted toward equity, which included 1) developing a type 1 diabetes clinic, 2) conducting social needs assessments and management, 3) training support staff to place trial CGMs at the point of care, 4) optimizing prescription workflows, and 5) educating providers on CGM. Transformations were prioritized based on feasibility, acceptability, and sustainability. To examine effect on prescribing behaviors, we collected monthly aggregate data from the electronic medical record and performed multiple linear regression to examine and compare change in CGM prescriptions over the 3 years of transformation.
RESULTS
In total, we included 1,357 adults with type 1 diabetes in the analysis (mean ± SD age 38 ± 18 years; 30% Black [n = 406], 45% Hispanic [n = 612], 12% White [n = 164]; and 74% publicly insured [n = 1,004]). During the period of transformation, CGM prescription rates increased overall from 15% to 69% (P < 0.001). Improvements were seen equally among Black (12% to 72%), Hispanic (15% to 74%), and White adults (20% to 48%) (between-group P = 0.053).
CONCLUSIONS
Diabetes practice transformations that target equity, offload provider burdens, and focus on feasible sustainable stakeholder-driven solutions can have powerful effects on provider prescribing behaviors to reduce root causes of inequity in CGM among underserved adults with type 1 diabetes. Continued focus is needed on upstream determinants of downstream CGM use.
Introduction
Continuous glucose monitoring (CGM) has become the standard of care in type 1 diabetes, demonstrating reductions in HbA1c levels, diabetic ketoacidosis, and severe hypoglycemia (1,2). Several trials have demonstrated the efficacy and benefits of CGM in type 1 diabetes outcomes across the life span (2–5). Importantly, CGM has also demonstrated beneficial effects on quality of life, diabetes self-management, and diabetes distress (6,7). These established benefits of CGM could have great implications for those at highest risk for complications, such as underserved populations.
Nevertheless, despite ease of use and established benefit of CGM, universal uptake remains limited. Non-Hispanic Black (Black) and Hispanic populations are among those with the lowest levels of diabetes technology use (8–11), regardless of insurance status or age, compared with non-Hispanic White (White) groups (12,13). In a study of young adults with type 1 diabetes, we showed that Black participants reported using CGM at one-fifth the rate of White participants, which was associated with 2.3% higher mean HbA1c levels, higher levels of diabetes distress, and lower levels of self-reported diabetes self-management (9). In another study, Black children with type 1 diabetes who were not on CGM had greater numbers of emergency visits and hospitalizations, compared with White children (14). As disparities widen due to the rapid pace of technological innovation, interventions are urgently needed to address root causes of inequity in CGM use.
Drawing from the socio-ecological model, there are known barriers to CGM use at the individual, health care, societal/social determinant, and policy levels that ultimately impact diabetes outcomes (15). We have previously published extensive literature on barriers to CGM use that outline individual-level (8,9), provider-level (16), and health care system–level factors (16). Furthermore, we recently conducted in-depth user-centered design workshops to elicit perspectives from a variety of diabetes health care providers who co-created interventions to increase technology use in underserved populations with type 1 diabetes (16). Our findings revealed that while provider bias was acknowledged as a key component of CGM inequity, the systems and structures within which providers practiced remained a fixed issue even for well-meaning providers who were aware of their own biases in prescribing. Practice limitations, such as short visits, lack of support staff, clinic resources, and burden of time for training and support were cited as major decision points for prescribing CGM (11), leading to the majority of co-created interventions focusing heavily on system-structure changes that either removed barriers or better supported providers in efficiently prescribing and onboarding patients to CGM (16). This is supported by other work on providers in primary care and through patient perspectives (8,17,18).
Currently, gaps remain in the field regarding whether modifying clinic structures and care processes will promote equity in CGM prescribing among providers. Interventions such as bias training and increasing provider awareness have not traditionally been effective at reducing inequity in prescriptions. Moreover, most studies evaluating or targeting CGM inequity are focused on pediatric care systems (19,20), which differ significantly from adult care systems where there are less resources, less specialized staff, less funding, and shorter visit times. Additionally, intervention studies to date are small, may not include multilevel stakeholders to develop and implement the interventions, and either accept donations of CGM devices or are fully funded by grants. Feasible, acceptable, and sustainable solutions are needed in adult care paradigms to reduce inequity for the vast populations of underserved adults with type 1 diabetes. Unique strategies may necessitate repurposing roles of existing staff or leveraging resources that exist outside of diabetes centers, but this remains understudied.
In addressing this critical issue, our adult diabetes practices, which service a large cohort of underserved adults with type 1 diabetes in New York, underwent several non-grant-funded clinic transformations over a period of 3 years to reshape the way we prescribe and offer diabetes technology. To remain responsive to gaps identified, we specifically focused our interventions on improving equity for underserved populations, offloading burdens for health care providers, and implementing changes that are feasible and sustainable. Our previous work informed and justified the need to design interventions that influence race-ethnicity–based differences in CGM-prescribing behaviors in adult diabetes practices (8,16). We included multilevel stakeholders such as leadership, providers, staff, and patients to inform and implement the practice transformations. We hypothesized that focusing interventions to improve CGM prescription rates would eventually translate into supporting equitable CGM access and use. Thus, this study was designed as the first critical step to ultimately improving equity in type 1 diabetes outcomes, by focusing on a root cause of inequity, namely, provider prescribing behaviors. Targeting upstream links in the chain of inequity should have great potential for reducing downstream health inequity across generations.
Research Design and Methods
Setting and Participants
Montefiore Medical Center (MMC) is a safety net hospital system in the Bronx, NY, which is the poorest and most diverse county in New York City. Mirroring the demographics of the Bronx, the racial-ethnic breakdown of MMC patients is ∼56% Hispanic, 30% Black, and 10% White, with the majority covered by public insurance (>75% Medicaid or dual-eligible Medicaid/Medicare) (21,22). The Fleischer Institute for Diabetes and Metabolism at MMC consists of four clinics in the Bronx. In January 2019, before practice transformations were initiated, ∼100 type 1 diabetes visits were conducted per month by four diabetologist physicians, three nurse practitioners, and eight endocrine fellows (n = 15). Throughout the study period, all patients with type 1 diabetes in New York on managed or straight Medicaid insurance plans were universally covered for CGM devices, although lengthy and frequent authorization procedures are required to start and maintain CGM, especially for underserved populations covered by Medicaid plans (23–25).
Diabetes Practice Transformations
We developed a suite of interventions that focused on redesigning healthcare delivery and removing structural barriers to CGM prescribing. These practice transformations were based on our ongoing work and others’ (8,9,16) that highlighted key drivers of inequitable prescribing behaviors, such as lack of type 1 diabetes specialized care, lack of time and resources to support underserved groups, inefficient prescribing workflows, challenges related to authorization procedures, and provider prescribing biases. We included leadership, health care providers, clinic staff, patients, and company representatives in the development and implementation plans of our interventions to increase feasibility, acceptability, and sustainability. These practice transformations occurred from January 2019 to December 2021 and are detailed in chronological order below:
1) Specialty Type 1 Diabetes Center
In response to patient needs that aligned with leadership strategic plans, the diabetes center started a specialty clinic to focus on type 1 diabetes. Monthly diabetes practice meetings were initiated to improve communication between diabetes practices across the sites of the Fleischer Institute where best practices were unified and institution-specific gaps were identified.
2) Support for Social Needs
As a shared venture with primary care, we embedded a social needs–trained licensed practice nurse (LPN) in our diabetes practice to assist with social needs assessments and management. We anticipated that help with social needs would alleviate structural barriers to CGM acceptance, use, and maintenance.
3) Staff CGM Training and Device Trials
We expanded diabetes expertise among existing staff by leveraging our local CGM representatives to train our LPNs/medical assistants (MAs) to place CGM devices at the point of care. LPN/MAs were taught how to educate patients on starting CGM, download CGM reports for providers in real time, and add patients to our cloud-based clinic accounts for ease of data retrieval. Apart from offloading burden from providers for CGM placement and initial training, expanding our LPN/MA capacity allowed our practice to offer CGM device trials, which could be done at visits or as structured appointments outside of provider clinical visits and exposed more patients to risk-free experiences with CGM before decision-making.
4) Streamlined CGM Prescribing Workflows
Standardization of prescribing workflows was endorsed as a priority of our patients who stated that the insurance authorization process was opaque, confusing, and deterred them from continuing CGM use. We created relationships with local durable medical equipment (DME) suppliers and pharmacy representatives to streamline and adopt new workflows to reduce barriers to device authorization. DMEs and pharmacies identified specific company representatives who were accountable for our practice. Together with our practice managers and clinic staff, we created workflows that included weekly summaries for pending paperwork and approvals. In addition, DMEs/pharmacies trained our administrative staff on best practices to improve the likelihood of authorization. Despite universal coverage for CGM devices for patients with type 1 diabetes in NY, often lengthy and frequent authorization procedures are required to start and maintain CGM. As part of our workflow, we developed a system for the DMEs to complete new and refill CGM authorizations that could increase the success of continuation of CGM, as well as lowered barriers to entry for providers to prescribe devices for their patients. The DME was able to track the authorization process in real time to update providers and staff on approvals and delivery of devices to patients.
5) Provider CGM Education and Bias Training
We held endocrinology division-wide education sessions to offer providers updates on CGM technology and education on data retrieval and interpretation. We also discussed CGM prescribing workflows with providers before implementation to enhance acceptability. We gave didactic lectures on the emerging literature from our group and others to highlight technology inequity and barriers to CGM use, underscoring the role of provider bias and practice system issues.
Data Collection
To examine the effect of our practice transformations, we retrospectively reviewed monthly aggregate data from the electronic medical record (EMR) and measured CGM prescription rates from January 2019, when the transformations began, to December 2021. Adults 18 years of age or older were included if they had a clinical diagnosis of type 1 diabetes, defined by ICD-10 codes, and at least one in-person or telemedicine visit with an endocrine provider in the preceding 12 months. Participants were excluded if they had not been seen in the endocrine practice for >1 year, if they had ICD-10 codes associated with steroid-induced diabetes, maturity-onset diabetes of the young, or gestational diabetes mellitus, or if they had a ratio of type 1 diabetes to non–type 1 diabetes or type 2 diabetes ICD-10 codes of <50% in the EMR.
To collect CGM prescription data, we used visit and nonvisit prescription data in the EMR to identify participants who had an active CGM prescription in their chart. CGM was categorized as either a continued prescription from the prior month or as a new prescription with no preceding CGM prescription in the past 3 years. We were interested in collecting both continued prescriptions and new prescriptions as different measures of how robust our practice transformations were in changing CGM prescribing behaviors. CGM prescriptions included those for Dexcom G4/G5/G6, FreeStyle Libre 1/2, and Guardian. We also extracted age, sex, race/ethnicity, and insurance type from the EMR to characterize the patient population. For race-ethnicity groups, we collected data on patients of Hispanic, Black, and White race-ethnicity. Patients classified as “other” were patients of Asian race-ethnicity or patients whose race-ethnicity were not recorded in the EMR.
Statistical Analysis
We tabulated and summarized baseline patient characteristics as mean ± SD or n (%) for the overall cohort and by race-ethnicity. We calculated overall CGM prescription rates as number of people with an active continued CGM prescription or a new CGM prescription in the reporting month, divided by total number of type 1 diabetes visits in the same month. New CGM prescription rates were separately calculated as number of people with a new prescription and no preceding CGM prescription in the past 3 years divided by total number of people eligible for starting CGM, i.e., patients with a type 1 diabetes visit in the reporting month without a prior active CGM prescription. We used CGM prescription percentages to examine CGM prescriptions from January 2019 to December 2021 both for the overall cohort and by race-ethnicity. We performed multiple linear regression to examine change in CGM prescriptions within each group over time and between race-ethnicity (3 year period of practice transformation). P values of <0.05 were considered statistically significant.
Results
Participants
In total, 1,357 adults with type 1 diabetes were included. Patients had to have had at least one visit with an endocrine provider between January 2019 and December 2021. Table 1 describes baseline characteristics of the patients. For the overall cohort, mean ± SD age was 38 ± 18 years, 52% were female, 30% were Black (n = 406), 45% were Hispanic (n = 612), and 12% were White (n = 164). The majority of participants were publicly insured (74%, n = 1,004). By race-ethnicity, mean age of Black and Hispanic patients was 39.6 ± 17.7 and 34.7 ± 16.3 years, respectively, compared with White patients, who were older at 49.6 ± 20.4 years (P = 0.001). In addition, while the majority of Black and Hispanic patients were publicly insured (80.5% and 82.8%, respectively), White patients were equally split between public and private insurance coverage (50% vs. 43.9%, respectively) (P = 0.01).
Table 1.
Baseline participant characteristics overall and by race-ethnicity
| Overall (n = 1,357) | Black (n = 406 [29.9%]) | Hispanic (n = 612 [45.0%]) | White (n = 164 [12.0%]) | Other (n = 174 [12.8%])* | |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 38.0 ± 18.1 | 39.6 ± 17.7 | 34.7 ± 16.3 | 49.6 ± 20.4 | 36.5 ± 17.3 |
| Female sex | 52 (706) | 52 (211) | 54 (330) | 49 (80) | 48 (84) |
| Public Insurance | 74 (1,004) | 80.5 (327) | 82.8 (507) | 50.0 (82) | 71.4 (125) |
Data are % (N) unless otherwise indicated.
Other includes Asian race-ethnicity and people for whom race-ethnicity could not be identified.
CGM Prescriptions Over Period of Practice Transformation
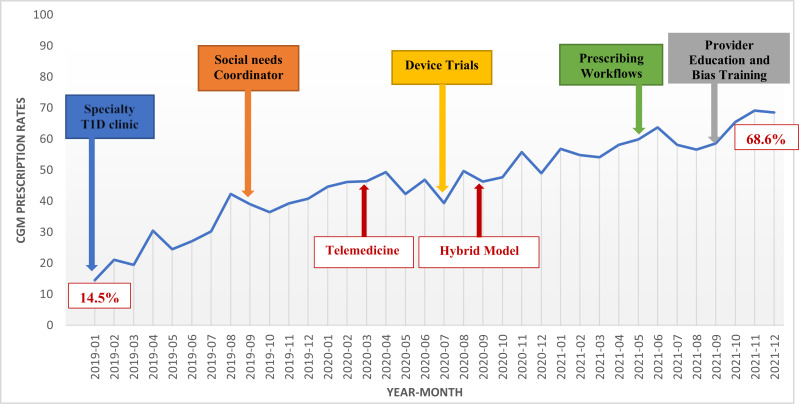
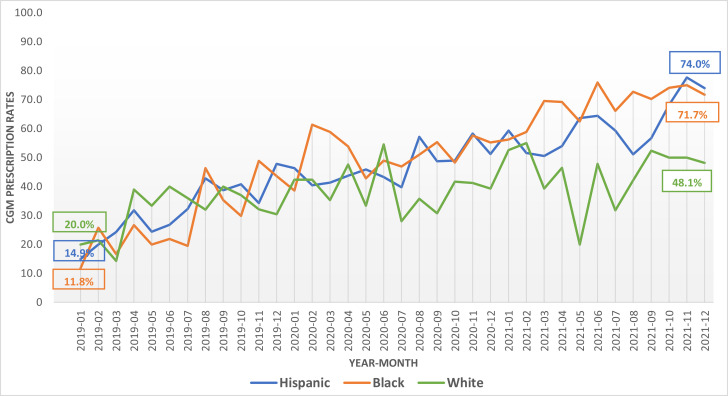
Figure 1 depicts the time of onset of various intervention components in relation to CGM prescription rates. Results of linear regression revealed that the rate of continued CGM prescriptions significantly increased from 15 to 69% in the overall cohort from January 2019 to December 2021 (P < 0.001) (Fig. 1). Equal improvements were observed in Black (12% to 72%), Hispanic (15% to 74%), and White patients (20% to 48%) (between-group Black vs. White and Hispanic vs. White P = 0.053 over the 3-year transformation period) (Fig. 2).
Figure 1.
Practice transformations and CGM prescriptions in adult type 1 diabetes cohort from January 2019 to December 2021 (n = 1, 357). T1D, type 1 diabetes.
Figure 2.
CGM prescriptions by race-ethnicity from January 2019 to December 2021.
Analysis of new prescriptions, i.e., patients with a new CGM prescription in the reporting month with no preceding CGM prescription in the past 3 years, also revealed an increase (22% to 38% overall, P = 0.04 over the 3-year transformation period).
Analyzing rates and interventions year by year, in 2019, when the specialty type 1 diabetes clinic, social needs coordinator, nurse training, and device trials began, there was a dramatic increase in continued CGM prescriptions, from 15% to 41% overall (Fig. 1) and from 12% to 44% in Black, 15% to 48% in Hispanic, and 20% to 30% in White patients (Fig. 2) (P < 0.001 for all groups over time). Likewise, new CGM prescriptions increased from 22% to 31%, overall (P = 0.01).
In 2020, with outpatient care in New York City severely affected by the coronavirus disease 2019 pandemic, and the majority of patient visits switched to telemedicine, prescription rates remained remarkably stable overall (44%–48%) (Fig. 1). However, new CGM prescriptions were reduced from 27% in January 2020 to 13% at the onset of the initial pandemic surge in March 2020. Prescriptions then recovered to pre-pandemic rates and were again reduced to 12% at the onset of the second surge in September–October 2020 (Fig. 1). No differences in rates were observed by race-ethnicity.
In 2021, with implementation of hybrid in-person and telemedicine care, institution of formal CGM prescription workflows, and technology and implicit bias training of diabetes providers, continued CGM prescription rates increased from 57% to 69% overall (P < 0.001 for all groups over time) (Fig. 1). By race-ethnicity, prescriptions increased from 56% to 71% in Black, 59% to 74% in Hispanic, and 48% to 53% in White patients (P < 0.05 between groups) (Fig. 2). New CGM prescriptions remained the same, from 35% to 38% (P = 0.35).
Conclusions
To improve equity in real-world diabetes care and extend diabetes technology to underserved adult populations with type 1 diabetes, our diabetes practice underwent several transformations to target health care system factors that may inadvertently enable inequitable CGM prescribing behaviors. These changes were made without grant funding and as joint practice decisions to enhance feasibility, acceptability, and sustainability. The transformations included: 1) starting a specialty type 1 diabetes clinic to centralize expertise, 2) embedding a social needs coordinator shared with primary care to address social barriers, 3) training support staff on CGM placement to offload provider burden and enable a device trial program, 4) changing prescribing workflows to become more efficient, and 5) expanding provider CGM education and awareness of bias. As a result, CGM prescription rates quadrupled from 15% to 69% and did not introduce new disparities by race-ethnicity, as evidenced by equivalent increases among Black, Hispanic, and White groups over time. Our study demonstrates the potential of targeting health care structural barriers to change provider prescribing behaviors, which is one of the most proximal steps in CGM use.
Our efforts at including multilevel stakeholder input provide an example of how to improve the likelihood of success and sustainability of health care delivery changes. Although an increase in CGM over the study period was to be expected, as is consistent with national trends, our rates of CGM prescription increases were faster than most clinic averages. This could reflect a low baseline of CGM prescription rates at the onset of transformations but, more importantly, suggests that the way in which we transformed the practice, focusing on co-created interventions from providers and patients, may have led to larger and longer-lasting gains over the 3 years. Of note, these increases were also achieved in a safety net hospital with a highly underresourced adult patient population, which poses additional burden on already stretched clinical staff. Moreover, our interventions did not introduce further disparity in the process, which is in contrast with prior studies focusing on improving technology use among children with type 1 diabetes that have shown widening disparities in CGM and insulin pump use with introduction of new clinic processes (26,27). Improving CGM prescriptions is a critical first step in eventually promoting CGM use. With sustained improvements in CGM prescribing as has been shown in our study, there is potential for clinical outcomes to improve over time with increased CGM use, reducing the risk of diabetes-related long-term complications in our underserved populations.
While health care interventions exist to increase CGM use among all populations with type 1 diabetes, few are targeted toward equity. Our interventions were designed to address gaps in the field, specifically, whether clinical transformations targeting equity can result in similar gains in CGM access across racial-ethnic groups and whether preliminary successes in pediatric clinics can be achieved in adult clinics. The Type 1 Diabetes Exchange Quality Improvement Collaborative (QIC) demonstrated a 21% increase in CGM use in adolescents and young adults with type 1 diabetes (ages 12–26 years) over 22 months after introducing CGM education for patients, staff awareness to promote CGM, state-level advocacy to improve insurance coverage, and improvement of clinical workflows (28). Similarly, there was a 13% improvement in pediatric insulin pump use among 12- to 26-year-olds after instituting provider pump education, integration of informed decision-making tools, and insurance approval workflows (20). The Pilot 4T study (Teamwork, Targets, Technology, and Tight Control in Newly Diagnosed Type 1 Diabetes), which included 135 youth with new-onset type 1 diabetes, showed that supplying CGM devices free of charge soon after diagnosis coupled with remote data review resulted in continued CGM use beyond the trial (29). Lastly, a study in a pediatric diabetes center in Alabama demonstrated a 12% decrease in inequity in CGM use between Black and White children over 13 months with interventions that included advocacy, provider education, and CGM trials (19). While such clinical interventions are laudable, it is unclear whether all race-ethnicities benefitted equally from these initiatives, especially in the earlier studies, and whether these largely pediatric clinic interventions could apply to adult care paradigms. In addition, insurance coverage issues and lack of patient access to technology when there was inadequate follow up in clinic likely affected racial/ethnic minority groups differentially, and could have introduced disparities inadvertently.
Our study, in contrast, was conducted in adults aged 18 years or older, and included multilevel stakeholders to design interventions targeted directly toward equity (11) to provide acceptable, feasible, and effective solutions to barriers that drive disparities in CGM use in adult care. Despite having limited resources and caring for a diverse population, the majority of whom are underserved with complex social and psychological needs, our practice transformations resulted in a fourfold increase in CGM prescription rates over 3 years. Importantly, these changes were not grant funded and, hence, were carried out through repurposing existing resources and leveraging outside support to enable and sustain these transformations over time. Our focus toward changing provider prescribing practices was initiated at the grassroots level using real-world diabetes clinicians. In addition, all interventions were developed with input from diabetes providers and, most importantly, based on feedback from our patient advisors, which likely enhanced sustainability and acceptability. We sought to address the root causes of inequities beyond managing or adjusting care for social determinants and socioeconomic status, to focus on barriers at the health care system and provider levels that have historically gone unnoticed. Several of these transformations are easily translatable to not only adult diabetes centers but also primary care settings with the potential to reduce disparities in CGM use in the real world on a larger scale. We do recognize that other centers in states outside of New York without universal CGM coverage likely have more difficulties with regard to access and authorization for devices, thereby limiting success. Advocacy for simplification of the insurance coverage criteria for CGM has been achieved in recent years in certain states and could be considered as a step in the transformation process (19).
Our study has several limitations. Due to limitations of EMR data, we were unable to collect data on real-time CGM use; thus, our prescription rates may overestimate actual CGM use. Nevertheless, we were interested in using practice transformations to first target prescribing behaviors, which was achieved in our study. The literature has demonstrated that there are multilevel barriers to obtaining and using CGM that span a somewhat chronological continuum, with one of the first steps including obtaining a prescription for CGM. At this proximal stage in the CGM process, provider bias has been shown to affect whether patients are informed of technology options and are ultimately prescribed technology (8,9,16,30). Focusing on equitable CGM prescribing will likely translate to improving CGM acceptance and sustainable use. Future initiatives will focus on modifying our data entry and collection methods to accurately capture patient use of CGM, to evaluate whether increased prescriptions translated to increased use. Another limitation of our study is that certain practice transformations were not uniformly disseminated to all of our clinic sites, due to staff shortages and limited resources. Nevertheless, success was seen at all sites despite variable penetrance of interventions. As an unintended benefit, since observing the success of these interventions at certain sites, our leadership and practice management have since decided to allocate extra resources to expand the practice transformations across the remaining sites. Another limitation is that new patient follow-up visits were severely affected during each coronavirus disease 2019 pandemic surge in New York, which could have negatively affected CGM prescription rates during those periods and overall.
Nevertheless, this study demonstrates that diabetes practice transformations can have powerful effects on provider prescribing behavior to promote equity in diabetes care. As a result of our interventions, CGM prescriptions for adults with type 1 diabetes increased dramatically and, more importantly, were equivalent across Hispanic and Black populations when compared with Whites. Use of multilevel stakeholders to enact clinic interventions as well as leveraging resources outside of the clinic obviated the need for grant funding and increased acceptability and sustainability of the transformation.
Our study emphasizes the importance of targeting barriers in the health care system to positively influence CGM prescribing behaviors in a system that does not itself usually facilitate change. In the larger scheme, reducing disparities in technology-prescribing patterns could positively impact sustainable access to technology, diabetes self-management, and quality of life, and eventually improve long-term outcomes in underserved populations with type 1 diabetes. Future studies will examine the impact of CGM prescriptions on actual use and the impact on longitudinal changes in HbA1C levels, hospitalizations, and psychosocial parameters. With the current wave of diversity, equity, and inclusion initiatives in the U.S. (31–33), there is great potential to translate our findings across different diabetes practices to improve equity in provider behaviors and care delivery among underserved populations with type 1 diabetes.
Article Information
Acknowledgments. The authors thank the diabetes providers, clinical/support staff, leadership (Y. Tomer, J. Crandall, E. Epstein), and the patient advisors at the Fleischer Institute for Diabetes and Metabolism at MMC, for their role in this study. They also thank the Type 1 Diabetes Exchange QI Collaborative for their efforts to help synthesize and catalyze transformation ideas.
Funding. Financial support for this study was provided by the National Institute of Diabetes and Digestive Kidney Diseases (5K23-DK115896, P30DK111022) for the senior author’s time and by the Type 1 Diabetes Exchange QI Collaborative and The Leona M. and Harry B. Helmsley Charitable Trust for the first author’s time.
None of the work described in the interventions was grant funded.
Duality of Interest. S.A. is a health disparities advisor to Medtronic, Inc. and Beta Bionics. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. P.M. and S.A. conceptualized, conducted, and analyzed the study and wrote and edited the manuscript. L.P.M. edited the manuscript. S.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 82nd Scientific Sessions of the American Diabetes Association, New Orleans, LA, 3–7 June 2022.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.20382990.
References
- 1. Beck RW, Hirsch IB, Laffel L, et al.; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 2009;32:1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care 2011;34:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pratley RE, Kanapka LG, Rickels MR, et al.; Wireless Innovation for Seniors With Diabetes Mellitus (WISDM) Study Group . Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA 2020;323:2397–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tamborlane WV, Beck RW, Bode BW, et al.; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 5. Ruedy KJ, Parkin CG, Riddlesworth TD; DIAMOND Study Group . Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: results from the DIAMOND trial. J Diabetes Sci Technol 2017;11:1138–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laffel LM, Kanapka LG, Beck RW, et al.; CGM Intervention in Teens and Young Adults with T1D (CITY) Study Group; CDE10 . Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA 2020;323:2388–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications 2015;29:572–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Agarwal S, Crespo-Ramos G, Long JA, Miller VA. “I didn’t really have a choice”: qualitative analysis of racial-ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diabetes Technol Ther 2021;23:616–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agarwal S, Kanapka LG, Raymond JK, et al. Racial-ethnic inequity in young adults with type 1 diabetes. J Clin Endocrinol Metab 2020;105:e2960–e2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Willi SM, Miller KM, DiMeglio LA, et al.; T1D Exchange Clinic Network . Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics 2015;135:424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McKergow E, Parkin L, Barson DJ, Sharples KJ, Wheeler BJ. Demographic and regional disparities in insulin pump utilization in a setting of universal funding: a New Zealand nationwide study. Acta Diabetol 2017;54:63–71 [DOI] [PubMed] [Google Scholar]
- 12. DeSalvo DJ, Noor N, Xie C, et al. Patient demographics and clinical outcomes among type 1 diabetes patients using continuous glucose monitors: data from T1D Exchange real-world observational study. J Diabetes Sci Technol. 9 October 2021 [Epub ahead of print]. DOI: 10.1177/19322968211049783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes in children and adults with type 1 diabetes: data from the T1D Exchange Quality Improvement Collaborative. Clin Diabetes 2021;39:278–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lipman TH, Smith JA, Patil O, Willi SM, Hawkes CP. Racial disparities in treatment and outcomes of children with type 1 diabetes. Pediatr Diabetes 2021;22:241–248 [DOI] [PubMed] [Google Scholar]
- 15. Lipman TH, Hawkes CP. Racial and socioeconomic disparities in pediatric type 1 diabetes: time for a paradigm shift in approach. Diabetes Care 2021;44:14–16 [DOI] [PubMed] [Google Scholar]
- 16. Agarwal S, Crespo-Ramos G, Leung SL, et al. Solutions to address inequity in diabetes technology use in type 1 diabetes: results from multidisciplinary stakeholder co-creation workshops. Diabetes Technol Ther 2022;24:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walker AF, Cuttriss N, Haller MJ, et al. Democratizing type 1 diabetes specialty care in the primary care setting to reduce health disparities: project extension for community healthcare outcomes (ECHO) T1D. BMJ Open Diabetes Res Care 2021;9:e002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mencher SR, Weinzimer SA, Nally LM, Van Name M, Nunez-Smith M, Sadler LS. Technology utilization in black adolescents with type 1 diabetes: exploring the decision-making process. Diabetes Technol Ther 2022;24:249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmitt J, Fogle K, Scott ML, Iyer P. Improving equitable access to continuous glucose monitors for Alabama’s children with type 1 diabetes: a quality improvement project. Diabetes Technol Ther 2022;24:481–491 [DOI] [PubMed] [Google Scholar]
- 20. Lyons SK, Ebekozien O, Garrity A, et al. Increasing insulin pump use among 12- to 26-year-olds with type 1 diabetes: results from the T1D Exchange Quality Improvement Collaborative. Clin Diabetes 2021;39:272–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. New York State Department of Health . Bronx County health indicators by race/ethnicity, 2016-2018. Accessed 1 April 2022. Available from https://www.health.ny.gov/statistics/community/minority/county/bronx.htm
- 22. United States Census Bureau . QuickFacts: Bronx County, New York. Accessed 10 April 2022. Available from https://www.census.gov/quickfacts/fact/table/bronxcountynewyork/PST045221
- 23. New York State Department of Health . Continuous glucose monitoring (CGM) systems for diabetes management, 2018. Accessed January 12, 2022. Available from https://www.health.ny.gov/health_care/medicaid/redesign/2018/2018-cgm_diabetes_mgmt.htm
- 24. New York State Department of Health . NYS Medicaid Preferred Diabetic Supply Program fact sheet, 2017. Accessed 12 January 2022. Available from https://newyork.fhsc.com/downloads/providers/NYRx_PDSP_fact_sheet.pdf
- 25. Office of Medicaid Management . Insulin pump therapy for people with diabetes, 2001. Accessed January 12, 2022. Available from https://www.health.ny.gov/health_care/medicaid/program/update/2001/nov2001.htm#:∼:text=Insulin
- 26. Lipman TH, Willi SM, Lai CW, Smith JA, Patil O, Hawkes CP. Insulin pump use in children with type 1 diabetes: over a decade of disparities. J Pediatr Nurs 2020;55:110–115 [DOI] [PubMed] [Google Scholar]
- 27. Lai CW, Lipman TH, Willi SM, Hawkes CP. Early racial/ethnic disparities in continuous glucose monitor use in pediatric type 1 diabetes. Diabetes Technol Ther 2021;23:763–767 [DOI] [PubMed] [Google Scholar]
- 28. Prahalad P, Ebekozien O, Alonso GT, et al. Multi-clinic quality improvement initiative increases continuous glucose monitoring use among adolescents and young adults with type 1 diabetes. Clin Diabetes 2021;39:264–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prahalad P, Ding VY, Zaharieva DP, et al. Teamwork, Targets, Technology, and Tight Control in Newly Diagnosed Type 1 Diabetes: the Pilot 4T study. J Clin Endocrinol Metab 2022;107:998–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Addala A, Hanes S, Naranjo D, Maahs DM, Hood KK. Provider implicit bias impacts pediatric type 1 diabetes technology recommendations in the United States: findings from the Gatekeeper Study. J Diabetes Sci Technol 2021;15:1027–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roberts BT, Rodgers GP. NIDDK initiatives addressing health disparities in chronic diseases. J Clin Invest 2020;130:5036–5038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thorlby R, Jorgensen S, Ayanian JZ, Sequist TD. Clinicians’ views of an intervention to reduce racial disparities in diabetes outcomes. J Natl Med Assoc 2011;103:968–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Golden SH, Joseph JJ, Hill-Briggs F. Casting a health equity lens on endocrinology and diabetes. J Clin Endocrinol Metab Inc. 2021;106:e1909–e1916 [DOI] [PubMed] [Google Scholar]